1. Introduction
Arterial hypertension is a complex disease that affects a large part of the world population. In most cases, this disease develops slowly, for a long time without noticeable symptoms. On the other hand, complications of untreated hypertension could be life threatening [
1,
2,
3].
In 90% of cases, hypertension is primary and its cause is unknown. In 10%, arterial hypertension occurs secondary to other disorders of the body’s functioning [
4,
5]. The heart is the organ most affected by the complications of hypertension. It can cause blood flow impaired, unfavorable cardiomyocyte remodeling and hypertrophy of the left ventricular wall, which leads to heart failure [
6,
7]. An increase in blood pressure may be genetically determined, as well as it may be associated with environmental factors.
In recent years, much attention has been paid to the Wnt/β-catenin signaling pathway, which is important for maintaining the homeostasis of the body [
8,
9]. The Wnt pathway is involved in many physiological as well as disease-related processes. The most important mediator in signal transduction in the canonical pathway is β-catenin, the increase of its concentration in the cytoplasm is associated with the activation of the pathway. In the absence of stimulation, the concentration of β-catenin is kept at a low level by its degradation in proteasomes [
10,
11]. Inhibition of the degradation complex, which includes: adenomatous polyposis coli protein (APC), casein kinase 1 (CK1) and glycogen synthase kinase 3ß (GSK-3ß), by activating the Frizzled (Fzd) receptor, leads to an increase in level of β-catenin in the cytoplasm of the cell and its translocation to the nucleus, where it induces the expression of Wnt-dependent genes [
10,
12,
13].
The Wnt/β-catenin signaling pathway participates in organogenesis and tissue homeostasis, and its deregulation is associated with the pathogenesis of many human diseases, including hypertension and heart disease [
12,
14,
15,
16,
17,
18,
