1. Introduction
Bacterial septicemia is widely prevalent in animals and characterized by severe illness and high mortality. The main symptoms of bacterial septicemia in fish are congestion at the base of the fins and gills; ascites; and swelling and bleeding in the liver, spleen, and kidney, which can lead to death (Qu, 2022). The peak incidence of bacterial septicemia is from June to September, when the water temperature is 25 ℃–32 ℃ and the infectivity rate is high, posing a great threat to freshwater fish aquaculture (Pękala-Safińska, 2018). The main pathogen responsible for bacterial septicemia is Aeromonas (Semwal et al., 2023), and the infection can be caused by a single or mixed species (Puneeth et al., 2022; Zepeda-Velázquez et al., 2015), with A.hydrophila, A. veronii, and A. sobria as the main pathogens. Aeromonas survives in diverse environmental conditions and possesses virulence factors that promote adhesion, colonization, and invasion of host cells. These virulence factors comprise membrane components, enzymes, and toxins and are differentially expressed among species, making some strains more virulent than others (Tomás, 2012). Aeromonas infection causes various disorders under conditions of high temperature, water pollution, and body injury, including systemic furunculosis in Salmonidae (Parker et al., 2011); ulcerative infections in catfish (Abdelhamed et al.,. 2017), carp ( Laltlanmawia et al., 2023), and wild-type zebrafish (Chandrarathna et al., 2018); and hemorrhagic sepsis in these species (Janda, 1991).
Esox lucius (order: Salmoniformes, family: Esocidae) are economically important carnivorous fish distributed in subcold water in the lakes and rivers of Asia, Europe, and North America (Larsson et al., 2015). A. salmonicida subsp. smithia were isolated and identified as the source of sepsis (manifested as skin and internal organ lesions) in fish farmed in Xinjiang and Xi’an (Gaoxue et al.,. 2006). The virulence gene detection in pathogenic bacteria isolated from the liver and kidney tissues of infected E. lucius in the areas surrounding Urumqi revealed dominant strains of pathogenic A. hydrophila (Qin et al.,. 2014). The above studies indicate that the range of bacterial pathogens responsible for causing septicemia in E. lucius is diverse, and different strains may exist in different regions.
This study was based on a previous epidemiological investigation of bacterial septicemia in E. lucius in ponds used for aquaculture, which showed that the disease was caused by various pathogens. Therefore, we used traditional pathogen isolation and identification methods combined with molecular biology techniques to identify the source of bacterial septicemia in the infected fish and determine its drug sensitivity profile. Furthermore, a rapid diagnostic method was established to identify the cause of bacterial septicemia and the presence of virulence genes. Our findings lay the foundation for diagnosing and controlling diseases of E. lucius in aquaculture.
2. Materials and Methods
2.1. Collection of Disease Materials
The bacterial septicaemia was suspected in 5 cultured fish ponds in Hutubi(86°N, 44°W) and Urumqi(87°N, 44°W), Xinjiang. A total of 20 E. lucius were collected, which 19 were diseased and 1 were healthy, with a body weight (158±66 g) and a body length (29.5±7.5 cm).
2.2. Main Reagents and Instruments
Brain–Heart Infusion (BHI) agar medium, Brain–Heart Infusion (BHI) broth medium, Rimler–Shotts (RS) medium, Aeromonas hydrophila Medium (AHM) and Gram stain were purchased from Qingdao Haibo Biotechnology. The bacterial genome extraction kit, mark D2000 was purchased from Beijing Tiangen Biochemical Technology; Biochemical microidentification tube and drug-sensitive paper were purchased from Hangzhou Binhe Microbial Reagent. 50×TEA electrophoresis buffer was purchased from Beijing Lanjieke Technology. agarose from Beijing Aoboxing Biotechnology. The 16s rDNA primers of
A. hydrophila,
A.veronii and
A.sobria,
A. hydrophila specific primers, virulence gene primers: Serine protease (
ahp), Hemolysin (
hly) and Aerolysin (
aer) were synthesized by Shanghai Bioengineering (
Table 1).
Main instruments and equipment: digital microscope and micro image acquisition and analysis processing system (MoticBA210); Purification table (Shanghai Boxun SW-CJ-2FD); PCR instrument (Hangzhou Bori TC-96); Electrophoresis apparatus (Beijing 61 DYY-11); Oakdam Water Quality Analyzer; Table high speed centrifuge (Shanghai Ebender MiniSpinplus); Biochemical incubator (Shanghai Qixin LRH-150); Electrophoresis tank (Beijing 61 DYCP-31DN); Ultraviolet analyzer (Shanghai Qinke ZF-2); Constant temperature culture oscillator (Shanghai ZWYR).
2.3. Test Methods
2.3.1. Water Quality Detection of Cultured Fish Ponds
The water quality of 5 fish ponds was tested, water samples were collected by water sampler, and 5 indexes including ammonia nitrogen, nitrite, pH, total alkalinity and total hardness were analyzed by the Oakdam Water Quality Analyzer and its description method. The water quality index of each fish pond was tested 3 times, and the average value was recorded. IBM® SPSS® Statistics, version 25.0., was used perform log (n+10) transformation of reference values and water quality data. Normal distribution was observed in reference values and water quality data, which the average value was calculated. The one-way analysis of variance were performed to test the differences between ammonia nitrogen, pH, total alkalinity, total hardness, nitrite of the five fish ponds and water quality reference values, with a confidence interval of 95%.
2.3.2. Bacterial Isolation and Identification
Gross changes in each organ were observed and recorded. Organs (liver, spleen, kidneys) with healthy and diseased fishes were quickly separated from the body and aseptically inoculated onto BHI agar medium, cultured at 28 ℃ for 24 h. Then, for purification, a single colony was inoculated onto BHI agar medium and cultured for 16−18 h at 28 °C. Next, a single colony was selected for Gram staining and observed under a microscope. The shape, color, size, and other characteristics of the colony were recorded. The morphological characteristics of the strains were observed. The purified strains were inoculated on RS medium, sheep blood agar medium and AHM, and cultured at 28 ℃ for 24 h.
2.3.3. Determination of Physicochemical Properties
The purified strains were inoculated onto 29 biochemical tubes for the identification of bacterial physicochemical properties, including the VogesProskauer (VP) test, the methyl red (MR) test, the oxidative-fermentative test, and so on,according to the instructions of the microbiochemical reaction tube, and the results were determined after culture at 37 ℃ for 24 h ~ 48 h.
2.3.4. Molecular Identification
The bacterial genome extraction kit was used to extract genomic DNA which was used as a template in all PCR reactions and 16s rDNA gene fragment was amplified. The PCR reaction system and conditions were shown in
Table 2. The PCR products were tested by 1.5% agarose gel electrophoresis.
2.3.5. Phylogenetic Tree Construction
Sequencing data returned, the NCBI web site to BLAST the sequence obtained sequence alignment (
https://blast.ncbi.nlm.nih.gov/Blast.cgi), screening and download sequence similarity was higher, use MEGA 11 software, The phylogenetic tree was constructed using the Maximum Likelihood method.
2.3.6. Pathogen Virulence Gene Detection
Three virulence genes,
ahp,
hly and
aer, were amplified by PCR. The reaction system and reaction conditions were shown in
Table 3. The PCR products were tested by 1.5% agarose gel electrophoresis.
2.3.7. Rapid Multiplex PCR Building of Pathogenic Aeromonas hydrophila
The specific primers of
A. hydrophila (
Ah) (Wang
et al.,. 2023) and three virulence gene primers (
ahp,
hly,
aer) were selected to establish a multiplex PCR rapid detection method.
A. hydrophila (
Ah) of the reaction system and reaction conditions were shown in
Table 4 (with alike
ahp, hly). Based on
Ah, ahp, hly, aer of the monoplex PCR results, multiplex PCR assays were further optimized where various parameters particularly concentrations of primers (0.3 to 0.9 μM), optimization of extension time (30 s to 2 min), annealing temperature (57 °C to 60 ℃) (
Table 4). The PCR products were tested by 1.5% agarose gel electrophoresis.
2.3.8. Drug Sensitivity Test
Antimicrobial susceptibility testing of the isolates were performed by Kirby–Bauer disk diffusion method. 16 types of antibiotics tested were flufenicol (30 µg/disk), ciprofloxacin (5 µg/disk), doxycycline (30 µg/disk), minocycline (30 µg/disk), penicillin (10 µg/disk), neomycin (30 µg/disk), enrofloxacin (10 µg/disk), kanamycin (30 µg/disk), tetracycline (30 µg/disk), levofloxacin (5 µg/disk), gentamicin (10±2.5 µg/disk), midecamycin (30 µg/disk), compound sulfamethoxazole (23.75 SMZ/1.25 TMP µg/disk), chloramphenicol (30 µg/disk), cephalothin (30 µg/disk), Vancomycin (30 µg/disk). The test strains were adjusted into 109 colony forming unit (CFU) using physiological saline. The suspensions were swabbed on BHI agar using sterile cotton bud. The inoculated media was left for 10 min before antibiotic discs were placed onto the agar surface. After 24 h of incubation period, diameter of the inhibition zone was measured in millimeter (mm) using a ruler and results were interpreted with reference to the standard provided by Clinical Laboratory Standards Institute.
3. Results and Analysis
3.1. Water Quality Statistical Analysis of Esox lucius Fish Culture
We analyzed the water quality of five fish ponds used for
E. lucius aquaculture in Hutubi and Urumqi (China). The ammonia nitrogen concentration in fish ponds 1 and 5 was significantly higher than the reference value (p<0.05). The pH and the total alkalinity concentration of fish pond 1, fish pond 3, fish pond 4 was significantly higher than the reference value (p<0.05). The total hardness concentration of fish pond 1, fish pond 3, fish pond 4 and fish pond 5 was significantly higher than the reference value (p<0.05). There was no significant difference between the nitrite concentration and the reference value in the five fish ponds (p<0.05)(
Table 5).
