Submitted:
20 May 2024
Posted:
21 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
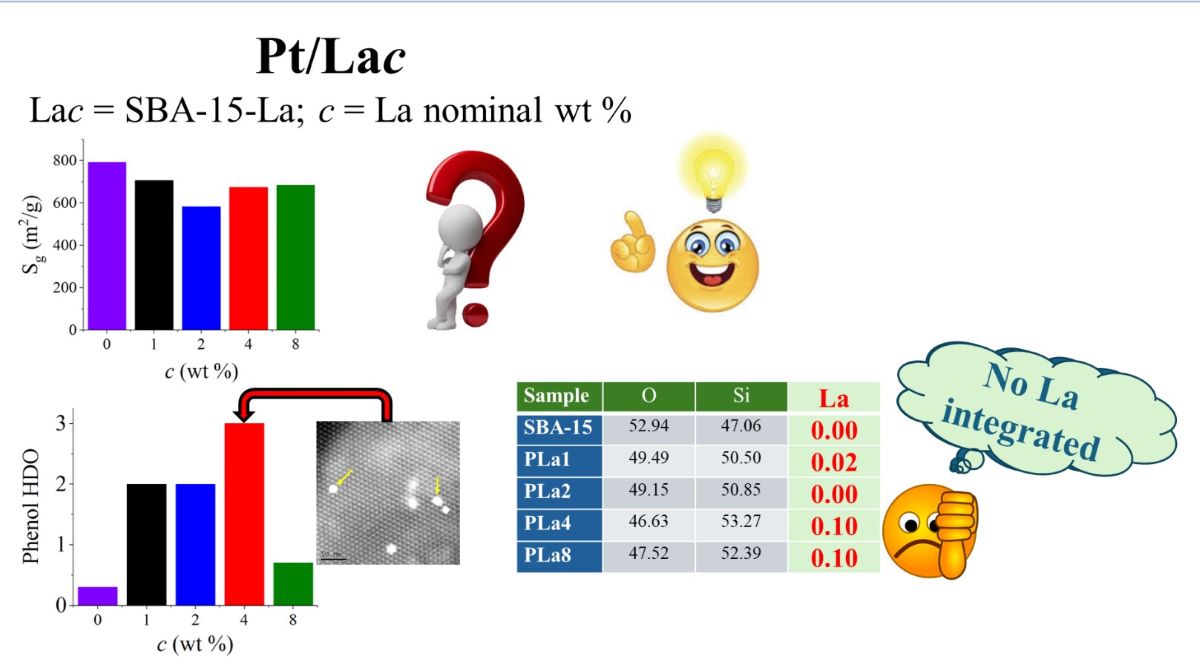
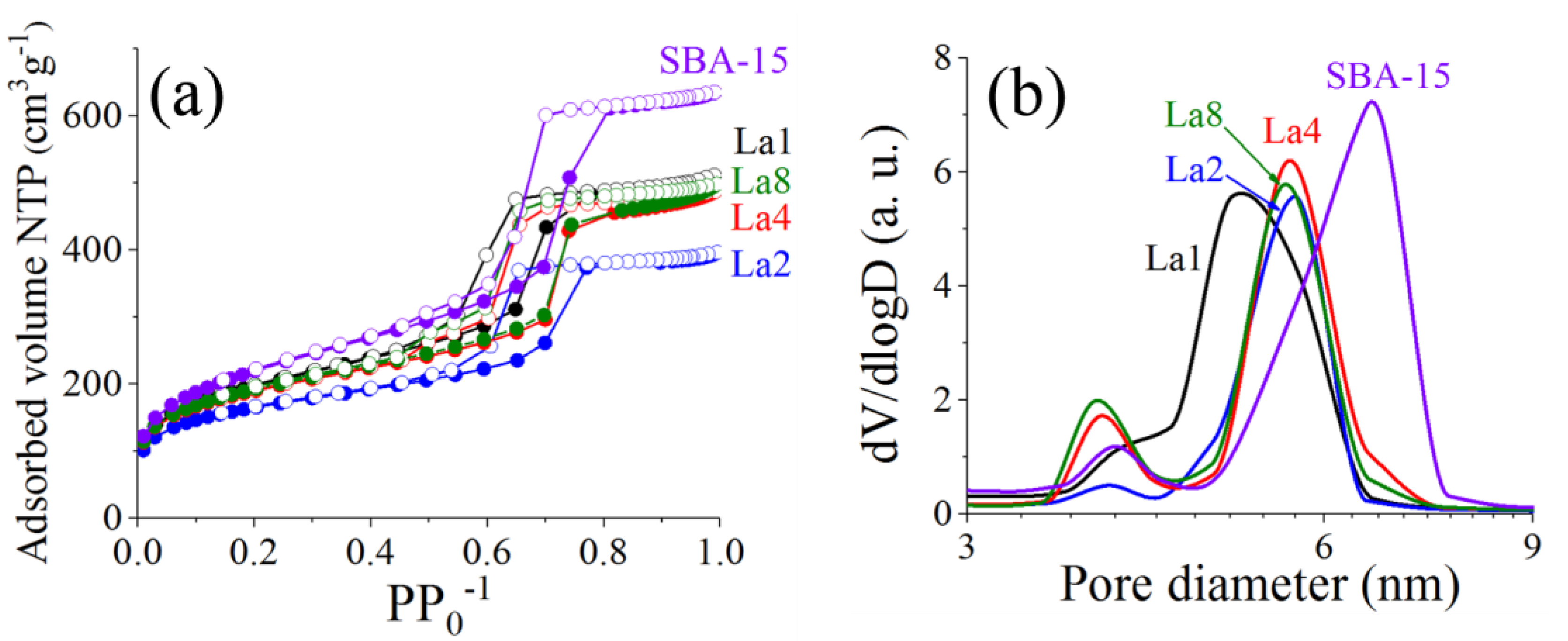
2.1. Materials Textural Properties
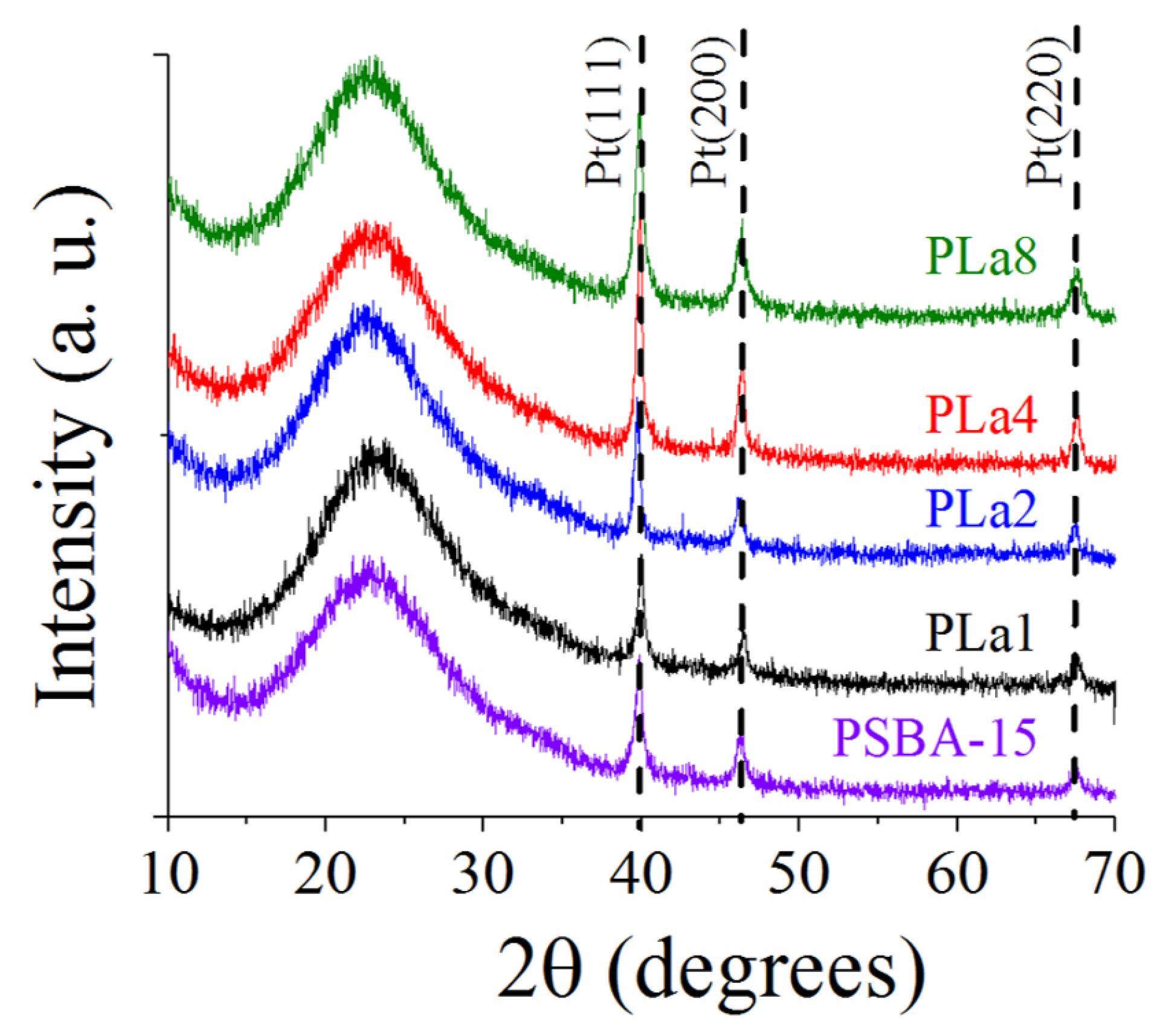
2.2. X-ray Diffraction
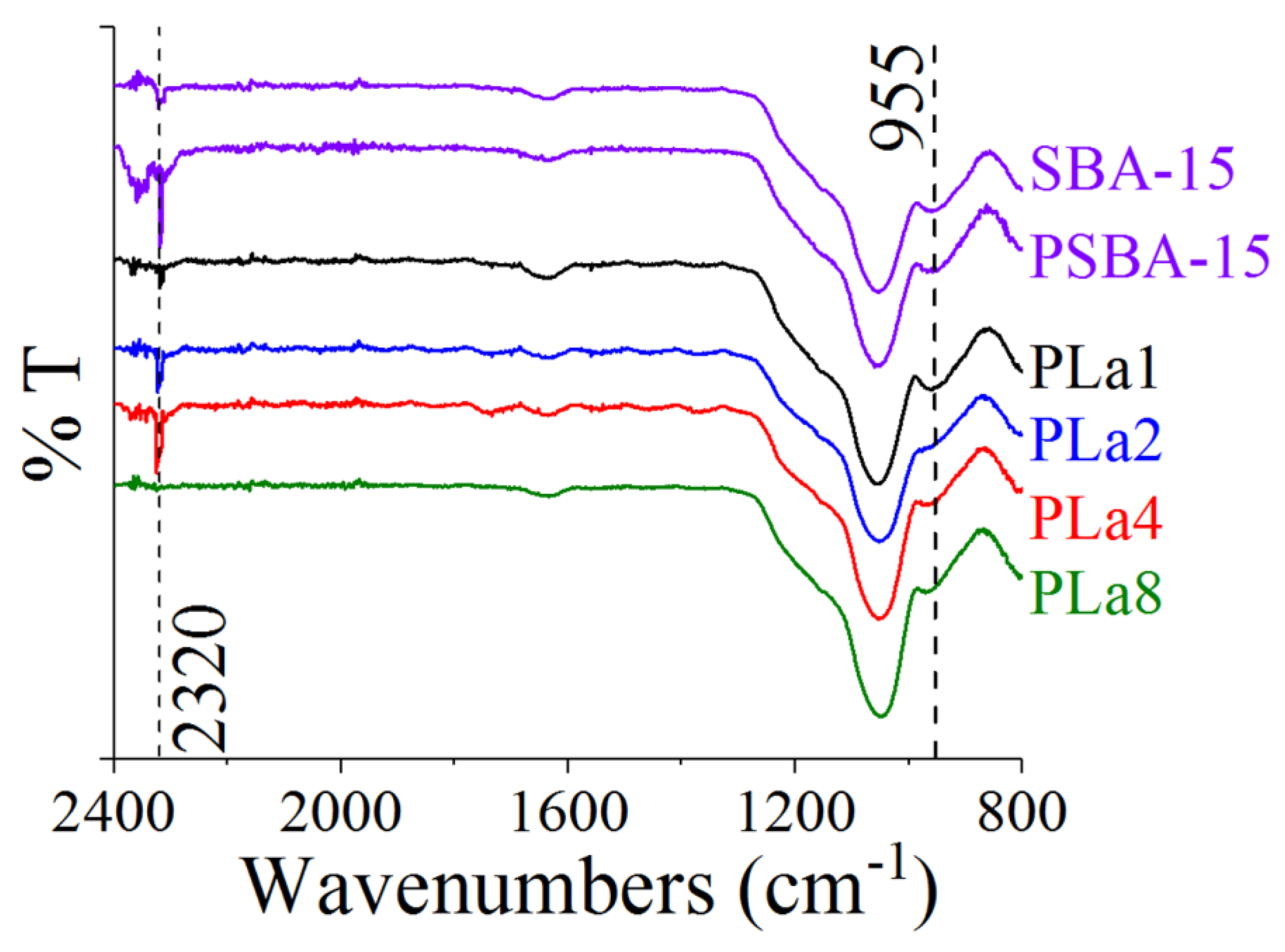
2.3. FTIR Characterization
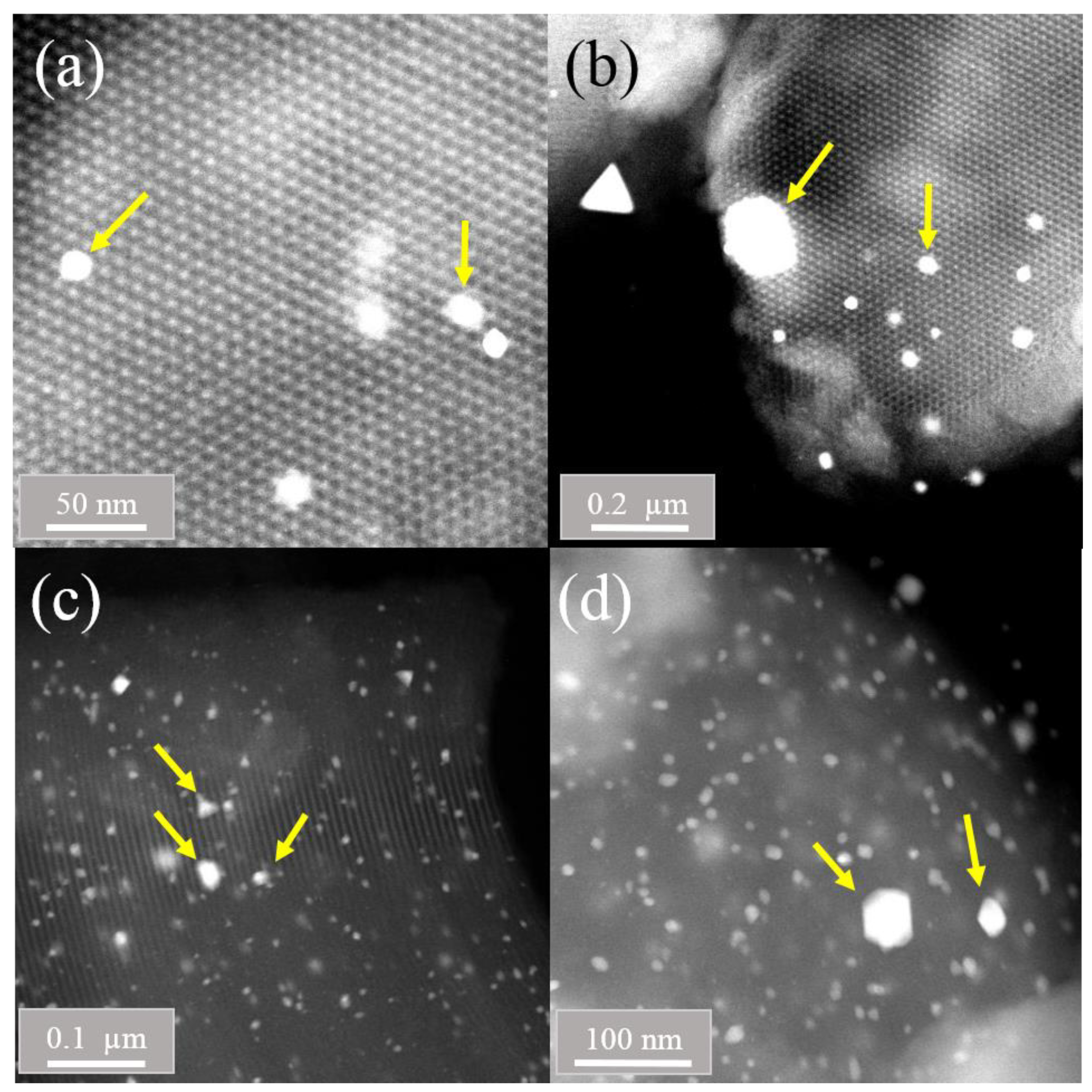
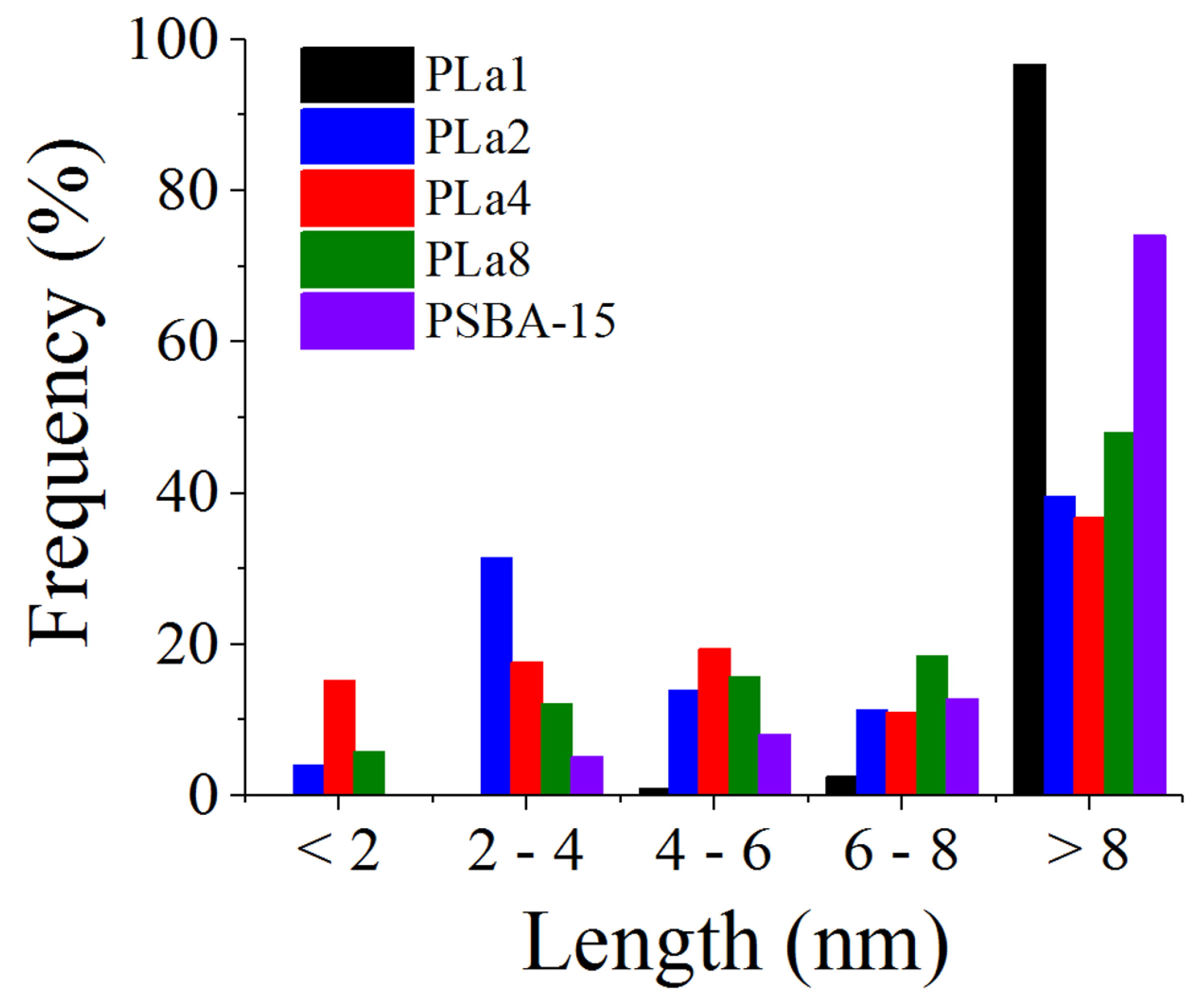
2.4. STEM (HAADF) Studies
2.5. HDO Reaction Test
2.6. NH3 and CO2 Temperature-Programmed Desorption (TPD)
2.7. Thermal Analysis
2.8. SEM-EDS Analysis
3. Experimental
3.1. Material Synthesis
3.1.1. La-Modified SBA-15
3.1.2. Pt-containing La-modified SBA-15
3.2. Materials Characterization
3.3. Phenol HDO Reaction Test
4. Conclusions
Acknowledgments
Supplementary Materials
Authors contributions
Funding
Data availability statement
Conflicts of interest
References
- Escobar, J.; Colín-Luna, J.A.; Barrera, M.C. Thioresistant PdPt/Al/SBA-15 for naphthalene hydrogenation. Ind. Eng. Chem. Res. 2024, 63(3), 1248–1260. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Fan, M.; Sun, W.; Jiang, P.; Dong, Y. Direct and postsynthesis of Ti-incorporated SBA-15 functionalized with sulfonic acid for efficient biodiesel production. Fuel 2019, 235, 426–432. [Google Scholar] [CrossRef]
