Submitted:
17 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Limnology Variables, Sampling and Plankton Analysis
2.3. Ciliates
2.4. Data Treatment
3. Results
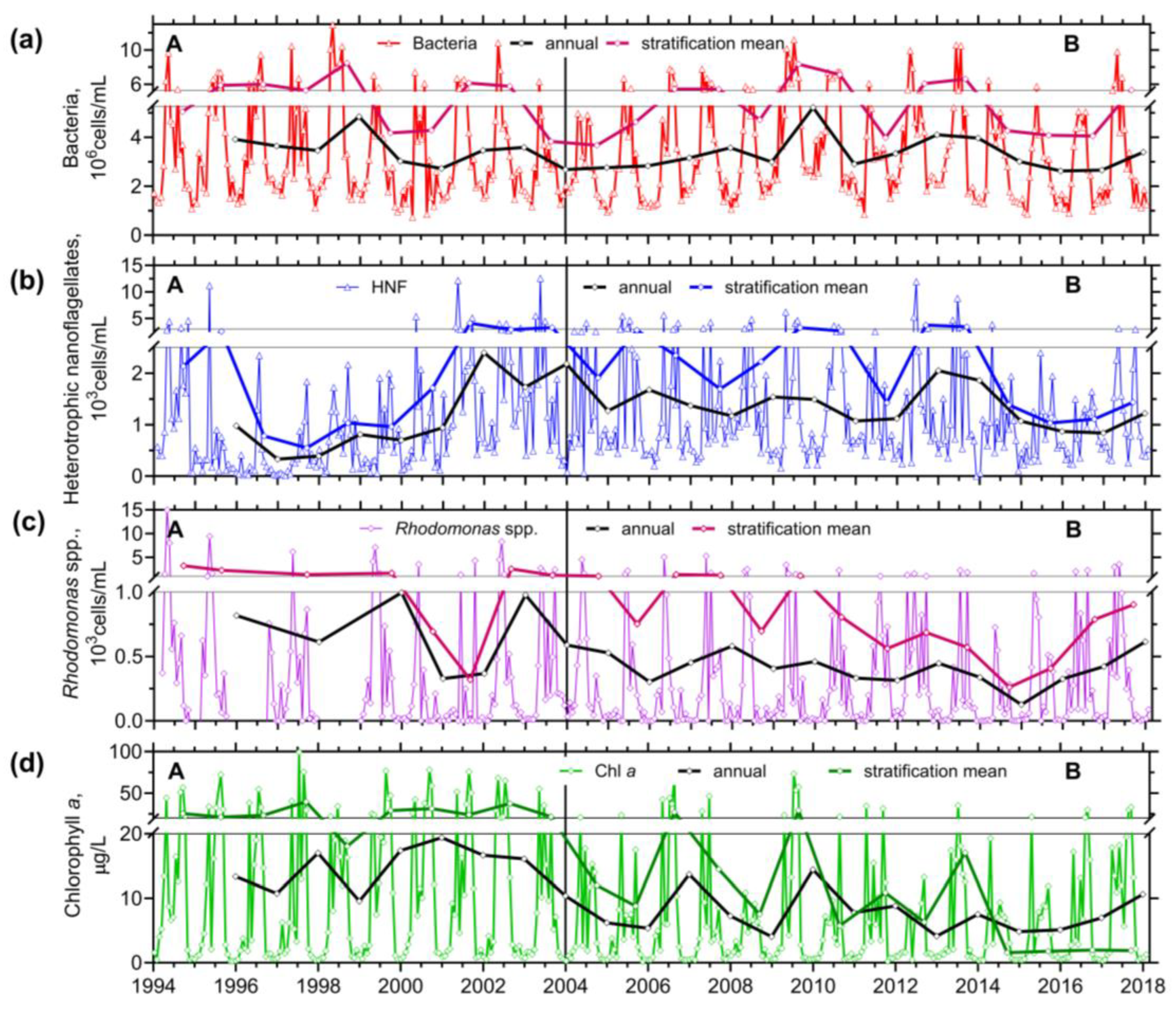
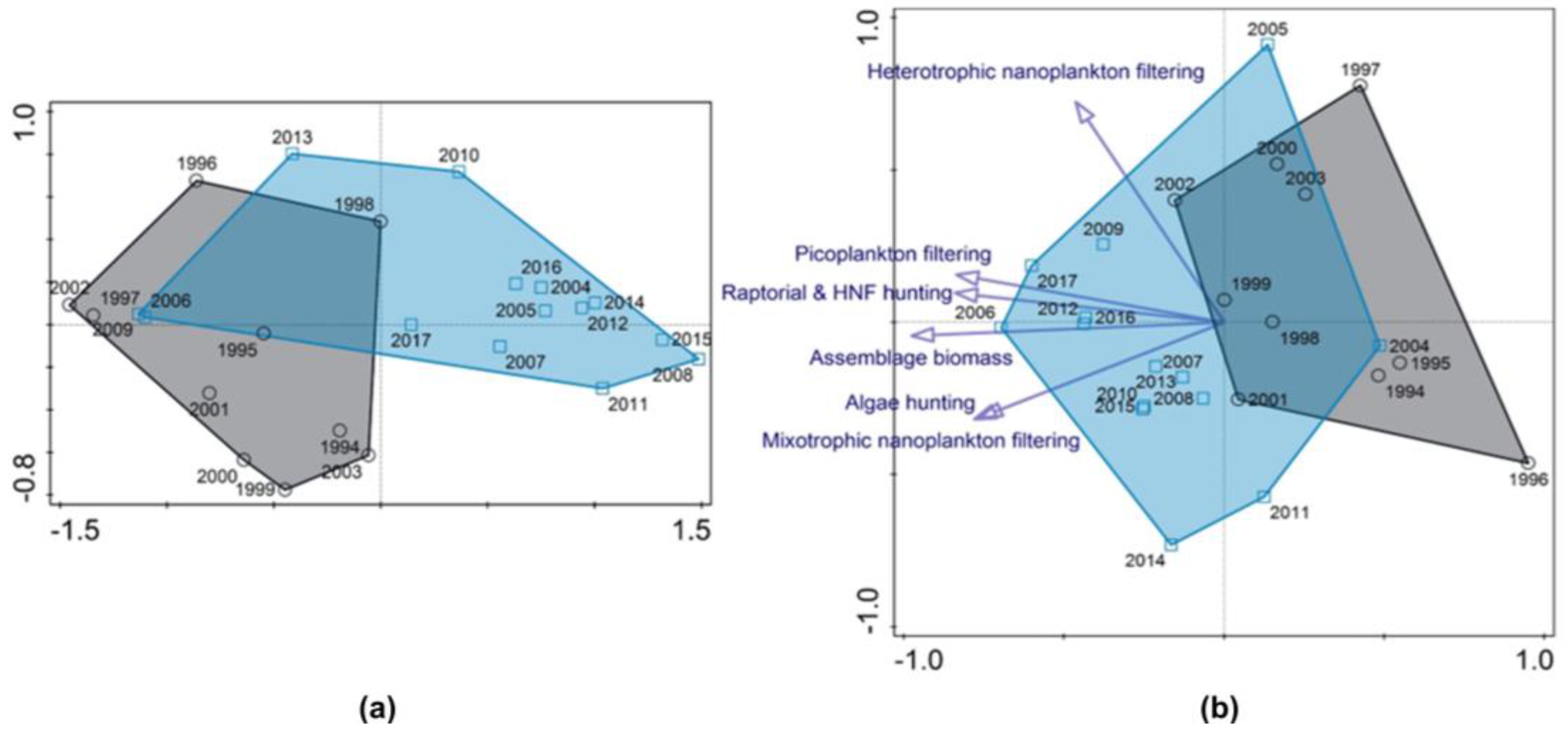
3.1. Long-Term Trends in the Reservoir
3.1.1. Physical-Chemical Variables
3.1.2. Plankton
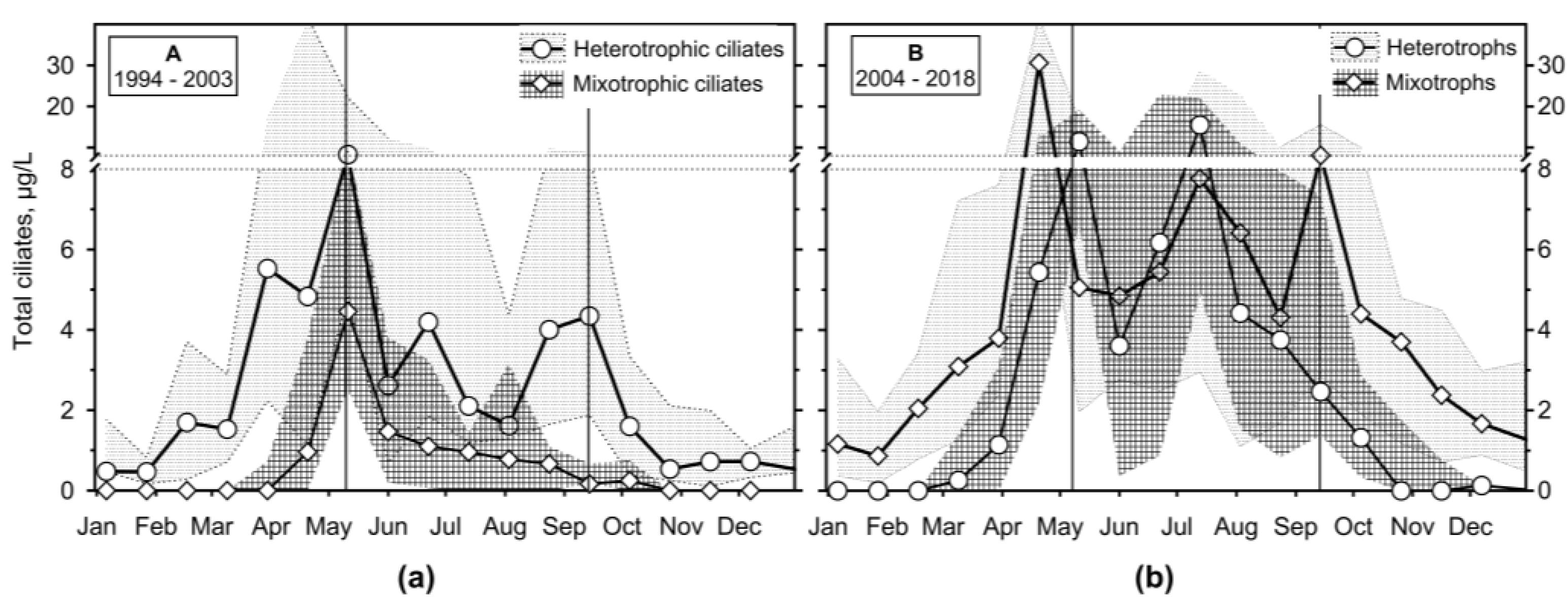
3.2. Seasonality
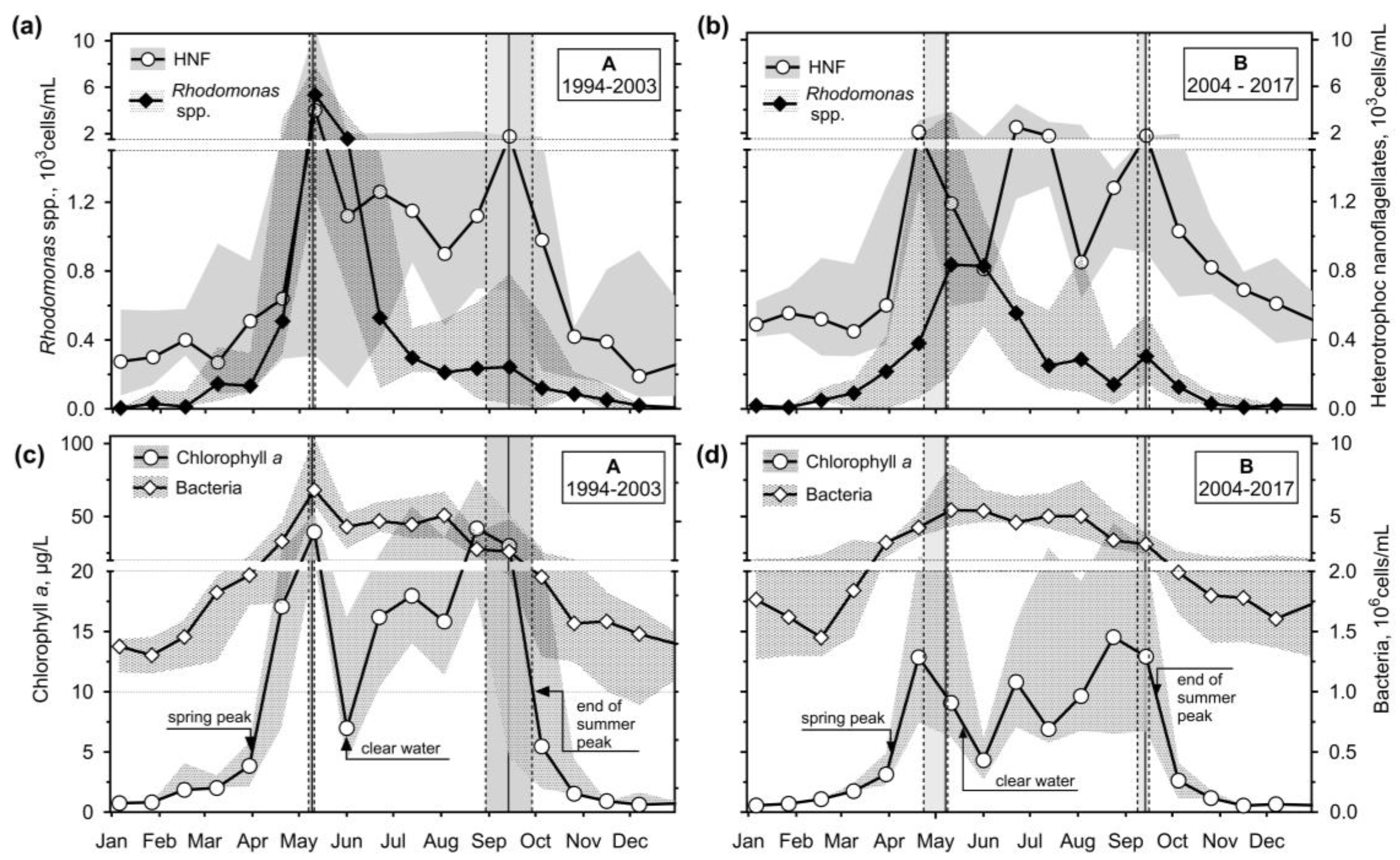
3.2.1. Physical-Chemical Variables
3.2.2. Chlorophyll a, Bacteria, Rhodomonads, and HETEROTROPHIC NANOFLAGELLATES
