Submitted:
21 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
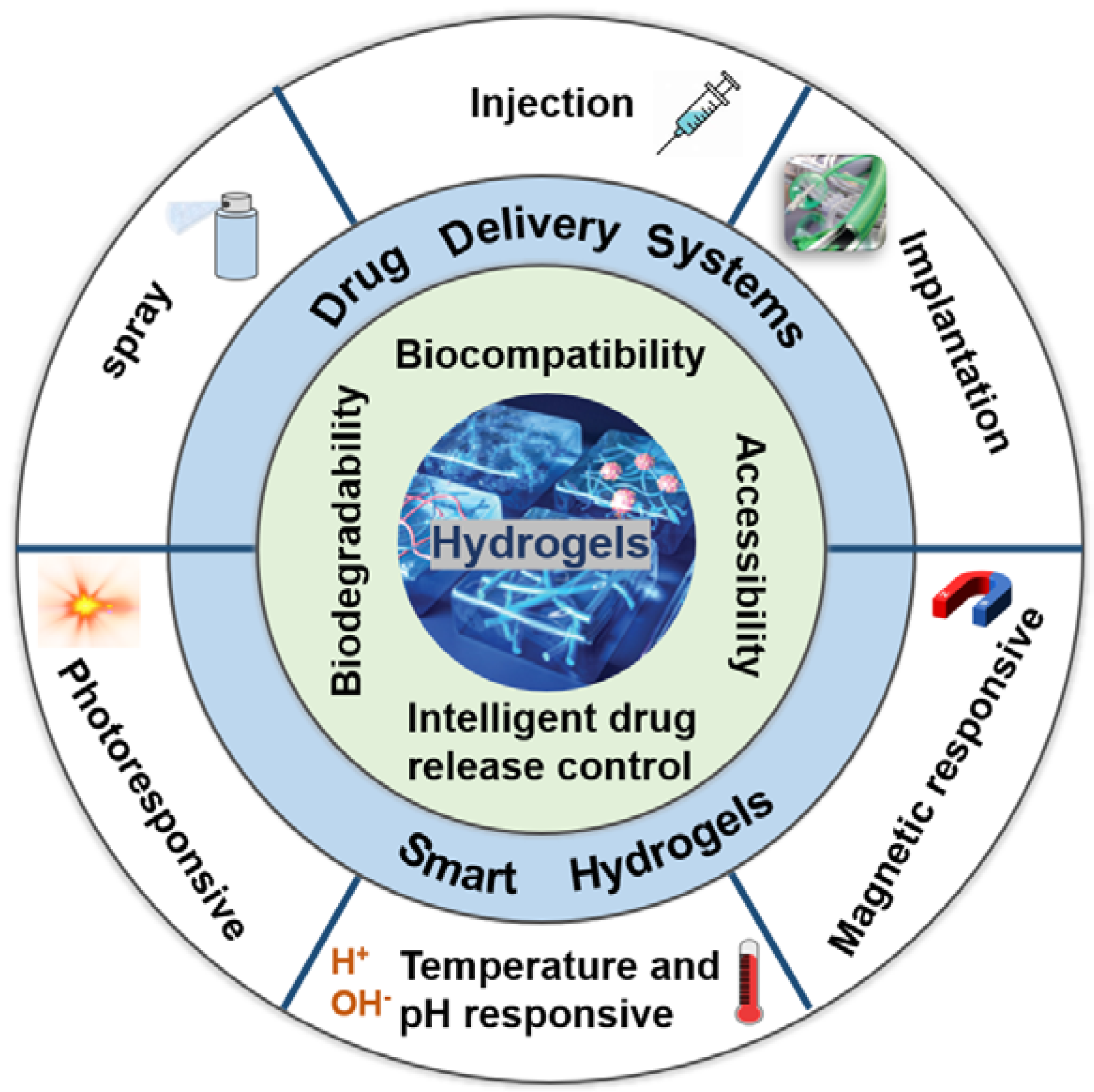
2. Different DDSs for Local Treatment of Brain Tumors
2.1. Injectable Hydrogels
2.2. Sprayable Hydrogels
2.3. Implantable Hydrogels
3. Smart Hydrogels for Local Treatment of Brain Tumors
3.1. Temperature and pH Responsive Hydrogels
3.2. Photoresponsive Hydrogels
3.3. Magnetic Responsive Hydrogels
4. Advantages of Hydrogels in the Treatment of Brain Tumors
5. Conclusions and Prospectives
Author Contributions
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- Anderson, A.R.; Segura, T. Injectable Biomaterials for Treatment of Glioblastoma. Advanced Materials Interfaces 2020, 7, 107-130. [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol Pharm 2020, 17, 373-391. [CrossRef]
- Zhao, Z.; Wang, Z.; Li, G.; Cai, Z.; Wu, J.; Wang, L.; Deng, L.; Cai, M.; Cui, W. Injectable Microfluidic Hydrogel Microspheres for Cell and Drug Delivery. Advanced Functional Materials 2021, 31, 2103339. [CrossRef]
- Yan, L.; Zhao, C.; Wang, Y.; Qin, Q.; Liu, Z.; Hu, Y.; Xu, Z.; Wang, K.; Jiang, X.; Han, L.; et al. Adhesive and conductive hydrogel-based therapy simultaneously targeting neuroinflammation and neurofunctional damage after brain injury. Nano Today 2023, 51, 150-167. [CrossRef]
- Kornev, V.A.; Grebenik, E.A.; Solovieva, A.B.; Dmitriev, R.I.; Timashev, P.S. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: A state-of-the-art review. Comput Struct Biotechnol J 2018, 16, 488-502. [CrossRef]
- Bharadwaj, V.N.; Nguyen, D.T.; Kodibagkar, V.D.; Stabenfeldt, S.E. Nanoparticle-Based Therapeutics for Brain Injury. Advanced Healthcare Materials 2017, 7, 68-82. [CrossRef]
- Brown, T.D.; Habibi, N.; Wu, D.; Lahann, J.; Mitragotri, S. Effect of Nanoparticle Composition, Size, Shape, and Stiffness on Penetration Across the Blood-Brain Barrier. ACS Biomater Sci Eng 2020, 6, 4916-4928. [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat Rev Mater 2016, 1, 190-228,. [CrossRef]
- Ren, E.; Wang, Y.; Liang, T.; Zheng, H.; Shi, J.; Cheng, Z.; Li, H.; Gu, Z. Local Drug Delivery Techniques for Triggering Immunogenic Cell Death. Small Methods 2023, 7, e2300347. [CrossRef]
