Submitted:
17 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
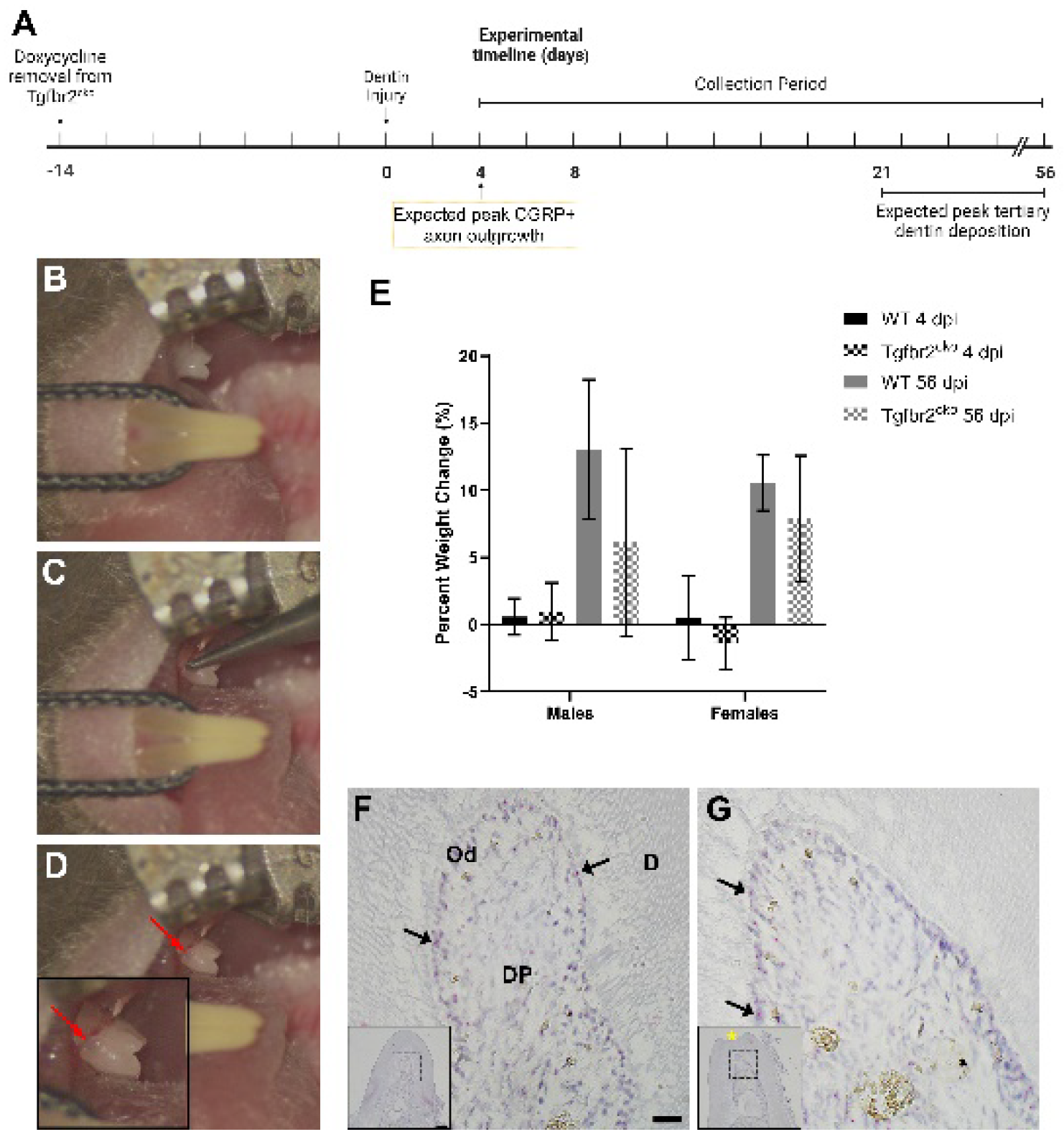
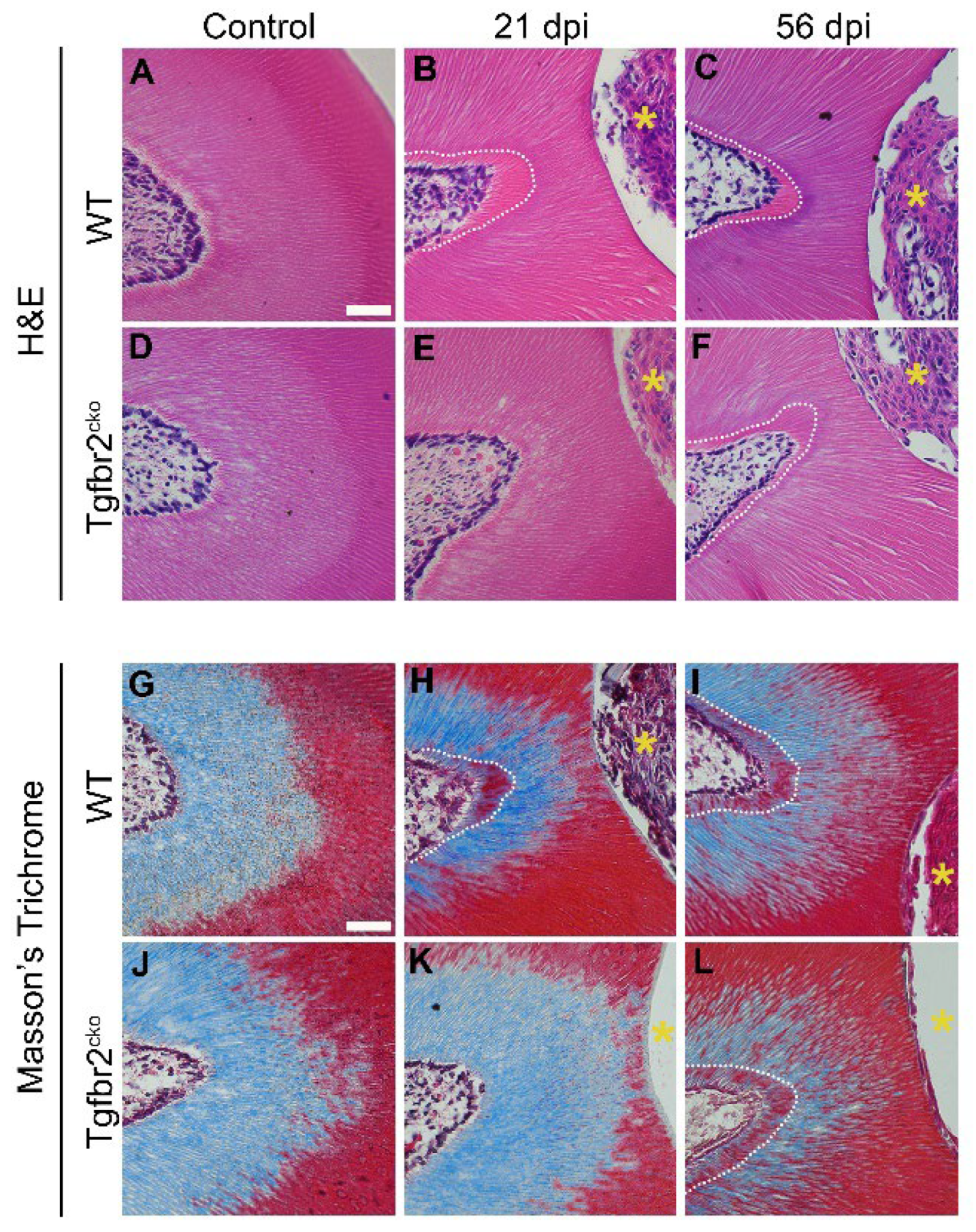
2.1.1. Tgfbr2cko model characterization
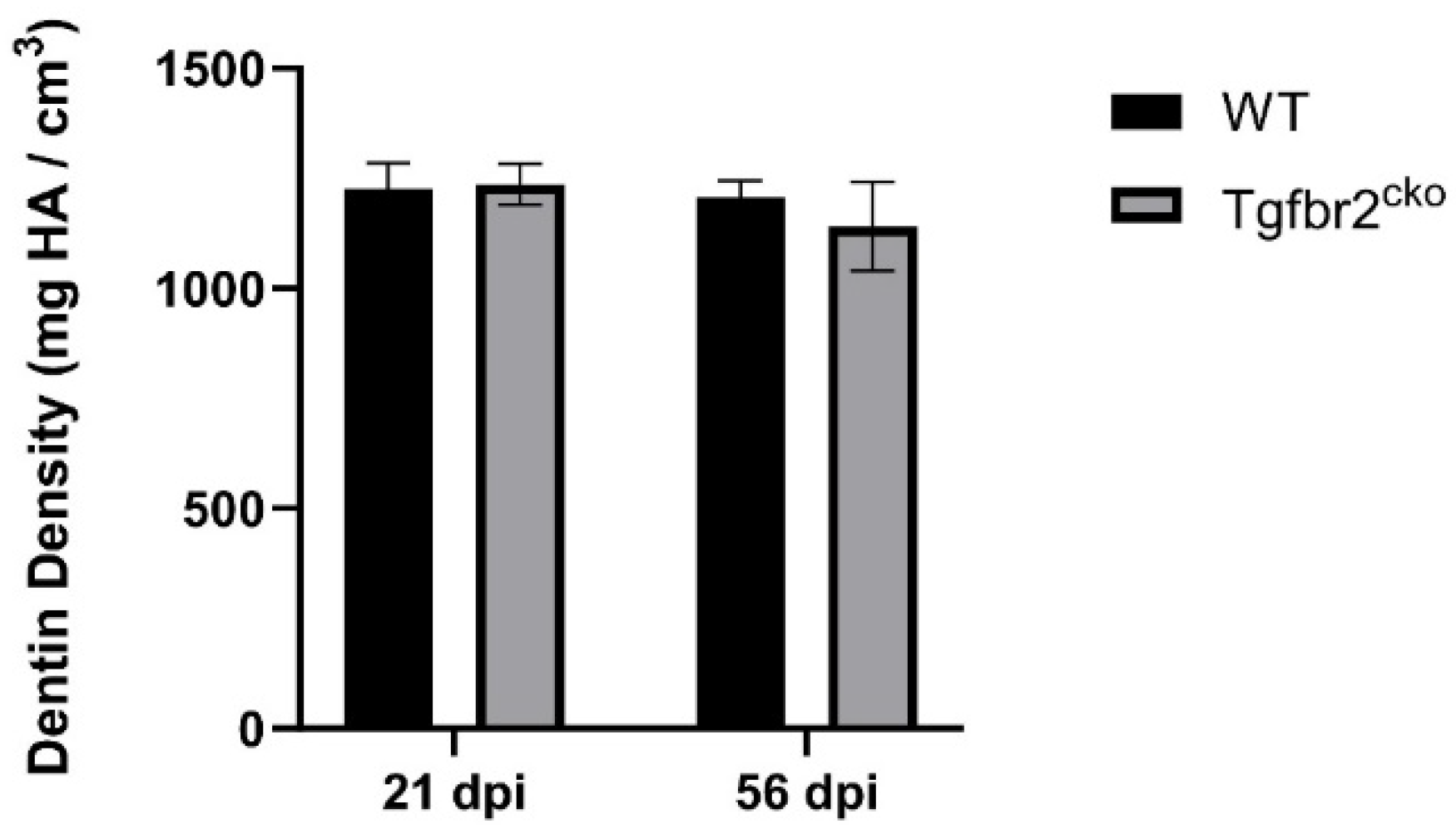
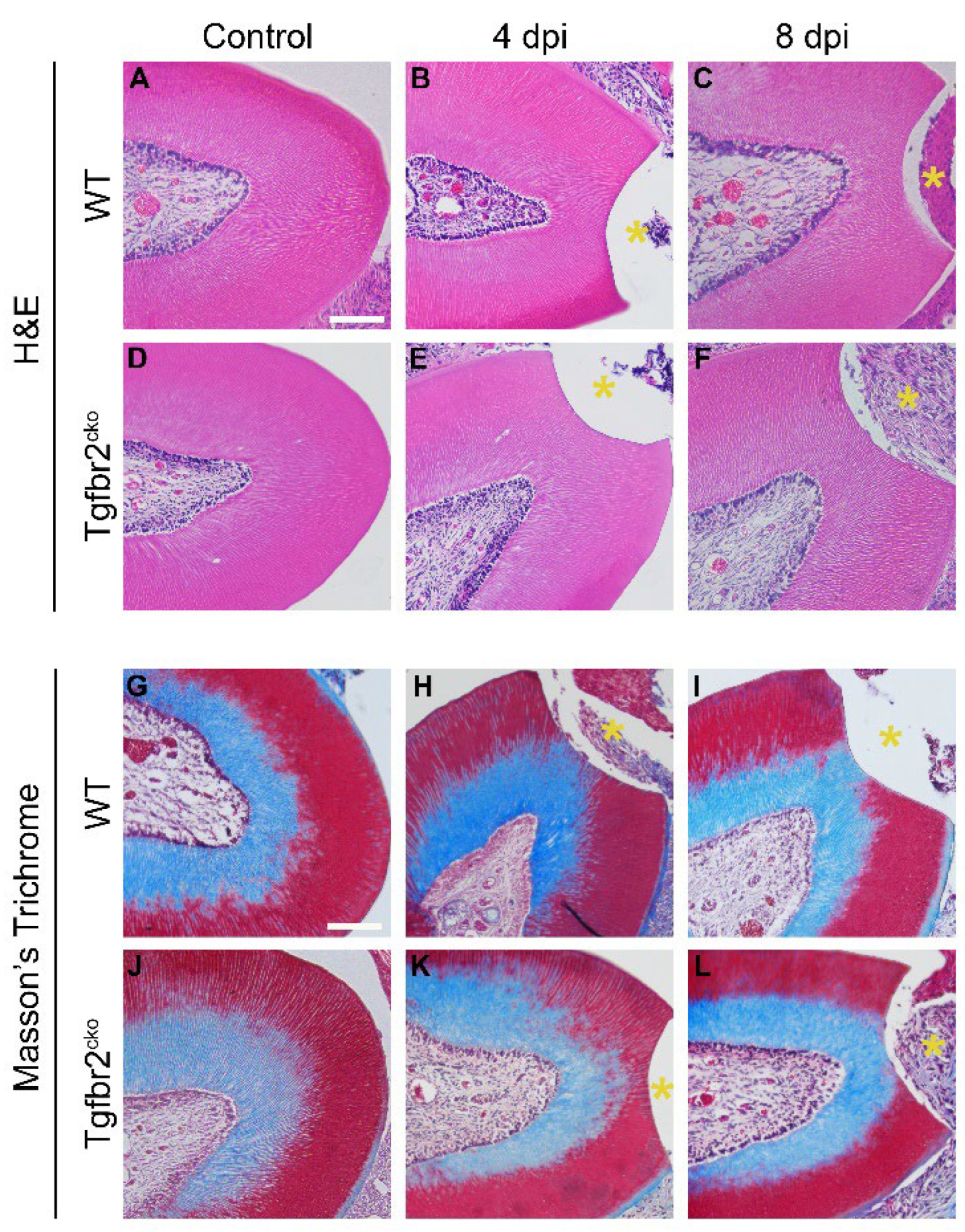
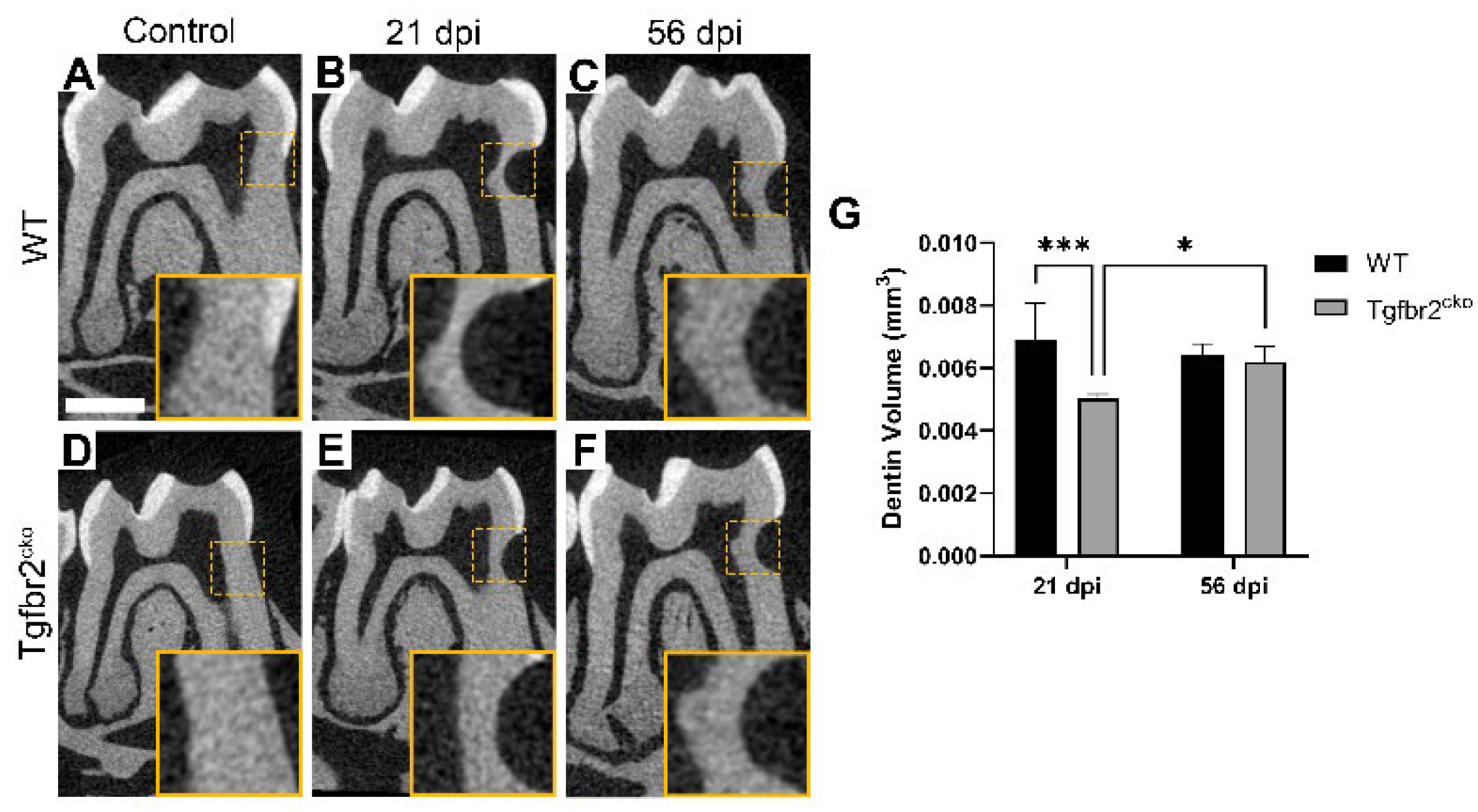
2.1.2. Tertiary dentin deposition is delayed in Tgfbr2cko M1s
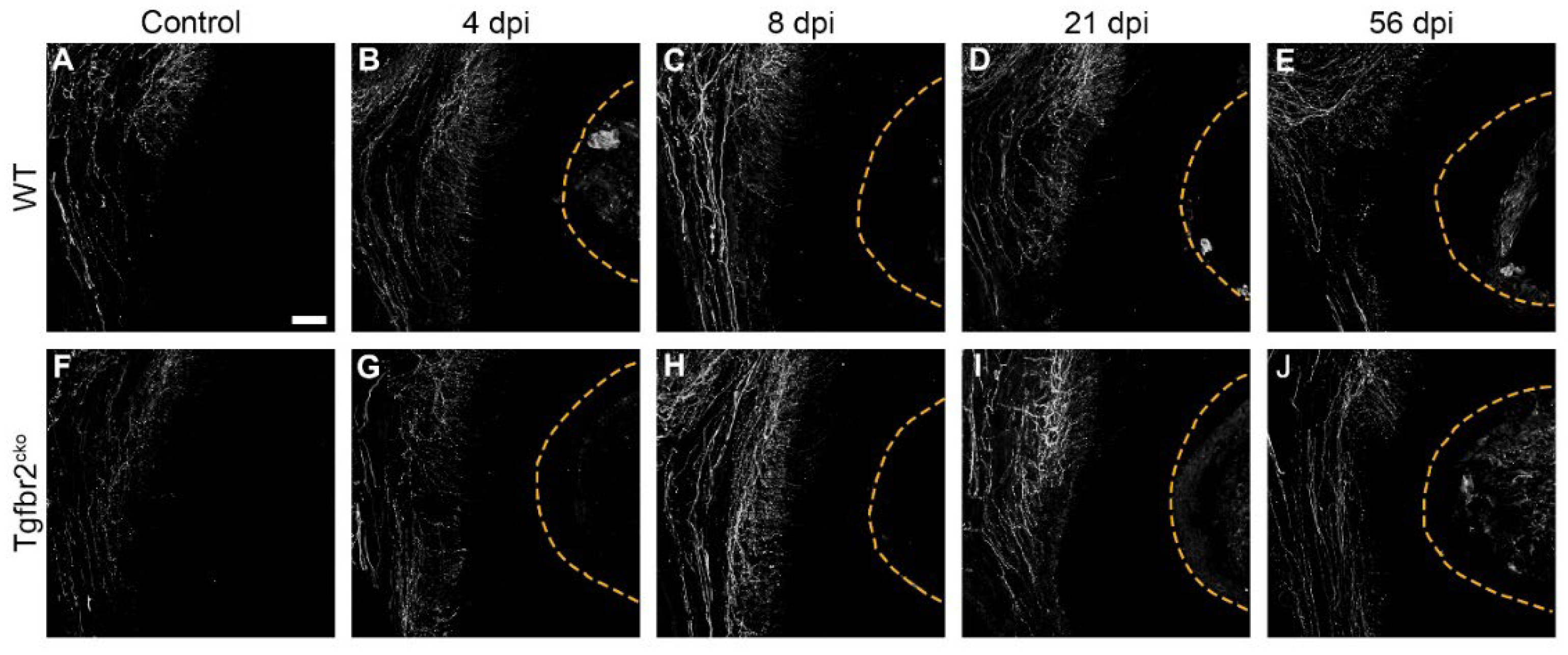
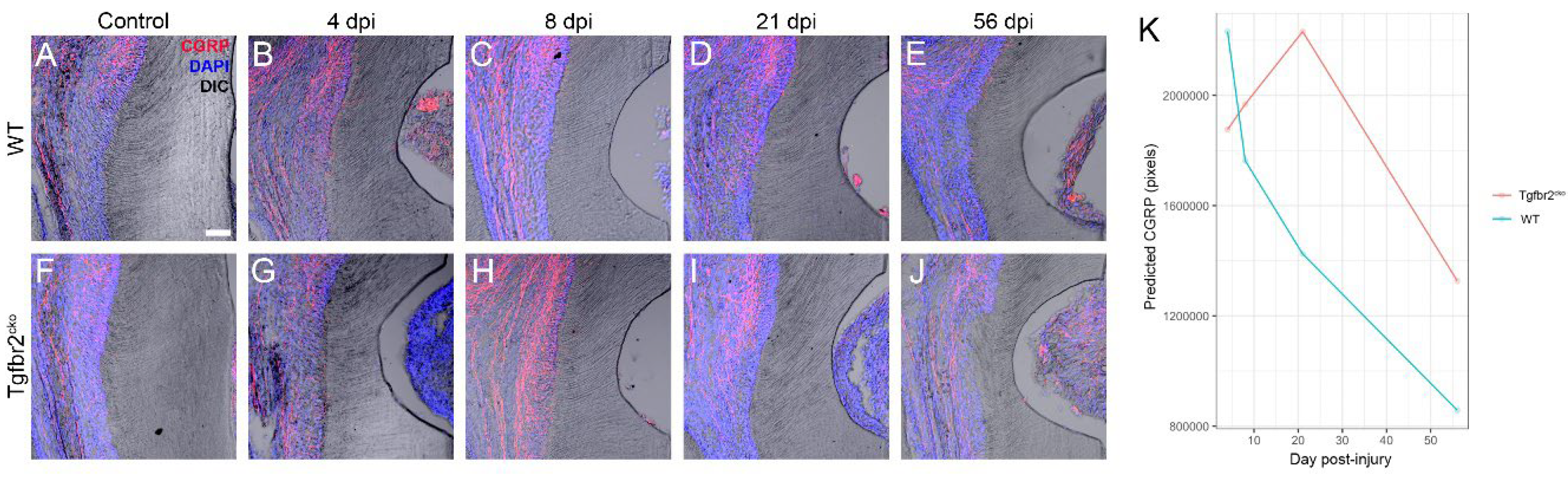
2.1.3. CGRP+ axon sprouting is elevated in Tgfbr2cko M1s
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Dentin Injury and Mandible Harvest
4.3. In Situ Hybridization (ISH) and Histological Staining
4.4. Micro-CT Scanning and Analysis
4.5. Immunofluorescence, Confocal Imaging, and Image Analysis
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| term | estimate | std.error | statistic | p.value |
|---|---|---|---|---|
