Submitted:
18 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Trichoderma as a Biocontrol Agent
3. Mechanism of Action of Trichoderma
3.1. Mycoparasitism
3.2. Mycovirus-Mediated Cross-Protection (MMCP)
3.3. Synthesis of Cell Wall Degrading Compounds (CWDCs)
3.4. Production of Antibiotics and Other Antifungal Compounds
3.5. Competition for Vital Nutrients and Space
3.6. Induction of Plant Resistance
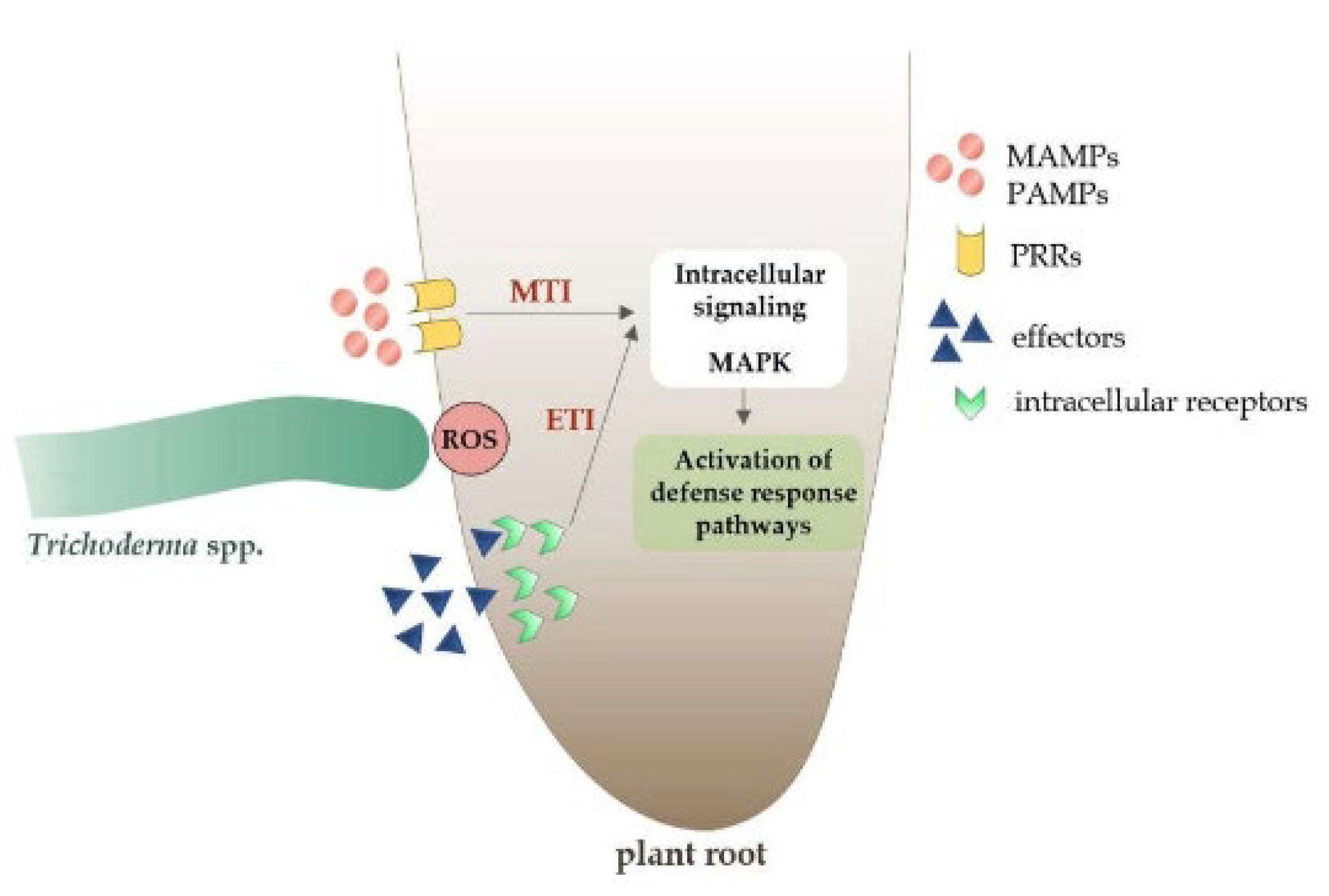
3.7. Plant Growth Promotion
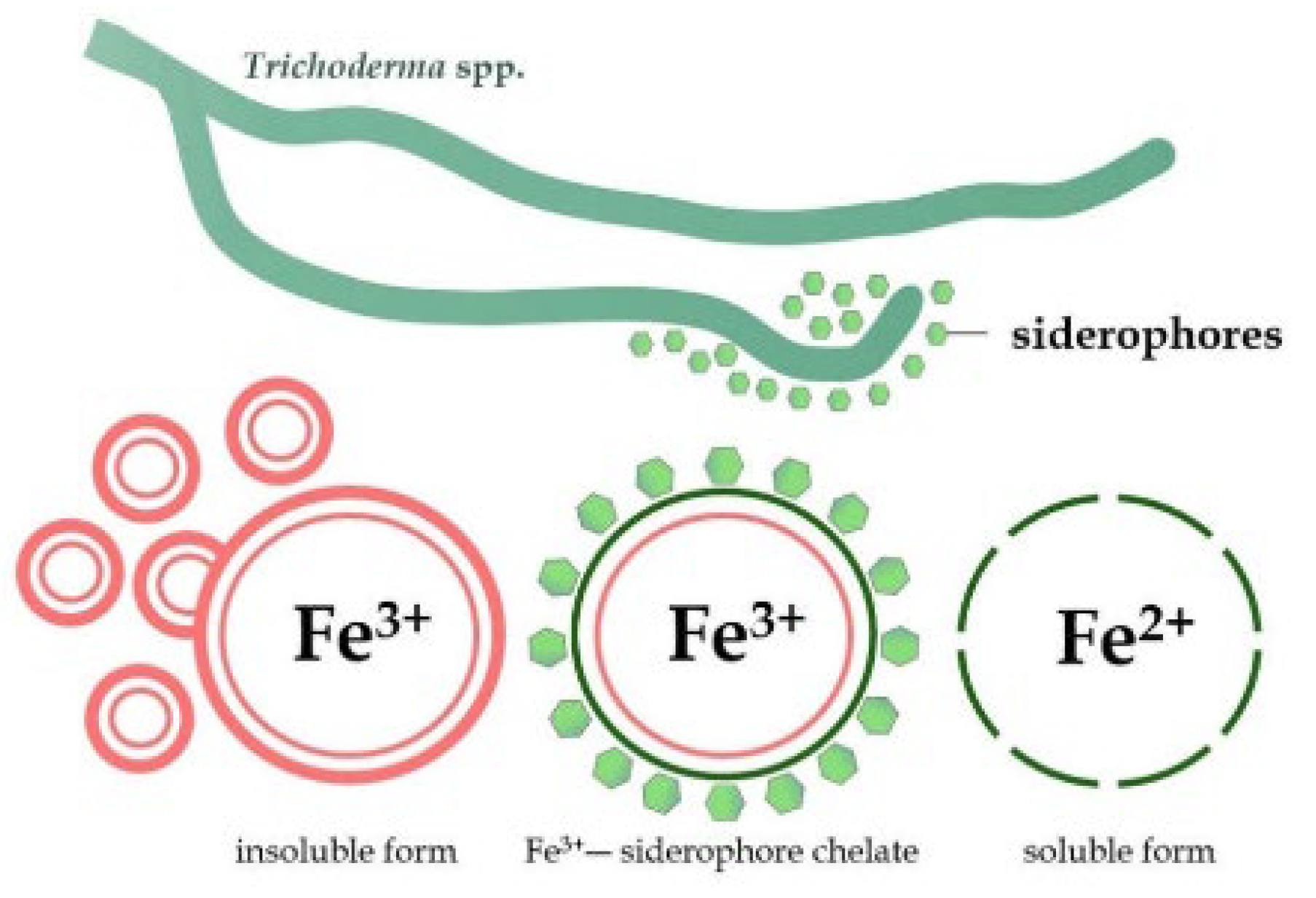
3.7.1. Mineral Solubilisation
3.7.2. Biological Nitrogen Fixation
3.7.3. Phytohormone Level Control
3.7.4. Modification of Plant's Environmental Conditions
4. Prospects of Trichoderma in Plant Disease Control
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abade, A.; Ferreira, P.A.; de Barros Vidal, F. Plant diseases recognition on images using convolutional neural networks: A systematic review. Computers and Electronics in Agriculture 2021, 185, 106125. [Google Scholar] [CrossRef]
- Oyesola, O.L.; Aworunse, O.S.; Oniha, M.I.; Obiazikwor, O.H.; Bello, O.; Atolagbe, O.M.; Obembe, O.O. Impact and Management of Diseases of Solanum tuberosum. IntechOpen 2021. [Google Scholar] [CrossRef]
- Ashwini, T.; Srinivas, A.; Mallikarjuna, G. CRISPR-Cas genome editing system: A versatile tool for developing disease resistant crops. Plant Stress 2022, 3, 100056. [Google Scholar] [CrossRef]
- Pooja, C.; Pooja, R.A.; Sumi, R.; Radhakrishnan, N.; Mehanathan, M. Molecular and metabolomic interventions for identifying potential bioactive molecules to mitigate diseases and their impacts on crop plants. Physiological and Molecular Plant Pathology 2021, 114, 101624. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Natural Ecology and Evolution 2019, 3, 430–439. [Google Scholar] [CrossRef]
- van Esse, R.; van der, D.; van Esse, T.L.; Reuber, D. Genetic modification to improve disease resistance in crops. New Phytology 2020, 225, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases - A review. Ecological Complexity 2022, 49, 100978. [Google Scholar] [CrossRef]
- Menka, T.; Debasish, P.; Reecha, M.; Binod, B.S.; Prashant, S. The Impact of Microbes in Plant Immunity and Priming Induced Inheritance: A Sustainable Approach for Crop protection. Plant Stress 2022, 4, 100072. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. The Egyptian Journal of Aquatic Research 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Saheem, R.; Tanveer, R.; Khalid, M.G. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chemical Engineering Journal Advances 2022, 11, 100301. [Google Scholar] [CrossRef]
- Tina, R.; Anuradha, B.; Parshuram, J.S.; Sukanta, M.; Nitish, R.M.; Shariful, A.; Nirmalendu, D. Bio-effective disease control and plant growth promotion in lentil by two pesticide degrading strains of Bacillus sp. Biological Control 2018, 127, 55–63. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Figlan, S.; Ntushelo, K.; Mwadzingeni, L.; Terefe, T.; Tsilo, T.J.; Shimelis, H. Breeding Wheat for Durable Leaf Rust Resistance in Southern Africa: Variability, Distribution, Current Control Strategies, Challenges, and Future Prospects. Frontier of Plant Science 2020, 11, 549. [Google Scholar] [CrossRef]