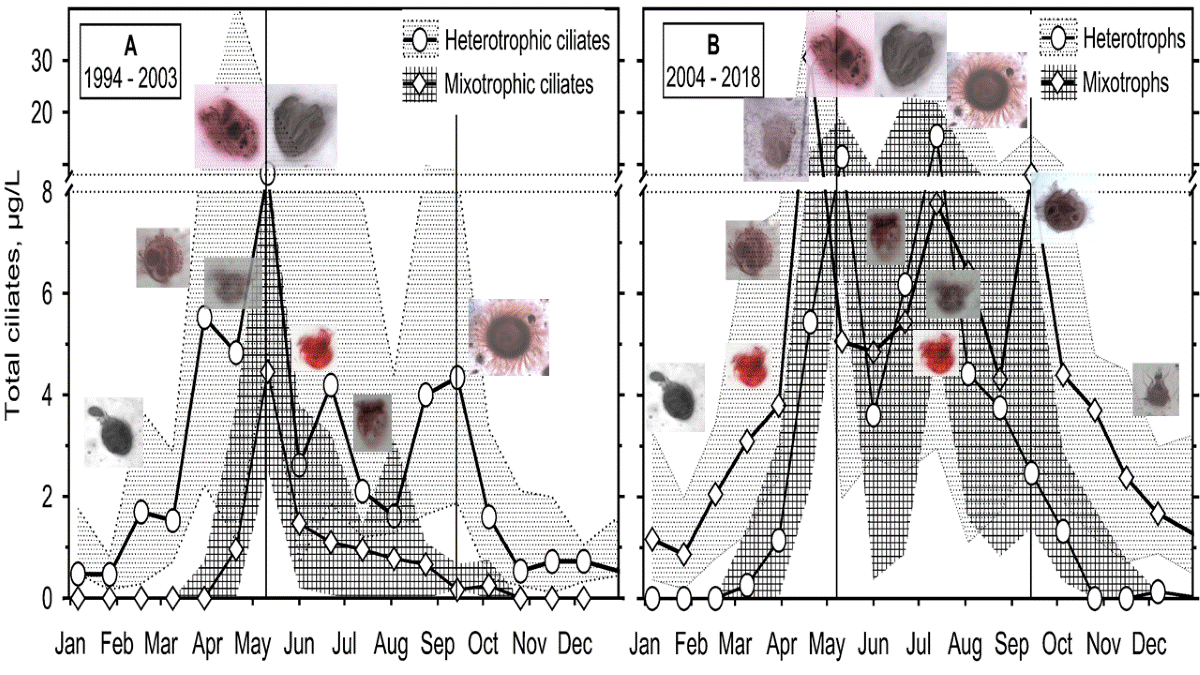
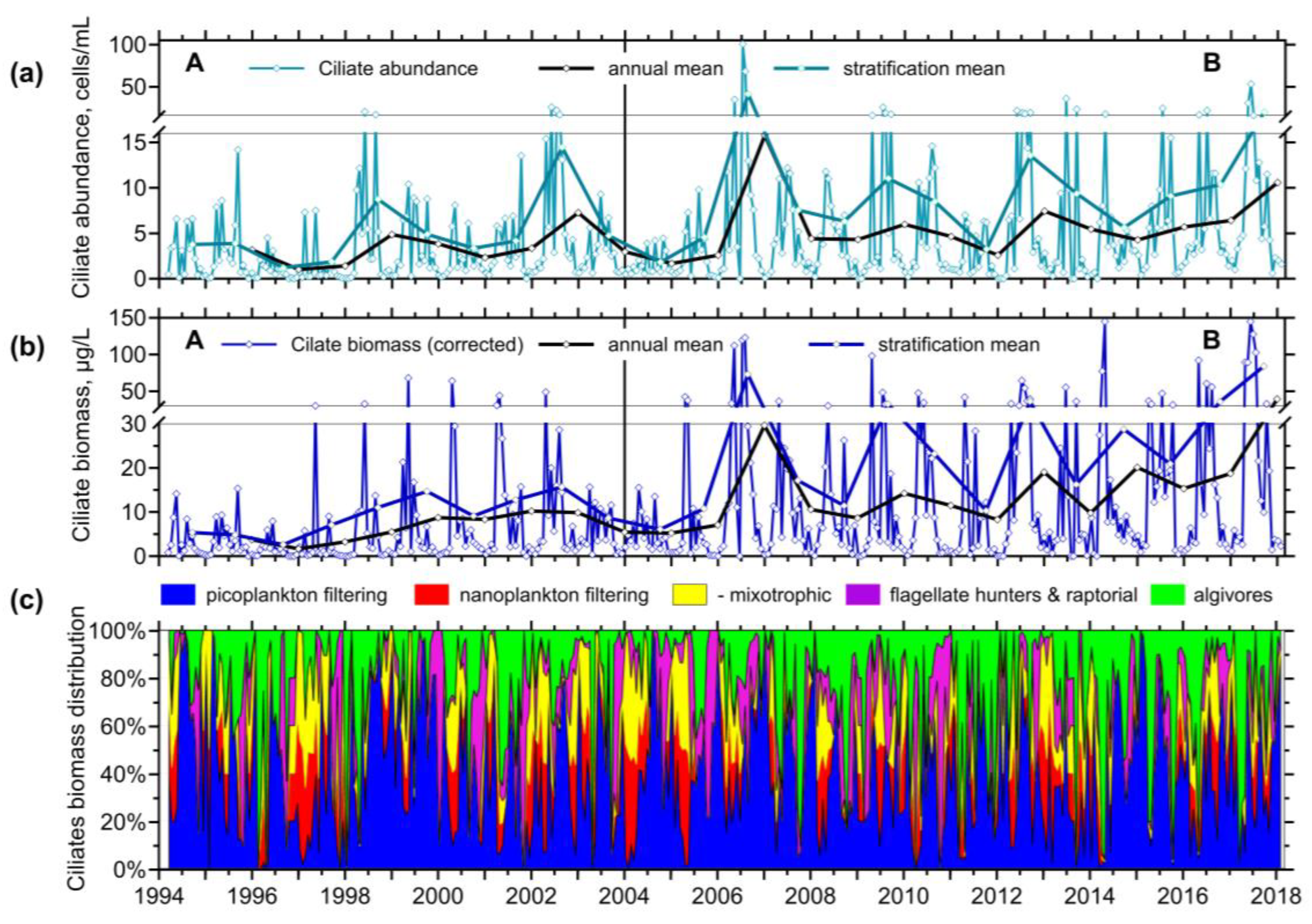
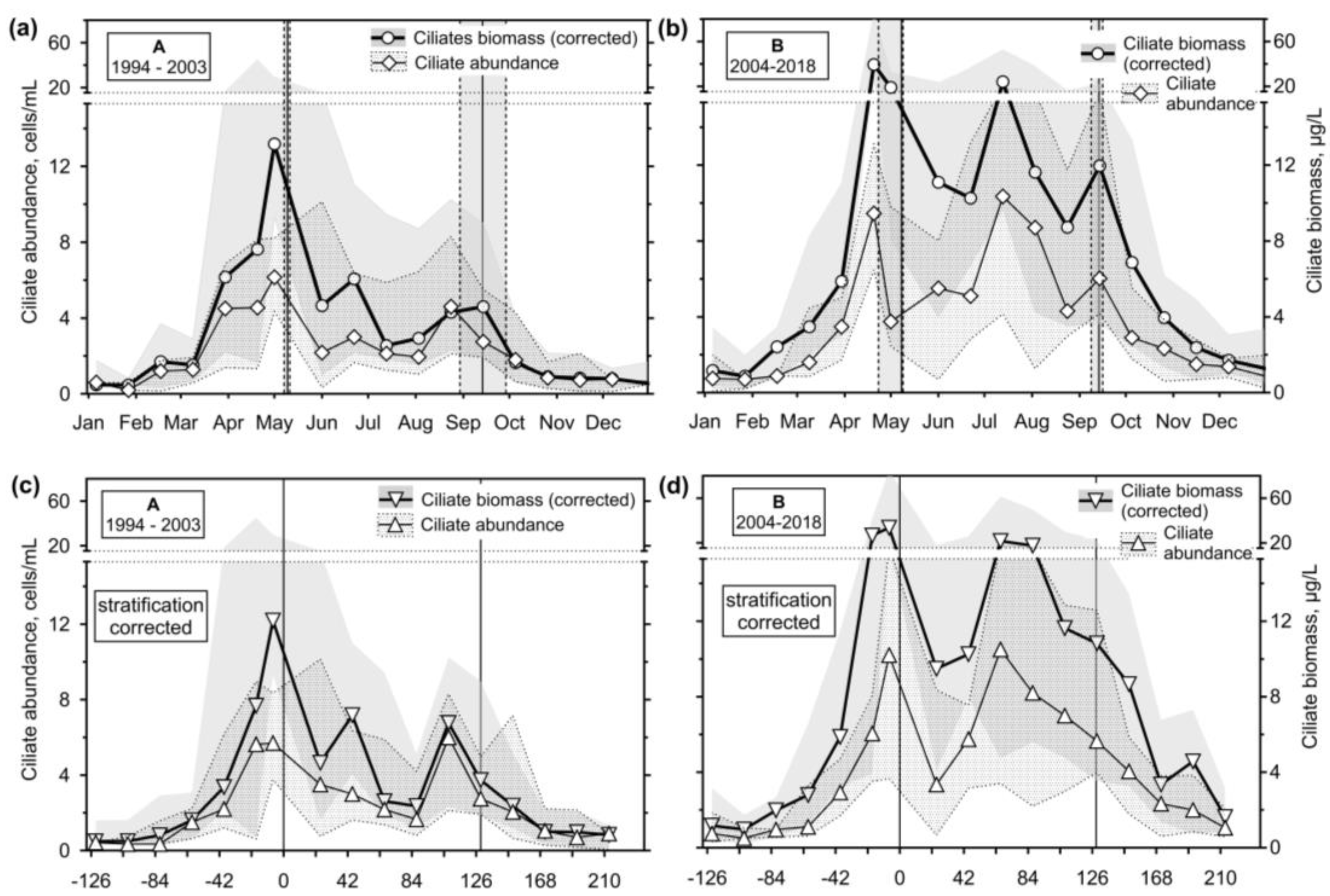
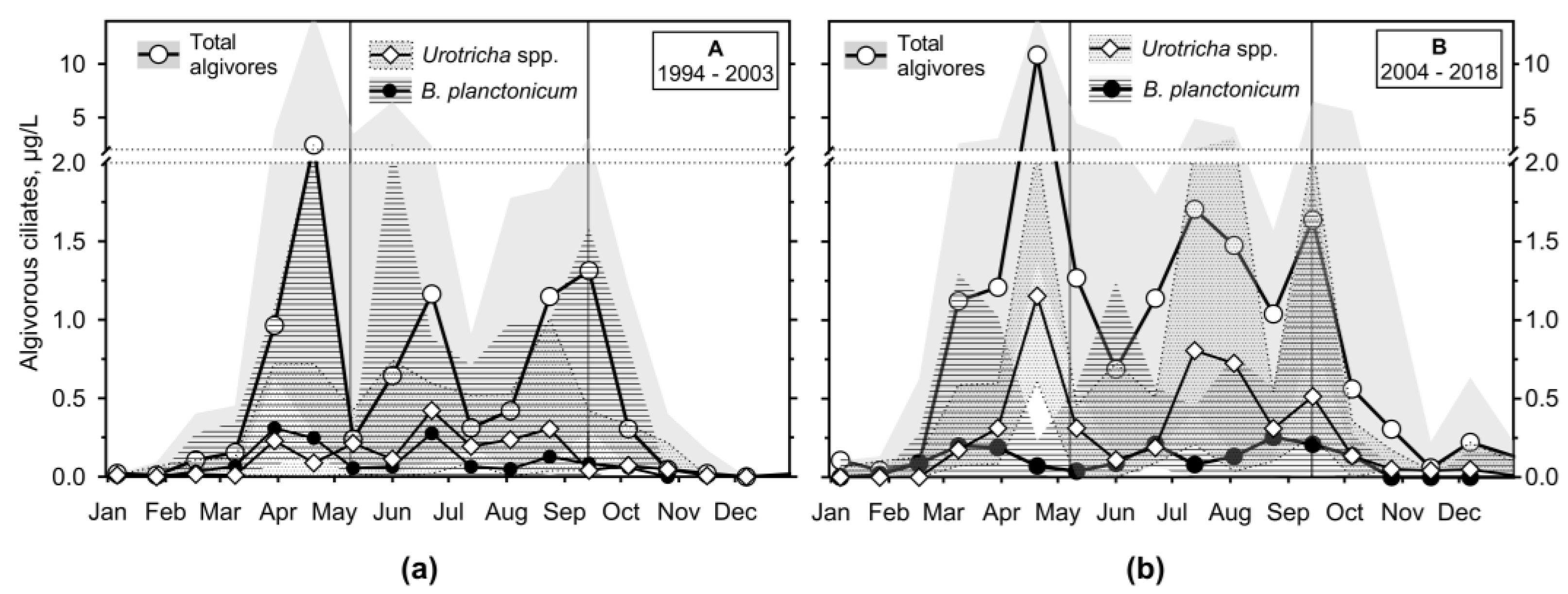
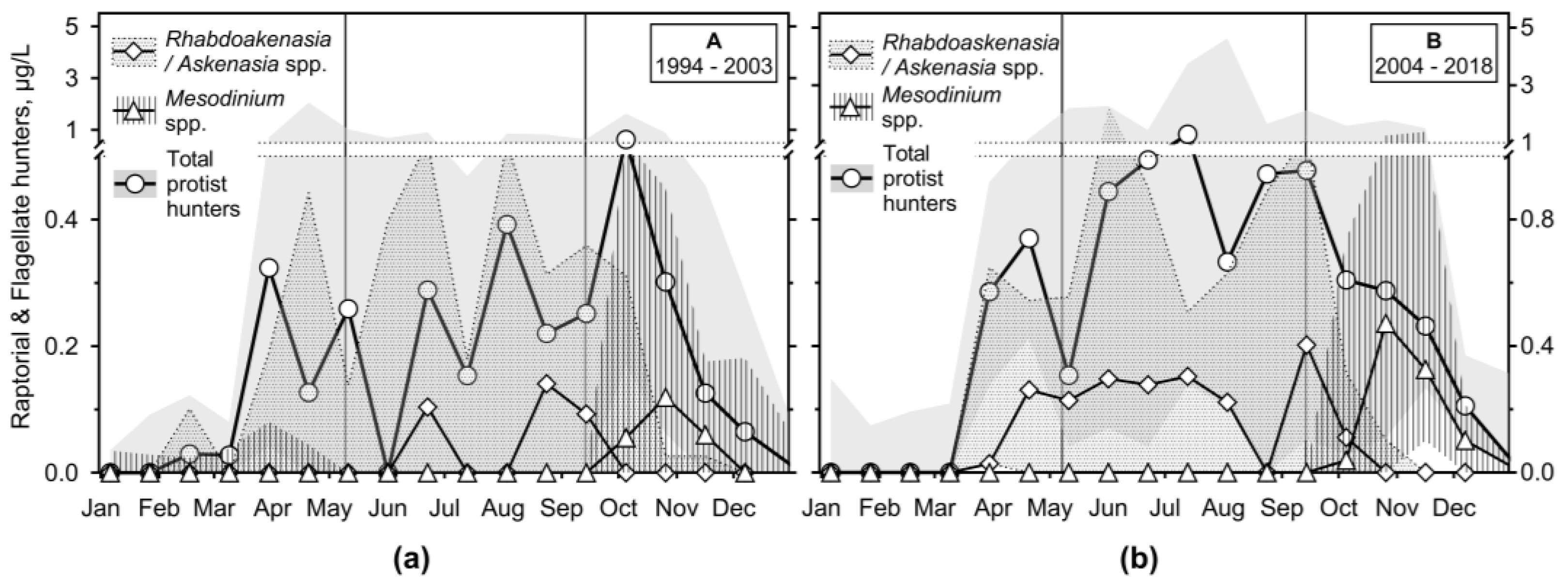
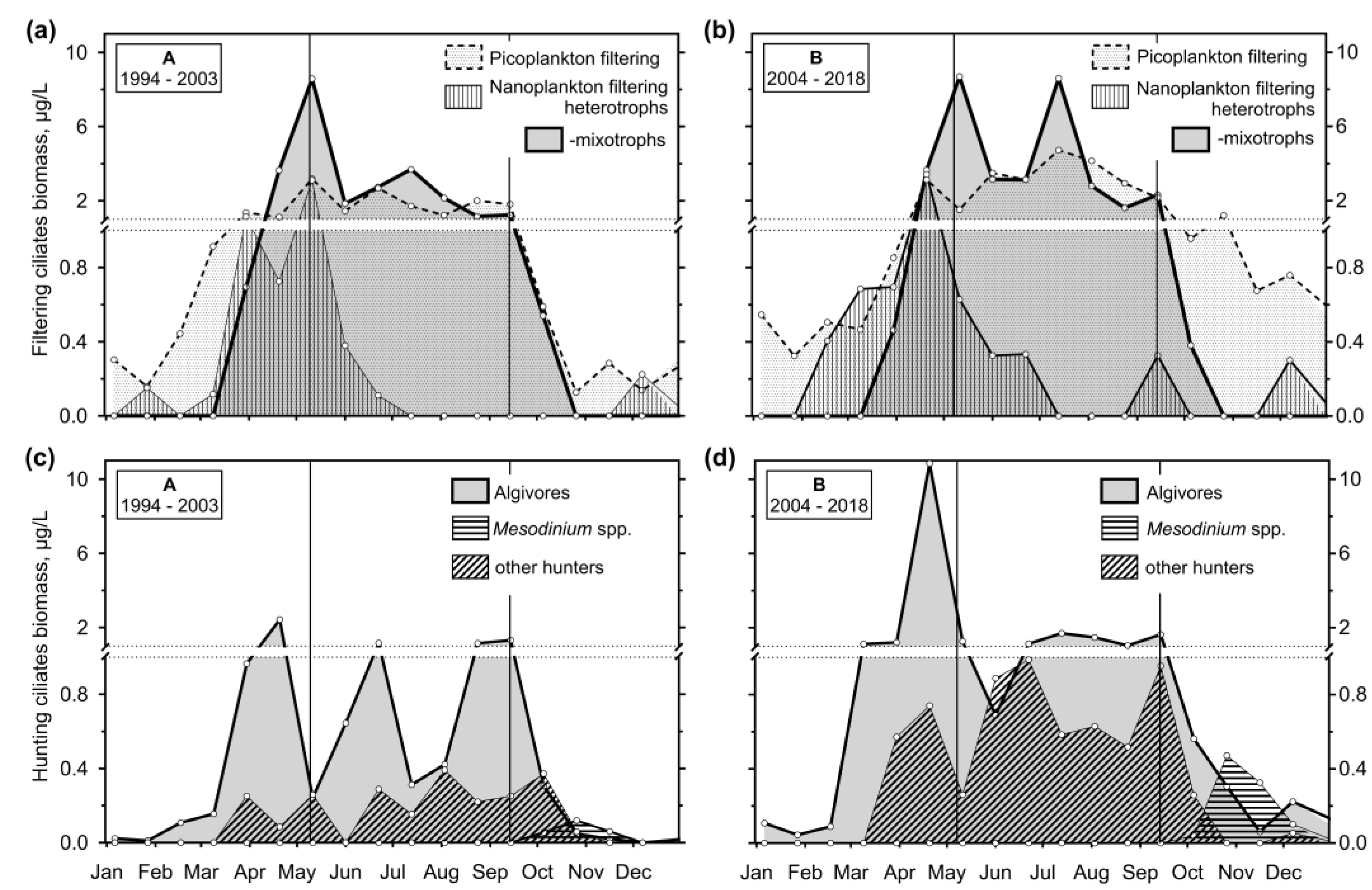
3.2.3. Ciliates’ Assemblage Abundance and Biomass
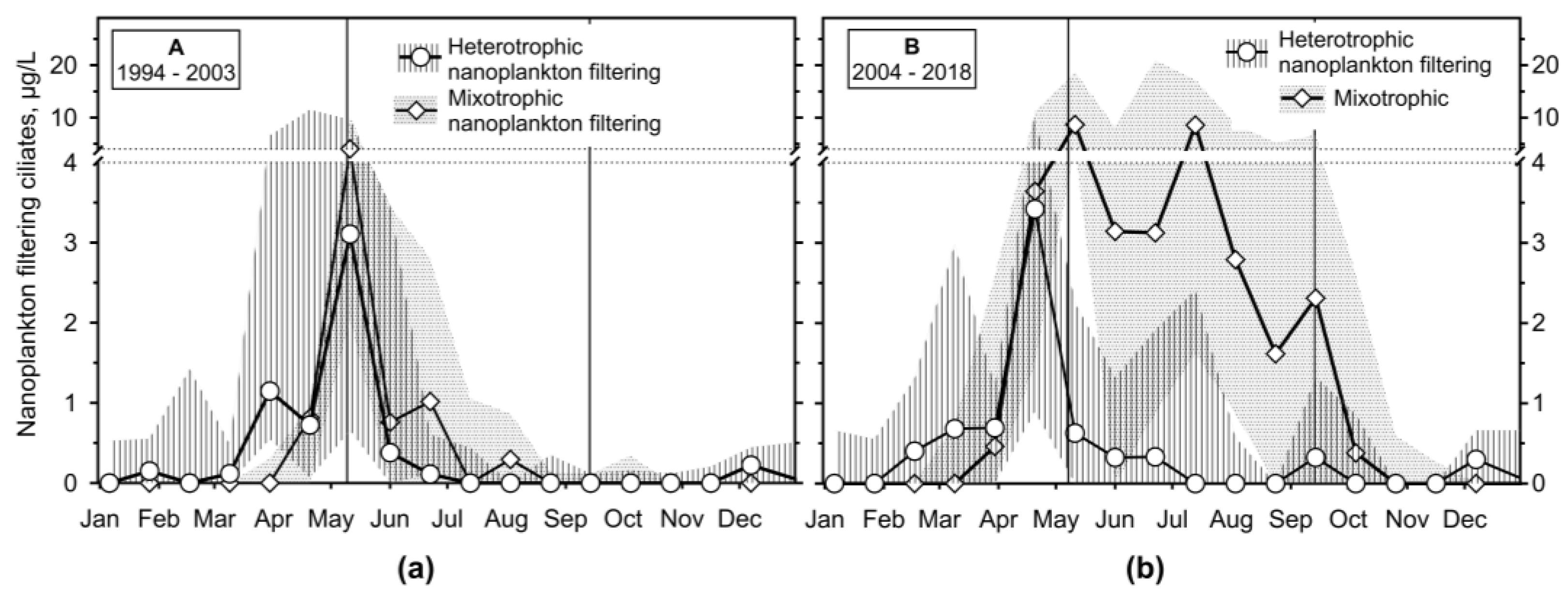
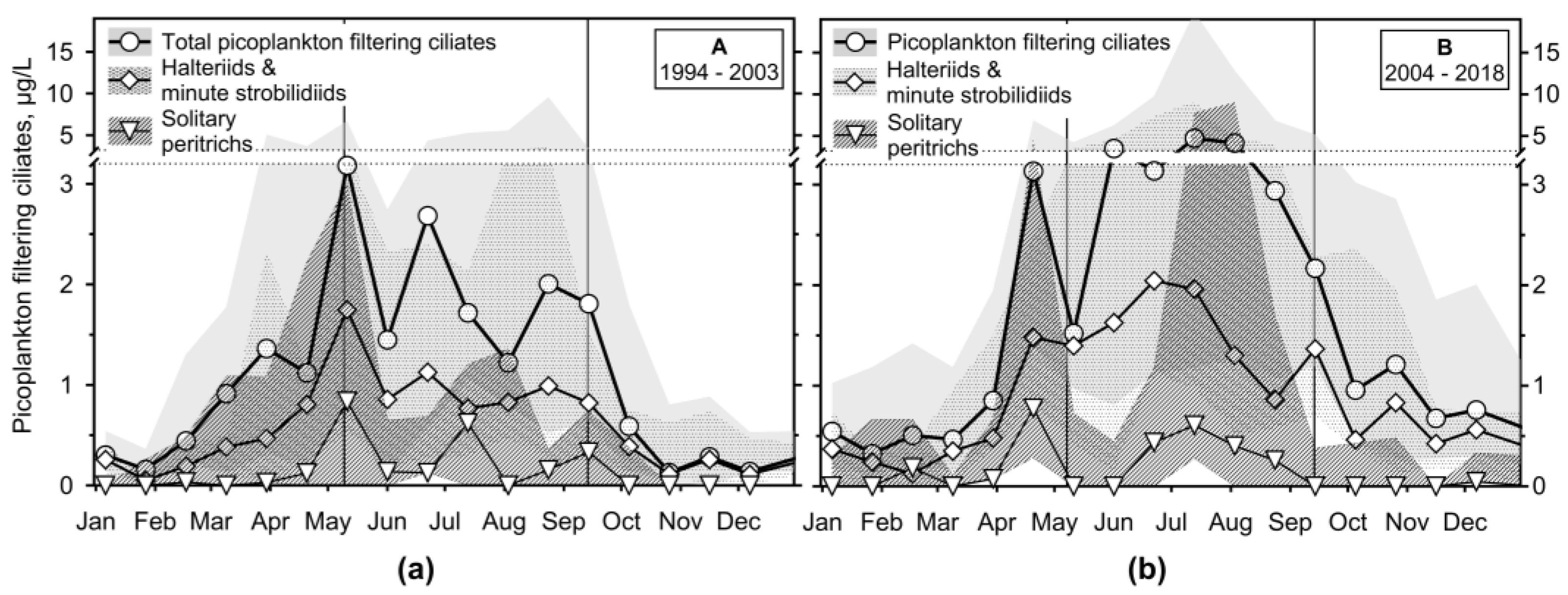
3.2.4. Annual Growth of Feeding-Behaviour Ciliate Groups
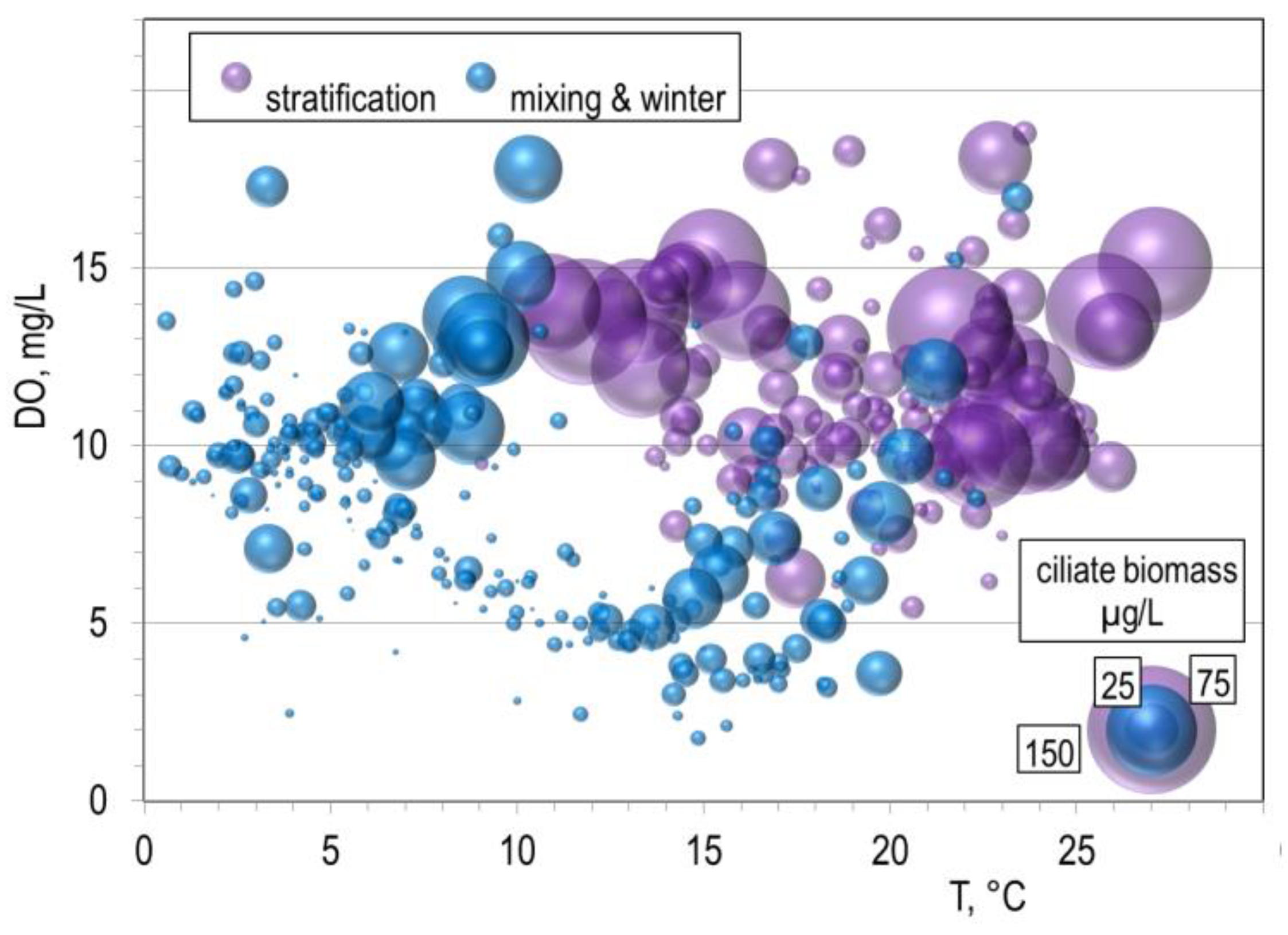