19]. The studies conducted so far show that the Wnt/ß-catenin signaling pathway plays a key role in the development of myocardial hypertrophy myocardial fibrosis, ventricular remodeling, heart failure and other pathophysiological processes [
20,
21].
Abnormalities in the functioning of organs directly responsible for blood pressure regulation may be related to dysregulated Wnt/β-catenin signaling [
22,
23].
Understanding the molecular mechanisms of structural and functional heart pathology in hypertension and identifying physiological and exogenous factors that can modulate the course of the disease and influence the patient's further prognosis seems particularly important for the development of modern and effective therapeutic strategies.
Considering the above, it was decided to conduct research aimed at evaluating the main elements of the Wnt/β-catenin pathway in the heart of hypertensive rats of various etiologies. The aim of the study was to immunohistochemically evaluate and compare the expression of Fzd8, WNT1, GSK-3ß and β-catenin genes in the hearts of rats with spontaneous hypertension (SHR) and DOCA-salt- induced hypertension.
2. Results
In rats from the study groups, systolic hypertension was found, which was respectively: SHR - 160.8 ± 3.3 (WKY - 122.3 ± 2.3), DOCA salt - 180.0 ± 13.0 (UNX - 126.0 ± 4.0).
2.1. Immunohistochemistry
A positive immunohistochemical reaction for Fzd8, WNT1, GSK-3β and β-catenin was observed in the hearts of all rats tested, although the severity of the reaction in control and hypertensive rats was different.
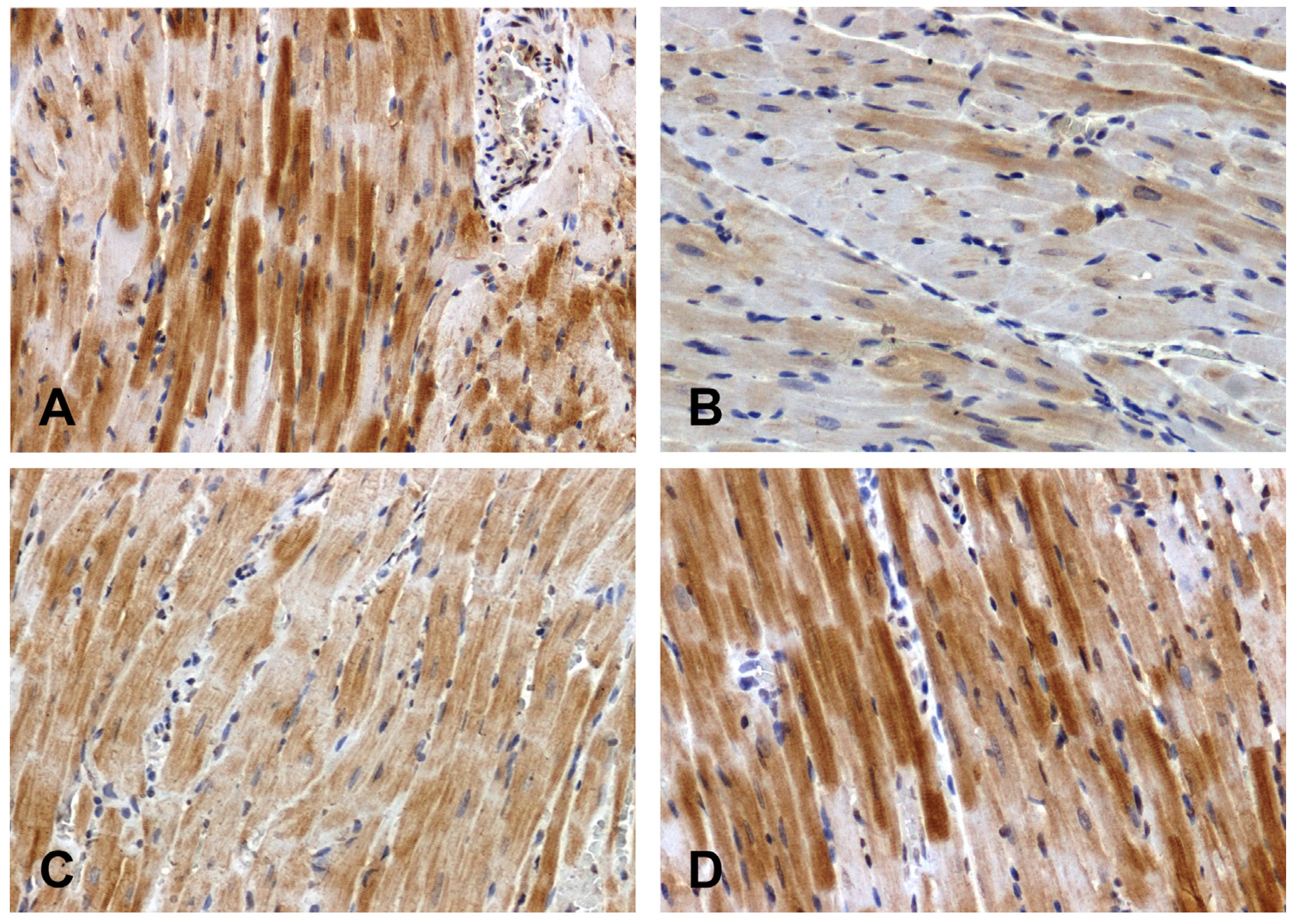
The intensity of the immunohistochemical reaction showing Fzd8 was weaker in the hearts of primary hypertensive rats (
Figure 1B) and stronger in secondary hypertensive rats (
Figure 1D) compared to control rats (
Figure 1A, 1C).
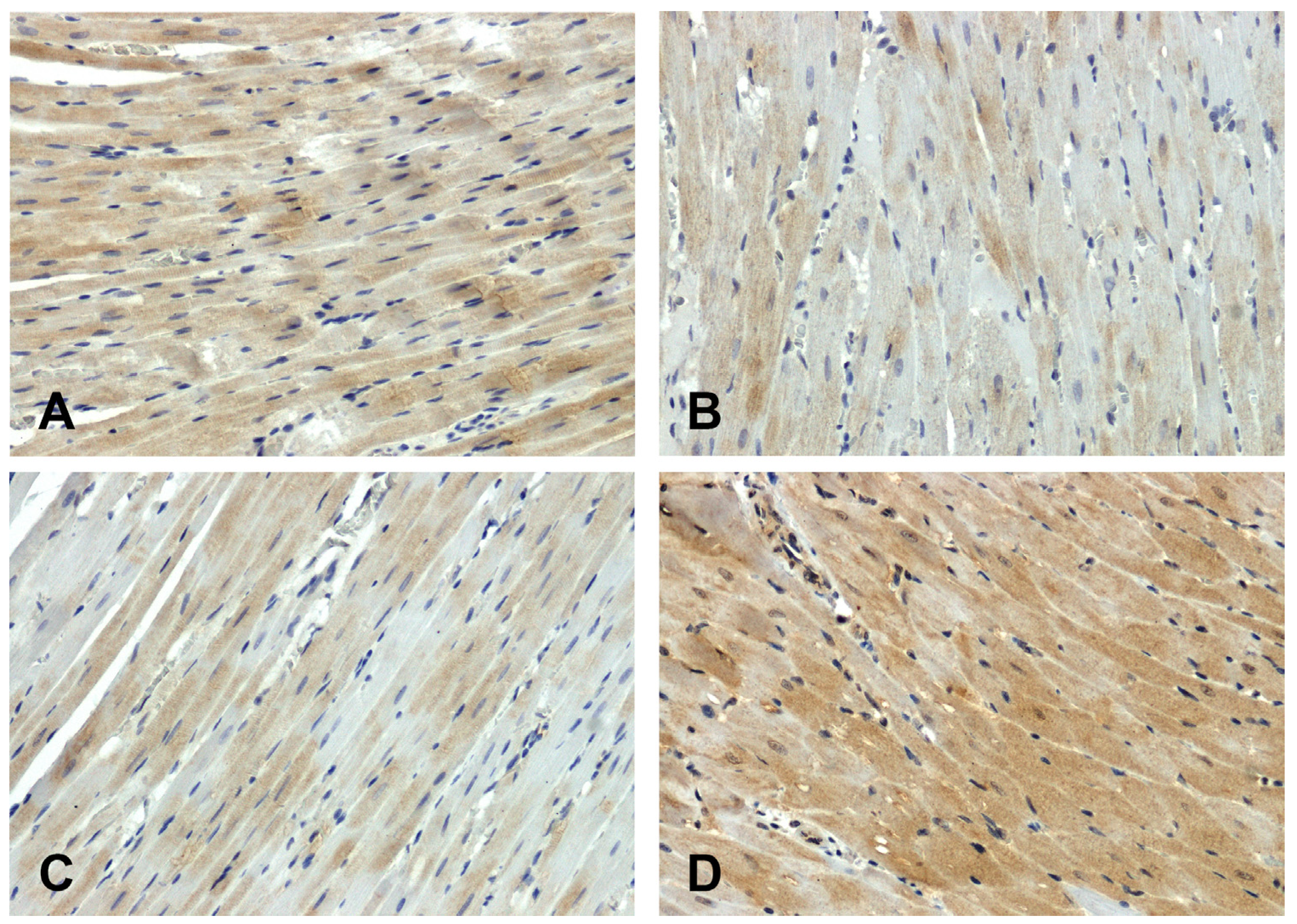
Wnt1 immunoreactivity in the hearts of normotensive rats was strong (
Figure 2A) or moderate (
Figure 2C). The intensity of the reaction in the hearts of SHR animals was attenuated (
Figure 2B) and enhanced in DOCA-salt group (
Figure 2D).
