Submitted:
21 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
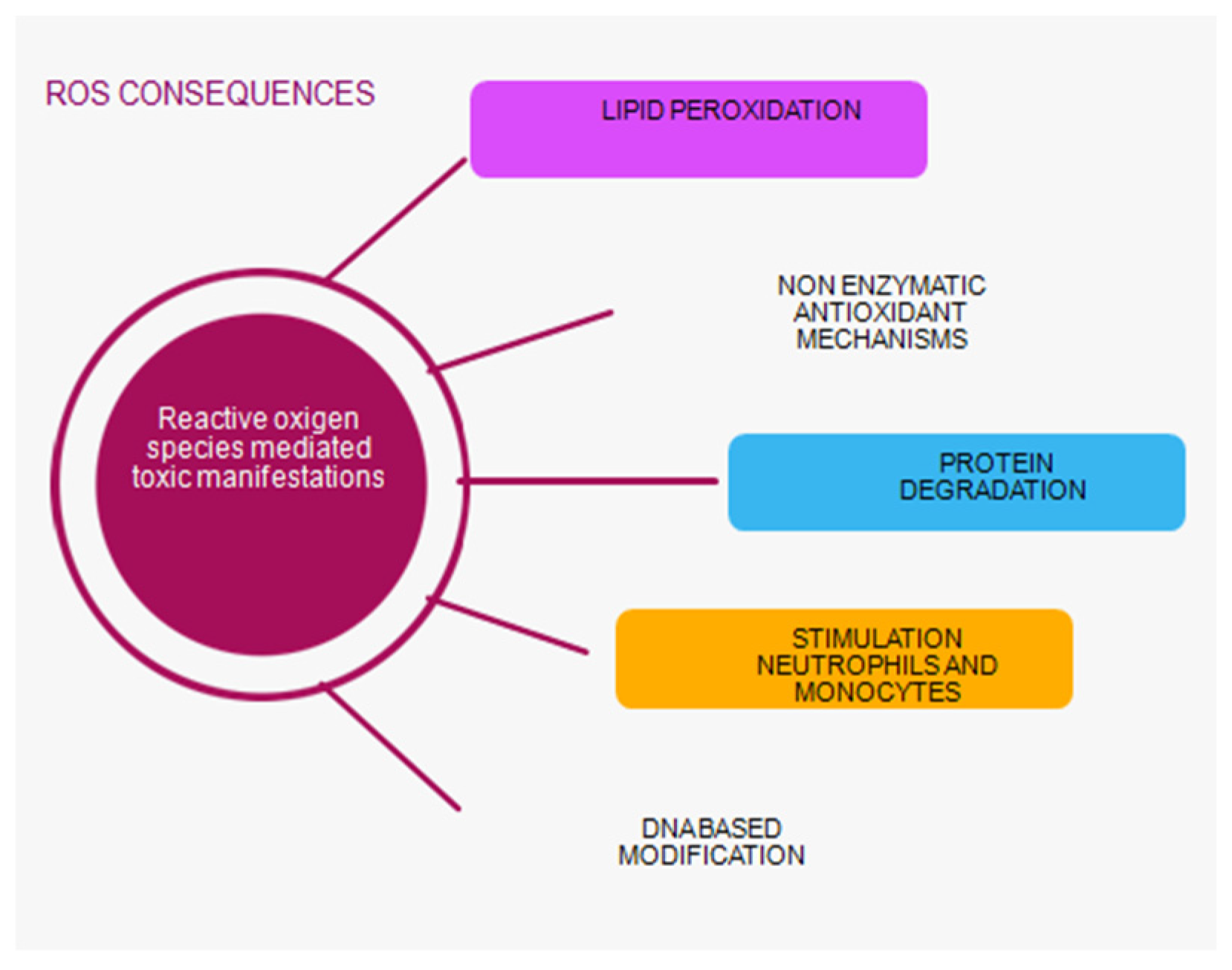
1.1. Oxidative Stress and Mechanisms of Its Occurrence
1.2. Oxidative Stress in Patients with AKI
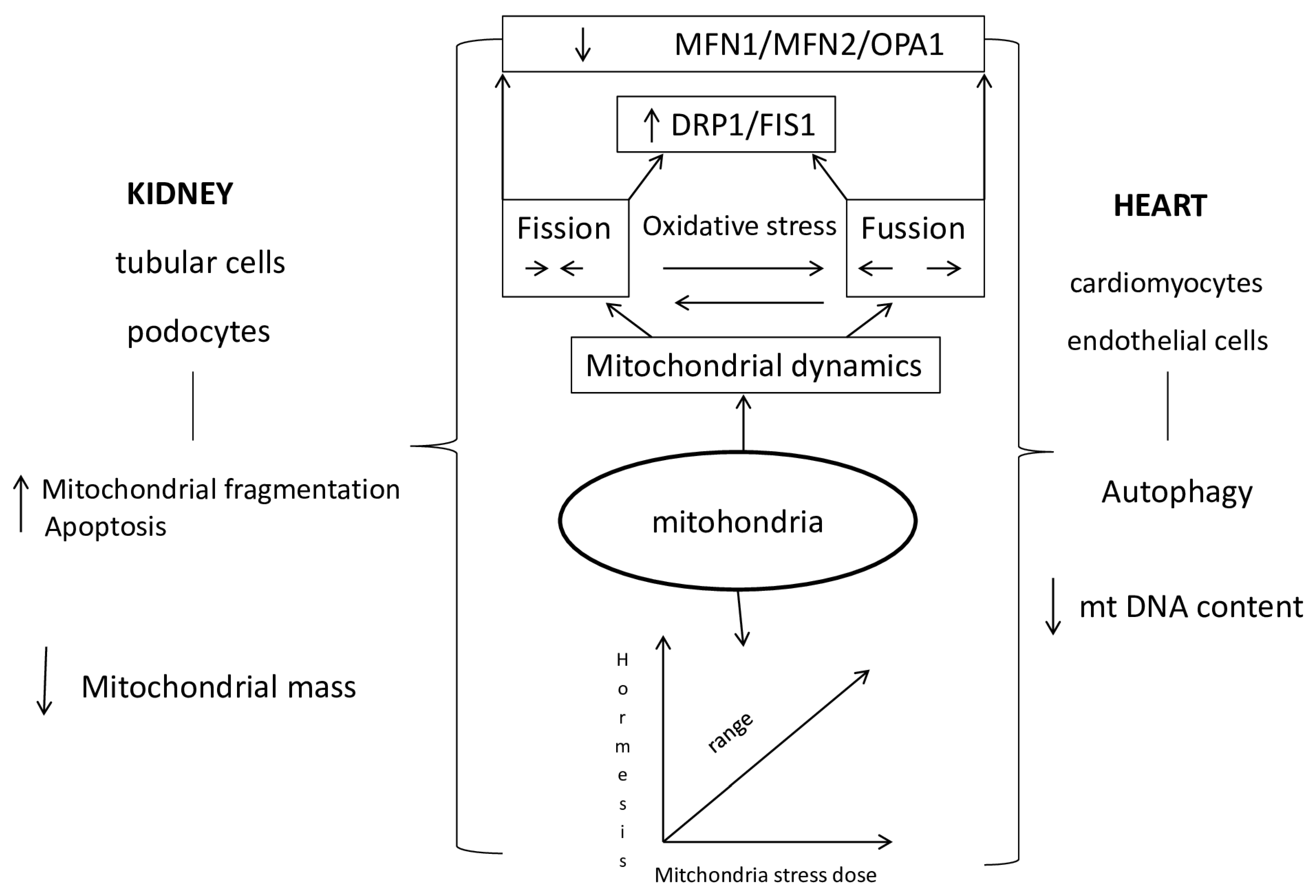
1.3. The Role of Mitochondria in Acute Renocardial Syndrome
1.4. Mechanisms of Mitochondrial Dysfunction in Cardiorenal Syndrome Type 3
2. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Declaration of Conflicting Interests
References
- K/DIGO AKI Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Intern Suppl. 2012, 2, S3–S106. [Google Scholar]
- Sawhney, S.; Fraser, D.S. Epidemiology of AKI: Utilizing Large Database to determine the Burden of AKI. ACKD 2017, 24, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Topete, L.M.; Rosner, M.H.; Ronco, C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team. Blood Purif. 2016, 43, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Macedo, E.; Mehta, R.L. Preventing Acute Kidney Injury. Crit Care Clin. 2015. [Google Scholar] [CrossRef]
- K/DIGO AKI Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Intern Suppl. 2012, 2, S3–S106. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Kellum, J.A.; Ronco, C.; Bellomo, R. Acute kidney disease and the community. Lancet 2016, 387, 1974–1976. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, D.; Rydén, L.; Pickering, J.W.; Sartipy, U.; Holzmann, M.J. Acute kidney injury-an overview of diagnostic methods and clinical management. Clin. Kidney J. 2017, 10, 323–331. [Google Scholar] [CrossRef]
- Feltes, C.M.; Van Eyk, J.; Rabb, H. Distant-organ changes after acute kidney injury. Nephron Physiol. 2008, 109, 80–84. [Google Scholar] [CrossRef]
- Tecson, K.M.; Hashemi, H.; Afzal, A.; Gong, T.A.; Kale, P.; McCullough, P.A. Community-Acquired Acute Kidney Injury as a Risk Factor of de novo Heart Failure Hospitalization. Cardiorenal Med. 2019, 9, 252–260. [Google Scholar] [CrossRef]
- ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2021. Eur Heart J 2021, 42, 3599–3726. [CrossRef]
- Pliquett, R.U. Cardiorenal Syndrome: An Updated Classification Based on Clinical Hallmarks. J. Clin. Med. 2022, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Virzì, G.M.; Breglia, A.; Castellani, C.; Ankawi, G.; Bolin, C.; de Cal, M.; Cianci, V.; Angelini, A.; Vescovo, G.; Ronco, C. Lipopolysaccharide in systemic circulation induces activation of inflammatory response and oxidative stress in cardiorenal syndrome type 1. J. Nephrol. 