3.3. Predator-Prey Relation and Importance of Different Feeding Behaviour Patterns within Ciliates
4. Discussion
4.1. Hydrodynamics of the Surface Layer and a Legacy of Defined Periods A and B
4.2. Environment Ciliates’ Identification Issues
4.3. Ciliate Ecology in the Reservoir
5. Conclusions
- The presented results as part of the long-term monitoring of reservoir Slapy, to which the ciliate analysis was added in 1994, is one of the longest from freshwater bodies.
- An application of the most straightforward statistical approach of median and interquartile range on a two-decade monitoring data on ciliates in the Slapy reservoir gave results which confirmed ciliate position in the updated PEG model, showing two main peaks related to a spring and summer phytoplankton maximums.
- The observation period was divided into two sections, which differed in terms of the reservoir's nutrient load and were associated with different patterns. In the first period, with higher nutrient loading, the spring peak of ciliate biomass was much higher than the summer one. Apparently, the phytoplankton suppressed the ciliate growth. During the lower nutrient loading period, two similarly high peaks were observed, consisting mainly of mixotrophic species.
- If the ciliate biomass data were time-normalised using a calculated day of stratification, a spring peak and a clear water phase would be very well defined. However, no adequate improvement was observed at the end of the stratification as the duration of the stratification varied.
