1. Introduction
The production of aquafeed is increasing with the rapid development of aquaculture, which caused the shortage of high-quality protein sources. In the aquaculture feed industry, fish meal is recognized as the highest quality protein source for aquatic animals [
1]. Unfortunately, the price of fish meal is becoming higher and higher due to the production of fish meal is limited [
2]. The development of plant protein sources that can replace fish meal is of great importance for the sustainable development of aquaculture [
3,
4]. However, plant protein sources cannot completely replace fish meal due to the amino acid imbalance [
5]. It is an effective way to improve the fish meal replacement ratio by mixing several plant protein sources together to complement amino acids profile.
Cottonseed protein is a potential plant protein source in aquaculture due to its large yield, low price and relatively stable supply. But the antinutritional factors such as gossypol limited quality and use level in aquaculture. Fortunately, fermentation can effectively reduce or eliminate anti-nutritional factors and improve the palatability and digestibility of cottonseed meal. Studies have reported that fermentation not only increased the crude protein content of cottonseed meal, but also increased the essential amino acids such as lysine, methionine and threonine [
6,
7,
8]. In fish, fermented cottonseed meal can partially replace fish meal. It has been reported that fermented cottonseed meal can replace 16% fish meal in the black sea bream [
9]. The proportion of substitutions also varies between species. Fermented cottonseed meal can replace 25% fish meal in the
Trachinotus ovatus [
10]. In crustaceans, the percentage of fish meal substituted by fermented cottonseed meal was higher than fish. It has been reported that fermented cottonseed meal can replace 30% fish meal in the
Litopenaeus vannamei [
11]. In summary, fermented cottonseed meal is a potential plant protein source to partially replacing fish meal.
Rapeseed meal is also a common plant protein source. However, due to its low digestion and antinutritional factors, its use in aquafeed is limited [
12,
13]. After fermentation, the degradation rate of these anti-nutritional factors could be decreased more than 85% [
14,
15]. Some studies have reported that fermented rapeseed meal can successfully replace 56.25% of fish meal without negative effects on the growth, nutrient utilization, innate immune response and oxidative stress of juvenile fish [
16]. In crustaceans, 64.4 g/kg fermented rapeseed meal did not negatively affect the growth and survival of
L. vannamei [
17]. In summary, fermented rapeseed meal can partially replace fish meal in aquaculture.
The Chinese mitten crab (
Eriocheir sinensis) is an economic crustacean that has been widely farmed. The development of low fish meal diet is a hot topic in this species. In our previous study, we have found that he optimum amount of fermented cotton meal was 24% in Chinese mitten crab [
18]. It is a distinct characteristic that arginine content was higher in fermented cotton meal [
19]. While, lysine and methionine are abundant in fermented rapeseed meal. The combination of these two protein sources can form a good amino acid complementation. Unfortunately, the suitable mixing proportion is still unknown. Therefore, this study mixed fermented cotton meal and fermented rapeseed meal according to different proportions (2:1, 1:1, 1:2), and investigated the effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the growth performance, and antioxidant capacity of juvenile Chinese mitten crab, thereby to increase the proportion of substitute fish meal on the basis of the previous single substitution.
2. Materials and Methods
2.1. Experimental Diets
The ratio of fermented cotton meal (FCM) and fermented rapeseed meal (FRM) were set according to 1:2, 1:1, and 2:1, and were supplemented into 25% fish meal (FM-25) and 15% fish meal (FM-15) diets, respectively to formulated 6 kinds of experimental diets. The formulation and proximate composition of the experimental diets are shown in
Table 1. The amino acid composition of experimental diets is shown in
Table 2.
The fish meal, cottonseed meal, soybean meal, FCM and FRM were finely ground. Where after, these ingredients were sieved through a 60-mesh strainer. Then the ingredients were weighed and mixed according to the formula. The distilled water were added after repeated mixing to make a dough. The dough was pelleted using a screw-press pelletizer. The pellets were dried in a dryer at 45 °C. Finally, diets were stored at -20 °C for using.
2.2. Feeding Trial, Sampling and Growth Evaluation
Juvenile crabs were obtained from a local farm. Crabs were acclimatized to the experimental conditions in 300 L tanks (100 × 80 × 60 cm) before the feeding trial. The plastic nets were put into tanks as shelters to reduce attacking behavior. When experiment began, a total of 720 crabs (0.38 ±0.01 g) were randomly distribute into 24 tanks (100 × 80 × 60 cm) and each treatment was 4 parallel tanks. Each tank containing 30 crabs. Diets with a daily ration of 4% body weight were hand-fed to crabs twice daily (8:00 and 18:00). Feces were removed after feeding (09:00). Meanwhile, the 30% tank volume of water was exchanged. During the feeding trial, the water temperature varied from 25 °C to 28 °C, the ammonia nitrogen was lower than 0.05 mg/L, the dissolved oxygen concentration was under 7 mg/L.