- Morales Hernández, G.; Pacheco Sosa, J. G.; Escobar Aguilar, J.; Torres Torres, J.G.; Pérez Vidal, H.; Lunagómez Rocha, M.A.; De la Cruz Romero, D.; del Ángel Vicente, P. Improving platinum dispersion on SBA-15 by titania addition. Rev. Mex. Ing. Chem. 2020, 19(2), 997–1010. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Santes, V.F.; Fouconnier, B. Guaiacol HDO on La-modified Pt/Al2O3: Influence of rare-earth loading. Can. J. Chem. Eng. 2023, 101(10), 5772–5784. [Google Scholar] [CrossRef]
- Bendahou, K.; Cherif, L.; Siffert, S.; Tidahy, H.L.; Benaïssa, H.; Aboukaïs, A. The effect of the use of lanthanum-doped mesoporous SBA-15 on the performance of Pt/SBA-15 and Pd/SBA-15 catalysts for total oxidation of toluene. Appl, Catal. A-Gen. 2008, 351, 82–87. [Google Scholar] [CrossRef]
- Roy, S.; Newalkar, B.L.; Datta, S. Vanadium Substituted SBA-15 Supported Bimetallic Pt, Pd Catalysts for Hydrogenation of Toluene to Methylcyclohexane. Can. J. Chem. Eng. 2014, 92, 1034–1040. [Google Scholar] [CrossRef]