- Stawicki, B.; Schacher, T.; Cho, H. Nanogels as a Versatile Drug Delivery System for Brain Cancer. Gels 2021, 7, 223-238,. [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat Rev Mater 2020, 5, 20-43. [CrossRef]
- Zhu, Y.J.; Yang Z.J.; Pan, Z.J.; Hao, Y.; Wang, C.J.; Dong, Z.L. Metallo-alginate hydrogel can potentiate microwave tumor ablation for synergistic cancer treatment. Sci. Adv 2022, 8, 5285-5299. [CrossRef]
- Chen, D.; Ma, X.; Zhu, J.; Wang, Y.; Guo, S.; Qin, J. Pectin based hydrogel with covalent coupled doxorubicin and limonin loading for lung tumor therapy. Colloids Surf B Biointerfaces 2024, 234, 113670. [CrossRef]
- Ayoubi-Joshaghani, M.H.; Seidi, K.; Azizi, M.; Jaymand, M.; Javaheri, T.; Jahanban-Esfahlan, R.; Hamblin, M.R. Potential Applications of Advanced Nano/Hydrogels in Biomedicine: Static, Dynamic, Multi-Stage, and Bioinspired. Advanced Functional Materials 2020, 30, 198-220. [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Materials Today 2020, 36, 102-124. [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404-1454. [CrossRef]
- Sun, X.; Yang, X.; Chen, Y.; Sun, J.; He, Z.; Zhang, S.; Luo, C. In situ self-assembled nanomedicines for cancer treatment. Chemical Engineering Journal 2023, 466, 1503-1529. [CrossRef]
- Che, L.; Lei, Z.; Wu, P.; Song, D. A 3D Printable and Bioactive Hydrogel Scaffold to Treat Traumatic Brain Injury. Advanced Functional Materials 2019, 29, 228-243. [CrossRef]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat Commun 2019, 10, 5205-5227. [CrossRef]
- Gou, S.; Meng, W.; Panayi, A.C.; Wang, R.; Zhang, R.; Gao, P.; He, T.; Geng, W.; Hu, S.; Yu, Y.; et al. Bioresponsive Self-Reinforcing Sericin/Silk Fibroin Hydrogel for Relieving the Immune-Related Adverse Events in Tumor Immunotherapy. Advanced Functional Materials 2023, 33, 188-216. [CrossRef]
- Liang, Q.; Shen, Z.; Sun, X.; Yu, D.; Liu, K.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Electron Conductive and Transparent Hydrogels for Recording Brain Neural Signals and Neuromodulation. Adv Mater 2023, 35, e2211159. [CrossRef]