After 56 d feeding trial, crabs were euthanized, five crabs were sampled randomly from each tank for proximate nutrient composition. Following, six crabs were sampled randomly and the hepatopancreases were frozen in liquid nitrogen and kept at ultra-low temperature freezer for enzyme activity and gene expression analyses.
Weight gain, specific growth rate and hepatopancreas index were calculated using the formulas as below:
Weight gain (WG, %) = (final crab weight - initial crab weight) / initial crab weight × 100
Specific growth rate (SGR, %) = 100 × (LN final crab weight - LN initial crab weight) / days
Hepatopancreas index (HSI, %) = hepatopancreas weight /whole crab weight × 100
2.3. Chemical Composition Analysis
The proximate nutrient compositions of diets and crabs were measured according to the procedures described in the AOAC [
20]. Firstly, moisture was measured using an oven (105 °C, 8 h). Subsequently, the experimental diets and crabs were crushed for crude protein (kjeldah method), crude lipid (Soxhlet extraction) and Ash (Muffle furnace) analyses.
2.4. Analysis of Biochemical Parameters in the Hepatopancreas
The antioxidant parameters in the hepatopancreas were measured using the commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The kits used in this study were as follows: superoxide dismutase (SOD; Cat. No. A001-1), glutathione peroxidase (GPX; Cat. No. A005-1-2), glutathione s-transferase (GST; Cat. No. A004-1-1), peroxidase (POD; Cat. No. A084-3-1), malondialdehyde (MDA; Cat. No. A003-1-2). Total protein (TP; Cat. No. A045-2-2).
2.5. Analysis of Amino Acid Composition
The feed samples were digested with 6 N HCl for 24 h under 110 °C, and then use the nitrogen removal. Samples were redissolved in 0.1 N HCl loading buffer and filtered through 0.22 μm polyether sulfone ultrafiltration. The amino acid compositions of feed were determined using a Hitachi L-8900 amino acid analyzer, Japan.
2.6. Analysis of Gene Expression
Total RNA was extracted using the RNAiso Plus (CAT # 9109, Takara, Japan). Where after, the reverse transcription was performed using a PrimeScript™ RT master mix reagent kit (Takara, Japan). The specific primers were designed using NCBI Primer BLAST (
Table 3). The RT-PCR amplification reactions were performed using CFX96 Real-Time PCR system (Bio-rad, Richmond, CA). PCR conditions were set according to the commercial kits (Takara, Japan). The relative gene expression levels were calculated by geometric averaging of multiple internal control genes [
21].
2.7. Statistical Analysis
Statistical analysis was performed using the SPSS 26.0 for Windows (SPSS, Michigan Avenue, Chicago, IL, USA). Two-way ANOVA was used to determine if there was any interaction between fish meal level and FCM:FRM ratio. At the same fish meal condition, one-way ANOVA was used to analyze the significant differences among crabs fed the diets with different FCM:FRM ratio. At the same FCM:FRM ratio, independent-samples T test was used to determine significant differences between crabs fed with different fish meal levels. Significance was set at P < 0.05.