- Flodström, K.; Alfredsson, V. Influence of the block length of triblock copolymers on the formation of mesoporous silica. Microporous and Mesoporous Mater. 2003, 59, 167–176. [Google Scholar] [CrossRef]
- Pinzón-Ramos, I.; Castillo-Araiza, C.O.; Tavizón-Pozos, J.A.; de los Reyes, J.A. On a Response Surface Analysis: Hydrodeoxygenation of Phenol over a CoMoS-Based Active Phase. Catalysts 2022, 12(10), 1139. [Google Scholar] [CrossRef]
- Leofanti, S.G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–210. [Google Scholar] [CrossRef]
- Palcheva, R.; Kaluža, L.; Dimitrov, L.; Tyuliev, G.; Avdeev, G.; Jirátová, K.; Spojakina, A. NiMo catalysts supported on the Nb modified mesoporous SBA-15 and HMS: effect of thioglycolic acid addition on HDS. Appl. Catal. A-Gen, 520, 24–34. [CrossRef]
- Miller, J.T.; Schreier, M.; Kropf, A.J.; Regalbuto, J. R. A fundamental study of platinum tetraammine impregnation of silica 2. The effect of method of preparation, loading, and calcination temperature on (reduced) particle size. J. Catal. 2004, 225, 203–212. [Google Scholar] [CrossRef]