The hearts of WKY rats showed stronger GSK-3β immunodetection (
Figure 3A) compared to the observed in SHR group (
Figure 3B). The increase in secondary pressure (
Figure 3D) led to an increase in GSK-3β immunoreactivity compared to normotensive UNX (
Figure 3C).
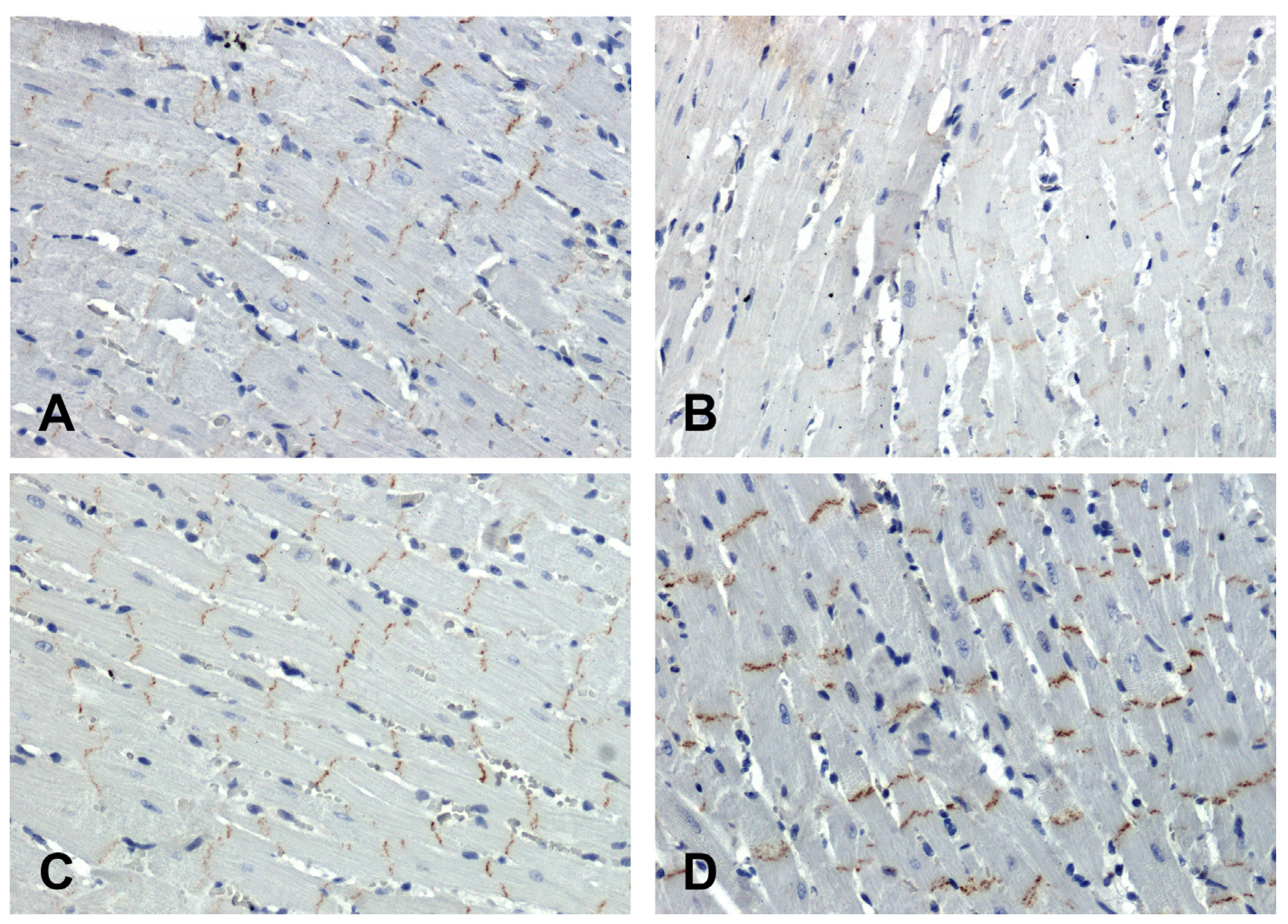
In the hearts of all rats, the immunohistochemical reaction using an antibody against β-catenin is positive in intercalated discs (
Figure 4). In SHR hearts, β-catenin immunoreactivity was attenuated (
Figure 4B) in relation to WKY (
Figure 4A), while in the hearts of secondary hypertensive rats (
Figure 4D), the reaction was enhanced compared to UNX (
Figure 4C).
Image morphometric analysis results are presented in
Table 1.