3. Results
3.1. Growth Performance
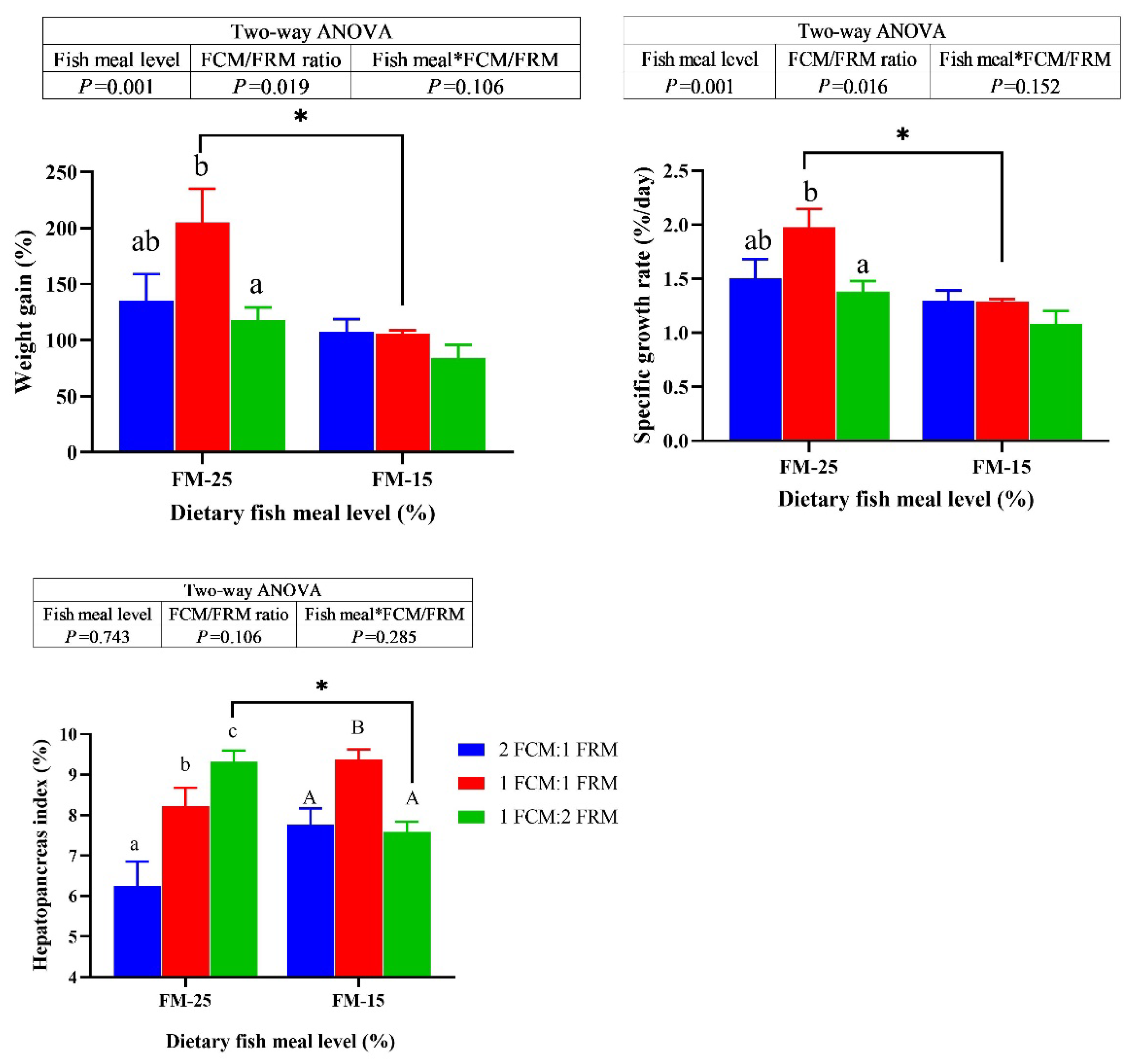
There were significant main effects of dietary fish meal levels on the weight gain (WG) and specific growth rate (SGR) (P < 0.05). the WG and SGR of crabs fed the diets containing 25% fish meal were significantly higher than crabs fed 15% fish meal diets (P < 0.05). At 25% fish meal level, the WG and SGR of crabs fed the diet with 1FCM:1FRM were significantly higher than those fed the 1FCM:2FRM and 2FCM:1FRM diets (P < 0.05). At 15% fish meal level, the ratio of FCM:FRM did not significantly affect the WG and SGR (P > 0.05). At 25% fish meal level, the hepatopancreas index (HSI) of crabs fed the diet with 1FCM:1FRM were significantly higher than those fed the 2FCM:1FRM (P < 0.05). Meanwhile, the HSI of crabs fed the diet with 1FCM:2FRM were significantly higher than those fed the 2FCM:1FRM and 1FCM:1FRM diets (P < 0.05). At 15% fish meal level, the HSI of crabs fed the diet with 1FCM:1FRM were significantly higher than those fed the 1FCM:2FRM and 2FCM:1FRM diets (P < 0.05).
Figure 1.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the growth performance of juvenile Chinese mitten crab. The asterisk (*) indicates that there are significant differences among different protein level groups with the same amount of glutamate, the same below.
Figure 1.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the growth performance of juvenile Chinese mitten crab. The asterisk (*) indicates that there are significant differences among different protein level groups with the same amount of glutamate, the same below.
3.2. Proximate Composition
At 25% fish meal level, the crude protein (CP) content of crabs fed the diet with 1FCM:1FRM were significantly higher than those fed the 2FCM:1FRM and 1FCM:2FRM (P < 0.05). At 15% fish meal level, the CP content of crabs fed the diet with 2FCM:1FRM were significantly higher than those fed the 1FCM:1FRM (P < 0.05). At 25% fish meal level, the crude lipid (CL) content of crabs fed the diet with 1FCM:2FRM were significantly higher than those fed the 2FCM:1FRM and 1FCM:1FRM (P < 0.05). At 15% fish meal level, the CL content of crabs fed the diet with 2FCM:1FRM were significantly higher than those fed the 1FCM:1FRM and 1FCM:2FRM (P < 0.05). At both 25% and 15% fish meal levels, the Ash content of crabs fed the diet with 2FCM:1FRM were significantly lower than those fed the 1FCM:1FRM and 1FCM:2FRM (P < 0.05).
Table 4.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the proximate composition of juvenile Chinese mitten crab.