3.2. Preliminary Clinical Diagnosis of Suspected Bacterial Septicemia in E. lucius
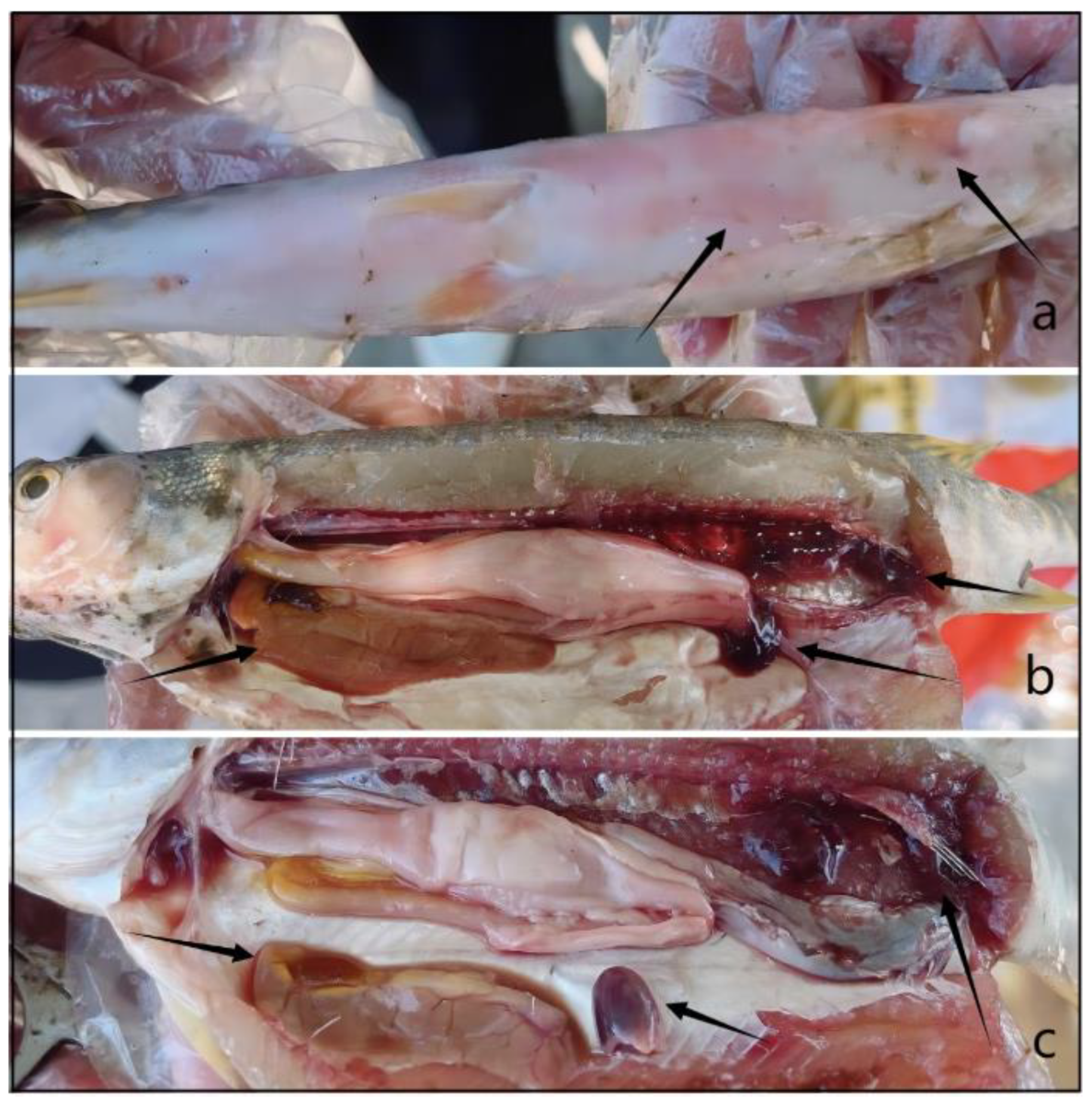
The onset of bacterial septicemia generally occurred in the summer (July–August), and the infection rate was ~30%. At the beginning of the disease, the fish swam slowly, often moved around the water outlet, and were lethargic. The mortality rate at the peak of the disease was high, showing a trend toward acute death. Microscopic examination of the body surface, gills, and intestines of the dead infected fish did not reveal the presence of parasites. Local skin and muscle tissue hyperemia and bleeding on the body surface were observed and were most serious at the edges of the gill covers, the base of the fins, and the abdomen (
Figure 1a). Dissection revealed a small amount of light yellow ascites in the abdominal cavity, an enlarged, earth-yellow liver (
Figure 1b), hemorrhagic spots on some liver surfaces, and a black–purple spleen (
Figure 1c). Bacterial septicemia was diagnosed based on the clinical symptoms and pathological changes.
3.3. Isolation and Morphological Identification
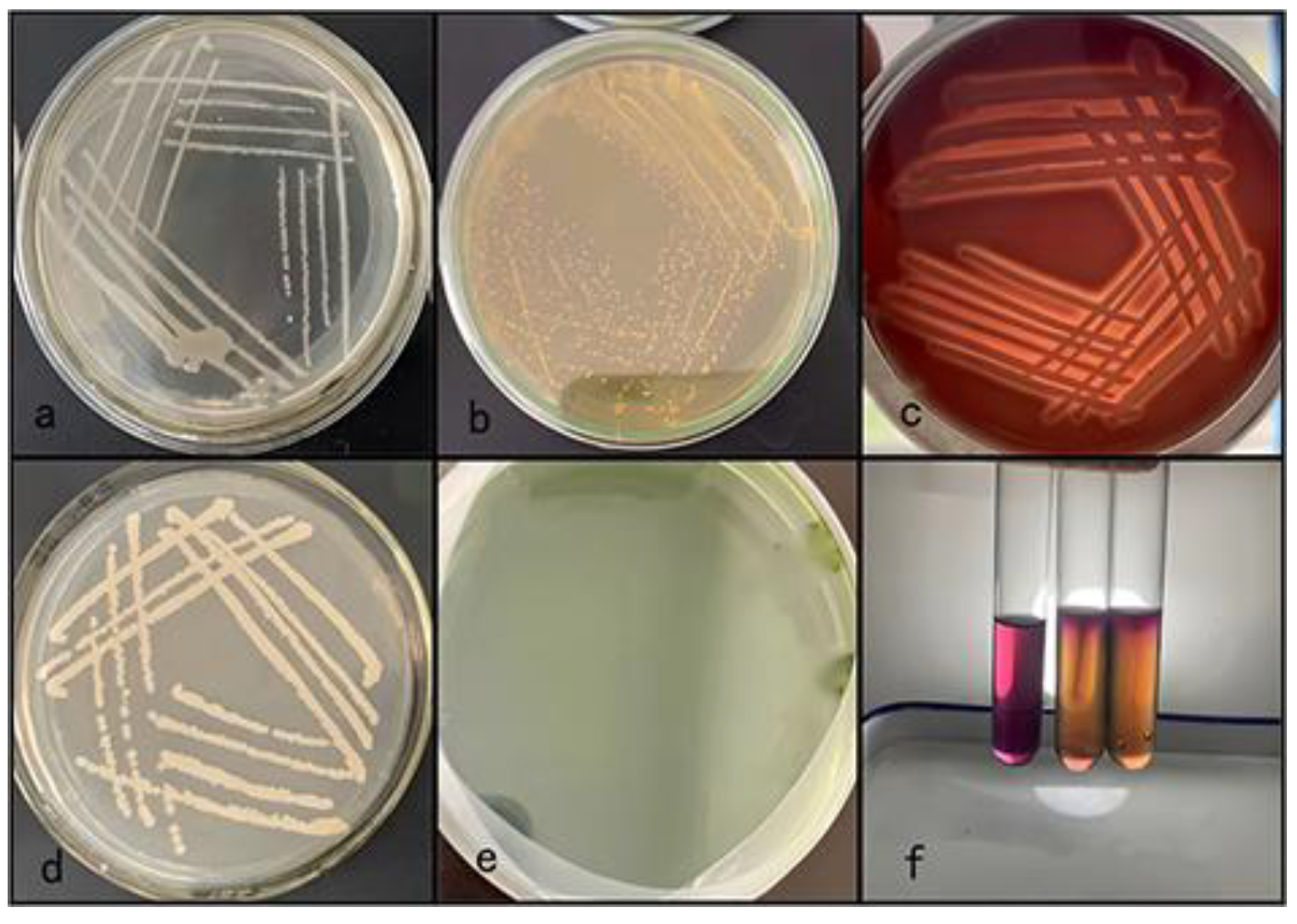
Five dominant strains were isolated from the liver, spleen, and kidney tissues of dead infected fish. Culture on BHI agar revealed round colonies with moist, smooth, gray and white translucent surfaces. The colony center was raised, and the edge was smooth. The colony diameter was 1.0–3.0 mm after 24 h (
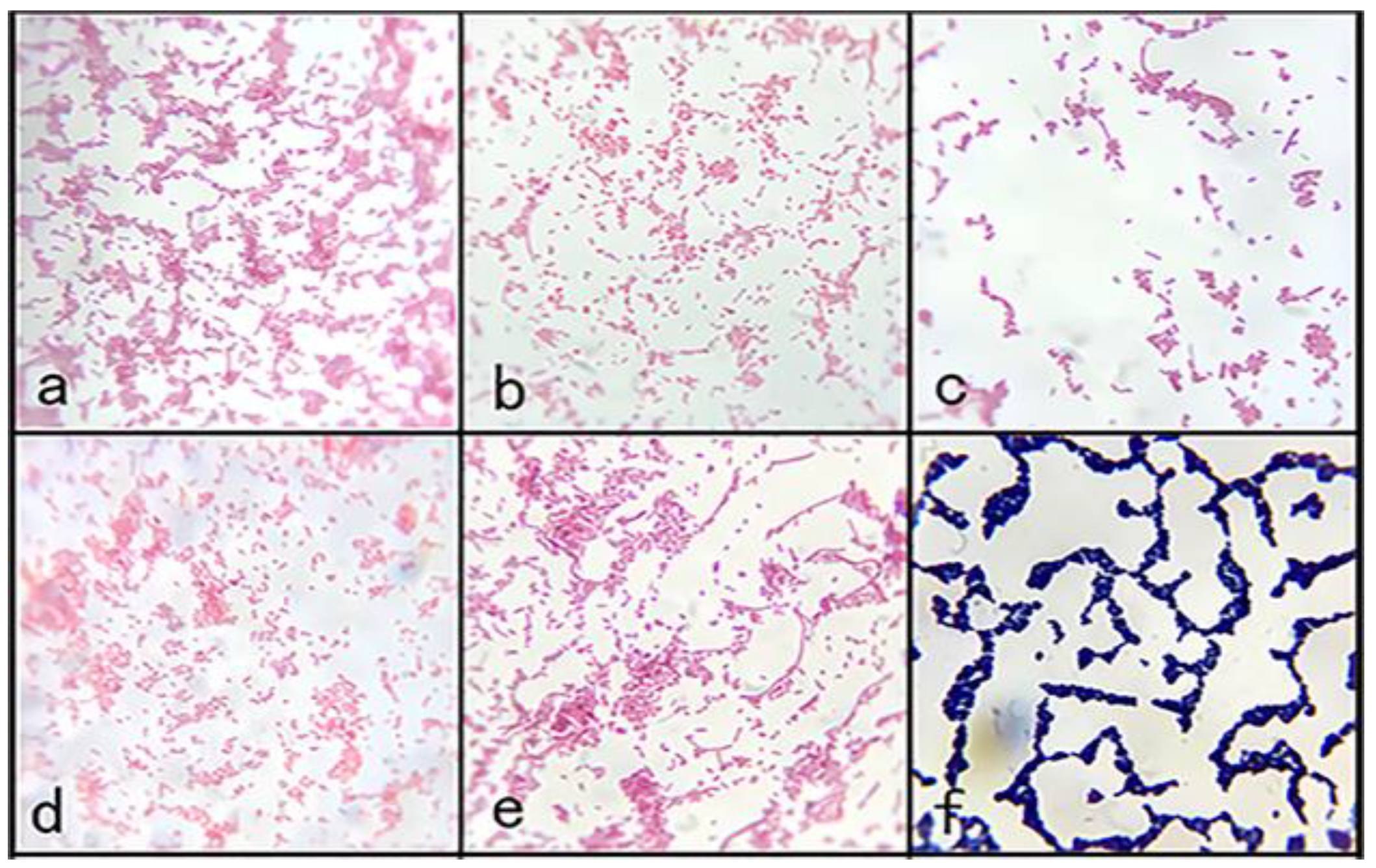
Figure 2a). Staining revealed gram-negative bacteria with blunt round ends, some of which were clustered into chains, consistent with the morphological characteristics of
Aeromonas (
Figure 3). On the RS medium, the colonies of the five strains were round and smooth with a central bulge and were yellow and opaque in color. The colony diameter was 0.8–1.0 mm after 24 h (
Figure 2b). The five strains were preliminarily identified as
Aeromonas. The colonies grew well on sheep blood agar and were grayish white, showing typical β-hemolysis. The diameter of the colonies was 1–3 mm after 24 h (
Figure 2c). Strain 1 was stabbed in AHM. The top of the semisolid medium was purple, the bottom was light yellow, and the growth along the puncture line showed a diffuse brush-like appearance (
Figure 2f). Thus, Five strains were preliminarily identified as
Aeromonas, among which strain 1 was
A. hydrophila. Dominant strains were isolated from the visceral tissue of healthy fish. Culture on BHI agar revealed round colonies with moist, rough, pale yellow translucent surfaces. The colony center was raised, and the edge was smooth. The colony diameter was 1.0–4.0 mm after 24 h (
Figure 2d). No colony growth observed on the RS medium(
Figure 2e).