- Olowe, O.M.; Lidia, N.; Michael, D.A.; Akinlolu, O.A.; Olubukola, O.B. Trichoderma: Potential bio-resource for the management of tomato root rot diseases in Africa. Microbiological Research 2022, 257, 126978. [Google Scholar] [CrossRef]
- Oyesola, O.L.; Sobowale, A.A.; Obembe, O.O. Effectiveness of Trichoderma koningii Extract on Aspergillus Species Isolated from Rotting Tomato (Solanum lycopersicum Mill). Tropical Journal of Natural Products and Research 2020, 4, 961–965. [Google Scholar] [CrossRef]
- Kuzmanovska, B.; Rusevski, R.; Jankulovska, M.; Oreshkovikj, K. Antagonistic activity of Trichoderma asperellum and Trichoderma harzianum against genetically diverse Botrytis cinerea isolates. Chilean Journal of Agricultural Research 2018, 78, 391–399. [Google Scholar] [CrossRef]
- Filizola, P.R.B.; Luna, M.A.C.; de Souza, A.F.; Coelho, I.L.; Laranjeira, D.; Campos–Takaki, G.M. Biodiversity and phylogeny of novel Trichoderma isolates from mangrove sediments and potential of biocontrol against Fusarium strains. Microbial Cell Fact 2019, 18, 89. [Google Scholar] [CrossRef]
- Manandhar, S.; Pant, B.; Manandhar, C.; Baidya, S. In-vitro evaluation of biocontrol agents against soil borne plant pathogens. J. Nep. Agric. Res. Counc 2019, 5, 68–72. [Google Scholar] [CrossRef]
- Kohl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Frontier of Plant Science 2019, 10, 845. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases-A Review. Plants (Basel, Switzerland) 2023, 12, 432. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. International journal of molecular sciences 2022, 23, 2329. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases - A review. Ecological Complexity 2022, 49, 100978. [Google Scholar] [CrossRef]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as alternative fungicides for controlling plant diseases: A comprehensive review of their efficacy, commercial representatives, advantages, challenges for adoption, and possible solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef]
- Grovermann, C.; Schreinemachers, P.; Berger, T. Quantifying pesticide overuse from farmer and societal points of view: An application to Thailand. Crop Protection 2013, 53, 161–168. [Google Scholar] [CrossRef]
- Srinivasan, R.; Tamò, M.; Subramanian, S. The case for integrated pest management in Africa: Transition from a pesticide-based approach. Current Opinion in Insect Science 2022, 54, 100970. [Google Scholar] [CrossRef]
- Pimentão, A.R.; Cuco, A.P.; Pascoal, C.; Cássio, F.; Castro, B.B. Current trends and mismatches on fungicide use and assessment of the ecological effects in freshwater ecosystems. Environmental Pollution 2024, 347, 123678. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Y.; Jiang, M.; You, Q.; Ma, X.; Xu, Z.; Bo, X. Unveiling the importance of VOCs from pesticides applicated in main crops for elevating ozone concentrations in China. Journal of Hazardous Materials 2024, 465, 133385. [Google Scholar] [CrossRef]
- Anuagasi, C.L.; Okigbo, R.N.; Anukwuorji, C.A.; Okereke, C.N. The Impact of Biofungicides on Agricultural Yields and Food Security in Africa. International Journal of Agricultural Technology 2017, 13, 953–978. [Google Scholar]