Table 4.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the proximate composition of juvenile Chinese mitten crab.
| Diets |
Crude protein |
Crude lipid |
Ash |
| (FM25) 2FCM:1FRM |
40.52±0.35a
|
8.81±0.59a
|
38.97±0.83a
|
| (FM25) 1FCM:1FRM |
41.1±0.14b
|
9.17±0.54a
|
40.11±0.76b
|
| (FM25) 1FCM:2FRM |
40.84±0.28a
|
10.03±0.82b
|
40.75±0.58b
|
| (FM15) 2FCM:1FRM |
42.25±0.22b
|
9.41±1.09b
|
38.67±0.79a
|
| (FM15) 1FCM:1FRM |
40.18±0.85a
|
6.58±1.15a
|
42.18±1.23b
|
| (FM15) 1FCM:2FRM |
41.38±1.07ab
|
7.39±0.51a
|
41.49±0.2b
|
| Two-way ANOVA |
| Fish meal level |
<0.01 |
<0.01 |
<0.01 |
| FCM/FRM ratio |
0.502 |
<0.01 |
<0.01 |
| Fish meal*FCM/FRM |
0.289 |
0.027 |
<0.05 |
3.3. The Activities of Enzymes Related to Antioxidant Capacity
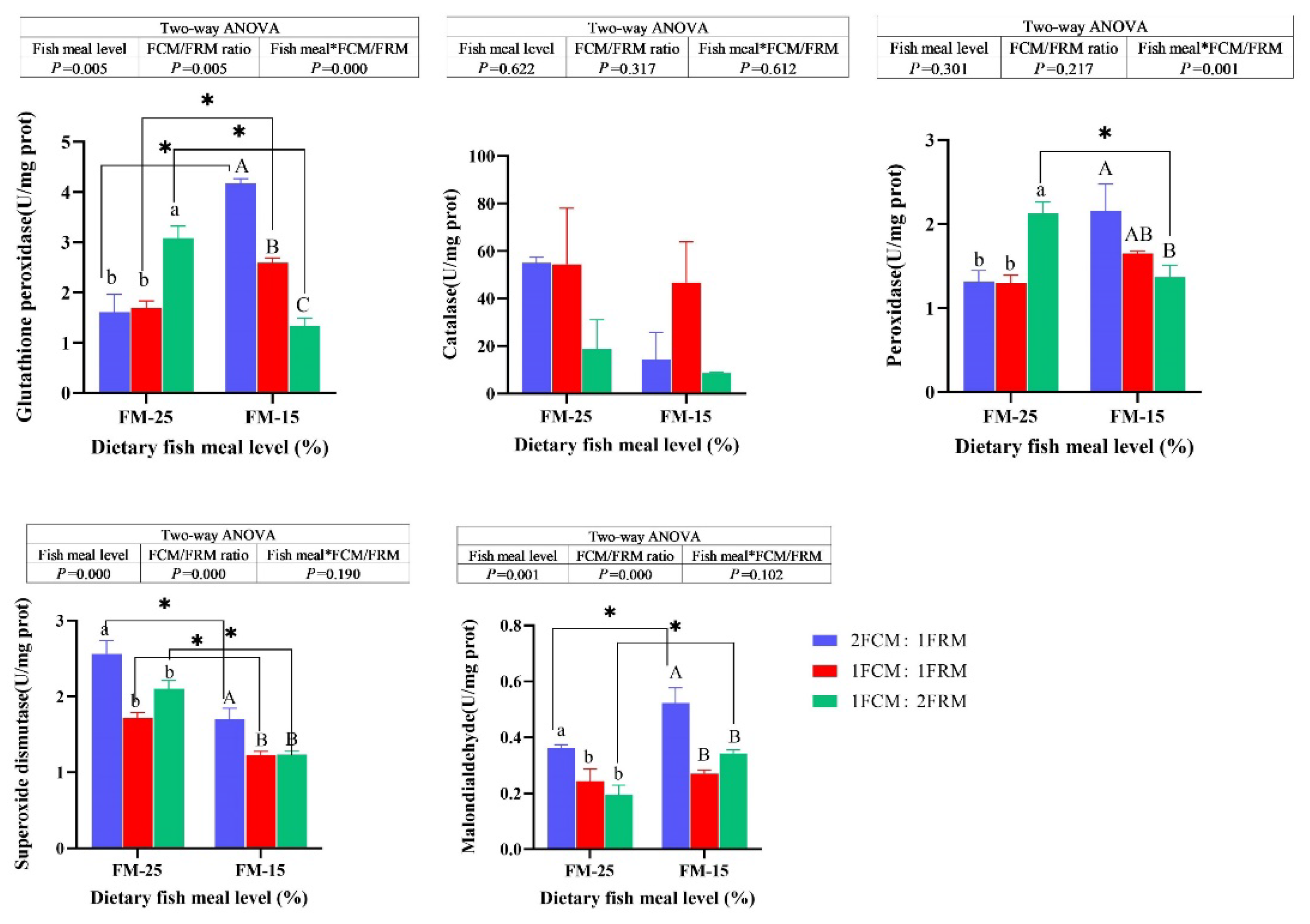
At 25% fish meal level, the activities of glutathione peroxidase (GPX) and peroxidase (POD) of crabs fed the diet with 1FCM:2FRM were significantly higher than those fed the 2FCM:1FRM and 1FCM:1FRM (P < 0.05). At 15% fish meal level, the GPX and POD of crabs fed the diet with 2FCM:1FRM were significantly higher than those fed the 1FCM:2FRM (P < 0.05). Dietary FCM/FRM ratio did not significantly affect the activities of catalase of Chinese mitten crab (P > 0.05). At both 25% and 15% fish meal levels, the activity of superoxide dismutase (SOD) and malondialdehyde (MDA) content of crabs fed the diet with 2FCM:1FRM were significantly higher than those fed the 1FCM:1FRM and 1FCM:2FRM (P < 0.05). There was significant main effect of dietary fish meal levels on the MDA (P < 0.05). the MDA content of crabs fed the diets containing 25% fish meal were significantly lower than crabs fed 15% fish meal diets (P < 0.05).