- Wang, H.; Zhang, L.-M. Intelligent biobased hydrogels for diabetic wound healing: A review. Chemical Engineering Journal 2024, 484, 1417-1432. [CrossRef]
- Bastiancich, C.; Vanvarenberg, K.; Ucakar, B.; Pitorre, M.; Bastiat, G.; Lagarce, F.; Preat, V.; Danhier, F. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Control Release 2016, 225, 283-293. [CrossRef]
- Tao, J.; Zhang, J.; Hu, Y.; Yang, Y.; Gou, Z.; Du, T.; Mao, J.; Gou, M. A conformal hydrogel nanocomposite for local delivery of paclitaxel. J Biomater Sci Polym Ed 2017, 28, 107-118. [CrossRef]
- Wang, F.; Su, H.; Lin, R.; Chakroun, R.W.; Monroe, M.K.; Wang, Z.; Porter, M.; Cui, H. Supramolecular Tubustecan Hydrogel as Chemotherapeutic Carrier to Improve Tumor Penetration and Local Treatment Efficacy. ACS Nano 2020, 14, 10083-10094. [CrossRef]
- Wang, K.; Wang, J.; Li, L.; Xu, L.; Feng, N.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. Novel Nonreleasing Antibacterial Hydrogel Dressing by a One-Pot Method. ACS Biomater Sci Eng 2020, 6, 1259-1268. [CrossRef]
- Gartner, Z.J.; Hu, J.L. Guiding tissue-scale self-organization. Nat Mater 2021, 20, 2-13. [CrossRef]
- Guan, Q.F.; Han, Z.M.; Zhu, Y.; Xu, W.L.; Yang, H.B.; Ling, Z.C.; Yan, B.B.; Yang, K.P.; Yin, C.H.; Wu, H.; et al. Bio-Inspired Lotus-Fiber-like Spiral Hydrogel Bacterial Cellulose Fibers. Nano Lett 2021, 21, 952-958. [CrossRef]
- Cha, G.D.; Lee, W.H.; Sunwoo, S.H.; Kang, D.; Kang, T.; Cho, K.W.; Kim, M.; Park, O.K.; Jung, D.; Lee, J.; et al. Multifunctional Injectable Hydrogel for In Vivo Diagnostic and Therapeutic Applications. ACS Nano 2022, 16, 554-567. [CrossRef]
- Lee, C. Injectable glucose oxidase-immobilized gelatin hydrogel prevents tumor recurrence via oxidation therapy. Colloids Surf B Biointerfaces 2023, 232, 113581. [CrossRef]
- Cheng, Z.; Xue, C.; Liu, M.; Cheng, Z.; Tian, G.; Li, M.; Xue, R.; Yao, X.; Zhang, Y.; Luo, Z. Injectable microenvironment-responsive hydrogels with redox-activatable supramolecular prodrugs mediate ferroptosis-immunotherapy for postoperative tumor treatment. Acta Biomater 2023, 169, 289-305. [CrossRef]