- Patrick, B.; Paul, E.; Gerardina, U. Incorporation of microorganisms to reduce chemical fungicide usage in black sigatoka control programs in Costa Rica by use of biological fungicides. Crop Protection 2021, 146, 105657. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability. 2022, 14, 12786. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; María, D.P.; Vianey, O.; Alfredo, H. Trichoderma species: versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: potential applications for management of Next-Gen agricultural challenges. Journal of Chemical Technology & Biotechnology 2018, 93, 2056–2063. [Google Scholar]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biological Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Rauf, A.; Subhani, M.N.; Siddique, M.; Shahid, H.; Chattha, M.B.; Alrefaei, A.F.; Hasan Naqvi, S.A.; Ali, H.; Lucas, R.S. Cultivating a greener future: Exploiting trichoderma derived secondary metabolites for fusarium wilt management in peas. Heliyon 2024, 10, e29031. [Google Scholar] [CrossRef] [PubMed]
- Prasun, K.; Mukherjee, A.M.; Susanne, Z.; Benjamin, A.H. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biology Reviews 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Frontiers of Microbiology 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal Viruses Unveiled: A Comprehensive Review of Mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The "secrets" of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; Del-Val, E.; Larsen, J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiological Research 2020, 240, 126552. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biological Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; Voltan, A.R.; Carraro, C.B.; Steindorff, A.S.; Goldman, G.H.; Silva, R.N.; Ulhoa, C.J.; Monteiro, V.N. Endo-β-1,3-glucanase (GH16 Family) from Trichoderma harzianum Participates in Cell Wall Biogenesis but Is Not Essential for Antagonism Against Plant Pathogens. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Ha, T.T.T. Characterization and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2020, 11, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Goughenour, K.D.; Whalin, J.; Slot, J.C.; Rappleye, C.A. Diversification of Fungal Chitinases and Their Functional Differentiation in Histoplasma capsulatum. Molecular Biology and Evolution 2021, 38, 1339–1355. [Google Scholar] [CrossRef] [PubMed]
- Vicente, I.; Baroncelli, R.; Hermosa, R.; Monte, E.; Vannacci, G.; Sarrocco, S. Role and genetic basis of specialised secondary metabolites in Trichoderma ecophysiology. Fungal Biology Reviews 2022, 39, 83–99. [Google Scholar] [CrossRef]
- Volkov, P.; Rubtsova, E.; Rozhkova, A.; Sinitsyna, O.; Zorov, I.; Kondratyeva, E.; Sinitsyn, A. Properties of recombinant endo-β-1,6-glucanase from Trichoderma harzianum and its application in the pustulan hydrolysis. Carbohydrate Research 2020, 499, 108211. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Frontiers of Microbiology 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Gueye, N.; Kumar, G.K.; Ndiaye, M.; Sall, S.Y.D.; Ndiaye, M.A.F.; Diop, T.A.; Ram, M.R. Factors affecting the chitinase activity of Trichoderma asperellum isolated from agriculture field soils. Joural of Applied Biology and Biotechnology. 2020, 8, 41–44. [Google Scholar] [CrossRef]
- Olasehinde, G.I.; Okolie, Z.V.; Oniha, M.I.; Adekeye, B.T.; Ajayi, A.A. In vitro antibacterial and antifungal activities of Chrysophyllum albidum and Diospyros monbuttensis leaves. Journal of Pharmacognosy and Phytotherapy 2016, 8, 1–7. [Google Scholar] [CrossRef]