- Vít, Z.; Gulková, D.; Kaluža, L.; Boaro, M. Effect of catalyst precursor and its pretreatment on the amount of β-Pd hydride phase and HDS activity of Pd-Pt/silica-alumina. Appl. Catal. B. Environ. 2014, 146, 213–220. [Google Scholar] [CrossRef]
- Hernández Morales, R.; Pacheco Sosa, J.G.; Torres Torres, J.G.; Pérez Vidal, H.; Lunagómez Rocha, M.A.; De la Cruz Romero, D. Pt/Ga-SBA-15 composites synthesis and characterization. Superf. y Vacío 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wen, B.; Zhu, M.; Dai, B. Lanthanum incorporated in MCM-41 and its application as a support for a stable Ni-based methanation catalyst. J. Rare Earths 2018, 36(4), 367–373. [Google Scholar] [CrossRef]
- Giraldo, L.; Bastidas-Barranco, M.; Moreno-Piraján, J.C. Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16. Molecules 2014, 19, 20594–20612. [Google Scholar] [CrossRef] [PubMed]
- Gregg, S.J.; Ramsay, J.D.F. A Study of the Adsorption of Carbon Dioxide by Alumina Using Infrared and Isotherm Measurements. J. Phys. Chem. 1969, 73, 1243–1247. [Google Scholar] [CrossRef]
- Parkyns, N.D. The Influence of Thermal Pretreatment on the Spectrum of Carbon Dioxide Adsorbed on Alumina. J. Phys. Chem. 1971, 76, 526–531. [Google Scholar] [CrossRef]