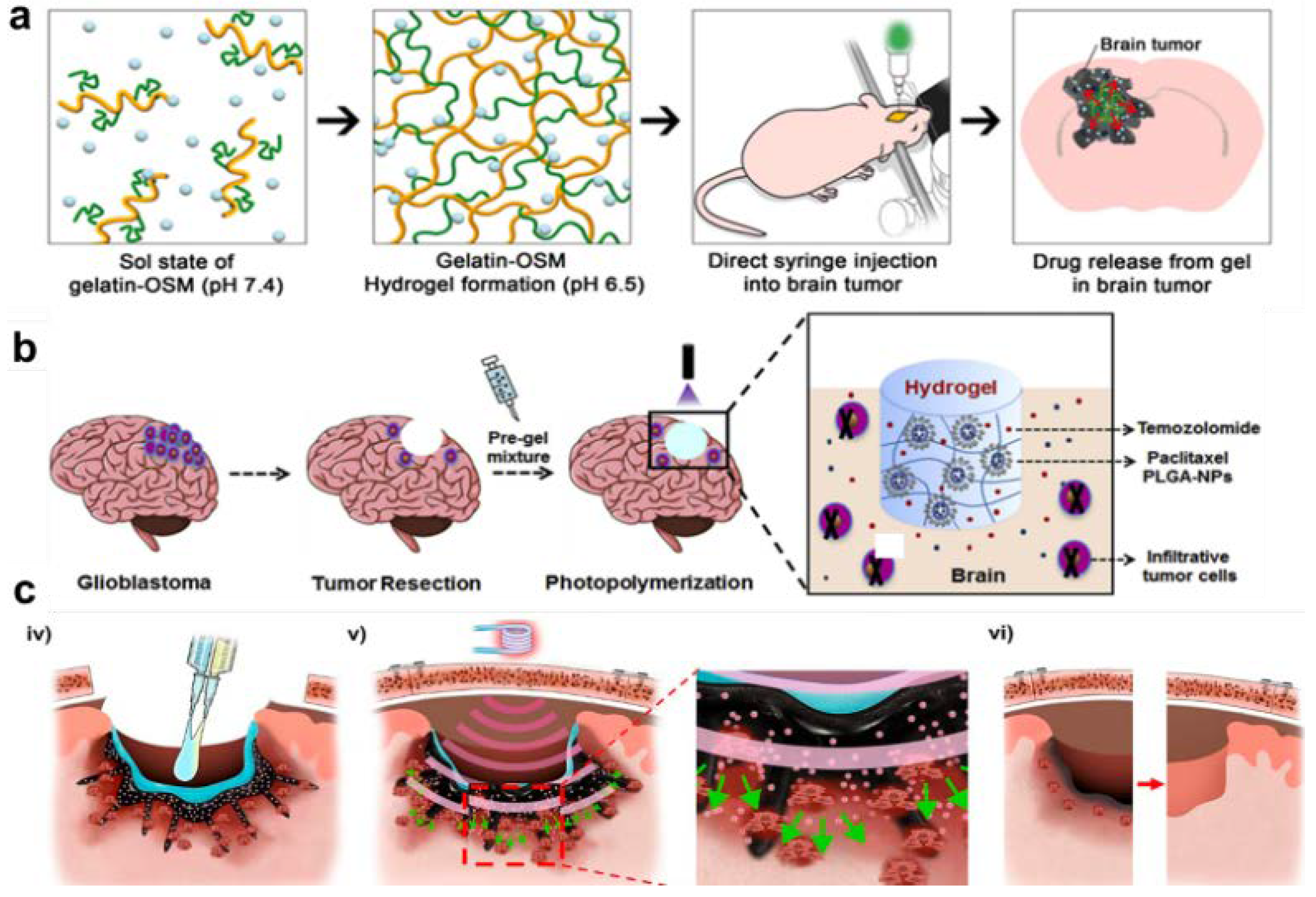
- Kang, T.; Cha, G.D.; Park, O.K.; Cho, H.R.; Kim, M.; Lee, J.; Kim, D.; Lee, B.; Chu, J.; Koo, S.; et al. Penetrative and Sustained Drug Delivery Using Injectable Hydrogel Nanocomposites for Postsurgical Brain Tumor Treatment. ACS Nano 2023, 17, 5435-5447. [CrossRef]
- Li, J.; Luo, G.; Zhang, C.; Long, S.; Guo, L.; Yang, G.; Wang, F.; Zhang, L.; Shi, L.; Fu, Y.; et al. In situ injectable hydrogel-loaded drugs induce anti-tumor immune responses in melanoma immunochemotherapy. Mater Today Bio 2022, 14, 100238. [CrossRef]
- Puente, P.; Fettig, N.; Luderer, M.J.; Jin, A.; Shah, S.; Muz, B.; Kapoor, V.; Goddu, S.M.; Salama, N.N.; Tsien, C.; et al. Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors. J Pharm Sci 2018, 107, 922-933. [CrossRef]
- Pradhan, K.; Das, G.; Khan, J.; Gupta, V.; Barman, S.; Adak, A.; Ghosh, S. Neuro-Regenerative Choline-Functionalized Injectable Graphene Oxide Hydrogel Repairs Focal Brain Injury. ACS Chem Neurosci 2019, 10, 1535-1543. [CrossRef]
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr Polym 2019, 203, 302-309. [CrossRef]
- Wang, Z.; Zeng, W.; Chen, Z.; Suo, W.; Quan, H.; Tan, Z.J. An intratumoral injectable nanozyme hydrogel for hypoxia-resistant thermoradiotherapy. Colloids Surf B Biointerfaces 2021, 207, 112026. [CrossRef]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf B Biointerfaces 2021, 200, 111581. [CrossRef]
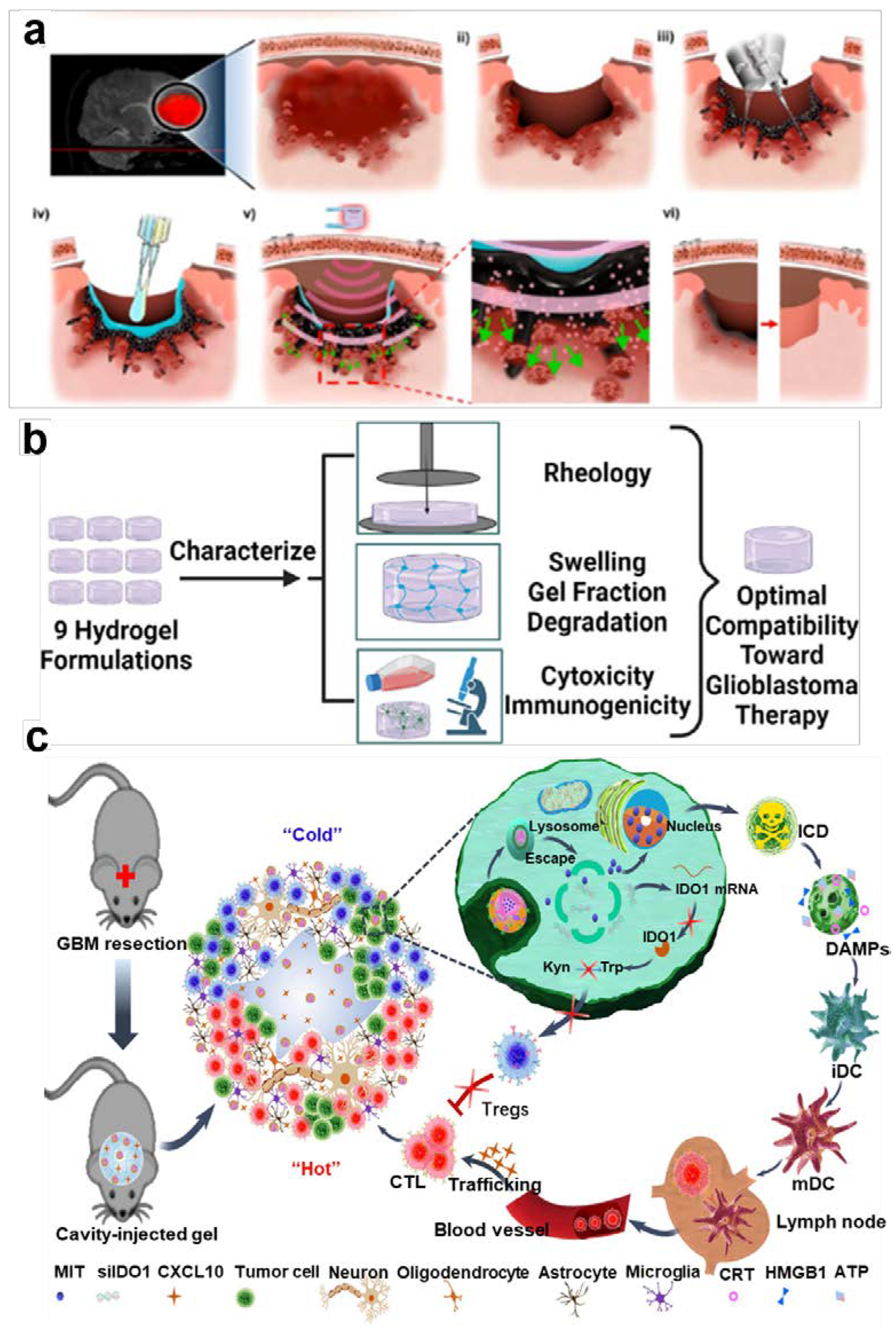
- Khan, Z.M.; Wilts, E.; Vlaisavljevich, E.; Long, T.E.; Verbridge, S.S. Characterization and structure-property relationships of an injectable thiol-Michael addition hydrogel toward compatibility with glioblastoma therapy. Acta Biomater 2022, 144, 266-278. [CrossRef]
- Zhang, D.; Chang, R.; Ren, Y.; He, Y.; Guo, S.; Guan, F.; Yao, M. Injectable and reactive oxygen species-scavenging gelatin hydrogel promotes neural repair in experimental traumatic brain injury. Int J Biol Macromol 2022, 219, 844-863. [CrossRef]