- De Zotti, M.; Sella, L.; Bolzonello, A.; Gabbatore, L.; Peggion, C.; Bortolotto, A.; Elmaghraby, I.; Tundo, S.; Favaron, F. Targeted amino acid substitutions in a Trichoderma peptaibol confer activity against fungal plant pathogens and protect host tissues from Botrytis cinerea infection. International Journal Molecular Sciences 2020, 21, 7521. [Google Scholar] [CrossRef]
- Deng, J.; Huang, W.; Li, Z.; Lu, D.; Zhang, Y.; Luo, X. Biocontrol activity of recombinant aspartic protease from Trichoderma harzianum against pathogenic fungi. Enzyme and Microbial Technology 2018, 112, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.; Hirpara, D.G.; Savaliya, D.D.; Golakiya, B. Extracellular metabolomics of Trichoderma biocontroller for antifungal action to restrain Rhizoctonia solani Kuhn in cotton. Physiological and Molecular Plant Pathology 2020, 112, 101547. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, Q.; Li, W.; Gao, L.; Liu, G. Strain improvement of Trichoderma harzianum for enhanced biocontrol capacity: Strategies and prospects. Frontiers in Microbiology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Pablo, J. Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. Journal of Fungi 2022, 8, 1037. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Marik, T.; Zafari, D.; Balázs, D.; Vágvölgyi, C.; Szekeres, A.; Kredics, L. Changes in Peptaibol Production of Trichoderma Species during In Vitro Antagonistic Interactions with Fungal Plant Pathogens. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Dias, L.; Oliveira-Pinto, P.R.; Fernandes, J.O.; Regalado, L.; Mendes, R.; Teixeira, C.; Mariz-Ponte, N.; Gomes, P.; Santos, C. Peptaibiotics: Harnessing the potential of microbial secondary metabolites for mitigation of plant pathogens. Biotechnology Advances 2023, 68, 108223. [Google Scholar] [CrossRef] [PubMed]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A.; Salonen, H.; Druzhinina, I.S.; Vágvölgyi, C.; Kredics, L. Structural Diversity and Bioactivities of Peptaibol Compounds From the Longibrachiatum Clade of the Filamentous Fungal Genus Trichoderma. Frontiers of Microbiology 2019, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S. Fungal volatile organic compounds: Emphasis on their plant growth-promoting. In Volatiles and Food Security; Choudhary, D., Sharma, A., Agarwal, P., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2017; pp. 313–333. [Google Scholar]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biology Review 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Cardoza, R.E.; McCormick, S.P.; Izquierdo-Bueno, I. Identification of polyketide synthase genes required for aspinolide biosynthesis in Trichoderma arundinaceum. Applied Microbiology and Biotechnology 2022, 106, 7153–7171. [Google Scholar] [CrossRef]
- Mary, L.S.; Russell, J.C. ; Molecular methods unravel the biosynthetic potential of Trichoderma species. RSC Advances 2021, 11, 6–3622. [Google Scholar] [CrossRef]
- Sobowale, A. Probable Effects of Dual Inoculation of Maize (Zea mays) Stem with Fusarium verticillioides and Certain Trichoderma Species on Fumonisin Content of Maize Seeds. American Journal of Plant Sciences 2019, 10, 752–759. [Google Scholar] [CrossRef]