2.2. Real-Time PCR
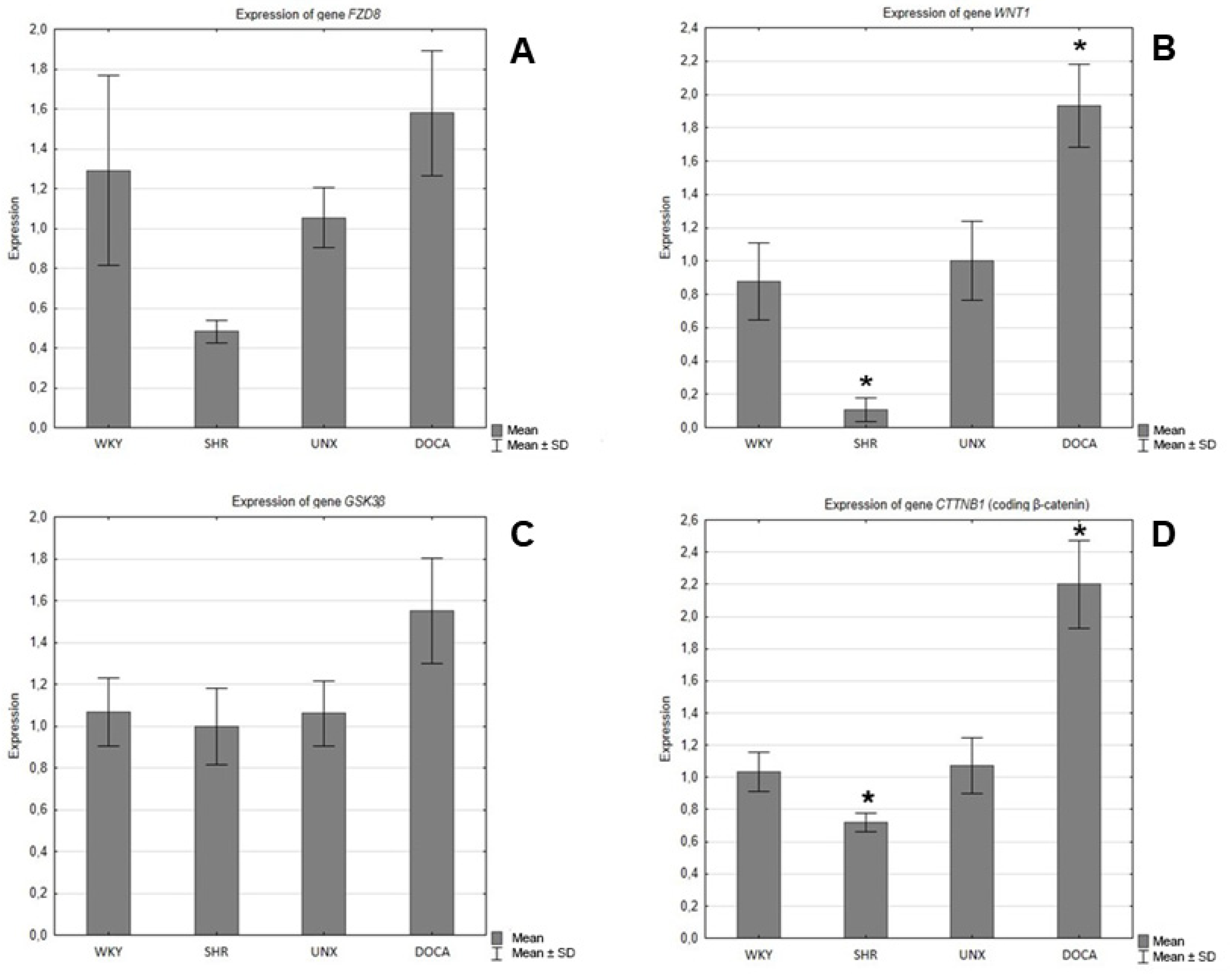
In the hearts of SHR rats, a decrease in the expression of genes encoding Fzd8, WNT1, GSK-3ß and β-catenin was observed compared to the control group (WKY) (
Figure 5 and
Table 2), and the difference was statistically significant in the case of WNT1 (
Figure 5B) and β-catenin (
Figure 5D). However, in the hearts of rats with secondary hypertension, an increase in gene expression of all tested proteins was found in relation to the control group (UNX). Statistical significance was found in the expression of genes encoding Wnt1 (
Figure 5B) and β-catenin (
Figure 5D).
3. Discussion
Many factors and molecular pathways are involved in the pathogenesis of hypertension, the determination and explanation of which is an ongoing challenge for scientists and clinicians. Understanding the mechanisms of the multifactorial origin of hypertension and various interactive regulations aimed at compensating for the action of vasoactive mediators is important in setting appropriate and effective treatment [
1]. Untreated or ineffectively treated hypertension leads to damage to many organs. An important complication of hypertension is the development of ischemic heart disease, the occurrence of cardiac arrhythmias or heart failure, which pose a serious risk of death [
6].
In this study, we examined and compared the expression pattern of Fzd8, WNT1, GSK-3β and β-catenin in the hearts of hypertensive rats of different etiology. The results of the presented studies indicate differences in the expression of elements of the canonical Wnt pathway depending on the type of hypertension. In SHR hearts, the expression of all examined parameters was reduced (statistical significance was found for Wnt1 and β-catenin), while secondary hypertension caused an increase in the expression of the investigated elements of Wnt/β-catenin signaling, especially significant for the Wnt1 ligand and β-catenin.