| Intercept | 1875690 | 199702 | 88.218 | <0.0001 |
| WT | 355742 | 272975 | 1.698 | 0.1925 |
| Ctl | -301958 | 302287 | 0.998 | 0.3178 |
| 8dpi | 92072 | 308701 | 0.089 | 0.7655 |
| 21dpi | 355390 | 335635 | 1.121 | 0.2897 |
| 56dpi | -548734 | 463553 | 1.401 | 0.2365 |
| WT:Ctl | -719330 | 412341 | 3.043 | 0.0811 |
| WT:8dpi | -560670 | 413896 | 1.835 | 0.1755 |
| WT:21dpi | -1161959 | 407350 | 8.137 | 0.0043 |
| Ctl:8dpi | -93531 | 414278 | 0.051 | 0.8214 |
| Ctl:21dpi | -18368 | 448516 | 0.002 | 0.9673 |
| WT:56dpi | -824827 | 521689 | 2.5 | 0.1139 |
| WT:Ctl:8dpi | 1354414 | 581367 | 5.428 | 0.0198 |
| WT:Ctl:21dpi | 921794 | 576407 | 2.557 | 0.1098 |
| Reference group: Tgfbr2cko, Day 4, injured | ||||
- WT:21dpi: The change from 4 dpi CGRP to 21 dpi CGRP in Wild Type mice is significantly different than the change from 4 dpi CGRP to 21 dpi CGRP in Tgfbr2cko mice. In other words, the effect of changing dpi from 4 to 21 is significantly different between genotypes, with a p-value of 0.00434.