- Bruns, J.; Egan, T.; Mercier, P.; Zustiak, S.P. Glioblastoma spheroid growth and chemotherapeutic responses in single and dual-stiffness hydrogels. Acta Biomater 2023, 163, 400-414. [CrossRef]
- Liu, J.; Qi, C.; Tao, K.; Zhang, J.; Zhang, J.; Xu, L.; Jiang, X.; Zhang, Y.; Huang, L.; Li, Q.; et al. Sericin/Dextran Injectable Hydrogel as an Optically Trackable Drug Delivery System for Malignant Melanoma Treatment. ACS Applied Materials & Interfaces 2016, 8, 6411-6422. [CrossRef]
- Zhang, J.; Chen, C.; Li, A.; Jing, W.; Sun, P.; Huang, X.; Liu, Y.; Zhang, S.; Du, W.; Zhang, R.; et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat Nanotechnol 2021, 16, 538-548. [CrossRef]
- Jiang, X.; Zeng, F.; Yang, X.; Jian, C.; Zhang, L.; Yu, A.; Lu, A. Injectable self-healing cellulose hydrogel based on host-guest interactions and acylhydrazone bonds for sustained cancer therapy. Acta Biomater 2022, 141, 102-113. [CrossRef]
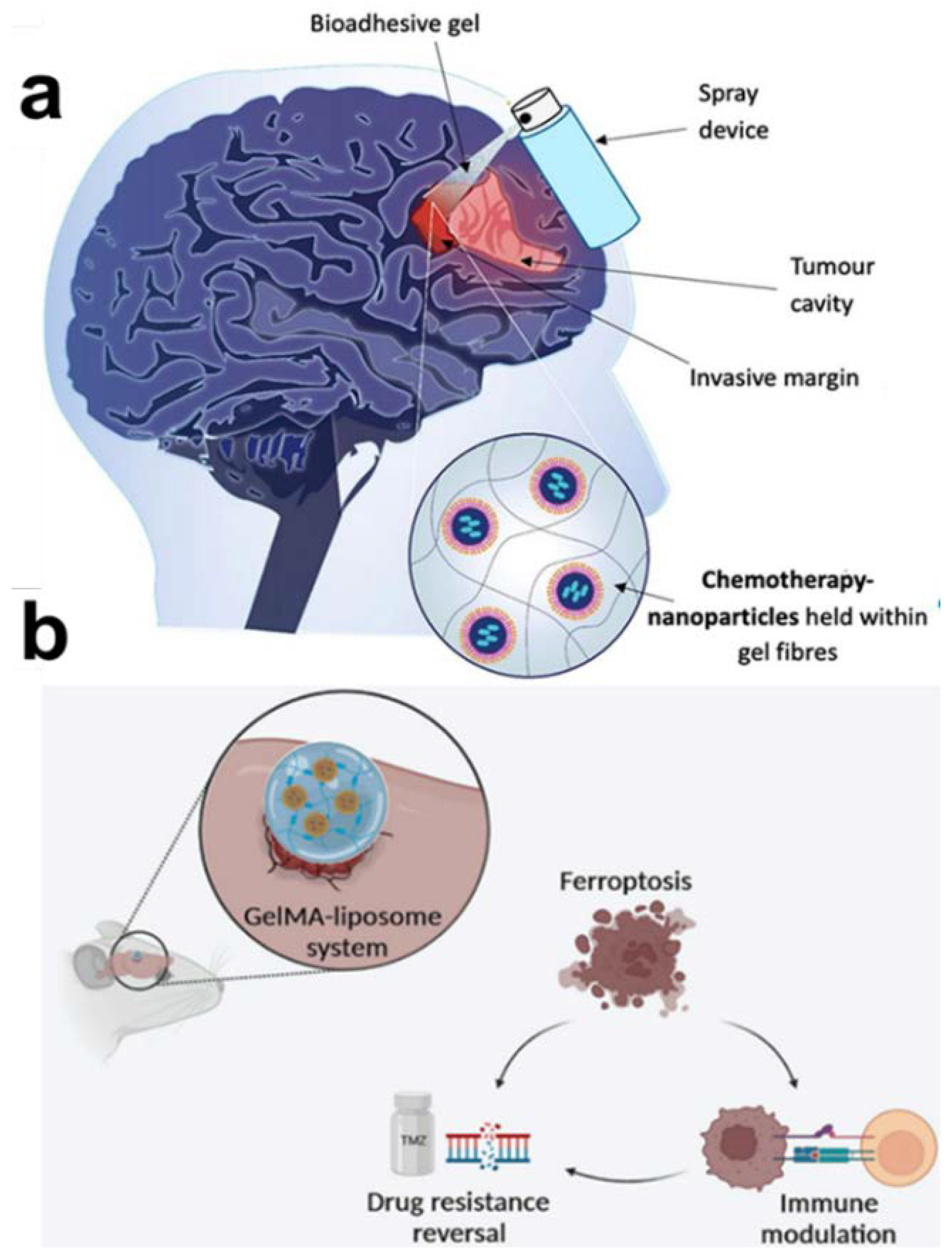
- McCrorie, P.; Mistry, J.; Taresco, V.; Lovato, T.; Fay, M.; Ward, I.; Ritchie, A.A.; Clarke, P.A.; Smith, S.J.; Marlow, M.; et al. Etoposide and olaparib polymer-coated nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours. Eur J Pharm Biopharm 2020, 157, 108-120. [CrossRef]
- Bozzato, E.; Tsakiris, N.; Paquot, A.; Muccioli, G.G.; Bastiancich, C.; Preat, V. Dual-drug loaded nanomedicine hydrogel as a therapeutic platform to target both residual glioblastoma and glioma stem cells. Int J Pharm 2022, 628, 122341. [CrossRef]
- Bakhrushina, E.O.; Mikhel, I.B.; Buraya, L.M.; Moiseev, E.D.; Zubareva, I.M.; Belyatskaya, A.V.; Evzikov, G.Y.; Bondarenko, A.P.; Krasnyuk, I.I., Jr.; Krasnyuk, II. Implantation of In Situ Gelling Systems for the Delivery of Chemotherapeutic Agents. Gels 2024, 10, 1004-1032. [CrossRef]
- Lyu, J.; Liu, H.; Chen, L.; Liu, C.; Tao, J.; Yao, Y.; Li, L.; Huang, Y.; Zhou, Z. In situ hydrogel enhances non-efferocytic phagocytosis for post-surgical tumor treatment. J Control Release 2023, 363, 402-414. [CrossRef]
- Wang, Z.; Liu, Z.; Wang, S.; Bing, X.; Ji, X.; He, D.; Han, M.; Wei, Y.; Wang, C.; Xia, Q.; et al. Implantation of hydrogel-liposome nanoplatform inhibits glioblastoma relapse by inducing ferroptosis. Asian J Pharm Sci 2023, 18, 100800. [CrossRef]