- Deepa, N.; Achar, P.N.; Sreenivasa, M.Y. Current Perspectives of Biocontrol Agents for Management of Fusarium verticillioides and Its Fumonisin in Cereals—A Review. Journal of Fungi 2021, 7, 776. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, L.; Knox, B.P.; Kubicek, C.P.; Druzhinina, I.S.; Baker, S.E. The polyketide synthase gene pks4 of Trichoderma reesei provides pigmentation and stress resistance. Eukaryotic Cell 2013, 12, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, B.; Liu, M.; Qin, L.; Dong, Z. Understanding the Role of Trichoderma reesei Vib1 in Gene Expression during Cellulose Degradation. Journal of Fungi 2021, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, M.L.; Ambilika, M.; Skellam, E.; Cox, R.J. Heterologous Expression of Secondary Metabolite Genes in Trichoderma reesei for Waste Valorization. Journal of Fungi 2022, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Sehim, A.E.; Hewedy, O.A.; Altammar, K.A.; Alhumaidi, M.S.; Abd Elghaffar, R.Y. Trichoderma asperellum empowers tomato plants and suppresses Fusarium oxysporum through priming responses. Frontiers in microbiology 2023, 14, 1140378. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.M.A.; Fernandes Fraceto, L.; Dashora, K.; Gupta, V.K. Chrysophanol: A Natural Anthraquinone with Multifaceted Biotherapeutic Potential. Biomolecules 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Pilarska, A.A.; Niewiadomska, A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Applied Sciences 2022, 13, 6434. [Google Scholar] [CrossRef]
- Scharf, D.H.; Brakhage, A.A.; Mukherjee, P.K. Gliotoxin--bane or boon? Environmental microbiology 2016, 18, 1096–1109. [Google Scholar] [CrossRef]
- Kumar, V.; Koul, B.; Taak, P.; Yadav, D.; Song, M. Journey of Trichoderma from Pilot Scale to Mass Production: A Review. Agriculture 2023, 13, 2022. [Google Scholar] [CrossRef]
- Zaid, R.; Koren, R.; Kligun, E.; Gupta, R.; Leibman-Markus, M.; Mukherjee, P.K.; Kenerley, C.M.; Bar, M.; Horwitz, B.A. Gliotoxin, an Immunosuppressive Fungal Metabolite, Primes Plant Immunity: Evidence from Trichoderma virens-Tomato Interaction. Microbiology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of Two Trichoderma Strains on Plant Growth, Rhizosphere Soil Nutrients, and Fungal Community of Pinus sylvestris var. mongolica Annual Seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. International Journal of Molecular Sciences 2019, 21, 4235. [Google Scholar] [CrossRef] [PubMed]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.M.R. Biotechnological development of Trichoderma-based formulations for biological control. Appl Microbiol Biotechnol 2023, 107, 5595–5612. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Letter of Applied Microbiology 2006, 43, 143–148. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Kong, S. Effects of Trichoderma asperellum and its siderophores on endogenous auxin in Arabidopsis thaliana under iron-deficiency stress. International Microbiology 2020, 23, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.; Shafiq, M.; Noman, F.; Abbas, S.A.A.A.; Ali, N. Structure and potential applications of bacterial siderophores. Bacterial Secondary Metabolites 2023, 159–175. [Google Scholar] [CrossRef]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–Metal Interactions: A Review of Toxicity and Homeostasis. Journal of Fungi 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore – A boon to agricultural sciences. Biological Control 2020, 144, 104214. [Google Scholar] [CrossRef]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and Characterization of Antibacterial Siderophores Secreted by Endophytic Fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Zeng, G.; Wu, G.; Qi, L.; Chen, G.; Fang, W.; Yin, W.