- It has been shown that the empirical chlorophyll a concentrations marking the beginning of the spring peak at 5 µg/L and the end of the summer peak at 7 µg/L coincide with the main peak of the ciliates: The spring peak of algae-hunting ciliates (Balanion planctonicum, urotrichs; more recently also Histiobalantium spp.) and heterotrophic nanoplankton-filtering tintinnids before stratification, and a peak of mixotrophic nanoplankton filtering ciliates in the stratification event, which lasted longer during the period of lower nutrient load. However, the ciliates showed a higher biomass before the summer peak of chlorophyll a. Only one ciliate, the genus Mesodinium, reached its maximum during the autumn mixing.
- Using the hydrodynamic model to calculate the water age in the epilimnion/surface layer proved useful for understanding ciliate growth there; the layer was well separated, which explains the straightforward behaviour of the ciliates and the composition assemblage changes with increasing nutrient limitation. On the other hand, no information can be extrapolated about the composition and activity of the ciliate assemblage in other parts of the water column.
- By monitoring the ciliate assemblages in the surface layers of different freshwater bodies, we gained valuable insights into their role within the microbial loop of the plankton food web. Our findings revealed an annual periodicity and long-term variations. It is recommended that future monitoring cover additional layers, such as deep chlorophyll a maximum or oxygen local minimum, if present, to capture the complexity of water bodies such as reservoirs or lakes with continuous river flow.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Informationsberichte; Informationberichte des Bayerischen Landesamtes Institut für Wasserwirtschaft: Munich, 1999; ISBN 978-3-930253-79-1.
- Weisse, T.; Montagnes, D.J.S. Ecology of Planktonic Ciliates in a Changing World: Concepts, Methods, and Challenges. J. Eukaryot. Microbiol. 2022, 69, e12879. [CrossRef]
- Plattner, H. Ciliate Research: From Myth to Trendsetting Science. J. Eukaryot. Microbiol. 2022, 69, e12926. [CrossRef]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) Model: Mechanisms Driving Plankton Succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [CrossRef]
- Zimmermann, R.; Meyer-Reil, L.-A. A New Method for Fluorescence Staining of Bacterial Populations on Membrane Filters. 30, 24–27. https://oceanrep.geomar.de/id/eprint/56019.
- Caron, D.A. Technique for Enumeration of Heterotrophic and Phototrophic Nanoplankton, Using Epifluorescence Microscopy, and Comparison with Other Procedures. Appl. Environ. Microbiol. 1983, 46, 491–498. [CrossRef]
- Porter, K.G.; Feig, Y.S. The Use of DAPI for Identifying and Counting Aquatic Microflora1. Limnol. Oceanogr. 1980, 25, 943–948. [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.; Gray, J.; Meyer-Reil, L.; Thingstad, F. The Ecological Role of Water-Column Microbes in the Sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [CrossRef]
- Sherr, E.B.; Sherr, B.F. Protistan Grazing Rates via Uptake of Fluorescently Labeled Prey. In Handbook of Methods on Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; Lewis Publishers: London, 1993; pp. 695–701.
- Sherr, E.B.; Sherr, B.F.; Berman, T.; Hadas, O. High Abundance of Picoplankton-Ingesting Ciliates during Late Fall in Lake Kinneret, Israel. J. Plankton Res. 1991, 13, 789–799. [CrossRef]
- Gifford, D.J. Consumption of Protozoa by Copepods Feeding on Natural Microplankton Assemblages. In Handbook of Methods on Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; Lewis Publishers: London, 1993; pp. 723–729.
- Stoecker, D.; Pierson, J. Predation on Protozoa: Its Importance to Zooplankton Revisited. J. Plankton Res. 2019, 41, 367–373. [CrossRef]
- Hecky, R.E.; Kling, H.J. The Phytoplankton and Protozooplankton of the Euphotic Zone of Lake Tanganyika: Species Composition, Biomass, Chlorophyll Content, and Spatio-temporal Distribution1. Limnol. Oceanogr. 1981, 26, 548–564. [CrossRef]
- Sherr, E.B.; Sherr, B.F. Preservation and Storage of Samples for Enumeration of Heterotrophic Protists. In Handbook of Methods on Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; Lewis Publishers: London, 1993; pp. 207–212.
- Stoecker, D.K.; Gifford, D.J.; Putt, M. Preservation of Marine Planktonic Ciliates: Losses and Cell Shrinkage during Fixation. Mar. Ecol. Prog. Ser. 1994, 110, 293–299. [CrossRef]
- Müller, H. The Relative Importance of Different Ciliate Taxa in the Pelagic Food Web of Lake Constance. Microb. Ecol. 1989, 18, 261–273. [CrossRef]
- Gaedke, U.; Wickham, S.A. Ciliate Dynamics in Response to Changing Biotic and Abiotic Conditions in a Large, Deep Lake (Lake Constance). Aquat. Microb. Ecol. 2004, 34, 247–261. [CrossRef]
- Šimek, K.; Bobková, J.; Macek, M.; Nedoma, J.; Psenner, R. Ciliate grazing on Picoplankton in a Eutrophic Reservoir during the Summer Phytoplankton Maximum: A Study at the Species and Community Level. Limnol. Oceanogr. 1995, 40, 1077–1090. [CrossRef]
- Hadas, O.; Berman, T. Seasonal Abundance and Vertical Distribution of Protozoa (Flagellates, Ciliates), and Bacteria in Lake Kinneret, Israel. Aquat. Microb. Ecol. 1998, 14, 161–170. [CrossRef]
- Peštová, D.; Macek, M.; Martínez-Pérez, M.E. Ciliates and Their Picophytoplankton-Feeding Activity in a High-Altitude Warm-Monomictic Saline Lake. Eur. J. Protistol. 2008, 44, 13–25. [CrossRef]
- Bautista-Reyes, F.; Macek, M. Ciliate Food Vacuole Content and Bacterial Community Composition in the Warm-Monomictic Crater Lake Alchichica, México. FEMS Microbiol. Ecol. 2012, 79, 85–97. [CrossRef]
- Montagnes, D.J.S.; Lynn, D.H. A Quantitative Protargol Stain (QPS) for Ciliates and Other Protists. In Handbook of Methods on Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; Lewis Publishers: London, 1993; pp. 229–240.
- Skibbe, O. An Improved Quantitative Protargol Stain for Ciliates and Other Planktonic Protists. Arch. Hydrobiol. 1994, 130, 339–347. [CrossRef]
- Pfister, G.; Sonntag, B.; Posch, T. Comparison of a Direct Live Count and an Improved Quantitative Protargol Stain (QPS) in Determining Abundance and Cell Volumes of Pelagic Freshwater Protozoa. Aquat. Microb. Ecol. 1999, 18, 95–103. [CrossRef]
- Sonntag, B.; Posch, T.; Klammer, S.; Griebler, C.; Psenner, R. Protozooplankton in the Deep Oligotrophic Traunsee (Austria) Influenced by Discharges of Soda and Salt Industries. Water Air Soil Pollut. Focus 2002, 2, 211–226. [CrossRef]
- Sonntag, B.; Posch, T.; Klammer, S.; Teubner, K.; Psenner, R. Phagotrophic Ciliates and Flagellates in an Oligotrophic, Deep, Alpine Lake: Contrasting Variability with Seasons and Depths. Aquat. Microb. Ecol. 2006, 43, 193–207. [CrossRef]
- Macek, M.; Callieri, C.; Šimek, K.; Lugo-Vázquez, A. Seasonal Dynamics, Composition and Feeding Patterns of Ciliate Assemblages in Oligotrophic Lakes Covering a Wide pH Range. Arch. Für Hydrobiol. 2006, 166, 261–287. [CrossRef]
- Posch, T.; Eugster, B.; Pomati, F.; Pernthaler, J.; Pitsch, G.; Eckert, E.M. Network of Interactions Between Ciliates and Phytoplankton During Spring. Front. Microbiol. 2015, 6. [CrossRef]
- Sánchez Medina, X.; Macek, M.; Bautista-Reyes, F.; Perz, A.; Bonilla Lemus, P.; Chávez Arteaga, M. Inter-Annual Ciliate Distribution Variation within the Late Stratification Oxycline in a Monomictic Lake, Lake Alchichica (Mexico). J. Limnol. 2016, 75. [CrossRef]
- Pitsch, G.; Bruni, E.P.; Forster, D.; Qu, Z.; Sonntag, B.; Stoeck, T.; Posch, T. Seasonality of Planktonic Freshwater Ciliates: Are Analyses Based on V9 Regions of the 18S rRNA Gene Correlated with Morphospecies Counts? Front. Microbiol. 2019, 10, 248. [CrossRef]
- Dirren-Pitsch, G.; Bühler, D.; Salcher, M.M.; Bassin, B.; Le Moigne, A.; Schuler, M.; Pernthaler, J.; Posch, T. FISHing for Ciliates: Catalyzed Reporter Deposition Fluorescence in Situ Hybridization for the Detection of Planktonic Freshwater Ciliates. Front. Microbiol. 2022, 13, 1070232. [CrossRef]
- Finlay, B.J.; Esteban, G.F. Planktonic Ciliate Species Diversity as an Integral Component of Ecosystem Function in a Freshwater Pond. Protist 1998, 149, 155–165. [CrossRef]
- Taylor, W.D.; Heynen, M.L. Seasonal and Vertical Distribution of Ciliophora in Lake Ontario. Can. J. Fish. Aquat. Sci. 1987, 44, 2185–2191. [CrossRef]
- Madoni, P. The Ciliated Protozoa of the Monomictic Lake Kinneret (Israel): Species Composition and Distribution during Stratification. Hydrobiologia 1990, 190, 111–120. [CrossRef]
- Macek, M. Distribution of Ciliates in the Římov Reservoir. Ergeb. Limnol. 1994, 40, 137–141.