2019, 32, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Virzì, G.M.; Breglia, A.; Brocca, A.; de Cal, M.; Bolin, C.; Vescovo, G.; Ronco, C. Levels of Proinflammatory Cytokines, Oxidative Stress, and Tissue Damage Markers in Patients with Acute Heart Failure with and without Cardiorenal Syndrome Type 1. Cardiorenal Med. 2018, 8, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Virzì, G.M.; Clementi, A.; de Cal, M.; Brocca, A.; Day, S.; Pastori, S.; Bolin, C.; Vescovo, G.; Ronco, C. Oxidative Stress: Dual Pathway Induction in Cardiorenal Syndrome Type 1 Pathogenesis. Oxidative Med. Cell. Longev. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Matheny, M.E.; Greevy, R.A.; Eden, S.K.; Perkins, A.M.; Parr, S.K.; Fly, J.; Abdel-Kader, K.; Himmelfarb, J.; Hung, A.M.; et al. Acute Kidney Injury and Risk of Incident Heart Failure Among US Veterans. Am. J. Kidney Dis. 2018, 71, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Gammelager, H.; Christiansen, C.F.; Johansen, M.B.; Tønnesen, E.; Jespersen, B.; Sørensen, H.T. Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit. Care 2014, 18, 492–492. [Google Scholar] [CrossRef] [PubMed]
- Dieter, B.P.; Daratha, K.B.; McPherson, S.M.; Short, R.; Alicic, R.Z.; Tuttle, K.R. Association of Acute Kidney Injury with Cardiovascular Events and Death in Systolic Blood Pressure Intervention Trial. Am. J. Nephrol. 2019, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sohel, B.M.; Rumana, N.; Ohsawa, M.; Turin, T.C.; Kelly, M.A.; Al Mamun, M. Renal function trajectory over time and adverse clinical outcomes. Clin. Exp. Nephrol. 2016, 20, 379–393. [Google Scholar] [CrossRef]
- Di Lullo, L.; Reeves, P.B.; Bellasi, A.; Ronco, C. Cardiorenal Syndrome in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 31–40. [Google Scholar] [CrossRef]
- Mehta, S.; Chauhan, K.; Patel, A.; Patel, S.; Pinotti, R.; Nadkarni, G.N.; Parikh, C.R.; Coca, S.G. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G. Ptolemy and Copernicus Revisited. Clin. J. Am. Soc. Nephrol. 2018, 13, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Hsu, C.; Parikh, R.V.; Leong, T.K.; Tan, T.C.; Walia, S.; et al. Non-recovery from dialzsis-requiring acute kidneyinjurz and short-term mortality and cardiovascular risk: a cohort study. BMC Nephrol. 2018, 19, 134. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Zelnick, L.R.; Chinchilli, V.M.; Moledina, D.G.; Coca, S.G.; Parikh, C.R.; Garg, A.X.; Hsu, C.-Y.; Go, A.S.; Liu, K.D.; et al. Association Between Early Recovery of Kidney Function After Acute Kidney Injury and Long-term Clinical Outcomes. JAMA Netw. Open 2020, 3, e202682–e202682. [Google Scholar] [CrossRef]