- The difference in the change from Day 4 dpi CGRP to Day 8 dpi CGRP between Wild Type mice and Tgfbr2cko mice in control teeth is significantly different than the difference in the change from Day 4 dpi CGRP to Day 8 dpi CGRP between Wild Type mice and Tgfbr2cko mice in injured teeth, with a p-value of 0.0198.
References
- Linde, A.; Goldberg, M. Dentinogenesis. Critical Reviews in Oral Biology & Medicine 1993, 4, 679.
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front Biosci (Elite Ed) 2011, 3, 711–735.
- Stanwick, M.; Barkley, C.; Serra, R.; Kruggel, A.; Webb, A.; Zhao, Y.; Pietrzak, M.; Ashman, C.; Staats, A.; Shahid, S.; et al. Tgfbr2 in Dental Pulp Cells Guides Neurite Outgrowth in Developing Teeth. Frontiers in Cell and Developmental Biology 2022, 0, 215. [CrossRef]
- Sloan, A.J.; Couble, M.L.; Bleicher, F.; Magloire, H.; Smith, A.J.; Farges, J.C. Expression of TGF-Beta Receptors I and II in the Human Dental Pulp by in Situ Hybridization. Adv Dent Res 2001, 15, 63–67. [CrossRef]
- Ahn, Y.H.; Kim, T.H.; Choi, H.; Bae, C.H.; Yang, Y.M.; Baek, J.A.; Lee, J.C.; Cho, E.S. Disruption of Tgfbr2 in Odontoblasts Leads to Aberrant Pulp Calcification. Journal of dental research 2015, 94, 828–835. [CrossRef]
- Niwa, T.; Yamakoshi, Y.; Yamazaki, H.; Karakida, T.; Chiba, R.; Hu, J.C.C.; Nagano, T.; Yamamoto, R.; Simmer, J.P.; Margolis, H.C.; et al. The Dynamics of TGF-β in Dental Pulp, Odontoblasts and Dentin. Scientific Reports 2018, 8. [CrossRef]
- Wang, Y.; Cox, M.K.; Coricor, G.; MacDougall, M.; Serra, R. Inactivation of Tgfbr2 in Osterix-Cre Expressing Dental Mesenchyme Disrupts Molar Root Formation. Developmental biology 2013, 382, 27–37. [CrossRef]
- Peters, S.B.; Wang, Y.; Serra, R. Tgfbr2 Is Required in Osterix Expressing Cells for Postnatal Skeletal Development. Bone 2017, 97, 54–64. [CrossRef]
- Corps, K.; Stanwick, M.; Rectenwald, J.; Kruggel, A.; Peters, S.B. Skeletal Deformities in Osterix-Cre;Tgfbr2f/f Mice May Cause Postnatal Death. Genes 2021, 12. [CrossRef]
- Neves, V.C.M.; Sharpe, P.T. Regulation of Reactionary Dentine Formation. Journal of Dental Research 2018, 97, 416–422. [CrossRef]
- Zhan, C.; Huang, M.; Chen, J.; Lu, Y.; Yang, X.; Hou, J. Sensory Nerves, but Not Sympathetic Nerves, Promote Reparative Dentine Formation after Dentine Injury via CGRP-Mediated Angiogenesis: An in Vivo Study. Int Endod J 2024, 57, 37–49. [CrossRef]
- Sarram, S.; Lee, K.F.; Byers, M.R. Dental Innervation and CGRP in Adult P75-Deficient Mice. The Journal of comparative neurology 1997, 385, 297–308.