-B. Transcriptional Differences Guided Discovery and Genetic Identification of Coprogen and Dimerumic Acid Siderophores in Metarhizium robertsii. Frontiers of Microbiology 2021, 12, 783609. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, L.; Wang, X.; Shah, D.; Song, X.; Kumar, V.; Shakoor, A.; Tripathi, K.; Ramteke, P.W.; Rani, R. Biosynthesis Pathways, Transport Mechanisms and Biotechnological Applications of Fungal Siderophores. Journal of Fungi 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Deb, L.; Pandey, A.K. Trichoderma- from lab bench to field application: Looking back over 50 years. Frontiers in Agronomy 2022, 4, 932839. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; Hamss, H.E.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Patkowska, E.; Mielniczuk, E.; Jamiołkowska, A. The Influence of Trichoderma harzianum Rifai T-22 and Other Biostimulants on Rhizosphere Beneficial Microorganisms of Carrot. Agronomy 2020, 10, 1637. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Wang, M. Isolation and molecular identification of Trichoderma species from wetland soil and their antagonistic activity against phytopathogens. Physiological and Molecular Plant Pathology 2019, 109, 101458. [Google Scholar] [CrossRef]
- López-Coria, M.; Guzmán-Chávez, F.; Carvente-García, R.; Muñoz-Chapul, D.; Sánchez-Sánchez, T.; Arciniega-Ruíz, J.M.; King-Díaz, B.; Sánchez-Nieto, S. Maize plant expresses SWEET transporters differently when interacting with Trichoderma asperellum and Fusarium verticillioides, two fungi with different lifestyles. Frontiers in Plant Science 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. International Journal of Molecular Sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Current Research in Microbial Sciences 2021, 2. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Frontiers in Plant Science 2019, 10, 845. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Journal of Insect Science 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nature Review Microbiology 2021, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Dangl, J. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (Auxin, Gibberellin) and ACC Deaminase In Vitro Synthesized by the Mycoparasitic Trichoderma DEMTkZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with this Strain Conidia. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Van Damme, E.J.M. Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules 2015, 20, 9029–9053. [Google Scholar] [CrossRef] [PubMed]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signalling. Frontiers in Plant Science 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, N.A.; Segretin, M.E.; Hernandez, I.; Mirkin, F.G.; Cha ́con, O.; Lopez, Y.; Borrás-Hidalgo, O.; Bravo-Almonacid, F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens underfield trials. Scientific Reports 2019, 9, 2791. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.L.; Pawlowski, M.L.; Chang, H.-X.; Hill, C.B. Successful technologies and approaches used to develop and manage resistance against crop diseases and pests. In Woodhead Publishing Series in Food Science, Technology and Nutrition. Emerging Technologies for Promoting Food Security; Madramootoo, C., Ed.; Woodhead Publishing: Sawston, UK 2016. [Google Scholar]