3.4. Physiological and Biochemical Identification
The five strains were identified using 20 physiological and biochemical tests. According to the established specifications (Fang
et al., 2010), the results of strain 1 were consistent with
A. hydrophila, and those of strain 3 were consistent with
A. sobria (esculin-negative, H
2S-negative). The results of strains 2, 4, and 5 were consistent with
A. veronii, with strain 4 positive for phenylalanine and strains 2 and 5 negative for H
2S and positive for ornithine and phenylalanine (
Table 6). Based on these biochemical results, the five strains were preliminarily identified as follows: strain 1,
A. hydrophila; strain 3,
A. sobria; and strains 2, 4, and 5,
A. veronii.
3.5. Molecular Identification and Sequence Analysis
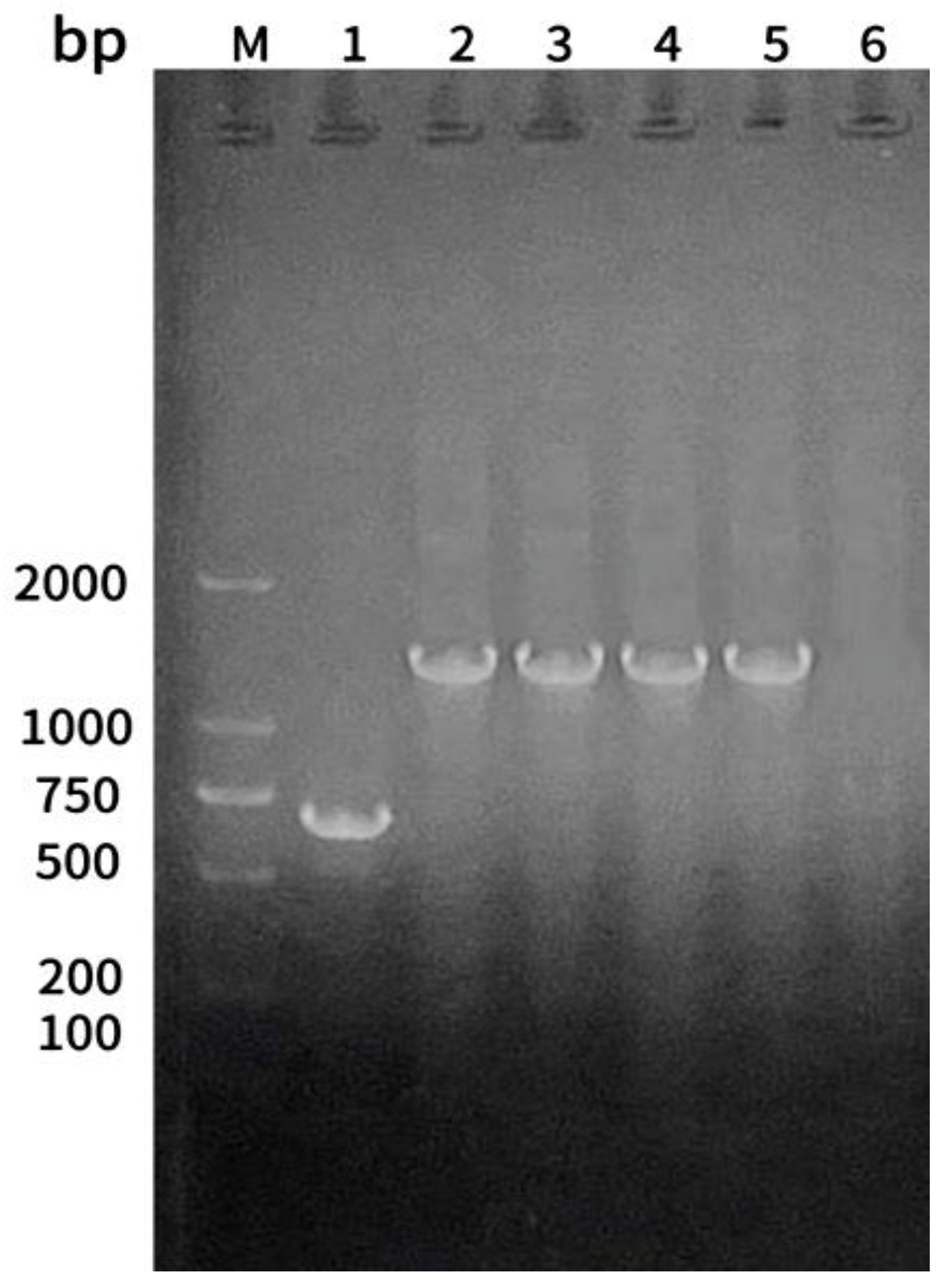
The 16S RNA universal primers of
A. hydrophila,
A. sobria, and
A. veronii were used for polymerase chain reaction (PCR) amplification using the DNA of five strains as the template, and the amplified products were analyzed by 1.5% agarose gel electrophoresis. Strains 1 had a single band at 685 bp, and strains 2, 4, and 5 had a single band at 1,500 bp. Strain 3 consisted of a single band at 1,430 bp, consistent with the expected size (
Figure 4).
The PCR amplicons were sequenced and compared with those in the NCBI database using BLAST (
https://blast.ncbi.nlm.nih.gov/Blast.cgi). The isolate sequences exhibited high homology (>99.63 %) with the reference strains of
A. hydrophila,
A. veronii, and
A. sobria in GenBank. We used MEGA 11.0to conduct multiple sequence alignment and for the construction of a phylogenetic tree using the maximum likelihood method. The five isolates were named JZ1–JZ5. JZ1 was closely related to
A. hydrophila (MK426644.1, MG984624.1, and MT384379.1). JZ2, JZ4, and JZ5 clustered together on the same branch and were closely related to
A. veronii (KY767505.1 and OR687219.1), forming a clade. JZ3 was closely related to
A. sobria (MN216284.1 and MF185225.1, KY683201.1) and clustered to form a clade. All isolates were closely related to
A. salmonicida subsp.
smithia (KC244782.1 and AB027544.2),
Edwardsiella piscicida (MN203719.1),
Yersinia ruckeri (JQ657818.1),
Plesiomonas shigelloides (BY160513), and
Vibrio cholerae (KJ725364.1) and were grouped into different clades. Considering these along with the above experimental results, the isolated strains were confirmed as
A. hydrophila,
A. veronii, and
A. sobria (
Figure 5).