- Mehta, R.L. Renal Recovery After Acute Kidney Injury and Long-term Outcomes Is Time of the Essence? JAMA Netw. Open 2020, 3, e202676–e202676. [Google Scholar] [CrossRef]
- Vaara, S.T.; Bhatraju, P.K.; Stanski, N.L.; McMahon, B.A.; Liu, K.; Joannidis, M.; Bagshaw, S.M. Subphenotypes in acute kidney injury: a narrative review. Crit. Care 2022, 26, 1–10. [Google Scholar] [CrossRef]
- Perinel, S.; Vincent, F.; Lautrette, A.; Dellamonica, J.; Mariat, C.; Zeni, F.; et al. Transient and Persistent Acute Kidney Injury and the Risk of Hospital Mortality in Critically Ill Patients: Results of a Multicenter Cohort Study. Crit Care Med. 2015, 43, e269–e275. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Mukherjee, P.; Robinson-Cohen, C.; O’keefe, G.E.; Frank, A.J.; Christie, J.D.; Meyer, N.J.; Liu, K.D.; Matthay, M.A.; Calfee, C.S.; et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit. Care 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Flannery, A.H.; Bosler, K.; Ortiz-Soriano, V.M.; Gianella, F.; Prado, V.; Lambert, J.; Toto, R.D.; Moe, O.W.; Neyra, J.A. Kidney Biomarkers and Major Adverse Kidney Events in Critically Ill Patients. Kidney360 2021, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Zager, R.A. Catalytic iron mediated renal stress responses during experimental cardiorenal syndrome 1 (“CRS-1”). Transl. Res. 2021, 237, 53–62. [Google Scholar] [CrossRef]
- Johnson, A.C.; Delrow, J.J.; Zager, R.A. Tin protoporphyrin activates the oxidant-dependent NRF2-cytoprotective pathway and mitigates acute kidney injury. Transl. Res. 2017, 186, 1–18. [Google Scholar] [CrossRef]
- Virzì, G.M.; Clementi, A.; Brocca, A.; de Cal, M.; Ronco, C. Molecular and Genetic Mechanisms Involved in the Pathogenesis of Cardiorenal Cross Talk. Pathobiology 2016, 83, 201–210. [Google Scholar] [CrossRef]
- Himmelfarb, J.; McMonagle, E.; Freedman, S.; Klenzak, J.; McMenamin, E.; Le, P.; Pupim, L.B.; Ikizler, T.A. The PICARD Group Oxidative Stress Is Increased in Critically Ill Patients with Acute Renal Failure. J. Am. Soc. Nephrol. 2004, 15, 2449–2456. [Google Scholar] [CrossRef]