Figure 2.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the activities of enzymes related to antioxidant capacity of juvenile Chinese mitten crab.
Figure 2.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the activities of enzymes related to antioxidant capacity of juvenile Chinese mitten crab.
3.4. The Relative Expressions of Genes Related to Antioxidant Capacity
At 25% fish meal level, the relative expression of peroxiredoxin (PRX) of crabs fed the diet with 1FCM:1FRM were significantly higher than those fed the 2FCM:1FRM and 1FCM:2FRM (P < 0.05). At 15% fish meal level, dietary FCM/FRM ratio did not significantly affect the relative expression of PRX of Chinese mitten crab (P > 0.05). At 15% fish meal level, the relative expression of glutathione peroxidase (GPX) and catalase (CAT) of crabs fed the diet with 2FCM:1FRM were significantly higher than those fed the 1FCM:2FRM (P < 0.05). At both 25% and 15% fish meal levels, dietary FCM/FRM ratio did not significantly affect the relative expression of glutathione S-transferase (GST) of Chinese mitten crab (P > 0.05).
Figure 3.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the relative expressions of genes related to antioxidant capacity of juvenile Chinese mitten crab. Peroxiredoxin, PRX; Glutathione Peroxidase, GPX; Catalase, CAT; Glutathione S-transferase, GST.
Figure 3.
Effects of fish meal replacement with a mixture of fermented cotton meal and fermented rapeseed meal on the relative expressions of genes related to antioxidant capacity of juvenile Chinese mitten crab. Peroxiredoxin, PRX; Glutathione Peroxidase, GPX; Catalase, CAT; Glutathione S-transferase, GST.
4. Discussion
A lot of previous studies reported, fermented plant protein sources can partially replace fish meal [
22,
23,
24,
25]. In the present study, dietary supplemented with a mixture of fermented cotton meal and fermented rapeseed meal according to the ratio of 1:1 significantly improved the growth performance of juvenile Chinese mitten crab fed with 25% fish meal diet. This result indicate that fermented plant protein sources can promote the growth of aquatic animals. Some similar results were also reported in the
L. vannamei and
Procambarus clarkia [
26,
27]. However, in the low fish meal condition (15%), the proportion of fermented cotton meal and fermented rapeseed meal did not affect the growth performance of juvenile crabs, which might be due to the low fish meal diets disturbed the normal growth of crab, thereby, the fermented plant protein sources could not improve the growth under unhealthy conditions. The hepatopancreas is an important organ for nutrient storage, detoxification and metabolism in Chinese mitten crab [
28,
29,
30,
31]. In the present study, dietary supplemented with a mixture of fermented cotton meal and fermented rapeseed meal according to the ratio of 1:1 significantly improved the hepatopancreas index of juvenile Chinese mitten crab fed with 25% or 15% fish meal diets, which showed that proper mixed ratio of fermented cottonseed meal and fermented rapeseed meal can improve the hepatopancreas nutrient accumulation in Chinese mitten crab. We speculate that this may be one of the reasons for the fermentation plant protein to promote growth performance of Chinese mitten crab.