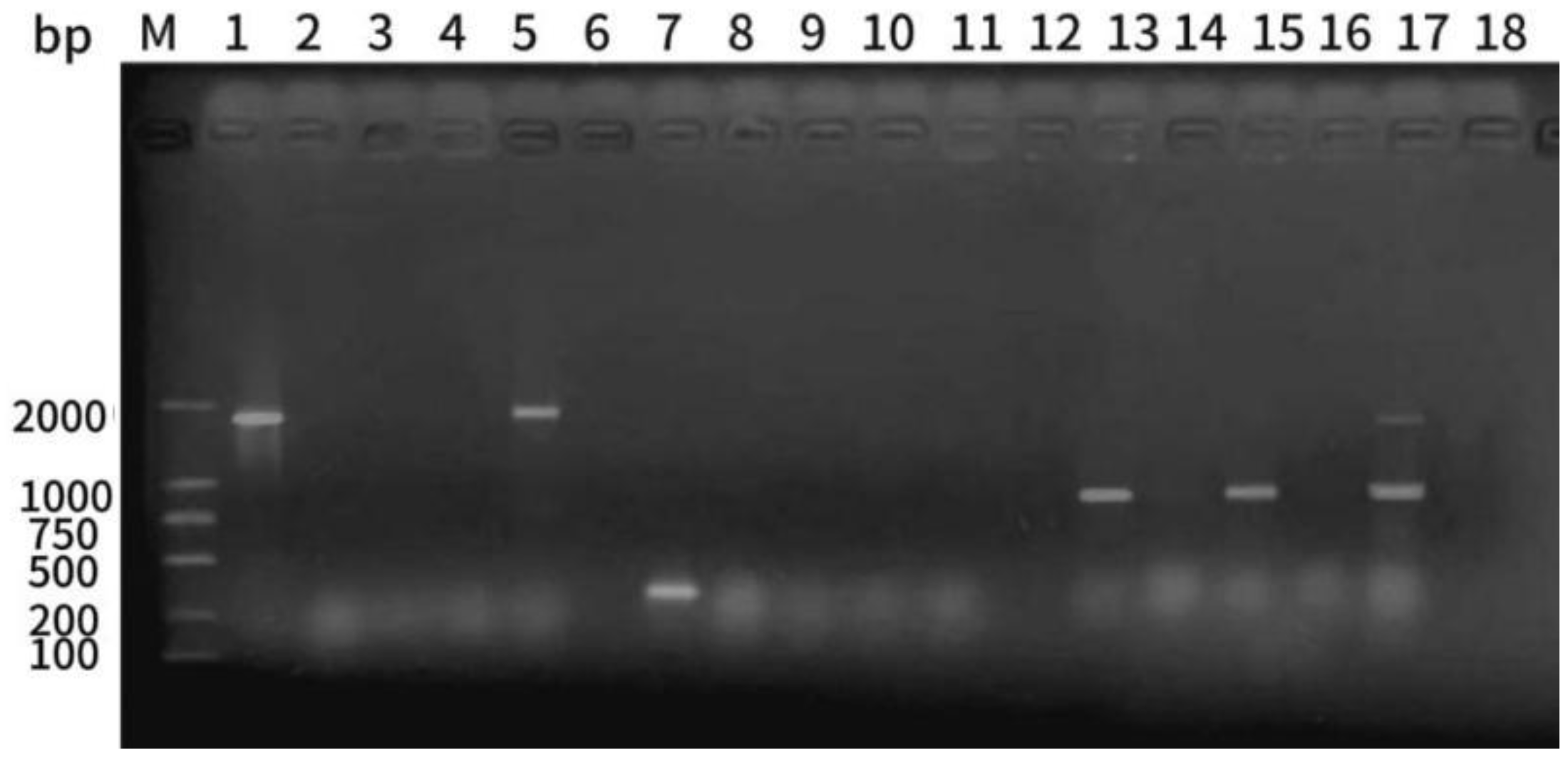
3.6. Detection of Virulence Genes in the Isolated Pathogenic Bacteria
The five isolated strains underwent PCR amplification to determine the presence of the virulence genes
hly,
aer, and
ahp. Electrophoresis analysis revealed that strain 1 harbored
hly,
aer, and
ahp, as evidenced by the bands of 1,800, 309, and 870 bp. Strain 2 harbored
hly,
aer, and
ahp virulence genes at 1,800 bp. Strain 3 harbored the
hly virulence gene, and the bands at 870 bp were consistent with the target bands. Strain 5 harbored the
ahp virulence gene, and the bands at 1,800 and 870 bp were consistent with the target bands, and the two virulence genes were
hly and
ahp (
Figure 6).
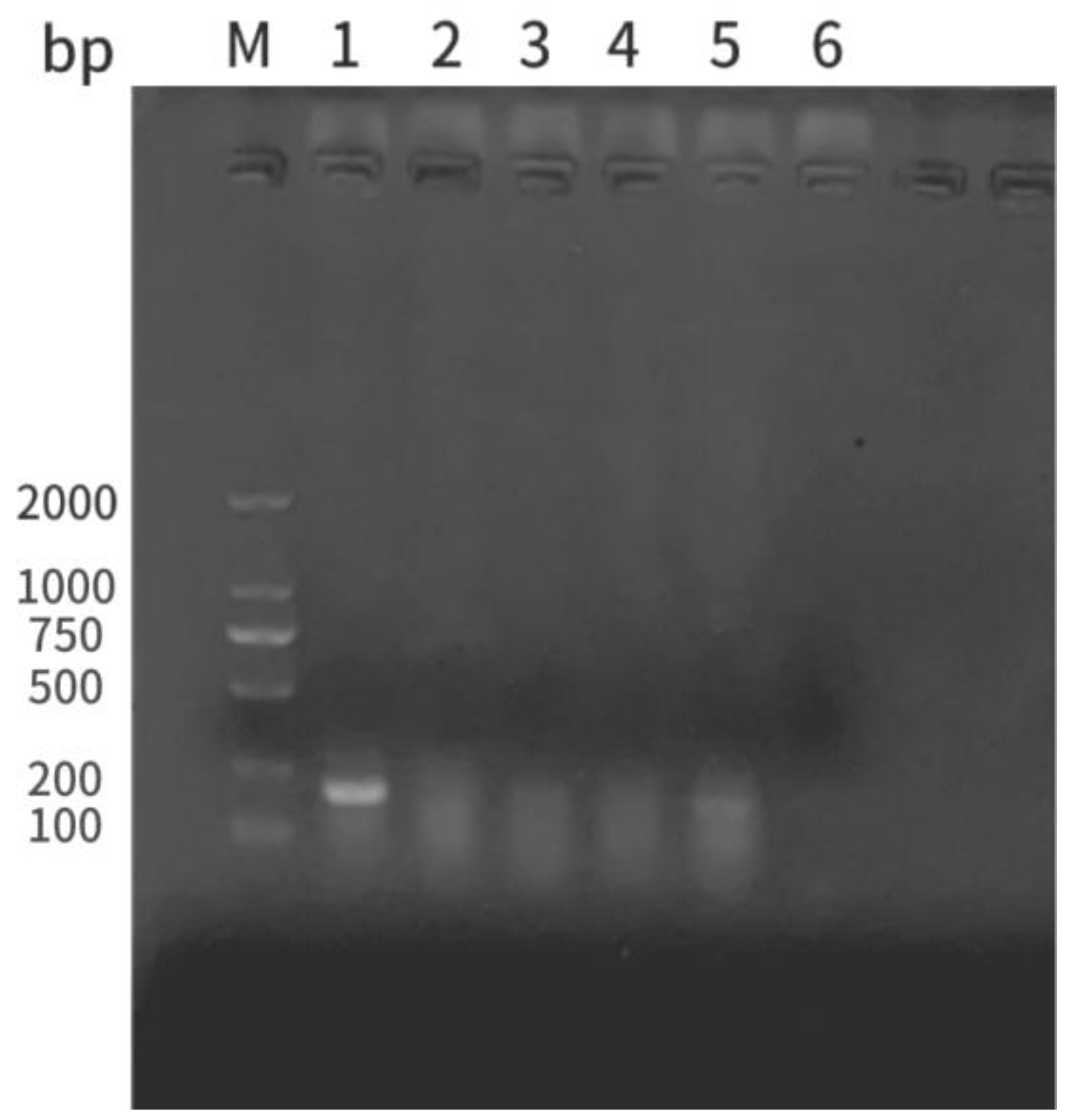
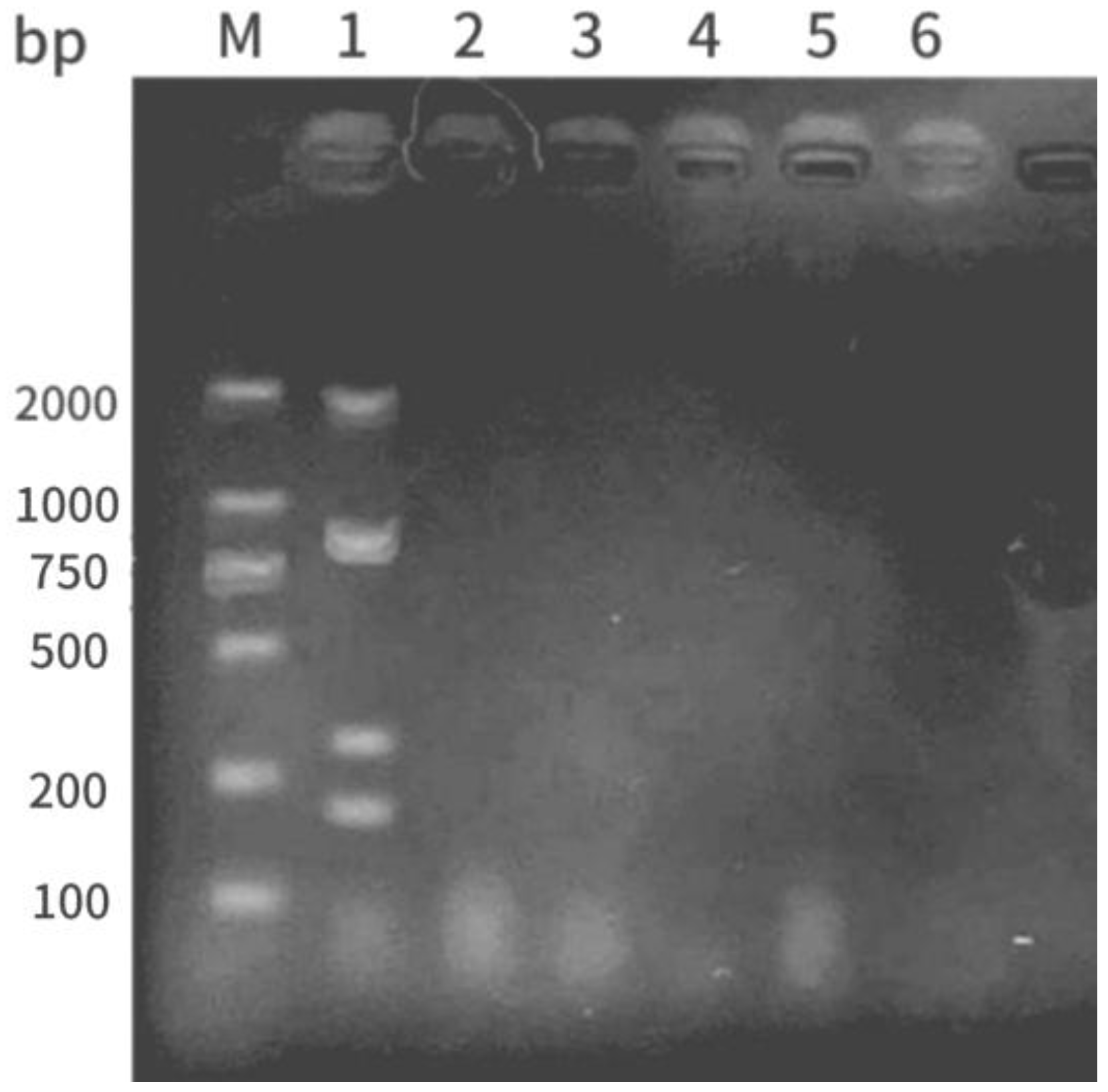
3.7. Establishment of Multiple PCR Rapid Detection Method
The five isolated strains were amplified via PCR using an
A. hydrophila-specific primer (
Ah). The resultant bands of strain 1 were consistent with the target band (202 bp), whereas those of the other four strains were not. Therefore, the Ah primer specifically identified
A. hydrophila (
Figure 7). To establish a multiplex PCR rapid detection method for pathogenic
A. hydrophila, four primers, including
Ah and one each specifically targeting
hly,
aer, and
ahp virulence genes, were used. The annealing temperature, extension time, and primer concentration was optimized. Amplification was optimal at an annealing temperature of 57℃, extension time of 2 min, and primer concentrations of 0.5 μM (
Figure 8). The multiplex PCR results revealed that only strain 1 had bands consistent with the target sizes for