- Zhou, B.; Fan, K.; Li, T.; Luan, G.; Kong, L. A biocompatible hydrogel-coated fiber-optic probe for monitoring pH dynamics in mammalian brains in vivo. Sensors and Actuators B: Chemical 2023, 380, 1334-1348. [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Advanced Functional Materials 2020, 31, 411-429. [CrossRef]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J Agric Food Chem 2022, 70, 6300-6316. [CrossRef]
- Li, X.; Duan, L.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels 2022, 8, 188-194. [CrossRef]
- Liu, H.; Deng, Z.; Li, T.; Bu, J.; Wang, D.; Wang, J.; Liu, M.; Li, J.; Yang, Y.; Zhong, S. Fabrication, GSH-responsive drug release, and anticancer properties of thioctic acid-based intelligent hydrogels. Colloids Surf B Biointerfaces 2022, 217, 112703. [CrossRef]
- Albor-Ramirez, E.; Reyes-Alberto, M.; Vidal-Flores, L.M.; Gutierrez-Herrera, E.; Padilla-Castaneda, M.A. Agarose Gel Characterization for the Fabrication of Brain Tissue Phantoms for Infrared Multispectral Vision Systems. Gels 2023, 9, 78-96,. [CrossRef]
- Fitzpatrick, D.P.; Kealey, C.; Brady, D.; Gately, N. Adapted sterilisation for the production of thermoresponsive hydrogels for downstream wound healing applications. Polymer Testing 2024, 132, 108379. [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv Mater 2016, 28, 86-91. [CrossRef]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.K.; Stevens, M.M. Tailoring Gelation Mechanisms for Advanced Hydrogel Applications. Advanced Functional Materials 2020, 30, 1102-1132. [CrossRef]
- Zhao, X.; Javed, B.; Tian, F.; Liu, K. Hydrogel on a Smart Nanomaterial Interface to Carry Therapeutics for Digitalized Glioma Treatment. Gels 2022, 8, 66-85. [CrossRef]
- Zhao, Y.; Ran, B.; Xie, X.; Gu, W.; Ye, X.; Liao, J. Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy. Gels 2022, 8, 112-130. [CrossRef]