- Escobar, J.; Ramírez, J.; Cuevas, R.; Ángeles, C.; Barrera, M.C.; Gutiérrez, A. Thiophene HDS on La-Modified CoMo/Al2O3 Sulfided Catalysts. Effect of Rare-Earth Content. Top. Catal. 2020, 63, 529–545. [Google Scholar] [CrossRef]
- Hungría, A.B.; Calvino, J.J.; Hernández-Garrido, J.C. HAADF STEM Electron Tomography in Catalysis Research. Top. Catal. 2019, 62, 808–821. [Google Scholar] [CrossRef]
- Ballesteros-Plata, D.; Infantes-Molina, A.; Rodríguez-Castellón, E. Study of bifunctionality of Pt/SBA-15 catalysts for HDO of Dibenzofuran reaction: Addition of Mo or use of an acidic support. Appl. Catal. A-Gen 2019, 580, 93–101. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ra, J.; Shen, K.; Vohs, J.M.; Gorte, R.J. Hydrodeoxygenation of m-Cresol over WOx-Pt/SBA-15 Using Alkanes as Hydrogen Carriers. ACS Catal. 2023, 13(16), 10908–10915. [Google Scholar] [CrossRef]
- Ballesteros-Plata, D.; Barroso-Martín, I.; Medina Cervantes, J.A.; Maciel, C.; Huirache-Acuña, R.; Rodríguez-Castellón, E.; Infantes-Molina, A. Bimetallic Niobium-Based Catalysts Supported on SBA-15 for Hydrodeoxygenation of Anisole. Ind. Eng. Chem. Res. 2021, 60(51), 18831–18840. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A.; Szczyglewska, P.; Nowak, I. The effect of metal (Nb, Ru, Pd, Pt) supported on SBA-16 on the phenol Hydrodeoxygenation. Catal. Today 2019, 325, 61–67. [Google Scholar] [CrossRef]
- Shivhare, A.; Hunns, J.A.; Durndell, L.J.; Parlett, C.M.A.; Isaacs, M.A.; Lee, A.F.; Wilson, K. Metal-Acid Synergy: Hydrodeoxygenation of Anisole over Pt/Al-SBA-15. ChemSusChem 2020, 13, 4945–4953. [Google Scholar] [CrossRef] [PubMed]
- Novodarszki, G.; Lónyi, F.; Csík, B.; Mihályi, M.R.; Barthos, R.; Valyon, J.; Vikár, A.; Deka, D.; Pászti, Z.; Shi, Y.; Solt, H.E. Hydroconversion of lignin-derived platform compound guaiacol to fuel additives and value-added chemicals over alumina-supported Ni catalysts. Appl. Catal. A-Gen 2024, 680, 119757. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Pawelec, B.; Fierro, J.L.G.; Loricera, C.V.; Jiménez-López, A.; Rodríguez-Castellón. Effect of Ir and Pt Addition on the HDO Performance of RuS2/SBA-15 Sulfide Catalysts. Top. Catal. 2015, 58, 247–257. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Jeon, J.-K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic pyrolysis of biomass components over mesoporous catalysts using Py-GC/MS. Catal. Today 2013, 204, 170–178. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Valente, J.S.; Solís-Casados, D.A.; Santes, V.; Terrazas, J.E.; Fouconnier, B.A.R. Dibenzothiophene Hydrodesulfurization over P-CoMo on Sol-Gel Alumina Modified by La Addition. Effect of Rare-Earth Content. Catalysts 2019, 9(4), 359. [Google Scholar] [CrossRef]
- Cui, J.-W.; Massoth, F.E.; Topsøe, N.Y. Studies of Molybdena-Alumina Catalysts XVIII. Lanthanum-Modified Supports. J. Catal. 1992, 136, 361–377. [Google Scholar] [CrossRef]
- Terrab, I.; Ouargli, R.; Boukoussa, B.; Ghomari, K.; Hamacha, R; Roy, R.; Azzouz, A.; Bengueddach, A. Assessment of the intrinsic interactions of mesoporous silica with carbon dioxide. Res Chem. Intermed. 2017, 43, 3775–3786. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Nunes-Beltrao, A. P.; Hamacha, R.; Azzouz, A. Assessment of the intrinsic interactions of nanocomposite polyaniline/SBA-15 with carbon dioxide: Correlation between the hydrophilic character and surface basicity. J. CO2 UTIL. 2018, 26, 171–178. [Google Scholar] [CrossRef]