Ah,
hly,
aer, and
ahp at 1,800, 870, 309, and 202 bp, respectively (
Figure 9), indicating that strain 1 was the pathogenic
A. hydrophila strain.
3.8. Drug Sensitivity Outcomes of the Isolated Pathogenic Bacteria
We used the Kirby–Bauer disk diffusion method to determine the sensitivity of the isolated pathogenic strains to 16 antibiotics. According to the inhibition zone diameter, strain 1 was resistant to medemycin and cotrimoxazole but sensitive to cephalothiophene. Strains 2, 4, and 5 were resistant to cotrimoxazole and vancomycin but sensitive to medemycin and cephalothiophene. Strain 3 was resistant to cephalosporin but sensitive to vancomycin. All five strains were resistant to penicillin and neomycin but sensitive to flufenicol, ciprofloxacin, doxycycline, minocycline, enrofloxacin, kanamycin, tetracycline, levofloxacin, gentamicin, and chloramphenicol (
Table 7).
4. Discussion
4.1. Correlation between the Incidence of Bacterial Septicemia and Water Quality
The main etiologies of bacterial septicemia include pathogenic microorganisms, deterioration in environmental water quality, and secondary parasitic infection. Severe water pollution is one of the main causes of bacterial septicemia in fish. When water temperature exceeds 25 ℃, the levels of toxic pollutants, such as ammonia nitrogen, nitrite, and hydrogen sulfide, increase, considerably changing the physical and chemical composition of the water for a long time and increasing the likelihood of sepsis outbreak. When the water temperature drops, the disease resolves (Abu-Elala, 2016). In this study, we analyzed the water quality of five fish ponds used for the aquaculture of Esox lucius. Sepsis outbreak occurred in the summer season (June–September) when the water temperature was 25 ℃–32 ℃.The ammonia nitrogen concentration in some of the fish ponds were much higher than normal, possibly due to the high water temperature and large amount of ammonia nitrogen produced by Esox lucius through excrement or in leftover bait (Sriyasak et al., 2015). Excessive ammonia nitrogen reduces the tolerance of cultivated fish to adverse environmental conditions and diseases; this is the main factor inducing diseases. Furthermore, the pH, total alkalinity, and total hardness concentration of most fish ponds are relatively high. Moreover, the total hardness is related to the regional environment, water source, and the service life of fish ponds (Saglam et al., 2013). If the water hardness is too high, it can deteriorate water quality, affect the osmotic pressure regulation of fish, and inhibit fish growth (Dias et al., 2012).If the water total alkalinity is high, the water pH becomes unstable and affects the activities of microorganisms and plankton in it, also leading to insufficient oxygen in severe cases (Rosenfeld et al., 2022). As a cold-water fish, the most suitable temperature for E. lucius is 18 ℃–23 ℃. In the high-temperature season, attention should be paid to maintaining a suitable water temperature, keeping the water clean, and maintaining water quality indicators as per the freshwater aquaculture standards. The required standards for E. lucius aquaculture can be maintained by changing the water, adding oxygen aeration devices, and using organic fertilizers and water softeners (Hu et al.,. 2010).
4.2. Analysis of Pathogenic Bacterial Infections in Bacterial Septicemia
Bacterial septicemia can be caused by a single bacterial species or a combination of species. Septicemia in Esox lucius is caused by a single pathogen, such as A. salmonicida subsp. Smithia (Vincent et al.,. 2017) or A. hydrophila (Lee et al., 2017). However, this study identified a mixed infection of A. hydrophila, A. veronii, and A. sobria, which differed from the above reports. Tests for bacterial virulence revealed that A. hydrophila harbored three virulence genes, ahp, aer, and hly, which are similar to the virulence genes contained in A. hydrophila and more pathogenic than A. vickerii and A. sobria. Although the main pathogen causing bacterial septicemia in freshwater fish is A. hydrophila, other pathogens may be involved, such as A. veronii (Legario et al., 2023), E. ictalurii (Chen et al., 2020), and A. jandaei (Mazumder et al., 2021). Mixed pathogenic infections have been reported in other cultivated fish; e.g., outbreak of bacterial septicemia in Indian carp caused by mixed infection by A. hydrophila and Pseudomonas aeruginosa (Laltlanmawia et al., 2023) and in wild-type zebrafish caused by A. hydrophila and A. veronii, with A. hydrophila being more pathogenic than A. veronii (Chandrarathna et al., 2018).A. hydrophila, A. jandaei, A. dhakensis isolated from freshwater ornamental fish showing signs of septicaemia (Jagoda et al., 2014). Ours is the first study to report bacterial septicemia in Esox lucius caused by mixed infection with three Aeromonas species. Thus, bacterial septicemia is more complicated than initially presumed and may depend on the interaction between the culture environment, the fish itself, and bacterial characteristics, such as virulence genes.
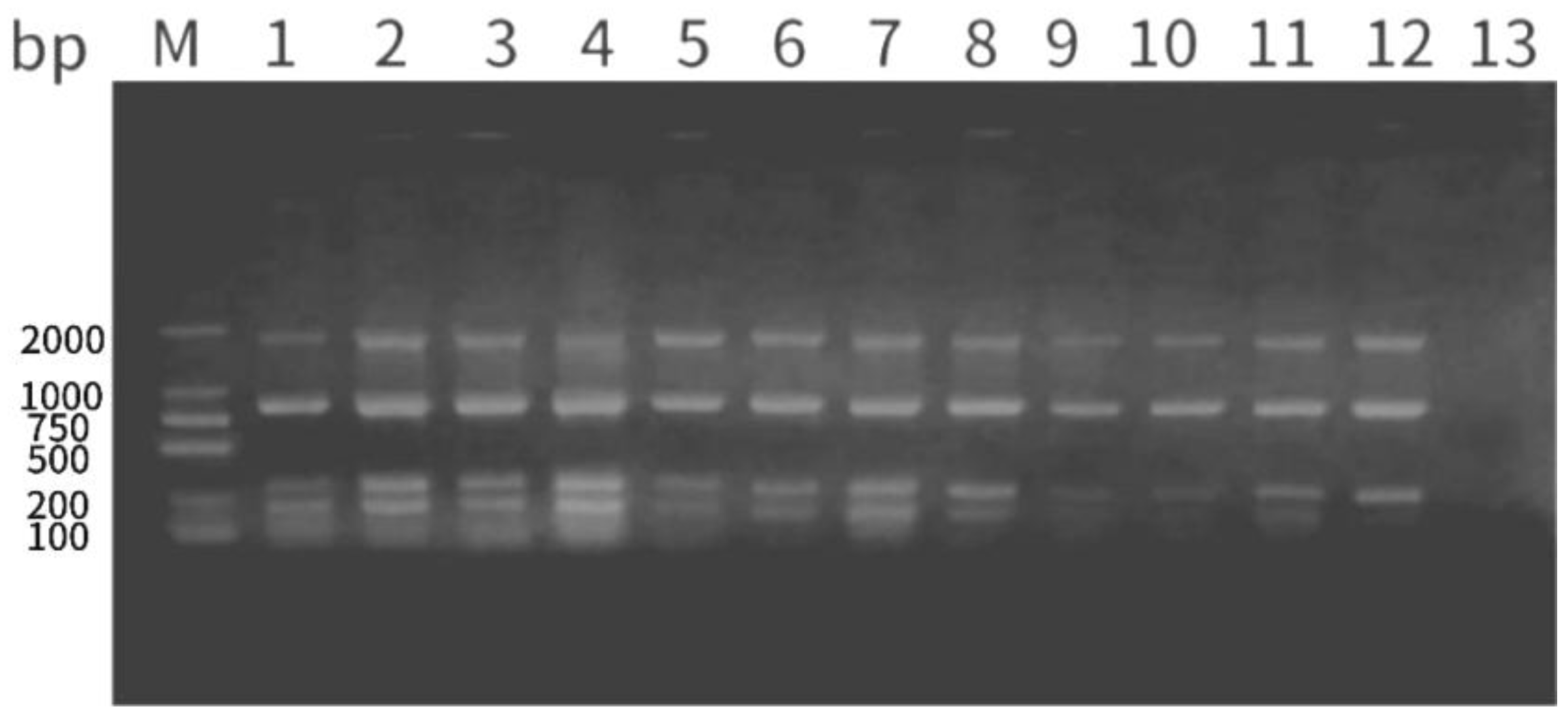
4.3. Analysis of Multiplex PCR Rapid Detection Method
Multiplex PCR, or composite PCR, simultaneously amplifies multiple target genes by adding more than two primers to the same reaction system (Zaher et al.,. 2021).In its practical application, the efficiency of target gene detection is improved while maintaining the detection accuracy, particularly in the identification of mixed infections(Heymans et al., 2018). For example, multiplex PCR was used to detect Arcobacter faecis and A. Lanthieri's specific primers and independent genes are assessed for specificity and sensitivity. (Zambri et al., 2019). A multiplex PCR method was established to detect A. hydrophila and its associated virulence genes; it was found to be more sensitive and practical than traditional bacterial isolation and identification(Zaher et al.,. 2021).Additionally, a multiplex PCR method was established to detect three pathogens of culture fisheries (Onuk et al.,. 2010). In our study, the use of primers specific for A. hydrophila in conventional PCR revealed the presence of the target sequence in strain 1, with no bands being detected in the other four strains. Thus, strain 1 was identified as A. hydrophila. We then established a multiplex PCR detection method using primers targeting the 16S rRNA gene and the virulence factor genes ahp, aer, and hly in the same PCR reaction to identify whether the strain under test was pathogenic A. hydrophila. Primer design and optimization of experimental conditions are crucial to establishing multiplex PCR assays. In our study, the single PCR amplification reaction used to detect A. hydrophila involveda primer annealing temperature of 59 ℃ and an extension time of 1 min. The four primers were optimized using concentrations of 0.3, 0.5, 0.7, and 0.9 μL at annealing temperatures of 57 ℃, 59 ℃, and 60 ℃ and extension times of 2 min, 1 min, and 30 s, respectively. At an annealing temperature of 57 ℃, elongation time of 2 min, and primer concentration of 0.5 μL, the target bands were amplified clearly. Therefore, the multiplex PCR established in this study is suitable for rapidly detecting pathogenic A. hydrophila, leading to the early administration of appropriate antibiotics and effectively reducing associated economic losses.