- Kim, S.H.; Lee, Y.; Balaraju, K.; Jeon, Y. Evaluation of Trichoderma atroviride and Trichoderma longibrachiatum as biocontrol agents in controlling red pepper anthracnose in Korea. Frontiers Plant Science 2023, 14, 1201875. [Google Scholar] [CrossRef]
- Yadav, M.; Divyanshu, K.; Dubey, M.K.; Rai, A.; Kumar, S.; Tripathi, Y.N.; Shukla, V.; Upadhyay, R.S. Plant growth promotion and differential expression of defense genes in chilli pepper against Colletotrichum truncatum induced by Trichoderma asperellum and T. Harzianum. BMC Microbiology 2023, 23. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatumH9 in cucumber. BMC Genomics 2019, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiological Research 2020, 239, 126569. [Google Scholar] [CrossRef]
- Ayub, J.; Tahir, A.; Iqbal, U.; Ayub, H.; Hyder, M.Z.; Kiyani, A.; Hafeez, F.Y.; Ilyas, M.K.; Ghafoor, A.; Yasmin, T. Effective biological control of Lentil (Lens culinaris) Fusarium wilt and plant growth promotion through native Rhizobacteria. Physiological and Molecular Plant Pathology 2023, 129, 102203. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Microbiological Research 2024, 281, 127621. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of Phosphates and Micronutrients by the Plant-Growth-Promoting and Biocontrol Fungus Trichoderma harzianum Rifai 1295-22. Applied and Environmental Microbiology 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Amoo, A.E.; Olanrewaju, O.S.; Babalola, O.O.; Ajilogba, C.F.; Chukwuneme, C.F.; Ojuederie, O.B.; Omomowo, O.I. The functionality of plant-microbe interactions in disease suppression. Journal of King Saud University – Science 2023, 35, 102893. [Google Scholar] [CrossRef]
- Alshammari, W.; Bairum, R.; Sulieman, A.M.; Alshammari, N.; Elamin, H. In vitro and in vivo Study of Antagonistic and Biocontrol of Trichoderma harzianum Strains Against Wood Decay Pathogens. Polish Journal of Environmental Studies 2024, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-X.; Cai, F.; Pang, G.; Shen, Q.-R.; Li, R.; Chen, W. Solubilisation of Phosphate and Micronutrients by Trichoderma harzianum and Its Relationship with the Promotion of Tomato Plant Growth. PLoS ONE 2015, 10, e0130081. [Google Scholar] [CrossRef]
- Tandon, A.; Fatima, T.; Shukla, D.; Tripathi, P.; Srivastava, S.; Singh, P.C. Phosphate solubilisation by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. Journal of King Saud University – Science 2019, 32, 791–798. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. From Amazon soils improve soybean plant growth. Scientific Reports 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Boat Bedine, M.A.; Iacomi, B.; Tchameni, S.N.; Sameza, M.L.; Fekam, F.B. Harnessing the phosphate-solubilising ability of Trichoderma strains to improve plant growth, phosphorus uptake and photosynthetic pigment contents in common bean (Phaseolus vulgaris). Biocatalysis and Agricultural Biotechnology 2022, 45, 102510. [Google Scholar] [CrossRef]