- Qu, Z.; Forster, D.; Bruni, E.P.; Frantal, D.; Kammerlander, B.; Nachbaur, L.; Pitsch, G.; Posch, T.; Pröschold, T.; Teubner, K.; et al. Aquatic Food Webs in Deep Temperate Lakes: Key Species Establish through Their Autecological Versatility. Mol. Ecol. 2021, 30, 1053–1071. [CrossRef]
- Abdullah Al, M.; Wang, W.; Jin, L.; Chen, H.; Xue, Y.; Jeppesen, E.; Majaneva, M.; Xu, H.; Yang, J. Planktonic Ciliate Community Driven by Environmental Variables and Cyanobacterial Blooms: A 9-Year Study in Two Subtropical Reservoirs. Sci. Total Environ. 2023, 858, 159866. [CrossRef]
- Straškraba, M.; Hrbáček, J.; Javornický, P. Effect of an Upstream Reservoir on the Stratification Conditions in Slapy Reservoir. In Hydrobiological Studies 2; Hrbáček, J., Straškraba, M., Eds.; Academia, Prague, 1973; pp. 7–82.
- Kopáček, J.; Hejzlar, J.; Porcal, P.; Posch, M. Trends in Riverine Element Fluxes: A Chronicle of Regional Socio-Economic Changes. Water Res. 2017, 125, 374–383. [CrossRef]
- Kopáček, J.; Hejzlar, J.; Porcal, P.; Znachor, P. Biogeochemical Causes of Sixty-Year Trends and Seasonal Variations of River Water Properties in a Large European Basin. Biogeochemistry 2021, 154, 81–98. [CrossRef]
- Fott, J.; Nedbalová, L.; Brabec, M.; Kozáková, R.; Řeháková, K.; Hejzlar, J.; Šorf, M.; Vrba, J. Light as a Controlling Factor of Winter Phytoplankton in a Monomictic Reservoir. Limnologica 2022, 95, 125995. [CrossRef]
- Lewis Jr., W.M. A Revised Classification of Lakes Based on Mixing. Can. J. Fish. Aquat. Sci. 1983, 40, 1779–1787. [CrossRef]
- Vystavna, Y.; Hejzlar, J.; Kopáček, J. Long-Term Trends of Phosphorus Concentrations in an Artificial Lake: Socio-Economic and Climate Drivers. PLoS ONE 2017, 12, e0186917. [CrossRef]
- Cole, T.M. CE-QUAL-W2: A Two-Dimensional, Laterally Averaged, Hydrodynamic and Water Quality Model 2016.
- Monsen, N.E.; Cloern, J.E.; Lucas, L.V.; Monismith, S.G. A Comment on the Use of Flushing Time, Residence Time, and Age as Transport Time Scales. Limnol. Oceanogr. 2002, 47, 1545–1553. [CrossRef]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-Pigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [CrossRef]
- Müller, H.; Schlegel, A. Responses of Three Freshwater Planktonic Ciliates with Different Feeding Modes to Cryptophyte and Diatom Prey. Aquat. Microb. Ecol. 1999, 17, 49–60. [CrossRef]
- Jerome, C.A.; Montagnes, D.J.S.; Taylor, F.J.R. The Effect of the Quantitative Protargol Stain and Lugol’s and Bouin’s Fixatives On Cell Size: A More Accurate Estimate of Ciliate Species Biomass. J. Eukaryot. Microbiol. 1993, 40, 254–259. [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [CrossRef]
- Straškrábová, V. Long-Term Development of Reservoir Ecosystems - Changes in Pelagic Food Webs and Their Microbial Component. Limnetica 2005, 24, 9–20. [CrossRef]
- Hyndman, R.J.; Fan, Y. Sample Quantiles in Statistical Packages. Am. Stat. 1996, 50, 361–365. [CrossRef]
- Motulsky, H.J. GraphPad Statistics Guide.; 2016.
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.1x.; Microcomputer Power: Ithaca, USA, 2018.
- Weisse, T.; Müller, H.; Pinto-Coelho, R.M.; Schweizer, A.; Springmann, D.; Baldringer, G. Response of the Microbial Loop to the Phytoplankton Spring Bloom in a Large Prealpine Lake. Limnol. Oceanogr. 1990, 35, 781–794. [CrossRef]
- Macek, M.; Jezbera, J.; Sed’a, J.; Straškrábová, V.; Šimek, K.; Han, D. Distribution and Growth Rates of Ciliates along a Longitudinal Profile of the Římov Reservoir. In Proceedings of the ResLim 2002; Hydrobiol. Inst. Acad. Sci. Czech Rep. & Fac. Biol. Sci. Univ. South Bohemia: České Budějovice, Czech Republic, 2002; pp. 196–199.
- Jezbera, J.; Nedoma, J.; Šimek, K. Longitudinal Changes in Protistan Bacterivory and Bacterial Production in Two Canyon-Shaped Reservoirs of Different Trophic Status. Hydrobiologia 2003, 504, 115–130. [CrossRef]
- Jeppesen, E.; Sørensen, P.B.; Johansson, L.S.; Søndergaard, M.; Lauridsen, T.L.; Nielsen, A.; Mejlhede, P. Recovery of Lakes from Eutrophication: Changes in Nitrogen Retention Capacity and the Role of Nitrogen Legacy in 10 Danish Lakes Studied over 30 Years. Hydrobiologia 2024. [CrossRef]
- Krainer, K.-H. Taxonomische Untersuchungen an neuen und wenig be kannten planktischen Ciliaten (Protozoa: Ciliophora) aus Baggerseen in Österreich. Luterbornia 1995, 21, 39–68.
- Pfister, G.; Auer, B.; Arndt, H. Pelagic Ciliates (Protozoa, Ciliophora) of Different Brackish and Freshwater Lakes — a Community Analysis at the Species Level. Limnologica 2002, 32, 147–168. [CrossRef]
- Frantal, D.; Agatha, S.; Beisser, D.; Boenigk, J.; Darienko, T.; Dirren-Pitsch, G.; Filker, S.; Gruber, M.; Kammerlander, B.; Nachbaur, L.; et al. Molecular Data Reveal a Cryptic Diversity in the Genus Urotricha (Alveolata, Ciliophora, Prostomatida), a Key Player in Freshwater Lakes, With Remarks on Morphology, Food Preferences, and Distribution. Front. Microbiol. 2022, 12, 787290. [CrossRef]
- Humby, P.L.; Lynn, D.H. Taxonomic Description of Six Species of Pelagic, Freshwater Ciliates from the Order Choreotrichida Collected from Lake Ontario, Canada. Aquat. Ecosyst. Health Manag. 2020, 23, 45–57. [CrossRef]
- Krainer, K.-H. Contributions to the Morphology, Infraciliature and Ecology of the Planktonic Ciliates Strombidium pelagicum n.sp., Pelagostrombidium mirabile (Penard, 1916) n.g., n. comb., and Pelagostrombidium fallax (Zacharias, 1896) n.g., n. comb. (Ciliophora, Oligotrichida). Eur. J. Protistol. 1991, 27, 60–70. [CrossRef]
- Gao, F.; Li, J.; Song, W.; Xu, D.; Warren, A.; Yi, Z.; Gao, S. Multi-Gene-Based Phylogenetic Analysis of Oligotrich Ciliates with Emphasis on Two Dominant Groups: Cyrtostrombidiids and Strombidiids (Protozoa, Ciliophora). Mol. Phylogenet. Evol. 2016, 105, 241–250. [CrossRef]
- Ganser, M.H.; Santoferrara, L.F.; Agatha, S. Molecular Signature Characters Complement Taxonomic Diagnoses: A Bioinformatic Approach Exemplified by Ciliated Protists (Ciliophora, Oligotrichea). Mol. Phylogenet. Evol. 2022, 170, 107433. [CrossRef]
- Agatha, S.; Weißenbacher, B.; Kirschner, M.; Ganser, M.H. Trichite Features Contribute to the Revision of the Genus Strombidium (Alveolata, Ciliophora, Spirotricha). J. Eukaryot. Microbiol. 2024, 71, e13001. [CrossRef]
- Krainer, K.; Foissner, W. Revision of the Genus Askenasia Blochmann, 1895, with Proposal of Two New Species, and Description of Rhabdoaskenasia minima N. G., N. Sp. (Ciliophora, Cyclotrichida). J. Protozool. 1990, 37, 414–427. [CrossRef]
- Macek, M.; Šimek, K.; Pernthaler, J.; Vyhnálek, V.; Psenner, R. Growth Rates of Dominant Planktonic Ciliates in Two Freshwater Bodies of Different Trophic Degree. J. Plankton Res. 1996, 18, 463–481. [CrossRef]
- Küppers, G.C.; Lopretto, E.C.; Claps, M.C. Morphological Aspects and Seasonal Changes of Some Planktonic Ciliates (Protozoa) from a Temporary Pond in Buenos Aires Province, Argentina. Pan-Am. J. Aquat. Sci. 2006.