- Kasuno, K.; Shirakawa, K.; Yoshida, H.; Mori, K.; Kimura, H.; Takahashi, N.; Nobukawa, Y.; Shigemi, K.; Tanabe, S.; Yamada, N.; et al. Renal redox dysregulation in AKI: application for oxidative stress marker of AKI. Am. J. Physiol. Physiol. 2014, 307, F1342–F1351. [Google Scholar] [CrossRef]
- Just, A. Nitric Oxide and Renal Autoregulation. Kidney Blood Press. Res. 1997, 20, 201–204. [Google Scholar] [CrossRef]
- Tomsa, A.M.; Alexa, A.L.; Junie, M.L.; Rachisan, A.L.; Ciumarnean, L. Oxidative stress as a potential target in acute kidney injury. PeerJ 2019, 7, e8046. [Google Scholar] [CrossRef]
- Dennis, J.M.; Witting, P.K. Protective role for antioxidants in acute kidney disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef]
- Araujo, M.; Welch, W.J. Oxidative stress and nitric oxide in kidney function. Curr. Opin. Nephrol. Hypertens. 2006, 15, 72–77. [Google Scholar] [CrossRef]
- Nilakantan, V.; Hilton, G.; Maenpaa, C.; Van Why, S.K.; Pieper, G.M.; Johnson, C.P.; Shames, B.D. Favorable balance of anti-oxidant/pro-oxidant systems and ablated oxidative stress in Brown Norway rats in renal ischemia-reperfusion injury. Mol. Cell. Biochem. 2007, 304, 1–11. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Anderstam, B.; Ann-Christin, B.-H.; Valli, A.; Stenvinkel, P.; Lindholm, B.; Suliman, M.E. Modification of the oxidative stress biomarker AOPP assay: Application in uremic samples. Clin. Chim. Acta 2008, 393, 114–118. [Google Scholar] [CrossRef]
- Selmeci, L.; Seres, L.; Antal, M.; Lukács, J.; Regöly-Mérei, A.; Acsády, G. Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients: a simple, fast and inexpensive automated technique. cclm 2005, 43, 294–7. [Google Scholar] [CrossRef]
- Mahzari, S.; Hosseinian, S.; Hadjzadeh, M.-A.; Mohebbati, R.; Noshahr, Z.S.; Rad, A.K. Kidney dysfunction and oxidative stress in doxorubicin-induced nephrotic rat: Protective role of sesame oil. Saudi Journal of Kidney Diseases and Transplantation 2021, 32, 1243–1252. [Google Scholar] [CrossRef]
- Irigaray, P.; Caccamo, D.; Belpomme, D. Oxidative stress in electrohypersensitivity self-reporting patients: Results of a prospective in vivo investigation with comprehensive molecular analysis. Int. J. Mol. Med. 2018, 42, 1885–1898. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Pfaff, A.R.; Beltz, J.; King, E.; Ercal, N. Medicinal Thiols: Current Status and New Perspectives. Mini-Reviews Med. Chem. 2020, 20, 513–529. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxidants Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Gillis, B.S.; Arbieva, Z.; Gavin, I.M. Analysis of lead toxicity in human cells. BMC Genom. 2012, 13, 344–344. [Google Scholar] [CrossRef]