In addition to the proven involvement of the canonical Wnt/β-catenin signaling pathway in the process of embryogenesis and proliferation of cardiac muscle cells [
15,
16], it also plays an important role in the remodeling of the heart muscle and cardiomyocytes in pathological conditions of this organ [
8,
9,
25]. In the study of the heart after ischemia-reperfusion injury, the involvement of the Wnt/β-catenin pathway in the processes of cardiac tissue remodeling in response to injury was demonstrated. Wnt1 was upregulated in the region of injury and induced cardiac fibroblasts to proliferate and express pro-fibrotic genes, what prevents ventricular dilatation [
26].
Mutual activation or inhibition of various pathways may be associated with the occurrence of hypertension. In hypertension, impaired activation of the renin-angiotensin-aldosterone system (RAAS), which is a critical regulator of blood volume, is often observed. Dysregulation of the Wnt signaling pathway may lead to disorders of the RAAS consequently leading to disturbances in blood pressure homeostasis [
27,
28]. Research by Xiao et al. in an animal model, showed an increase in the expression of Wnt pathway genes in rat kidney in response to angiotensin II infusion. Moreover, the use of an inhibitor of the Wnt/β-catenin pathway reduced blood pressure [
29]. According to the literature, in spontaneous hypertensive rats the plasma activity of the RAAS is increased and decreased in course of secondary hypertension (DOCA-salt) [
30]. In our study, we observed a decreased expression of the Wnt/β-catenin pathway in the heart of SHR and an intense activity of this pathway in DOCA-salt rats. Based on the obtained results, we can expect the existence of other, additional mechanisms regulating the activity of the Wnt pathway in hypertensive rat hearts.
The decrease in β-catenin expression in the SHR heart presented in the current publication is consistent with the reports of Zheng et al., who also found reduced β-catenin immunoreactivity in intercalated discs of cardiomyocytes in the SHR group [
31]. Another studies conducted on an experimental model of heart failure also showed a decrease in β-catenin levels in the heart tissue of guinea pigs and hamsters [
32,
33].
Hypertrophy of cardiomyocytes is one of the main consequences of arterial hypertension. There is a large amount of evidence for the involvement of the Wnt/β-catenin pathway in cardiomyocyte remodeling processes. Research by Hirschy et al. showed that the persistent, stable peptide level, reflecting the activity of the Wnt/β-catenin pathway, caused the development of dilated cardiomyopathy, and premature death of mice [
34]. Other study demonstrated that genetic depletion of β-catenin significantly enhanced left ventricular function and survival in mice experiencing myocardial infarction, whereas stabilization of β-catenin had the opposite effects [
35]. Xiang et al. proved that cardiac fibroblasts without β-catenin expression did not produce collagen when the Wnt/β-catenin pathway was activated under pressure overload conditions. In the transgenic mouse model, this absence of β-catenin resulted in neither fibrosis nor cardiomyocyte hypertrophy [
36]. Complications of primary hypertension in rat models, such as cardiomyocyte hypertrophy may be observed due to the long-lasting state of elevated blood pressure, so the decreased activity of β-catenin could be considered as an adaptive mechanism to minimize the hypertrophy. In this way, we could try to explain the decrease in the activity of the Wnt/β-catenin system in our study in the heart of rats with essential hypertension.
Taking into account a limited number of reports indicating the relationship of the Wnt/β-catenin pathway with arterial hypertension, it is important and justified to investigate its involvement in this disease. The present study shows different changes in the Wnt/β-catenin signaling pathway in the hearts of rats with primary and secondary hypertension. This study is the first to evaluate some components of the classical Wnt/β-catenin signaling pathway in various types of hypertension using immunohistochemical and molecular methods. In our work we demonstrated that changes in the activity of the Wnt/β-catenin pathway in hypertension may occur at every level of this pathway. It should be noted that the intensity of changes in the Wnt/β-catenin pathway in the heart depends on the etiology of hypertension. The presented results may constitute the basis for further research aimed at better understanding the role of the Wnt/β-catenin pathway in the functioning of the heart, as well as the pathophysiological mechanisms leading to its dysfunction and complications in the state of elevated blood pressure. A more detailed understanding of the role of the WNT/β-catenin pathway in hypertension-associated disturbances of heart functioning requires further investigation.
4. Materials and Methods
The research material comes from the Department of Experimental Physiology and Pathophysiology, Medical University of Bialystok, courtesy of Professor Barbara Malinowska. The procedures performed on experimental animals were approved by the Local Ethics Committee for Animal Research in Olsztyn. Six week-old male animals with an initial weight of 170-200 g were kept under constant humidity (60 ± 5%) and temperature (22 ± 1 °C). A 12/12 hour light/dark cycle was maintained. The rats had free access to standard pelleted food and drinking water.
The experimental animals were divided into four groups:
SHR – seven male rats with genetically determined systemic hypertension, an inbred strain established from Wistar rats selected for high blood pressure.
WKY – five male, normotensive Wistar Kyoto rats as a reference for SHR.
DOCA-salt – seven male, Wistar rats that were uninephrectomized, then rendered hypertensive through a salt-rich diet and Deoxycorticosterone Acetate (DOCA) injection.