- Sommer, F.; Sonntag, B.; Rastl, N.; Summerer, M.; Tartarotti, B. Ciliates in Man-Made Mountain Reservoirs. Front. Environ. Sci. 2022, 10, 903095. [CrossRef]
- Cleven, E. Pelagic Ciliates in a Large Mesotrophic Lake: Seasonal Succession and Taxon-Specific Bacterivory in Lake Constance. Int. Rev. Hydrobiol. 2004, 89, 289–304. [CrossRef]
- Tirok, K.; Gaedke, U. Regulation of Planktonic Ciliate Dynamics and Functional Composition during Spring in Lake Constance. Aquat. Microb. Ecol. 2007, 49, 87–100. [CrossRef]
- Lischke, B.; Weithoff, G.; Wickham, S.A.; Attermeyer, K.; Grossart, H.-P.; Scharnweber, K.; HIlt, S.; Gaedke, U. Large Biomass of Small Feeders: Ciliates May Dominate Herbivory in Eutrophic Lakes. J. Plankton Res. 2016, 38, 2–15. [CrossRef]
- Šimek, K.; Grujčić, V.; Nedoma, J.; Jezberová, J.; Šorf, M.; Matoušů, A.; Pechar, L.; Posch, T.; Bruni, E.P.; Vrba, J. Microbial Food Webs in Hypertrophic Fishponds: Omnivorous Ciliate Taxa Are Major Protistan Bacterivores. Limnol. Oceanogr. 2019, 64, 2295–2309. [CrossRef]
- Liu, Y.; Dong, Y.; Zhao, F.; Zheng, S.; Wang, C.; Zhang, W. Distinct Distribution Patterns of Planktonic Ciliate Communities along Environmental Gradients in a Semi-Enclosed Bay. Ecol. Indic. 2022, 144, 109513. [CrossRef]
- Šimek, K.; Macek, M.; Pernthaler, J.; Straškrabová, V.; Psenner, R. Can Freshwater Planktonic Ciliates Survive on a Diet of Picoplankton? J. Plankton Res. 1996, 18, 597–613. [CrossRef]
- Fenchel, T. Protozoan Filter Feeding. Prog. Protistol. 1986, 1, 65–113.
- Bernard, C.; Rassoulzadegan, F. Bacteria or Microflagellates as a Major Food Source for Marine Ciliates: Possible Implications for the Microzooplankton. Mar. Ecol. Prog. Ser. 1990, 64, 147–155. [CrossRef]
- Posch, T.; Pitsch, G.; Bruni, E.P. Protists: Ciliates. In Encyclopedia of Inland Waters; Elsevier, 2022; pp. 639–649 ISBN 978-0-12-822041-2.
- Lu, X.; Gao, Y.; Weisse, T. Functional Ecology of Two Contrasting Freshwater Ciliated Protists in Relation to Temperature. J. Eukaryot. Microbiol. 2021, 68, e12823. [CrossRef]
- Weisse, T.; Jezberová, J.; Moser, M. Picoplankton Feeding by the Ciliate Vorticella similis in Comparison to Other Peritrichs Emphasizes Their Significance in the Water Purification Process. Ecol. Indic. 2021, 121, 106992. [CrossRef]
- Müller, H.; Schöne, A.; Pinto-Coelho, R.M.; Schweizer, A.; Weisse, T. Seasonal Succession of Ciliates in Lake Constance. Microb. Ecol. 1991, 21, 119–138. [CrossRef]
- Kammerlander, B.; Koinig, K.A.; Rott, E.; Sommaruga, R.; Tartarotti, B.; Trattner, F.; Sonntag, B. Ciliate Community Structure and Interactions within the Planktonic Food Web in Two Alpine Lakes of Contrasting Transparency. Freshw. Biol. 2016, 61, 1950–1965. [CrossRef]
- Müller, H. Laboratory Study of the Life Cycle of a Freshwater Strombidiid Ciliate. Aquat. Microb. Ecol. 2002, 29, 189–197. [CrossRef]
- Stoecker, D.K.; Johnson, M.D.; de Vargas, C.; Not, F. Acquired Phototrophy in Aquatic Protists. Aquat. Microb. Ecol. 2009, 57, 279–310. [CrossRef]
- Schoener, D.; McManus, G. Plastid Retention, Use, and Replacement in a Kleptoplastidic Ciliate. Aquat. Microb. Ecol. 2012, 67, 177–187. [CrossRef]
- Müller, H.; Geller, W. Maximum Growth Rates of Aquatic Ciliated Protozoa: The Dependence on Body Size and Temperature Reconsidered. Arch. Hydrobiol. 1993, 126, 315–327. [CrossRef]
- Müller, H.; Weisse, T. Laboratory and Field Observations on the Scuticociliate Histiobalantium from the Pelagic Zone of Lake Constance, FRG. J. Plankton Res. 1994, 16, 391–401. [CrossRef]
- Macek, M.; Šimek, K.; Bittl, T. Conspicuous Peak of Oligotrichous Ciliates Following Winter Stratification in a Bog Lake. J. Plankton Res. 2001, 23, 353–363. [CrossRef]
- Chen, W.-L.; Chiang, K.-P.; Tsai, S.-F. Neglect of Presence of Bacteria Leads to Inaccurate Growth Parameters of the Oligotrich Ciliate Strombidium Sp. During Grazing Experiments on Nanoflagellates. Front. Mar. Sci. 2020, 7, 569309. [CrossRef]
| Feeding behaviour | Family | Genus / species |
|---|---|---|
| Picoplankton filtering PF |
Halteriidae Claparède & Lachmann 1858 |
Halteria grandinella (Müller, 1773) Halteria — mixotrophic Pelagohalteria viridis (Fromentel, 1876) |
| Strobilidiidae Kahl in Doflein & Reichenow 1929 |
Rimostrombidium brachykinetum Krainer, 1995 Rimostrombidium humile (Penard, 1922) Rimostrombidium— mixotrophic |
|
| Vorticellidae Ehrenberg 1838 |
Vorticella — minute Vorticella — large Vorticella aqua-dulcis-complex Stokes, 1887 Vorticella mayeri Faure-Fremiet, 1920 |
|
| Zoothamniidae Sommer, 1951 | Pseudohaplocaulus sp. | |
| Astylozoidae Kahl 1935 |
Astylozoon sp. Hastatella sp. |
|
| Trichodinidae Claus 1874 | Trichodina cf. diaptomi Basson and Van As, 1991 | |
| Cinetochilidae Perty 1852 | Cinetochilum margaritaceum Perty, 1852 | |
| Cyclidiidae Ehrenberg 1838 | Cyclidium spp. | |
| other minute scuticociliates | ||
| Heterotrophic nanoplankton filtering HF |
Strobilidiidae Kahl in Doflein & Reichenow 1929 | Rimostrombidium lacustris (Foissner, Skogstad & Pratt, 1988) |
| Tintinnidiidae Kofoid & Campbell 1929 |
Tintinnidium spp. Tintinnopsis spp. Codonella cratera Leidy, 1887 |
|
| Mixotrophic nanoplankton filtering MF |
Pelagostrombidiidae Agatha 2004 |
Pelagostrombidium spp. Limnostrombidium spp. |
| Strobilidiidae Kahl in Doflein & Reichenow 1929 | Rimostrombidium velox (Faurè-Fremiet, 1924) | |
| Algae hunters AH |
Balanionidae Small & Lynn 1985 | Balanion planctonicum (Foissner, Oleksiv &Müller, 1990) |
| Urotrichidae Small & Lynn 1985 |
Urotricha ~10µm Urotricha globosa Schewiakoff, 1892 Urotricha furcata Schewiakoff, 1892 Urotricha pseudofurcata Krainer 1995 Urotricha pelagica Kahl, 1932 Urotricha castalia Muñoz, Tellez & Fernandez-Galiano, 1987 |
|
| Histiobalantiidae de Puytorac & Corliss in Corliss 1979 | Histiobalantium spp. | |
| Chilodonellidae Deroux 1970 |
Phascolodon vorticella Stein, 1859 Trithigmostoma sp. |
|
| Raptorial & Flagellate hunters RH |
Colepidae Ehrenberg 1838 | Coleps spp. |
| Actinobolinidae Kahl 1930 | Actinobolina spp. | |
| Enchelyidae Ehrenberg 1838 | Enchelys spp. | |
| Trachelophyllidae Kent 1882 | Lagynophrya spp. — heterotrophicLagynophrya spp. — nixotrophic | |
| Didiniidae Poche 1913 |
Monodinium spp. Monodinium spp. — large |
|
| Incertae sedis CON-threeP |
Askenasia chlorelligera Krainer & Foissner, 1990 Askenasia volvox (Penard 1922) Askenasia spp. — minute Askenasia spp. — large Rhabdoaskenasia minima Krainer & Foissner, 1990 |
|
| Incertae sedis SAL |
Mesodinium spp. Litonotus spp. — minute |
|
| Oversized biomass | Epistylididae Kahl 1933 | Epistylis spp. — pelagic, colonial |
| Stentoridae Carus 1863 | Stentor sp. | |
| Holophryidae Perty 1852 | Holophrya spp. — large | |
| Cyclotrichiidae Jankowski 1980 | Cyclotrichium sp. | |
| Lacrymariidae de Fromentel 1876 | Lacrymaria spp. | |
| Dileptidae Jankowski 1980 | Pelagodileptus spp. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