- Bartz, W.; Górka, M.; Rybak, J.; Rutkowski, R.; Stojanowska, A. The assessment of effectiveness of SEM- EDX and ICP-MS methods in the process of determining the mineralogical and geochemical composition of particulate matter deposited on spider webs. Chemosphere 2021, 278, 130454. [Google Scholar] [CrossRef] [PubMed]
| Sample | SBET (m2g-1) |
Vp (cm3g-1) |
aDp Peak 1 |
(nm) Peak 2 |
bSBET (m2g-1) |
SBET/bSBET |
| SBA-15 | 793 | 0.98 | 3.98 | 6.6 | - | |
| La1 | 706 | 0.78 | 4.09 | 5.10 | 785 | 0.90 |
| La2 | 582 | 0.61 | 3.93 | 5.66 | 777 | 0.75 |
| La4 | 674 | 0.75 | 3.89 | 5.60 | 761 | 0.89 |
| La8 | 685 | 0.76 | 3.86 | 5.58 | 730 | 0.94 |
| Catalyst | Crystal size (nm) |
|---|---|
| PSBA-15 | 22.3 |
| PLa1 | 23.6 |
| PLa2 | 27.9 |
| PLa4 | 32.8 |
| PLa8 | 21.2 |
| Sample | Peak position (cm-1)) |
| SBA-15 | 955 |
| PSBA-15 | 959 |
| PLa1 | 961 |
| PLa2 | 964 |
| PLa4 | 968 |
| PLa8 | 971 |
| Sample |
kHDO (×10-5) (s-1) |
| PSBA-15 | 0.3 |
| PLa1 | 2.0 |
| PLa2 | 2.0 |
| PLa4 | 3.0 |
| PLa8 | 0.7 |
| Sample | O | Si | La |
| SBA-15 | 52.94 | 47.06 | 0.00 |
| La1 | 49.49 | 50.50 | 0.02 |
| La2 | 49.15 | 50.85 | 0.00 |
| La4 | 46.63 | 53.27 | 0.10 |
| La8 | 47.52 | 52.39 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