UNX - five male, normotensive Wistar rats that were uninephrectomized only, reference for DOCA-salt-induced hypertensive rats.
4.1. DOCA–Salt Hypertension
DOCA-salt animals were anesthetized by intraperitoneal injection of sodium pentobarbital (300 μmol or ~70 mg/kg body weight (bw)). The right kidney was removed through the right lateral abdominal incision. After a 1-week recovery period, hypertension was induced for 4 weeks by s.c. DOCA injections (67 μmol or ~25 mg/kg in 0.4 ml/kg dimethylformamide; DMF) twice weekly and replacing drinking water with 1% sodium chloride NaCl solution. Control unilateral nephrectomy (UNX) normotensive rats were also kidneyless but received DMF, 0.4 ml/kg, subcutaneous twice a week and drank tap water.
4.2. Indirect Blood Pressure Measurement
After 6 weeks, systolic blood pressure was measured in all animals by a non-invasive tail cuff method (rat tail blood pressure monitor, Hugo Sachs Elektronik-Harvard Apparatus, March-Hugstetten, Germany). Measurements were considered reliable if three consecutive measurements did not differ by more than 5 mmHg, then the average was taken. Hypertension (systolic blood pressure (SBP) values equal to or greater than 150 mmHg) was verified in SHR and DOCA-salt animals.
4.3. Collection and Fixation of Material
After 6 weeks, heart muscle fragments were collected from all rats under deep anesthesia with pentobarbital (50 mg/kg body weight). Cardiac tissue was immediately fixed in 4% buffered formalin and routinely embedded set in paraffin, or placed in RNAlater solution (AM7024 Thermo Fisher, Waltham, MA, USA) and stored at -80 °C. Paraffin blocks were cut into 4 μm sections, stained with hematoxylin and eosin (for general histological evaluation) and immunohistochemical reactions were performed to Fzd8, Wnt1, GSK-3β, and β-catenin detection. Samples stored in RNAlater solution were analyzed by real-time PCR to assess the expression of genes encoding Fzd8, Wnt1, GSK-3β and β-catenin.
4.4. Immunohistochemistry
Immunostaining was made by the following protocol (Kasacka et al., 2018) [
24]: paraffin-embedded sections were deparaffined and hydrated in pure alcohols. The sections of left ventricle of the heart tissue were subjected to pretreatment in a pressure chamber and heated using appropriate: Target Retrieval Solution Citrate pH=6.0 (S 2369; Agilent Technologies, Inc. Santa Clara, CA, USA) (for β-catenin) and TRS pH=9.0 (S 2367; Agilent Technologies, Inc. Santa Clara, CA, USA) (for Fzd8, Wnt1 and GSK-3β). After cooling down to room temperature, the sections were incubated with Dako REAL Peroxidase-Blocking Solution (S 2023; Agilent Technologies, Inc. Santa Clara, CA, USA). The sections with the primary antibodies: Fzd8 (1:400; ab155093, Abcam, Cambridge, UK), Wnt-1 (1:500; ab189001, Abcam, Cambridge, UK), GSK-3β (1:100; ab68476, Abcam, Cambridge, UK) β-catenin (1:2000; ab32572, Abcam, Cambridge, UK) and were incubated 24 hours at +4ºC in a humidified chamber. Procedure was followed by incubation with secondary antibody (REAL™ EnVision™ Detection System, Peroxidase/DAB, Rabbit/Mouse detection kit (K5007; Agilent Technologies Denmark Ap/S, Produktionsvej 42, 2600 Glostrup, Denmark). The bound antibodies were visualized by incubation with DAB Flex chromogen. Finally, the left ventricle of the heart sections were counterstained in hematoxylin QS (H-3404 Vector Laboratories, Burlingame, CA, USA) and observed under a light microscope. The sections were dehydrated and the specificity of the antibodies was confirmed using a negative control, where the antibodies were replaced by Antibody Diluent (S3022; Agilent Technologies Denmark Ap/S, Produktionsvej 42, 2600 Glostrup, Denmark). The staining results were evaluated in an Olympus BX43 light microscope (Olympus 114 Corp., Tokyo, Japan) with an Olympus DP12 digital camera (Olympus 114 Corp., Tokyo, Japan) and documented.
4.5. Real-Time PCR
Tissue fragments taken from the left ventricles of the rats' hearts were placed in an RNA-later solution. Total RNA was isolated using NucleoSpin® RNA Isolation Kit (Machery-Nagel). Quantification and quality control of total RNA was determined using a spectrophotometer - NanoDrop 2000 (ThermoScientific). An aliquot of 1 µg of total RNA was reverse transcribed into cDNA using iScript™ Advanced cDNA Synthesis Kit for RT-qPCR (BIO-RAD). Synthesis of cDNA was performed in a final volume of 20 μl using a Thermal Cycler (Model SureCycler 8800, Aligent Technologies). For reverse transcription, the mixtures were incubated at 46°C for 20 min, then heated to 95°C for 1 min and finally cooled quickly at 4°C. Quantitative real-time PCR reactions were performed using Stratagene Mx3005P (Aligent Technologies) with the SsoAdvanced™ Universal SYBER® Green Supermix (BIO-RAD). Specific primers for FZD8 (FZD8), Wnt1 (WNT1), Gsk3b (GSK3B), Ctnnb1 (CTNNB1) and GAPDH (GAPDH) were designed by BIO-RAD Company. The housekeeping gene GAPDH (GAPDH) was used as a reference gene for quantification. To determine the amounts of levels of test genes expression, standard curves were constructed for each gene separately with serially diluted PCR products. PCR products were obtained by cDNA amplification using specific primers as follows: FZD8 (qRnoCED0054913, BIO-RAD), WNT1 (qRnoCED0003949, BIO-RAD), GSK3β (qRnoCID0001683, BIO-RAD), CTNNB1 (qRnoCID0053256, BIO-RAD) and GAPDH (qRnoCID0057018, BIO-RAD). QRT-PCR was carried out in a doublet in a final volume of 20 μl under the following conditions: 2 min polymerase activation at 95°C, 5 s denaturation at 95°C, 30 s annealing at 60°C for 35 cycles. PCR reactions were checked, including no-RT-controls, omitting of templates, and melting curve to ensure only one product was amplified. The relative quantification of gene expression was determined by comparing Ct values using the ΔΔCt method. All results were normalized to GAPDH.