Animal growth is closely related to the accumulation of nutrients from the view of nutrient metabolism. Some studies reported that fermented plant proteins affected animal growth by affecting body nutrient composition. For example, the crude lipid content decreased with the increasing fermented cotton meal levels, which in turn affecting the growth of L. vannamei [
32]. The similar result was also found in the present study. It was observed that the low proportion of fermented cottonseed meal significantly reduced the lipid content of crabs fed the 25% fish meal diets. On the other hand, a study reported that high fermented rapeseed meal reduced the lipid content of L. vannamei [
33]. Therefore, we speculated that the increasing lipid level of crabs in the present study was also related to variation of fermented rapeseed meal. These results were highly consistent with the hepatopancreas, which is the major lipid storage site of crabs. However, the opposite results were found in crabs fed the low fish meal (25%) diets. It is difficult to explain the reason for the abnormal lipid changes under the low fish meal diets based on the available scientific reports. A further study is needed to figure out the possible reasons. In the present study, Dietary fermented plant protein source did not significantly change the protein content of crabs. These results are consistent with the previous studies. In summary, the mixture ratio of fermented cotton meal and fermented rapeseed meal affected growth performance maybe through influencing the lipid content, not protein, in the Chinese mitten crab.
The growth performance of an animal is closely related to its health status and antioxidant capacity is an important indicator of health status [
34]. Superoxide dismutase (SOD) and catalase (CAT) are important components of the antioxidant system [
35,
36]. SOD converts oxygen free radicals into hydrogen peroxide, following CAT and peroxidase (POD) decompose hydrogen peroxide into water, thereby these enzymes play a role in protecting organism from oxidative damage [
37]. In the present study, malondialdehyde (MDA) content of crabs fed the diet with 1FCM:1FRM and 1FCM:2FRM were significantly higher than those fed the 2FCM:1FRM diet, which indicated an appropriate proportion of FCM: FRM can reduced oxidative stress. However, the mixed ratios of 1FCM:1FRM and 1FCM:2FRM did not significantly increase the activities of SOD and CAT of crabs. These results suggest that fermented plant protein sources may not reduce oxidative stress through the SOD-CAT system. Glutathione peroxidase also plays an important role in the antioxidant system [
37]. A study reported that fermented cottonseed meal significantly increased the GSH content of L. vannamei [
38]. In the present study, the mixed ratio of 1FCM:2FRM significantly increased the activity of glutathione peroxidase (GPX), which indicated that it may be one of the ways in which fermented plant protein sources improve the antioxidant capacity of Chinese mitten crab. Besides, peroxiredoxin (PRX) can effectively regulate the level of ROS and play an antioxidant role in animals [
39,
40]. Previous studies have found that the relative expression of Prx6 were significantly up-regulated in the case of oxidative stress, resulting in a strong ability of scavenging free radicals [
41]. In the present study, the relative expression of PRX of crabs fed the diet with 1FCM:1FRM were significantly higher than the other group under 25% fish meal condition. This result indicated that it may be another way in which fermented plant protein sources improve the antioxidant capacity of Chinese mitten crab. In summary, the mixture of fermented cotton meal and fermented rapeseed meal can improve the antioxidant capacity of Chinese mitten crab by up-regulating the PRX expression and increasing the GPX activity.
5. Conclusions
At 25% fish meal level, diets supplemented with a mixture of fermented cotton meal and fermented rapeseed meal (1:1) can significantly improve the growth of Chinese mitten crab by increasing the body lipid content, the relative expression of PRX and the activity of GPX.
Author Contributions
Yisong He, writing – original draft and editing; Jiajun Zheng, investigation and writing—review and editing. He Lv, resources and software. Li Jia, investigation. Yang Xu, writing—review and editing. Yue Tan, writing—review and editing. Gengwu Gou, resources. Changle Qi, supervision. Jinyun Ye, supervision.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research was supported by grants from the Zhejiang Provincial Natural Science Foundation of China under Grant No. LTGN23C190003 and the Huzhou Natural Science Foundation (2021YZ14).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halver, J.E.; Hardy, R.W. Fish nutrition. Academic press, California, USA 2002.
- NRC. Nutrient requirements of fish and shrimp. National academies press, Washington 2011.
- Cabral, E.M.; Fernandes, T.J.R.; Campos, S.D.; Castro-Cunha, M.; Oliveira, M.; Cunha, L.M.; Valente, L.M.P. Replacement of fish meal by plant protein sources up to 75% induces good growth performance without affecting flesh quality in ongrowing Senegalese sole. Aquaculture 2013, 380, 130–138. [Google Scholar] [CrossRef]
- Mai, K. Aquatic Animal Nutrition and Feed Science. China Agriculture Press, Beijing 2011.