- Taylor, P.E.; Byers, M.R.; Redd, P.E. Sprouting of CGRP Nerve Fibers in Response to Dentin Injury in Rat Molars. Brain research 1988, 461, 371–376. [CrossRef]
- Moore, E.R.; Michot, B.; Erdogan, O.; Ba, A.; Gibbs, J.L.; Yang, Y. CGRP and Shh Mediate the Dental Pulp Cell Response to Neuron Stimulation. Journal of dental research 2022, 101. [CrossRef]
- de Almeida, J.F.A.; Chen, P.; Henry, M.A.; Diogenes, A. Stem Cells of the Apical Papilla Regulate Trigeminal Neurite Outgrowth and Targeting through a BDNF-Dependent Mechanism. Tissue engineering. Part A 2014, 20, 3089–3100. [CrossRef]
- Kline, L.W.; Yu, D.C. Effects of Calcitonin, Calcitonin Gene-Related Peptide, Human Recombinant Bone Morphogenetic Protein-2, and Parathyroid Hormone-Related Protein on Endodontically Treated Ferret Canines. Journal of Endodontics 2009, 35, 866–869. [CrossRef]
- Barkley, C.; Serra, R.; Peters, S.B. A Co-Culture Method to Study Neurite Outgrowth in Response to Dental Pulp Paracrine Signals. Journal of visualized experiments : JoVE 2020. [CrossRef]
- Hardin, J.W.; Hilbe, J.M. Generalized Estimating Equations; 2nd ed.; Chapman and Hall/CRC: New York, 2013; ISBN 978-0-429-11103-7.
- Paige, C.; Plasencia-Fernandez, I.; Kume, M.; Papalampropoulou-Tsiridou, M.; Lorenzo, L.E.; David, E.T.; He, L.; Mejia, G.L.; Driskill, C.; Ferrini, F.; et al. A Female-Specific Role for Calcitonin Gene-Related Peptide (CGRP) in Rodent Pain Models. Journal of Neuroscience 2022, 42, 1930–1944. [CrossRef]
- B, M.; SM, C.; JL, G. Effects of Calcitonin Gene-Related Peptide on Dental Pulp Stem Cell Viability, Proliferation, and Differentiation. Journal of endodontics 2020, 46, 950–956. [CrossRef]
- Saito, N.; Kimura, M.; Ouchi, T.; Ichinohe, T.; Shibukawa, Y. Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. Biomolecules 2022, 12, 1747. [CrossRef]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Grawish, M.E.; Scheven, B.A. Neurotrophic Effects of Dental Pulp Stem Cells on Trigeminal Neuronal Cells. Scientific Reports 2020 10:1 2020, 10, 1–13. [CrossRef]
- Pagella, P.; Miran, S.; Neto, E.; Martin, I.; Lamghari, M.; Mitsiadis, T.A. Human Dental Pulp Stem Cells Exhibit Enhanced Properties in Comparison to Human Bone Marrow Stem Cells on Neurites Outgrowth. The FASEB Journal 2020, 34, 5499–5511. [CrossRef]
- Zhan, C.; Huang, M.; Zeng, J.; Chen, T.; Lu, Y.; Chen, J.; Li, X.; Yin, L.; Yang, X.; Hou, J. Irritation of Dental Sensory Nerves Promotes the Occurrence of Pulp Calcification. Journal of Endodontics 2023. [CrossRef]
- Byers, M.R.; Swift, M.L.; Wheeler, E.F. Reactions of Sensory Nerves to Dental Restorative Procedures. Proc Finn Dent Soc 1992, 88 Suppl 1, 73–82.
- Woodnutt, D.A.; Wager-Miller, J.; O’Neill, P.C.; Bothwell, M.; Byers, M.R. Neurotrophin Receptors and Nerve Growth Factor Are Differentially Expressed in Adjacent Nonneuronal Cells of Normal and Injured Tooth Pulp. Cell and Tissue Research 2000, 299, 225–236. [CrossRef]
- Byers, M.R.; Wheeler, E.F.; Bothwell, M. Altered Expression of NGF and P75 NGF-Receptor by Fibroblasts of Injured Teeth Precedes Sensory Nerve Sprouting. Growth factors (Chur, Switzerland) 1992, 6, 41–52.