4.4. Analysis of Drug Prevention and Treatment of Bacterial Septicemia
The main methods for preventing and treating bacterial septicemia include using antibiotics (Cízek et al., 2010), bacteriophages (Dang et al., 2021), and vaccine (Sun et al.,. 2019) .Antibiotics are widely used for the prevention and control of various bacterial diseases in aquaculture (Moser et al., 2019). To avoid drug resistance, the Kirby–Bauer paper disk diffusion method is often used to determine the sensitivity of isolates to specific antibiotics. At present, China has approved the use of 13 antibiotics for aquatic products: sulfoxamycin powder, flufenicol powder, flufenicol injection, flumequine powder, enrofloxacin powder (for aquatic use), doxycycline hydrochloride powder (for aquatic use), ciprofloxacin hydrochloride premix, ciprofloxacin hydrochloride premix, neomycin sulfate powder (for aquatic use), sulfamethoxine sodium powder (for aquatic use), compound sulfadiazine powder (for water production), compound sulfadiazine powder (for aquatic use), and compound sulfamethoxazole powder (for aquatic use) (Zhang, 2023).
In our study, we selected 16 antibiotics for sensitivity testing against three strains. All three Aeromonas strains were sensitive to flufenicol, ciprofloxacin, doxycycline, minocycline, enrofloxacin, kanamycin, tetracycline, levofloxacin, gentamicin, and chloramphenicol but resistant to penicillin and neomycin. The A. hydrophila strain was resistant to medemycin and cotrimoxazole and sensitive to cephalothiophene, which differed from the reported sensitivity of A. hydrophila to penicillin (Thaotumpitak et al.,. 2023), indicating that the strain isolated in this study had developed penicillin resistance. The A. veronii strain was resistant to cotrimoxazole and vancomycin and sensitive to medemycin and cephalothiophene, similar to the reported sensitivity of A. veronii (Fauzi et al.,. 2021; De Silva et al.,. 2019).The A. sobria strain was resistant to cephalothiophene and sensitive to vancomycin, similar to the reported drug sensitivity of A. sobria (Yilmaz et al.,. 2018). Aquaculture drug use is guided by green development with infection prevention as the main objective. In some cases, prevention and control are combined, and green prevention and control of aquaculture diseases and standardized drug use are advocated. Therefore, to achieve precision medicine, we chose to scientifically select drugs using sensitivity testing to avoid drug misuse and abuse. Such a technique not only reduces drug dosage and improves fish quality but also provides a source of references to achieve therapeutic effects.
5. Conclusions
In this study, we isolated five strains from the liver, spleen, and kidney of dead E. lucius. One strain was identified as A. hydrophila, three as A. veronii, and one as A. sobria. We also established a multiplex PCR method to rapidly detect bacterial septicemia strains and virulence genes, which were found to be sensitive to flufenicol, ciprofloxacin, and doxycycline based on the drug sensitivity tests. Therefore, this study provides a theoretical basis for realizing early prediction and warning and effective prevention and control of bacterial septicemia in fish aquaculture.
Author Contributions
Xiaofei Yan: conceived and designed the experiments, performed the experiments, wrote the paper. Yiyang Zhao: conceived and designed the experiments, performed the experiments; analyzed the data, wrote the paper. Tao Wang: analyzed the data, contributed reagents/materials/analysis tools. Yuqi Wang: analyzed the data, contributed reagents/materials/analysis tools. Xinyu Cao: contributed reagents/materials/analysis tools.
Funding
This work was sponsored by the Basic research funds for colleges and universities in Xinjiang Autonomous Region from Department of Education, Xinjiang Autonomous Region (No. XJEDU2022 Z003).
Ethical Considerations
The care and use of experimental animals complied with Xinjiang Agricultural University (Xinjiang, China) animal welfare laws, guidelines and policies as approved by the Animal Ethics Committee of Xinjiang Agricultural University (Xinjiang, China) with the ethical code: 2023027.
Consent to participate and Consent for publication
All authors read and approved the final manuscript, and consent for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdelhamed H, Ibrahim I, Baumgartner W, Lawrence ML, Karsi A. (2017). Characterization of Histopathological and Ultrastructural Changes in Channel Catfish Experimentally Infected with Virulent Aeromonas hydrophila. ( 8, 1519. [CrossRef]
- Abu-Elala NM, Abd-Elsalam RM, Marouf S, Abdelaziz M, Moustafa M. (2016)Eutrophication, Ammonia Intoxication, and Infectious Diseases: Interdisciplinary Factors of Mass Mortalities in Cultured Nile Tilapia. Journal of aquatic animal health, 28, 187-198. [CrossRef]
- 3. Buján N, Toranzo AE, Magariños B. (2018). Edwardsiella piscicida: a significant bacterial pathogen of cultured fish. Dis Aquat Organ. [CrossRef]
- 4. Chandrarathna HPSU, Nikapitiya C, Dananjaya SHS, Wijerathne CUB, Wimalasena SHMP, Kwun HJ, Heo GJ, Lee J, De Zoysa M. (2018). Outcome of co-infection with opportunistic and multidrug resistant Aeromonas hydrophila and A. veronii in zebrafish: Identification, characterization, pathogenicity and immune responses. Fish Shellfish Immunol. [CrossRef]
- Chen H, Yuan G, Su J, Liu X. (2020). Hematological and immune genes responses in yellow catfish (Pelteobagrus fulvidraco) with septicemia induced by Edwardsiella ictaluri. ( 97, 531–539. [CrossRef] [PubMed]
- Cízek A, Dolejská M, Sochorová R, Strachotová K, Piacková V, Veselý T. (2010). Antimicrobial resistance and its genetic determinants in aeromonads isolated in ornamental (koi) carp (Cyprinus carpio koi) and common carp (Cyprinus carpio). ( 142, 435–439. [CrossRef]
- Chen, M. (2009). The study on the characteristics of Aeromonas sobria of pelteobagrus fulvidraco and the cloning and expression of its hemolysin gene. Southwest University.
- Dang THO, Xuan TT, Duyen LT, Le NP, Hoang HA. (2021). Protective efficacy of phage PVN02 against haemorrhagic septicaemia in striped catfish Pangasianodon hypophthalmus via oral administration. ( 44, 1255–1263. [CrossRef] [PubMed]
- 9. De Silva BCJ, Hossain S, Dahanayake PS, Heo GJ. (2019). Aeromonas spp. from marketed Yesso scallop (Patinopecten yessoensis): molecular characterization, phylogenetic analysis, virulence properties and antimicrobial susceptibility. Journal of Applied Microbiology. [CrossRef]
- 10. Dias JD, Simões NR, Bonecker CC. (2012). Zooplankton community resilience and aquatic environmental stability on aquaculture practices: a study using net cages. Brazilian Journal of Biology. [CrossRef]
- Fang H, Chen CZ, Zhang XJ. (2010) Aquacultural animal pathogenic bacteriology. Beijing: China Agricultural Press.