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. Replacement of fish meal with fermented plant proteins in the aquafeed industry: A systematic review and meta-analysis. Rev. Aquacult. 2023, 15, 62–88. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Cheng, W.; Huang, Y.Q.; Zhang, J.S.; Cheng, X.P.; Wang, B.Z.; Yang, Q.F. Experiment on feeding pigs with fermented cotton meal instead of soybean meal. Feed Ind. 2010, 31, 23–26. [Google Scholar]
- Ma, L.; Zhang, R.J.; Cheng, F.S. Effect of fermented cottonseed meal replacing part of soybean meal on growth performance of growing pigs. Zhejiang J. Anim. Sci. Vet. Med. 2012, 37, 3–6. [Google Scholar]
- Ding, X.L. Applied study on fermented rapeseed meal in animal production. Master’s Thesis, Anhui Agricultural University, Hefei, 2011. [Google Scholar]
- Sun, H.; Ye, Y.B.; Yao, X.H.; Wu, Y.F.; Wang, X.; Liu, Y. Effects of partial replacement of fish meal with fermented cottonseed meal on growth performance, body composition and plasma biochemical indexes of juvenile black sea bream. Chin. J. Anim. Nutr. 2014, 5, 1238–1245. [Google Scholar]
- Wu, G.D.; Lan, K.P.; Cheng, X.; Wang, Y.; Zhou, C.P.; Lin, H.Z.; Ma, Z.H.; Wang, J. Effects of replacement of fish meal by fermented cottonseed meal on growth performance, feed utilization and intestinal bacteria community of juvenile golden pompano (Trachinotus ovatus). South China Fish. Sci. 2023, 19, 126–138. [Google Scholar]
- Hong; Sun; Jiang, W; Tang, X. Effects of replacement of fish meal with fermented cottonseed meal on growth performance, body composition and hemolymph indexes of Pacific white shrimp, Litopenaeus vannamei Boone, 1931. Aquacult. Res. 2016, 47, 2623–2632. [Google Scholar] [CrossRef]
- Luo, Y.; Ai, Q.; Mai, K.; Zhang, W.; Xu, W.; Zhang, Y. Effects of dietary rapeseed meal on growth performance, digestion and protein metabolism in relation to gene expression of juvenile cobia (Rachycentron canadum). Aquaculture 2012, 69, 109–116. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.F.; Li, J.; He, S.; Dou, Y.Q. Effects of rapeseed meal instead of fish meal on intestinal absorption and amino acid metabolism of Siniperca chuatsi. J. Huazhong Agric. Univ. 2018, 37, 9. [Google Scholar]
- Gao, D.Y.; Li, L.M.; Xu, F.Z.; Zhang, B.H.; Wu, S.H. Study on microbial solid-state anaerobic fermentation of rapeseed meal. Food Ferment. Ind. 2010, 36, 75–79. [Google Scholar]
- Mazumder; Anisha, D. ; Plessis; Jeanetta; Dwivedi; Anupma. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.; Basuini, M.E.; El-Hais, A.M.; Olivier, A. Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquacult. 2018, 3, 26–37. [Google Scholar] [CrossRef]
- Dr, J.R.; Dayal, J.S.; Kondusamy, A.; Yuvaphuspa, R.; Muralidhar, M. Evaluation of fungal fermented rapeseed meal as a fishmeal substitute in the diet of Penaeus vannamei. J. Coastal. Res. 2019, 86, 82–89. [Google Scholar]
- Yang, X.; Ye, J.Y.; Zhang, Y.X.; Wu, C.L.; Liu, P.; Wang, W. Effects of fish meal replacement by common cottonseed meal and fermented cottonseed meal on growth performance, body composition and hepatopancreas digestive enzyme activities of juvenile Chinese mitten crab (Eriocheir sinensis). Chin. J. Anim. Nutr. 2014, 6, 11–19. [Google Scholar]
- Wu, Z.; Liu, J.; Chen, J.; Pirzado, S.A.; Liu, G. Effects of fermentation on standardized ileal digestibility of amino acids and apparent metabolizable energy in rapeseed meal fed to broiler chickens. Animals 2020, 10, 63–72. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official methods of analysis of AOAC international. Association of Official Analytical Chemists, Washington DC, USA 2005.