- Baylis, C.; Qiu, C. Importance of nitric oxide in the control of renal hemodynamics. Kidney Int. 1996, 49, 1727–1731. [Google Scholar] [CrossRef]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms—Biomarkers—Interventions, and Future Perspectives. Oxidative Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Lushchak, V.I. Classification of oxidative stress based on its intensity. EXCLI J. 2014, 13, 922–937. [Google Scholar]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Free Radic. Antioxid. 2008, 4, 89. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species - the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Verhaar, M.C.; Westerweel, P.E.; van Zonneveld, A.J.; Rabelink, T.J. Free radical production by dysfunctional eNOS. Heart 2004, 90, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Wagner, R. Mitochondrial Outer Membrane Channels: Emerging Diversity in Transport Processes. BioEssays 2018, 40, e1800013. [Google Scholar] [CrossRef] [PubMed]
- Krüger, V.; Becker, T.; Becker, L.; Montilla-Martinez, M.; Ellenrieder, L.; Vögtle, F.-N.; Meyer, H.E.; Ryan, M.T.; Wiedemann, N.; Warscheid, B.; et al. Identification of new channels by systematic analysis of the mitochondrial outer membrane. J. Cell Biol. 2017, 216, 3485–3495. [Google Scholar] [CrossRef]
- Baines, C.P.; Gutiérrez-Aguilar, M. The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 2018, 73, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Giorgi, C.; Bonora, M.; Punzetti, S.; Pavasini, R.; Wieckowski, M.R.; Campo, G.; Pinton, P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 142–153. [Google Scholar] [CrossRef]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.; Kazachenko, A.; Vyssokikh, M.; Vasileva, A.; Tcvirkun, D.; Isaev, N.; Kirpatovsky, V.; Zorov, D. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007, 72, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, B.; Li, Y.; Xu, X.; Lv, J.; Jia, Q.; Chai, R.; Xue, W.; Li, Y.; Wang, Y.; et al. Mithochondrial Dysfunction:An Emerging Link in the pathophysiology of Cardiorenal Syndrome. Front Cardiovasc. Med. 2022, 9, 837270. [Google Scholar] [CrossRef] [PubMed]