- Diogenes, A. Trigeminal Sensory Neurons and Pulp Regeneration. Journal of Endodontics 2020, 46, S71–S80. [CrossRef]
- Bowles, W.R.; Burke, R.; Sabino, M.; Harding-Rose, C.; Lunos, S.; Hargreaves, K.M. Sex Differences in Neuropeptide Content and Release from Rat Dental Pulp. J Endod 2011, 37, 1098–1101. [CrossRef]
- Buck, S.; Reese, K.; Hargreaves, K.M. Pulpal Exposure Alters Neuropeptide Levels in Inflamed Dental Pulp and Trigeminal Ganglia: Evaluation of Axonal Transport. J Endod 1999, 25, 718–721. [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. Nat Med 2016, 22, 1160–1169. [CrossRef]
- Appelt, J.; Baranowsky, A.; Jahn, D.; Yorgan, T.; Köhli, P.; Otto, E.; Farahani, S.K.; Graef, F.; Fuchs, M.; Herrera, A.; et al. The Neuropeptide Calcitonin Gene-Related Peptide Alpha Is Essential for Bone Healing. EBioMedicine 2020, 59. [CrossRef]
- Zhao, X.; Wu, G.; Zhang, J.; Yu, Z.; Wang, J. Activation of CGRP Receptor–Mediated Signaling Promotes Tendon-Bone Healing. Science Advances 2024, 10, eadg7380. [CrossRef]
- Wang, L.; Song, Y.; Yi, X.; Wu, C.; Guo, Q.; Zhou, X.; Song, D.; Zhang, L.; Huang, D. Semaphorin 7A Accelerates the Inflammatory Osteolysis of Periapical Lesions. Journal of Endodontics 2022, 48, 641-649.e2. [CrossRef]
- Yoshida, S.; Wada, N.; Hasegawa, D.; Miyaji, H.; Mitarai, H.; Tomokiyo, A.; Hamano, S.; Maeda, H. Semaphorin 3A Induces Odontoblastic Phenotype in Dental Pulp Stem Cells. Journal of Dental Research 2016, 95, 1282–1290. [CrossRef]
- Li, Y.; Yang, L.; He, S.; Hu, J. The Effect of Semaphorin 3A on Fracture Healing in Osteoporotic Rats. Orthop Sci 2015, 20, 1114–1121. [CrossRef]
- Kenan, S.; Onur, Ö.D.; Solakoğlu, S.; Kotil, T.; Ramazanoğlu, M.; Çelik, H.H.; Ocak, M.; Uzuner, B.; Fıratlı, E. Investigation of the Effects of Semaphorin 3A on New Bone Formation in a Rat Calvarial Defect Model. Journal of Cranio-Maxillofacial Surgery 2019, 47, 473–483. [CrossRef]
- Fukuda, T.; Takeda, S.; Xu, R.; Ochi, H.; Sunamura, S.; Sato, T.; Shibata, S.; Yoshida, Y.; Gu, Z.; Kimura, A.; et al. Sema3A Regulates Bone-Mass Accrual through Sensory Innervations. Nature 2013, 497, 490–493. [CrossRef]
- Mei, H.; Li, Z.; Lv, Q.; Li, X.; Wu, Y.; Feng, Q.; Jiang, Z.; Zhou, Y.; Zheng, Y.; Gao, Z.; et al. Sema3A Secreted by Sensory Nerve Induces Bone Formation under Mechanical Loads. Int J Oral Sci 2024, 16, 1–11. [CrossRef]
- Berkowitz, G.S.; Spielman, H.; Matthews, A.G.; Vena, D.; Craig, R.G.; Curro, F.A.; Thompson, V.P. Postoperative Hypersensitivity and Its Relationship to Preparation Variables in Class I Resin-Based Composite Restorations: Findings from the Practitioners Engaged in Applied Research and Learning (PEARL) Network. Part 1. Compend Contin Educ Dent 2013, 34, e44–e52.
- Younus, M.Z.; Ahmed, M.A.; Syed, A.U.Y.; Baloch, J.M.; Ali, M.; Sheikh, A. Comparison between Effectiveness of Dentine Desensitizer and One Bottle Self-Etch Adhesive on Dentine Hypersensitivity. Technol Health Care 2021, 29, 1153–1159. [CrossRef]
- Dammaschke, T. The History of Direct Pulp Capping. J Hist Dent 2008, 56, 9–23.
- Dammaschke, T.; Nowicka, A.; Lipski, M.; Ricucci, D. Histological Evaluation of Hard Tissue Formation after Direct Pulp Capping with a Fast-Setting Mineral Trioxide Aggregate (RetroMTA) in Humans. Clin Oral Investig 2019, 23, 4289–4299. [CrossRef]
- Kenneth M. Hargreaves / Harold E. Goodis / Franklin R. Tay (Editor) | Seltzer and Bender’s Dental Pulp Available online: https://www.quintessence-publishing.com/gbr/en/product/seltzer-and-benders-dental-pulp (accessed on 25 April 2024).
- Cox, C.F.; Sübay, R.K.; Ostro, E.; Suzuki, S.; Suzuki, S.H. Tunnel Defects in Dentin Bridges: Their Formation Following Direct Pulp Capping. Oper Dent 1996, 21, 4–11.