- Fauzi NNFNM, Hamdan RH, Mohamed M, Ismail A, Mat Zin AA, Mohamad NFA. (2021). Prevalence, antibiotic susceptibility, and presence of drug resistance genes in Aeromonas spp. isolated from freshwater fish in Kelantan and Terengganu states, Malaysia. ( 14(8), 2064–2072. [CrossRef] [PubMed]
- 13. Fu QF, Qiu JQ, Hu K, Yang TL, An J. (2011). The Analyse of Virulence Factors-Pathogenicity Relationships of Aeromonas hydrophila Strains Isolated from China. Journal of biology, (in Chinese). [CrossRef]
- 14. Gorgoglione B, Carpio Y, Secombes CJ, Taylor NG, Lugo JM, Estrada MP. (2015). Viral and bacterial septicaemic infections modulate the expression of PACAP splicing variants and VIP/PACAP receptors in brown trout immune organs. Fish Shellfish Immunol. [CrossRef]
- Heymans R, Vila A, van Heerwaarden CAM, Jansen CCC, Castelijn GAA, van der Voort M, Biesta-Peters EG. (2018). Rapid detection and differentiation of Salmonella species, Salmonella Typhimurium and Salmonella Enteritidis by multiplex quantitative PCR. ( 13, e0206316. [CrossRef] [PubMed]
- Hu PH, Jin YC, Qu XW, Cheng C, Shang XL, Zhang Y, Qu XC. (2010). Study on acute toxicity of ammonia nitrogen to adult Esox lucius. ( 3, 109–111. (In Chinese) [CrossRef]
- Janda, JM. (1991). Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. [CrossRef]
- Jagoda SS, Wijewardana TG, Arulkanthan A, Igarashi Y, Tan E, Kinoshita S, Watabe S, Asakawa S. (2014). Characterization and antimicrobial susceptibility of motile aeromonads isolated from freshwater ornamental fish showing signs of septicaemia. ( 109, 127–137. [CrossRef]
- Laltlanmawia C, Saha H, Ghosh L, Saha RK, Malla S. (2023). Identification and analysis of pathogenic bacteria causing outbreaks in Indian major carp aquaculture of Tripura. ( 35, 263–279. [CrossRef]
- Larsson P, Tibblin P, Koch-Schmidt P, Engstedt O, Nilsson J, Nordahl O, Forsman A. (2015). Ecology, evolution, and management strategies of northern pike populations in the Baltic Sea. ( 44, 451–61. [CrossRef]
- Lee SW, Wendy W. (2017). Antibiotic and heavy metal resistance of Aeromonas hydrophila and Edwardsiella tarda isolated from red hybrid tilapia (Oreochromis spp.) coinfected with motile aeromonas septicemia and edwardsiellosis. ( 10, 803–807. [CrossRef]
- Legario FS, Choresca CH, Jr Grace K, Turnbull JF, Crumlish M. (2023). Identification and characterization of motile Aeromonas spp. isolated from farmed Nile tilapia (Oreochromis niloticus) in the Philippines. ( 134, lxad279. [CrossRef]
- 23. Mazumder A, Choudhury H, Dey A, Sarma D. (2021). Isolation and characterization of two virulent Aeromonads associated with haemorrhagic septicaemia and tail-rot disease in farmed climbing perch Anabas testudineus. Sci Rep. [CrossRef]
- 24. Moser C, Lerche CJ, Thomsen K, Hartvig T, Schierbeck J, Jensen PØ, Oana C, Høiby N. (2019). Antibiotic therapy as personalized medicine–general considerations and complicating factors. Apmis. [CrossRef]
- Onuk EE, Ciftci A, Findik A, Durmaz Y. (2010). Development and evaluation of a multiplex PCR assay for simultaneous detection of Flavobacterium psychrophilum, Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida in culture fisheries. Journal of veterinary science. 11, 235–241. [CrossRef] [PubMed]
- Osman KM, Al-Maary KS, Mubarak AS, Dawoud TM, Moussa IMI, Ibrahim MDS, Hessain AM, Orabi A, Fawzy NM. (2017). Characterization and susceptibility of streptococci and enterococci isolated from Nile tilapia (Oreochromis niloticus) showing septicaemia in aquaculture and wild sites in Egypt. ( 13, 357. [CrossRef] [PubMed]
- Parker JL, Shaw JG. (2011). Aeromonas spp. clinical microbiology and disease. The Journal of infection 62, 109–118. [CrossRef] [PubMed]
- Pękala-Safińska, A. (2018). Contemporary Threats of Bacterial Infections in Freshwater Fish. Journal of veterinary research, 62, 261–267. [CrossRef]
- 29. Puneeth TG, Pallavi B, Vilasini U, Kushala KB, Nithin MS, Girisha SK, & SureshT. (2022). Large scale mortality in cultured Nile tilapia (Oreochromis niloticus): natural co-infection with Aeromonas hydrophila and Streptococcus iniae. Iranian journal of veterinary research. [CrossRef]
- Qin L, Yin JG, Zhang W, Jia S, Yue C. (2014). Isolation and identification of pathogenic Aeromonas hydrophila from Esox lucius. ( 35, 40–45. (in Chinese). [CrossRef]
- Qu, BZ. (2022). Diagnosis and treatment of two cases of bacterial septicemia in freshwater fish. Fishery Enrichment Guide, (in Chinese). [CrossRef]
- 32. Rosenfeld J, Lee R. (2022). Thresholds for Reduction in Fish Growth and Consumption Due to Hypoxia: Implications for Water Quality Guidelines to Protect Aquatic Life. Environ Manage. [CrossRef]
- Saglam D, Atli G, Canli M. (2013). Investigations on the osmoregulation of freshwater fish (Oreochromis niloticus) following exposures to metals (Cd, Cu) in differing hardness. ( 92, 79–86. [CrossRef] [PubMed]
- Semwal A, Kumar A, Kumar N. (2023). A review on pathogenicity of Aeromonas hydrophila and their mitigation through medicinal herbs in aquaculture. ( 9, e14088. [CrossRef]
- Song, HL. (2020). Isolation and identification of Aeromonas veronii from micropterus salmoides and construction of mutant strain of OmpA gene. He'nan Normal University, MA thesis.
- 36. Sriyasak P, Chitmanat C, Whangchai N, Promya J, Lebel L. (2015). Effect of water de-stratification on dissolved oxygen and ammonia in tilapia ponds in Northern Thailand. International Aquatic Research. [CrossRef]
- 37. Sun CW, Gong H, Lai YZ, Jiang XY, Ren Y, Chen ZH, Tao JF. (2019). Optimization of production process of inactivated vaccine against Aeromonas vickerii CA07 strain. Chinese Journal of Biologics, (in Chinese). [CrossRef]
- Thaotumpitak V, Sripradite J, Atwill ER, Jeamsripong S. (2023). Emergence of colistin resistance and characterization of antimicrobial resistance and virulence factors of Aeromonas hydrophila, Salmonella spp., and Vibrio cholerae isolated from hybrid red tilapia cage culture. ( 11, e14896. [CrossRef]
- Tomás, JM. (2012). The main Aeromonas pathogenic factors. ISRN microbiology, 4, 256261. [CrossRef]
- Vincent AT, Paquet VE, Bernatchez A, Tremblay DM, Moineau S, Charette SJ. ( 7, 7054. [CrossRef]
- Wang LL, Hou TM, Li HM, Wei, Yuan HW, Lei LC, Zhang FX. (2023). Virulence and drug sensitivity of Mylopharyngodon piceus ST251 highly pathogenic Aeromonas hydrophila. ( 38, 573–583. (in Chinese). [CrossRef]
- 42. Yilmaz S, Sova M, Ergün S. (2018). Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. Journal of Applied Microbiology, 1727. [CrossRef]
- Zaher HA, Nofal MI, Hendam BM, Elshaer MM, Alothaim AS, Eraqi MM. (2021). Prevalence and Antibiogram of Vibrio parahaemolyticus and Aeromonas hydrophila in the Flesh of Nile Tilapia, with Special Reference to Their Virulence Genes Detected Using Multiplex PCR Technique. Antibiotics (Basel, Switzerland). 10, 654. [CrossRef] [PubMed]
- 44. Zambri M, Cloutier M, Adam Z, Lapen DR, Wilkes G, Sunohara M, Topp E, Talbot G, Khan IUH. (2019). Novel virulence, antibiotic resistance and toxin gene-specific PCR-based assays for rapid pathogenicity assessment of Arcobacter faecis and Arcobacter lanthieri. BMC microbiology. [CrossRef]
- Zhang, SM. (2023). Application and precautions of antibiotics for aquaculture. Heilongjiang Fisheries, (in Chinese). [CrossRef]
- Zepeda-Velázquez AP, Vega-Sánchez V, Salgado-Miranda C, & Soriano-Vargas E. (2015). Histopathological findings in farmed rainbow trout (Oncorhynchus mykiss) naturally infected with 3 different Aeromonas species. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire, 79(3), 250–254.
- 47. Zhu DL, Li AH, Wang JG, Li M, Cai TZ, Hu J. (2006). Correlation between virulence and virulence gene distribution in Aeromonas hydrophila. Journal of Sun Yat-sen University (Natural Science Edition), (in Chinese). [CrossRef]
Figure 1.
The clinical symptoms and pathological changes of E. lucius diagnosed with bacterial septicemia. (a) Hyperemia of abdomen and fin base. (b) Earth-yellow, enlarged liver. (c) Black–purple spleen.
Figure 1.
The clinical symptoms and pathological changes of E. lucius diagnosed with bacterial septicemia. (a) Hyperemia of abdomen and fin base. (b) Earth-yellow, enlarged liver. (c) Black–purple spleen.
Figure 2.
The colonies morphology. BHI agar medium of diseased fish strains. (b) RS medium of diseased fish strains. (c) Sheep blood agar medium showing β-hemolysis of diseased fish strains. (d) BHI agar medium of healthy fish strains. (e) RS medium of healthy fish strains. (f) AHM of diseased fish strains. From left to right: negative control, strain 1 of diseased fish, strain 2 of healthy fish.
Figure 2.
The colonies morphology. BHI agar medium of diseased fish strains. (b) RS medium of diseased fish strains. (c) Sheep blood agar medium showing β-hemolysis of diseased fish strains. (d) BHI agar medium of healthy fish strains. (e) RS medium of healthy fish strains. (f) AHM of diseased fish strains. From left to right: negative control, strain 1 of diseased fish, strain 2 of healthy fish.
Figure 3.
Gram staining results. (a–e) Gram staining results of the diseased fish isolates. (f)Gram staining results of the healthy fish isolates.
Figure 3.
Gram staining results. (a–e) Gram staining results of the diseased fish isolates. (f)Gram staining results of the healthy fish isolates.
Figure 4.
Results of agarose gel electrophoresis of 16S rRNA gene PCR amplification products of isolated strains. M: DNA ladder; lanes 1–5: strains 1–5; lane 6: negative control.
Figure 4.
Results of agarose gel electrophoresis of 16S rRNA gene PCR amplification products of isolated strains. M: DNA ladder; lanes 1–5: strains 1–5; lane 6: negative control.
Figure 5.
Maximum likelihood phylogenetic tree constructed based on 16S rRNA gene sequences of isolated strains JZ1–JZ5.
Figure 5.
Maximum likelihood phylogenetic tree constructed based on 16S rRNA gene sequences of isolated strains JZ1–JZ5.
Figure 6.
Electrophoretic map of PCR amplification products of hly, aer, and ahp virulence genes. M: MarkD2000; 1–5: results of strains 1–5 hly virulence gene amplification; 7–11: results of strains 1–5 aer virulence gene amplification; 13–17: results of strains 1–5 ahp virulence gene amplification; 6, 12, and 18: negative controls.
Figure 6.
Electrophoretic map of PCR amplification products of hly, aer, and ahp virulence genes. M: MarkD2000; 1–5: results of strains 1–5 hly virulence gene amplification; 7–11: results of strains 1–5 aer virulence gene amplification; 13–17: results of strains 1–5 ahp virulence gene amplification; 6, 12, and 18: negative controls.
Figure 7.
Electrophoresis results of Ah PCR. M: DMA ladder; lanes 1-5: strains 1-5; lane 6: negative control.
Figure 7.
Electrophoresis results of Ah PCR. M: DMA ladder; lanes 1-5: strains 1-5; lane 6: negative control.
Figure 8.