4.6. Measurement of the Intensity of the Immunohistochemical Reaction
Sections of the heart were made from each animal for immunohistochemistry showing Fzd8, Wnt1, GSK-3β and β-catenin. Five randomly selected microscopic fields (each field 0.785 mm2, 200× magnification (20× lens and 10× eyepiece)) from each heart section were documented using an Olympus DP12 microscope camera. Nikon’s NIS Elements Advanced Research microscopy image analysis software, version 3.10 was used to evaluate digital images of the heart samples. The average optical density of the examined objects was measured to assess the intensity of the immunohistochemical reaction. The intensity of the immunohistochemical reaction for Fzd8, Wnt1, GSK-3β and β-catenin was measured in each image and quantified using a grayscale level of 0–256. A value of 0 means a completely white pixel, i.e., minimum light saturation, while 256 means a completely black pixel, i.e., maximum light saturation.
4.7. Statistical Analysis
All data collected for individual rats were collected and assigned to two control groups (WKY, UNX) and two treatment groups (SHR, DOCA-salt). For measurable features, the arithmetic mean and standard error (SE) were calculated. Then, using the STATISTICA 13.3 computer package, a statistical analysis was performed using a one-way ANOVA test. Fisher's Least Significant Differences test was used to perform post-hoc analysis. The level of statistical significance was assumed to be p<0.05.
Author Contributions
Conceptualization, I.K.; methodology, I.K. M.M. and N.D.; validation, I.K., M.M. and N.D.; formal analysis, I.K.; investigation, I.K., M.M. and N.D.; resources, I.K.; data curation, I.K., M.M., N.D.; writing—original draft preparation, M.M.; writing—review and editing, I.K.; visualization, I.K., M.M.; supervision, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Medical University of Bialystok grant number SUB/1/DN/22/001/2232 and B.SUB.23.146
Institutional Review Board Statement
The procedures performed on experimental animals were approved by the Local Ethics Committee for Animal Research in Olsztyn.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
APC, adenomatous polyposis coli protein; CK1, casein kinase 1; GSK-3ß, glycogen synthase kinase 3ß; FZD receptors, Frizzled receptors; SHR, Spontaneously Hypertensive Rat; DOCA, Deoxycorticosterone Acetate; WKY rats, Wistar Kyoto rats; UNX. uninephrectomy/unilateral nephrectomy; DMF, Dimethyloformamide; SBP, systolic blood pressure; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PCR, polymerase chain reaction; RAAS, renin-angiotensin-aldosterone system;
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F. et al. Hypertension. Nat Rev Dis Primers. 2018 Mar 22;4:18014.
- Iqbal, A.M.; Jamal, S.F. Essential Hypertension. 2023 Jul 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology (Bethesda). 2017 Mar;32(2):112-125. [CrossRef]
- Charles, L.; Triscott, J.; Dobbs, B. Secondary Hypertension: Discovering the Underlying Cause. Am Fam Physician. 2017 Oct 1;96(7):453-461.
- Kasacka, I.; Piotrowska, Z.; Lewandowska A. Alterations of rat stomach endocrine cells under renovascular hypertension. Adv Med Sci. 2014 Sep;59(2):190-5. [CrossRef]
- Nadar, S.K.; Lip, G.Y.H. The heart in hypertension. J Hum Hypertens. 2021 May;35(5):383-386.
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017 Aug;5(8):543-551.
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol Rev. 2018 Jan;70(1):68-141.
- Arnold, A.C.; Robertson, D. Defective Wnt Signaling: A Potential Contributor to Cardiometabolic Disease? Diabetes. 2015 Oct;64(10):3342-4.
- Koziński, K.; Dobrzyń, A. Szlak sygnałowy Wnt i jego rola w regulacji metabolizmu komórki. Postepy Hig Med Dosw 11. 2013; 67.
- Park, H.B.; Kim, J.W.; Baek, K.H. Regulation of Wnt Signaling through Ubiquitination and Deubiquitination in Cancers. Int J Mol Sci. 2020 May 30;21(11):3904. [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009 Jul;17(1):9-26.
- Młynarczyk, M.; Kasacka, I. The role of the Wnt / β-catenin pathway and the functioning of the heart in arterial hypertension - a review. Adv Med Sci. 2022 Mar;67(1):87-94.