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fenglu, H.; Junzhe, Q.; Yayu, Q.; Zhao, L.; Hu, C.; Chang, X.; Haitao, Z.; G., Q.J.; Liqiao, C.; Erchao, L. Partial replacement of soybean meal with fermented cottonseed meal in a low fishmeal diet improves the growth, digestion and intestinal microbiota of juvenile white shrimp Litopenaeus vannamei. Aquacult. Rep. 2022, 27, 128–162. [Google Scholar]
- Stanislav, S.; Olga, K.; Svetlana, I.; Alexander, P.; Olesia, K.; Olga, K.; Nataly, F.; Olga, B. Evaluating the influence of microbial fermentation on the nutritional value of soybean meal. Fermentation 2022, 8, 458–458. [Google Scholar] [CrossRef]
- Bi, H.; Zhao, H.; Lu, F.; Zhang, C.; Bie, X.; Lu, Z. Improvement of the nutritional quality and fibrinolytic enzyme activity of soybean meal by fermentation of bacillus subtilis. J. Food. Process. Pres. 2015, 35, 1235–1242. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Li, Z.Z.; Liu, B.; Liu, Z.H.; Sun, C.X.; Zhou, Q.L.; Zheng, X.C. Fermented feed on growth performance, antioxidant capacity and intestinal microorganisms of Macrobrachium nipponense. J. Hydrobio. 2023, 47, 1435–1445. [Google Scholar]
- Yao, H.X.; Cheng, X.R.; Yuan, H.W.; Luo, K.; Fang, L.; Cheng, Y.; Gao, W.H.; Tian, J.; Liu, Y.S. Effects of fermented feed on growth performance, muscle quality, antioxidant capacity and intestinal microbial community of Procambarus clarkii. J. Fish. China. 2023, 47, 123–136. [Google Scholar]
- Yong-Jun, D.; Guang-Zhen, J.; Wen-Bin, L.; Prudence, A.K.; Ding-Dong, Z.; Xiang-Fei, L.; Cheng, C.; Wen-Bin, L. Evaluation of dietary linoleic acid on growth as well as hepatopancreatic index, lipid accumulation oxidative stress and inflammation in Chinese mitten crabs (Eriocheir sinensis). Aquacult. Rep. 2022, 22, 137–253. [Google Scholar]
- Chen, X.; Wang, J.; Yue, W.; Liu, J.; Wang, C. Hepatopancreas transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with white hepatopancreas syndrome. Fish. Shellfish. Immun. 2017, 70, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.K.; Zhu, J.; Cai, C.; Ye, Y.; He, J. Dietary protease modulates nutrient retention efficiency and hepatopancreatic protease activity in juvenile Chinese mitten crab Eriocheir sinensis. Aquacult. Nutr. 2018, 24, 911–917. [Google Scholar] [CrossRef]
- Hao, W.; Meng, G.; Xian, Z.; Shao, J.; Lin, L.; Jian, L. Nutritional qualities of normal and precocious adult male Chinese mitten crabs (Eriocheir sinensis). Aquacult. Rep. 2019, 50, 2267–2275. [Google Scholar]
- Feng, H.; Jun, Q.; Ya, Q.; Zhao, L.; Hu, C.; Chang, X.; Hai, Z.; G., Q.J.; Liqiao, C.; Erchao, L. Partial replacement of soybean meal with fermented cottonseed meal in a low fishmeal diet improves the growth, digestion and intestinal microbiota of juvenile white shrimp Litopenaeus vannamei. Aquacult. Rep. 2022, 27, 183–228. [Google Scholar]
- Jannathulla, R.; Dayal, J.S.; Ambasankar, K.; Yuvapushpa, R.; Kumar, J.A.; Muralidhar, M. Evaluation of fungal fermented rapeseed meal as a fishmeal substitute in the diet of Penaeus vannamei. J. Coastal. Res. 2019, 86, 82–89. [Google Scholar] [CrossRef]
- Parrillataylor, D.P.; Zentenosavín, T. Antioxidant enzyme activities in pacific white shrimp (Litopenaeus vannamei) in response to environmental hypoxia and reoxygenation. Aquaculture 2011, 318, 379–383. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Guo, Z.; Zhao, Y.; Gao, Y.; Yu, T.; Chen, Y.; Zhang, D.; Wang, G. Effects of dietary oxidized fish oil on growth performance and antioxidant defense mechanism of juvenile Rhynchocypris lagowski Dybowski. Aquaculture 2019, 512, 368–734. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Liu, Z.; Huo, Y.; Guo, T.; Fang, H.; Zhang, Y.; Liu, Y.; Niu, J.; Tian, L. Effects of dietary oxidized fish oil on growth performance, antioxidant defense system, apoptosis and mitochondrial function of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2018, 500, 347–358. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro-Psychoph. 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Qian, J.; Qu, Y.; Li, Z.; Chen, H.; Xu, C.; Zhang, H.; Qin, J.G.; Chen, L.; Li, E. Partial replacement of soybean meal with fermented cottonseed meal in a low fishmeal diet improves the growth, digestion and intestinal microbiota of juvenile white shrimp Litopenaeus vannamei. Aquacult. Rep. 2022, 27, 13–39. [Google Scholar] [CrossRef]
- Kang, S.W.; Rhee, S.G.; Chang, T.S.; Jeong, W.; Choi, M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends. Mol. Med. 2005, 11, 571–578. [Google Scholar] [CrossRef]
- Zhang, B.; Xiang, B.Y. Research progress of peroxiredoxin family of antioxidant proteins. Prog. Physiol. 2004, 6, 352–355. [Google Scholar]
- Mu, C.K. Cloning and expression of Peroxiredoxin 6 and Thioredoxin 1 genes of Eriocheir sinensis. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, 2009. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).