Electrophoresis results of Ah-hly-aer-ahp multiplex PCR optimization of strain 1. M: DNA ladder; lanes 1–12: primer concentrations of 0.3, 0.5, 0.7 and 0.9 µM at an annealing temperature of 57 ℃ and elongation time of 2 min (lanes 1–4), an annealing temperature 59 ℃ and elongation time of 1 min (lanes 5–8), and an annealing temperature of 60 ℃ and elongation time of 30 s (lanes 9–12); lane 13: negative control.
Figure 8.
Electrophoresis results of Ah-hly-aer-ahp multiplex PCR optimization of strain 1. M: DNA ladder; lanes 1–12: primer concentrations of 0.3, 0.5, 0.7 and 0.9 µM at an annealing temperature of 57 ℃ and elongation time of 2 min (lanes 1–4), an annealing temperature 59 ℃ and elongation time of 1 min (lanes 5–8), and an annealing temperature of 60 ℃ and elongation time of 30 s (lanes 9–12); lane 13: negative control.
Figure 9.
Electrophoresis results of Ah-hly-aer-ahp multiplex PCR products. M: DNA ladder; lanes 1–5: strains 1–5; lane 6: negative control.
Figure 9.
Electrophoresis results of Ah-hly-aer-ahp multiplex PCR products. M: DNA ladder; lanes 1–5: strains 1–5; lane 6: negative control.
Table 1.
Primers sequence information.
Table 1.
Primers sequence information.
| Name |
Primer sequence (5 '-3') |
Amplified sequence length /bp |
Document source |
|
A. hydrophila 16S rDNA |
F:GAGGAGGAAAGGTTGATGCC
R:CTTGAGTTCCCACCATTACG |
685 |
Li et al.,. 2014 |
|
A. veronii 16S rDNA |
F:AGAGTTTGATCCTGGCTCAG
R:GGTTACCTTGTTACGACTT |
1500 |
Song, 2020 |
|
A. sobria 16S rDNA |
F:AGAGTTTAGTCTGGCTCAG
R:TACGGTTACCTTGTTACGACTT |
1431 |
Chen, 2009 |
|
A. hydrophila specific primers(Ah) |
F:CCTGGTCACCAGATAGTG
R:GGTGATCGGAGAGGACTT |
202 |
Wang et al.,. 2023 |
| Serine protease (ahp) |
F:GTTAGCGTTGGCAATCTCG
R:CGCTGGAGTAGGAGGAACG |
874 |
Fu et al.,. 2011 |
| Hemolysin (hly) |
F:TGACAGGCAAGTAGAATAACGC
R:TGTCCGCCTTCCACTCCC |
1815 |
| Rerolysin (aer) |
F:CAAGAACAAGTTCAAGTGGCCA
R:ACGAAGGTGTGGTTCCAGT |
309 |
Zhu et al.,. 2006 |
Table 2.
PCR reaction system and reaction conditions.
Table 2.
PCR reaction system and reaction conditions.
| Primer name |
PCR amplification system (25 μL) |
PCR reaction system |
| A. hydrophila |
The PCR reaction had a total volume of 25.0 μL, including Reaction-mix (Taq 2.5 U/μL, dNTP Mixture 2.5 mM each) 12.5 μL, template of Bacterial strains DNA 1.0 μL, ddH2O 9.5 μL, forward primer (10 μM) 1 μL, and reverse primer (10 μM) 1 μL. |
Predenaturation at 94 ℃ for 5 min; 94 ℃ 30 s, 56 ℃ 30 s, 72 ℃ 1 min, 30 cycles; extend at 72 ℃ for 10 min |
| A. veronii |
Predenaturation at 94 ℃ for 5 min; 94 ℃ 30 s, 57 ℃ 30 s, 72 ℃ 90 s, 32 cycles; extend at 72 ℃ for 10 min |
| A. sobria |
Predenaturation at 94 ℃ for 5 min; 94 ℃ 1 min, 55 ℃ 1 min, 72 ℃ 1 min, 36 cycles; extend at 72 ℃ for 10 min |
Table 3.
Sequence of virulence gene primers and reaction system.
Table 3.
Sequence of virulence gene primers and reaction system.
| Virulence gene |
PCR amplification system (25 μL) |
PCR reaction system |
| ahp |
The PCR reaction had a total volume of 25.0 μL, including Reaction-mix (Taq 2.5 U/μL, dNTP Mixture 2.5 mM each) 12.5 μL, template of Bacterial strains DNA 1.0 μL, ddH2O 9.5 μL, forward primer (10 μM) 1 μL, and reverse primer (10 μM) 1 μL. |
Predenaturation at 94 ℃ for 5 min; 94 ℃ 30 s, 57 ℃ 30 s, 72 ℃ 30 s, 35 cycles; extend at 72 ℃ for 10 min. |
| hly |
| aer |
Predenaturation at 94 ℃ for 5 min; 94 ℃ 30 s, 60 ℃ 30 s, 72 ℃ 1 min, 35 cycles; extend for 2 min at 72 ℃. |
Table 4.
Optimization protocol for multiplex PCR.
Table 4.
Optimization protocol for multiplex PCR.
Target VAT
Gene |
Ah-ahp-hly-aer
|
| Assay group |
Assay 1 |
Assay 2 |
Assay 3 |
Assay 4 |
| Primer Concentration (μM) |
0.3 |
0.5 |
0.7 |
0.9 |
| Annealing Temperature (℃) |
57/59/60 |
| Extension time (s) |
30/60/120 |
Table 5.
Water quality test results.
Table 5.
Water quality test results.
| Variable |
Fish pond 1 |
Fish pond 2 |
Fish pond 3 |
Fish pond 4 |
Fish pond 5 |
Reference values |
| Ammonia nitrogen (mg/L) |
1.022±0.000A
|
1.000±0.001B
|
1.014±0.001B
|
1.002±0.000B
|
1.054±0.002A
|
1.007±0.012B
|
| pH |
1.272±0.000A
|
1.243±0.000B
|
1.273±0.000A
|
1.280±0.000A
|
1.243±0.001B
|
1.243±0.025B
|
| Total alkalinity (mg/L) |
2.525±0.003A
|
2.193±0.016B
|
2.471±0.001A
|
2.326±0.003A
|
2.236±0.046B
|
2.189±0.141B
|
| Total hardness (mg/L) |
2.433±0.002A
|
2.132±0.042B
|
2.582±0.003A
|
2.121±0.012A
|
2.108±0.032A
|
2.213±0.104B
|
| Nitrite (mg/L) |
1.001±0.000A
|
1.000±0.000A
|
1.000±0.000A
|
1.000±0.000A
|
1.001±0.000A
|
1.113±0.005A
|
Table 6.
Physiological and biochemical identification results for Aeromonas isolates.
Table 6.
Physiological and biochemical identification results for Aeromonas isolates.
| Test number |
Biochemical substance |
Result for Aeromonas hydrophila
|
Result for Aeromonas veronii
|
Result for Aeromonas sobria
|
1 |
2 |
3 |
4 |
5 |
| 1 |
Ornithine |
- |
+ |
- |
- |
+ |
- |
- |
+ |
| 2 |
Arginine |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 3 |
Lysine |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 4 |
Sucrose |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 5 |
Urea |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
| 6 |
Esculin |
+ |
+ |
- |
+ |
+ |
- |
+ |
+ |
| 7 |
Glucose |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 8 |
Hydrogen sulfide |
+ |
- |
- |
+ |
- |
- |
+ |
- |
| 9 |
ONPG |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 10 |
Mannitol |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 11 |
Ribitol |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
| 12 |
Xylose |
- |
- |
- |
- |
- |
- |
- |
- |
| 13 |
Adonitol |
- |
- |
- |
- |
- |
- |
- |
- |
| 14 |
Indigo substrate |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 15 |
Nitrate reduction |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 16 |
Phenylalanine |
- |
+ |
- |
- |
+ |
- |
+ |
+ |
| 17 |
Oxidase |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 18 |
Arginine dihydrolase |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| 19 |
Lactose |
- |
- |
- |
- |
- |
- |
- |
- |
| 20 |
VP |
- |
+ |
+ |
- |
+ |
+ |
+ |
+ |
Table 7.
Drug sensitivity outcomes of the isolated pathogenic bacteria.
Table 7.
Drug sensitivity outcomes of the isolated pathogenic bacteria.
| Test number |
Drug |
Strain 1 |
Strain 2 |
Strain 3 |
Strain 4 |
Strain 5 |
| 1 |
Flufenicol |
33 |
S |
34 |
S |
30 |
S |
35 |
S |
31 |
S |
| 2 |
Ciprofloxacin |
36 |
S |
30 |
S |
40 |
S |
30 |
S |
30 |
S |
| 3 |
Doxycycline |
25 |
S |
27 |
S |
26.5 |
S |
25.5 |
S |
27 |
S |
| 4 |
Minocycline |
24 |
S |
25.5 |
S |
24 |
S |
25.5 |
S |
26 |
S |
| 5 |
Penicillin |
6 |
R |
6 |
R |
6 |
R |
6 |
R |
6 |
R |
| 6 |
Neomycin |
17 |
R |
15 |
R |
15 |
R |
17 |
R |
15 |
R |
| 7 |
Enrofloxacin |
39 |
S |
30 |
S |
40 |
S |
30 |
S |
27 |
S |
| 8 |
Kanamycin |
19 |
S |
20.1 |
S |
22.5 |
S |
19 |
S |
17 |
I |
| 9 |
Tetracycline |
30 |
S |
31 |
S |
32.5 |
S |
27 |
S |
28 |
S |
| 10 |
Levofloxacin |
40 |
S |
35 |
S |
40 |
S |
31 |
S |
28 |
S |
| 11 |
Gentamicin |
16.5 |
S |
20 |
S |
23.5 |
S |
17 |
S |
17 |
S |
| 12 |
Midecamycin |
6 |
R |
18.5 |
S |
15 |
I |
19.5 |
S |
19 |
S |
| 13 |
Compound sulfamethoxazole |
15 |
R |
22 |
R |
24 |
I |
21 |
R |
21 |
R |
| 14 |
Chloramphenicol |
36 |
S |
35.5 |
S |
34 |
S |
32 |
S |
34 |
S |
| 15 |
Cephalothin |
25 |
S |
27.5 |
S |
10 |
R |
26.5 |
S |
23 |
S |
| 16 |
Vancomycin |
11 |
I |
9 |
R |
13 |
S |
6 |
R |
9 |
R |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).