- Ravarotto, V.; Bertoldi, G.; Innico, G.; Gobbi, L.; Calò, L.A. The Pivotal Role of Oxidative Stress in the Pathophysiology of Cardiovascular-Renal Remodeling in Kidney Disease. Antioxidants 2021, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Boncompagni, C.; Ramaccini, D.; Pedriali, G.; Bouhamida, E.; Tremoli, E.; Giorgi, C.; Pinton, P. Comprehensive Analysis of Mitochondrial Dynamics Alterations in Heart Diseases. Int. J. Mol. Sci. 2023, 24, 3414. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 401–414. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2021, 477, 15–38. [Google Scholar] [CrossRef]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-Chain Fatty Acids in the Metabolism of Heart Failure – Rethinking the Fat Stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef]
- Tang, C.; Cai, J.; Yin, X.-M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, M.; Xiong, L.; Fan, J.; Zhou, Y.; Li, H.; Peng, X.; Zhong, Z.; Wang, Y.; Huang, F.; et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Shao, X.; Zhu, X.; Wu, J.; Wu, B.; Zhang, M.; Zhou, W.; Zhou, Y.; Jin, H.; et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free. Radic. Biol. Med. 2020, 152, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M.; Kume, S.; Yasuda-Yamahara, M.; Kuwagata, S.; Yamahara, K.; Takeda, N.; Tanaka, Y.; Chin-Kanasaki, M.; Nakae, Y.; Yokoi, H.; et al. Inhibition of mitochondrial fission protects podocytes from albumin-induced cell damage in diabetic kidney disease. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2022, 1868, 166368. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharmacol. 2020, 178, 2060–2076. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; An, P.; Luo, J.; Luo, Y. Mitochondrial Homeostasis in VSMCs as a Central Hub in Vascular Remodeling. Int. J. Mol. Sci. 2023, 24, 3483. [Google Scholar] [CrossRef]
- Cooper, H.A.; Cicalese, S.; Preston, K.J.; Kawai, T.; Okuno, K.; Choi, E.T.; Kasahara, S.; Uchida, H.A.; Otaka, N.; Scalia, R.; et al. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovasc. Res. 2020, 117, 971–982. [Google Scholar] [CrossRef]
- Pabla, N.; Bajwa, A. Role of Mitochondrial Therapy for Ischemic-Reperfusion Injury and Acute Kidney Injury. Nephron 2021, 146, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders. Life 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-J.; Wang, Z.-Y.; Xu, L.; Chen, X.-H.; Li, X.-X.; Liao, W.-T.; Ma, H.-K.; Jiang, M.-D.; Xu, T.-T.; Xu, J.; et al. HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020, 36, 101671. [Google Scholar] [CrossRef]