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017 Jun 1;169(6):985-999. https://doi.org/10.1016/j.cell.2017.05.016. [CrossRef]
- Gessert, S.; Kühl, M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010 Jul 23;107(2):186-99. [CrossRef]
- Pahnke, A.; Conant, G.; Huyer, L.D.; Zhao, Y.; Feric, N.; Radisic, M. The role of Wnt regulation in heart development, cardiac repair and disease: A tissue engineering perspective. Biochem Biophys Res Commun. 2016 May 6;473(3):698-703. [CrossRef]
- Fu, W.B.; Wang, W.E.; Zeng, C.Y. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharmacol Sin. 2019 Jan;40(1):9-12. [CrossRef]
- Ruiz-Villalba, A.; Hoppler, S.; Van den Hoff, M.J. Wnt signaling in the heart fields: Variations on a common theme. Dev Dyn. 2016 Mar;245(3):294-306. [CrossRef]
- Ozhan, G.; Weidinger, G. Wnt/β-catenin signaling in heart regeneration. Cell Regen. 2015 Jul 8;4(1):3. [CrossRef]
- Umbarkar, P.; Ejantkar, S.; Tousif, S.; Lal, H. Mechanisms of Fibroblast Activation and Myocardial Fibrosis: Lessons Learned from FB-Specific Conditional Mouse Models. Cells. 2021 Sep 14;10(9):2412. [CrossRef]
- Zheng, X.; Gao, Q.; Liang, S.; Zhu, G.; Wang, D.; Feng, Y. Cardioprotective Properties of Ginkgo Biloba Extract 80 via the Activation of AKT/GSK3β/β-Catenin Signaling Pathway. Front Mol Biosci. 2021 Nov 3;8:771208. [CrossRef]
- Kasacka, I.; Piotrowska, Ż.; Niezgoda, M.; Lewandowska, A.; Łebkowski, W. Ageing-related changes in the levels of β-catenin, CacyBP/SIP, galectin-3 and immunoproteasome subunit LMP7 in the heart of men. PLoS One. 2020 Mar 2;15(3):e0229462. [CrossRef]
- Lee, C.Y.; Kuo, W.W.; Baskaran, R.; Day, C.H.; Pai, P.Y.; Lai, C.H. et al. Increased β-catenin accumulation and nuclear translocation are associated with concentric hypertrophy in cardiomyocytes. Cardiovasc Pathol. 2017 Nov-Dec;31:9-16. [CrossRef]
- Kasacka, I.; Piotrowska, Ż.; Weresa, J.; Filipek, A. Comparative evaluation of CacyBP/SIP protein, β-catenin, and immunoproteasome subunit LMP7 in the heart of rats with hypertension of different etiology. Exp Biol Med (Maywood). 2018 Nov;243(15-16):1199-1206. [CrossRef]
- Marinou, K.; Christodoulides, C.; Antoniades, C.; Koutsilieris, M. Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab. 2012 Dec;23(12):628-36. [CrossRef]
- Duan, J.; Gherghe, C.; Liu, D.; Hamlett, E.; Srikantha, L.; Rodgers, L. et al. Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012 Jan 18;31(2):429-42.
- Zhou, L.; Li, Y.; Hao, S.; Zhou, D.; Tan, R.J.; Nie, J. et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol. 2015 Jan;26(1):107-20. [CrossRef]
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System. 2023 Mar 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- Xiao, L.; Xu, B.; Zhou, L.; Tan, R.J.; Zhou, D.; Fu, H. et al. Wnt/β-catenin regulates blood pressure and kidney injury in rats. Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1313-1322. [CrossRef]
- Clozel, J.P.; Müller, R.K.; Roux S.; Fischli W.; Baumgartner H.R. Influence of the status of the renin-angiotensin system on the effect of cilazapril on neointima formation after vascular injury in rats. Circulation 1993 88 1222–1227. [CrossRef]
- Zheng, Q.; Chen, P.; Xu, Z.; Li, F.; Yi, X.P. Expression and redistribution of β-catenin in the cardiac myocytes of left ventricle of spontaneously hypertensive rat. J Mol Histol. 2013 Oct;44(5):565-73n and redistribution of β-catenin in the cardiac myocytes of left ventricle of spontaneously hypertensive rat. J Mol Histol. 2013 Oct;44(5):565-73. [CrossRef]
- Wang, X.; Gerdes, A.M. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol. 1999 Feb;31(2):333-43. [CrossRef]
- Yoshida, M.; Ohkusa, T.; Nakashima, T.; Takanari, H.; Yano, M.; Takemura, G. et al. Alterations in adhesion junction precede gap junction remodelling during the development of heart failure in cardiomyopathic hamsters. Cardiovasc Res. 2011 Oct 1;92(1):95-105. [CrossRef]
- Hirschy A.; Croquelois A.; Perriard E.; Schoenauer R.; Agarkova I.; Hoerstrup S.P. et al. Stabilised beta-catenin in postnatal ventricular myocardium leads to dilated cardiomyopathy and premature death. Basic Res Cardiol 2010;105:597–608. [CrossRef]
- Zelaraya ́n L.C.; Noack C.; Sekkali B.; Kmecova J.; Gehrke C.; Renger A. et al. Beta-Catenin down-regulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentia- tion. Proc Natl Acad Sci U S A 2008;105:19762–7.
- Xiang F.L.; Fang M.; Yutzey K.E. Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun 2017;8(1): 712. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).