- Song, M.; Wang, X.; Xu, H.; Zhou, X.; Mu, C. Effect of Trichoderma viride on insoluble phosphorus absorption ability and growth of Melilotus officinalis. Scientific Reports 2023, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; La Rocca, N. Biological Nitrogen Fixation. Encyclopedia of Ecology (Second Edition) 2012, 264–279. [Google Scholar] [CrossRef]
- Shirvani, M.; Yahaghi, Z. Role of Pb-solubilizing and plant growth-promoting bacteria in Pb uptake by plants. Advances in Microbe-Assisted Phytoremediation of Polluted Sites 2021, 231–270. [Google Scholar] [CrossRef]
- Mei, H.M.; Ruan, Y.N.; Zhang, J.X.; Cui, J.X.; Yan, K.; Dong, X.Y.; Bian, L.X.; Sun, Y.H. Effects of Trichoderma on nitrogen absorption and use efficiency in Lycium chinense roots under saline stress. Ying yong sheng tai xue bao = The journal of applied ecology 2022, 33, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Hill, R. Applications of Trichoderma in Plant Growth Promotion. Biotechnology and Biology of Trichoderma 2013, 415–428. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Frontiers in plant science 2021, 11, 614971. [Google Scholar] [CrossRef] [PubMed]
- Jha, C.K.; Sharma, P.; Shukla, A.; Parmar, P.; Patel, R.; Goswami, D.; Saraf, M. Microbial enzyme, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase: An elixir for plant under stress. Physiological and Molecular Plant Pathology 2021, 115, 101664. [Google Scholar] [CrossRef]
- Isabel, M.; Rubén, S. Plant Defensive Responses Triggered by Trichoderma spp. As Tools to Face Stressful Conditions. Horticulturae 2022, 8, 1181. [Google Scholar] [CrossRef]
- Bahadur, A.; Dutta, P. Trichoderma Spp.: Their Impact in Crops Diseases Management. IntechOpen, 2022. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Pelagio-Flores, R.; López-Bucio, J. Mechanism of plant immunity triggered by Trichoderma. New and Future Developments in Microbial Biotechnology and Bioengineering 2019, 57–73. [Google Scholar] [CrossRef]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; Romano, C.Z.; Moya-Elizondo, E.A.; Zhou, L. Trichoderma spp. Genes Involved in the Biocontrol Activity Against Rhizoctonia solani. Frontiers in Microbiology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. Journal of Fungi 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.I.; Prismantoro, D.; Permadi, N.; Rossiana, N.; Miranti, M.; Mispan, M.S.; Mohamed, Z.; Doni, F. Bioprospecting the roles of Trichoderma in alleviating plants' drought tolerance: Principles, mechanisms of action, and prospects. Microbiological Research 2024, 283, 127665. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Gorai, P.S.; Mandal, N.C. Trichoderma spp.—Application and future prospects in agricultural industry. Recent Advancement in Microbial Biotechnology 2020, 49–70. [Google Scholar] [CrossRef]
- Khan, M.R.; Mohiddin, F.A. Trichoderma: Its Multifarious Utility in Crop Improvement. Crop Improvement Through Microbial Biotechnology 2017, 263–291. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, M.M.M.; Yadav, S.; Sharma, P. Applications of Trichoderma virens and biopolymer-based biostimulants in plant growth and productions. Biostimulants in Plant Protection and Performance 2023, 349–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