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp Capping Materials Modulate the Balance between Inflammation and Regeneration. Dent Mater 2019, 35, 24–35. [CrossRef]
- Rabea, A.A. Assessment of Bone Marrow-Derived Mesenchymal Stem Cells Capacity for Odontogenic Differentiation and Dentin Regeneration in Methimazole-Treated Albino Rats (Light Microscopic Study). The Saudi Dental Journal 2022, 34, 27–35. [CrossRef]
- Rabea, A.A. Histological, Histochemical and Immunohistochemical Evaluation of the Role of Bone Marrow-Derived Mesenchymal Stem Cells on the Structure of Periodontal Tissues in Carbimazole-Treated Albino Rats. Archives of Oral Biology 2020, 119, 104887. [CrossRef]
- Frozoni, M.; Balic, A.; Sagomonyants, K.; Zaia, A.A.; Line, S.R.P.; Mina, M. A Feasibility Study for the Analysis of Reparative Dentinogenesis in pOBCol3.6GFPtpz Transgenic Mice. Int Endod J 2012, 45, 907–914. [CrossRef]
- Oka, S.; Oka, K.; Xu, X.; Sasaki, T.; Bringas, P.; Chai, Y. Cell Autonomous Requirement for TGF-Beta Signaling during Odontoblast Differentiation and Dentin Matrix Formation. Mechanisms of development 2007, 124, 409–415. [CrossRef]
- Yapijakis, C.; Davaria, S.; Gintoni, I.; Chrousos, G.P. The Impact of Genetic Variability of TGF-Beta Signaling Biomarkers in Major Craniofacial Syndromes. Adv Exp Med Biol 2023, 1423, 187–191. [CrossRef]
- Jani, P.; Nguyen, Q.C.; Almpani, K.; Keyvanfar, C.; Mishra, R.; Liberton, D.; Orzechowski, P.; Frischmeyer-Guerrerio, P.A.; Duverger, O.; Lee, J.S. Severity of Oro-Dental Anomalies in Loeys-Dietz Syndrome Segregates by Gene Mutation. Journal of Medical Genetics 2020, 57, 699–707. [CrossRef]
- Yamano, S.; Kuo, W.P.; Sukotjo, C. Downregulated Gene Expression of TGF-Βs in Diabetic Oral Wound Healing. Journal of cranio-maxillo-facial surgery : Official publication of the European Association for Cranio-Maxillo-Facial Surgery 2013, 41, e42-8. [CrossRef]
- Widbiller, M.; Austah, O.; Lindner, S.R.; Sun, J.; Diogenes, A. Neurotrophic Proteins in Dentin and Their Effect on Trigeminal Sensory Neurons. Journal of Endodontics2 2019, 45, 729–765. [CrossRef]
- Austah, O.; Widbiller, M.; Tomson, P.L.; Diogenes, A. Expression of Neurotrophic Factors in Human Dentin and Their Regulation of Trigeminal Neurite Outgrowth. Journal of Endodontics 2018, 45, 414–419. [CrossRef]
- Smith, A.J.; Cassidy, N.; Perry, H.; Bègue-Kirn, C.; Ruch, J.V.; Lesot, H. Reactionary Dentinogenesis. Int J Dev Biol 1995, 39, 273–280.
- Ivica, A.; Deari, S.; Patcas, R.; Weber, F.E.; Zehnder, M. Transforming Growth Factor Beta 1 Distribution and Content in the Root Dentin of Young Mature and Immature Human Premolars. Journal of Endodontics 2020, 46, 641–647. [CrossRef]
- Widbiller, M.; Eidt, A.; Lindner, S.R.; Hiller, K.-A.; Schweikl, H.; Buchalla, W.; Galler, K.M. Dentine Matrix Proteins: Isolation and Effects on Human Pulp Cells. International Endodontic Journal 2018, 51, e278–e290. [CrossRef]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Hoffer, P.C.; Schmalz, G. EDTA Conditioning of Dentine Promotes Adhesion, Migration and Differentiation of Dental Pulp Stem Cells. International Endodontic Journal 2016, 49, 581–590. [CrossRef]
- Mohammadi, Z.; Shalavi, S.; Jafarzadeh, H. Ethylenediaminetetraacetic Acid in Endodontics. Eur J Dent 2013, 7, S135–S142. [CrossRef]
- Dos Reis-Prado, A.H.; Abreu, L.G.; Fagundes, R.R.; Oliveira, S. de C.; Bottino, M.C.; Ribeiro-Sobrinho, A.P.; Benetti, F. Influence of Ethylenediaminetetraacetic Acid on Regenerative Endodontics: A Systematic Review. Int Endod J 2022, 55, 579–612. [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The Effect of Calcium Hydroxide on Solubilisation of Bio-Active Dentine Matrix Components. Biomaterials 2006, 27, 2865–2873. [CrossRef]
- Ferracane, J.L.; Cooper, P.R.; Smith, A.J. Dentin Matrix Component Solubilization by Solutions at pH Relevant to Self-Etching Dental Adhesives. The journal of adhesive dentistry 2013, 15, 407–40712. [CrossRef]
- Singh, K.K.; Rommel, K.; Mishra, A.; Karck, M.; Haverich, A.; Schmidtke, J.; Arslan-Kirchner, M. TGFBR1 and TGFBR2 Mutations in Patients with Features of Marfan Syndrome and Loeys-Dietz Syndrome. Human Mutation 2006, 27, 770–777. [CrossRef]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A Syndrome of Altered Cardiovascular, Craniofacial, Neurocognitive and Skeletal Development Caused by Mutations in TGFBR1 or TGFBR2. Nature Genetics 2005, 37, 275–281. [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr Osteoporos Rep 2015, 13, 327–335. [CrossRef]
- Xu, M.T.; Sun, S.; Zhang, L.; Xu, F.; Du, S.L.; Zhang, X.D.; Wang, D.W. Diabetes Mellitus Affects the Biomechanical Function of the Callus and the Expression of TGF-Beta1 and BMP2 in an Early Stage of Fracture Healing. Braz J Med Biol Res 2015, 49, e4736. [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease, and Therapeutics. Sig Transduct Target Ther 2024, 9, 1–40. [CrossRef]
- Nagasaki, A.; Nagasaki, K.; Kear, B.D.; Tadesse, W.D.; Thumbigere-Math, V.; Millán, J.L.; Foster, B.L.; Somerman, M.J. Delivery of Alkaline Phosphatase Promotes Periodontal Regeneration in Mice. J Dent Res 2021, 100, 993–1001. [CrossRef]
- Ao, M.; Chavez, M.B.; Chu, E.Y.; Hemstreet, K.C.; Yin, Y.; Yadav, M.C.; Millán, J.L.; Fisher, L.W.; Goldberg, H.A.; Somerman, M.J.; et al. Overlapping Functions of Bone Sialoprotein and Pyrophosphate Regulators in Directing Cementogenesis. Bone 2017, 105, 134–147. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