- Morarasu, S.; Morarasu, B.C.; Ghiarasim, R.; Coroaba, A.; Tiron, C.; Iliescu, R.; Dimofte, G.M. Targeted Cancer Therapy via pH-Functionalized Nanoparticles: A Scoping Review of Methods and Outcomes. Gels 2022, 8, 223-245. [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing. Colloids Surf B Biointerfaces 2022, 218, 112738. [CrossRef]
- Ma, Z.; Yang, X.; Ma, J.; Lv, J.; He, J.; Jia, D.; Qu, Y.; Chen, G.; Yan, H.; Zeng, R. Development of the mussel-inspired pH-responsive hydrogel based on Bletilla striata polysaccharide with enhanced adhesiveness and antioxidant properties. Colloids Surf B Biointerfaces 2021, 208, 112066. [CrossRef]
- Du, M.; Jin, J.; Zhou, F.; Chen, J.; Jiang, W. Dual drug-loaded hydrogels with pH-responsive and antibacterial activity for skin wound dressing. Colloids Surf B Biointerfaces 2023, 222, 113063. [CrossRef]
- Xue, X.; Feng, M.; Liang, K.; Wu, Z.; Zhao, C.; Chen, Y.; Pu, H. Mesh-size adjustable hydrogel via light and pH induction. Materials Letters 2024, 361, 136163. [CrossRef]
- Kang, J.H.; Turabee, M.H.; Lee, D.S.; Kwon, Y.J.; Ko, Y.T. Temperature and pH-responsive in situ hydrogels of gelatin derivatives to prevent the reoccurrence of brain tumor. Biomed Pharmacother 2021, 143, 112144. [CrossRef]
- Lee, Y.; Kang, T.; Cho, H.R.; Lee, G.J.; Park, O.K.; Kim, S.; Lee, B.; Kim, H.M.; Cha, G.D.; Shin, Y.; et al. Localized Delivery of Theranostic Nanoparticles and High-Energy Photons using Microneedles-on-Bioelectronics. Adv Mater 2021, 33, e2100425. [CrossRef]
- Yang, J.; Sun, Z.; Dou, Q.; Hui, S.; Zhang, P.; Liu, R.; Wang, D.; Jiang, S. NIR-light-responsive chemo-photothermal hydrogel system with controlled DOX release and photothermal effect for cancer therapy. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2023, 667, 131407. [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv Mater 2019, 31, e1807333. [CrossRef]
- Chen, M.; Quan, G.; Wen, T.; Yang, P.; Qin, W.; Mai, H.; Sun, Y.; Lu, C.; Pan, X.; Wu, C. Cold to Hot: Binary Cooperative Microneedle Array-Amplified Photoimmunotherapy for Eliciting Antitumor Immunity and the Abscopal Effect. ACS Appl Mater Interfaces 2020, 12, 32259-32269. [CrossRef]