- Yao, M.; Liu, Y.; Sun, M.; Qin, S.; Xin, W.; Guan, X.; Zhang, B.; He, T.; Huang, Y. The molecular mechanisms and intervention strategies of mitophagy in cardiorenal syndrome. Front. Physiol. 2022, 13, 1008517. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Egea, V.; Bidzhekov, K.; Natarelli, L.; Mourão, A.; Blanchet, X.; Wichapong, K.; Aslani, M.; Brunßen, C.; Horckmans, M.; et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci. Transl. Med. 2020, 12, eaaz2294. [Google Scholar] [CrossRef]
- Bugga, P.; Alam, J.; Kumar, R.; Pal, S.; Chattopadyay, N.; Banerjee, S.K. Sirt3 ameliorates mitochondrial dysfunction and oxidative stress through regulating mitochondrial biogenesis and dynamics in cardiomyoblast. Cell. Signal. 2022, 94, 110309. [Google Scholar] [CrossRef]
- Yu, R.; Lendahl, U.; Nistér, M.; Zhao, J. Regulation of Mammalian Mitochondrial Dynamics: Opportunities and Challenges. Front. Endocrinol. 2020, 11, 374. [Google Scholar] [CrossRef]
- Merry, T.L.; Chan, A.; Woodhead, J.S.T.; Reynolds, J.C.; Kumagai, H.; Kim, S.-J.; Lee, C. Mitochondrial-derived peptides in energy metabolism. Am. J. Physiol. Metab. 2020, 319, E659–E666. [Google Scholar] [CrossRef]
- Gyuraszova, M.; Gurecka, R.; Babickova, J.; Tothova, L. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Agborbesong, E.; Li, X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int J Mol Sci 2021, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xia, F.; Li, L.; Peng, X.; Liu, W.; Zhang, Y.; Fang, H.; Zeng, Z.; Chen, Z. Novel Insights into the Molecular Features and Regulatory Mechanisms of Mitochondrial Dynamic Disorder in the Pathogenesis of Cardiovascular Disease. Oxidative Med. Cell. Longev. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Graciani, K.M.; Chapa-Dubocq, X.R.; MacMillan-Crow, L.A.; Javadov, S. Association Between L-OPA1 Cleavage and Cardiac Dysfunction During Ischemia-Reperfusion Injury in Rats. Cell. Physiol. Biochem. 2020, 54, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Toan, S.; Li, R.; Zhou, H. Melatonin fine-tunes intracellular calcium signals and eliminates myocardial damage through the IP3R/MCU pathways in cardiorenal syndrome type 3. Biochem. Pharmacol. 2020, 174, 113832. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Wang, X.; Cui, S.; Liu, R.; Liu, J.; Fu, B.; Gong, M.; Wang, C.; Shi, Y.; et al. Grb2 Induces Cardiorenal Syndrome Type 3: Roles of IL-6, Cardiomyocyte Bioenergetics, and Akt/mTOR Pathway (Publication with Expression of Concern. See vol. 10, 2022). Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