- Gan, S.; Wu, Y.; Zhang, X.; Zheng, Z.; Zhang, M.; Long, L.; Liao, J.; Chen, W. Recent Advances in Hydrogel-Based Phototherapy for Tumor Treatment. Gels 2023, 9, 176-190. [CrossRef]
- Hwang, J.; Jin, J.O. Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma. Biomolecules 2020, 10, 1124-1142. [CrossRef]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Preat, V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J Control Release 2019, 309, 72-81. [CrossRef]
- Wang, Y.; Pan, H.; Meng, Z.; Zhang, C. In Situ Biosynthesis of Photothermal Parasite for Fluorescence Imaging-Guided Photothermal Therapy of Tumors. Gels 2022, 8, 55-75. [CrossRef]
- Zheng, D.; Huang, C.; Hu, Y.; Zheng, T.; An, J. Constructions of synergistic photothermal therapy antibacterial hydrogel based on polydopamine, tea polyphenols and polyvinyl alcohol and effects on wound healing in mouse. Colloids Surf B Biointerfaces 2022, 219, 112831. [CrossRef]
- Chen, L.; Chen, G.; Hu, K.; Chen, L.; Zeng, Z.; Li, B.; Jiang, G.; Liu, Y. Combined photothermal and photodynamic therapy enhances ferroptosis to prevent cancer recurrence after surgery using nanoparticle-hydrogel composite. Chemical Engineering Journal 2023, 468, 1130-1148. [CrossRef]
- Ye, J.; Jiang, J.; Zhou, Z.; Weng, Z.; Xu, Y.; Liu, L.; Zhang, W.; Yang, Y.; Luo, J.; Wang, X. Near-Infrared Light and Upconversion Nanoparticle Defined Nitric Oxide-Based Osteoporosis Targeting Therapy. ACS Nano 2021, 15, 13692-13702. [CrossRef]
- Chang, L.; Liu, X.; Zhu, J.; Rao, Y.; Chen, D.; Wang, Y.; Zhao, Y.; Qin, J. Cellulose-based thermo-responsive hydrogel with NIR photothermal enhanced DOX released property for anti-tumor chemotherapy. Colloids Surf B Biointerfaces 2022, 218, 112747. [CrossRef]
- Jia, Y.P.; Shi, K.; Yang, F.; Liao, J.F.; Han, R.X.; Yuan, L.P.; Hao, Y.; Pan, M.; Xiao, Y.; Qian, Z.Y.; et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Advanced Functional Materials 2020, 30, 1115-1138. [CrossRef]
- Singh, B.; Kumar, A.; Rohit. Gamma radiation formation of sterculia gum-alginate-carbopol hydrogel dressing by grafting method for use in brain drug delivery. Chemical Physics Letters 2021, 779, 138875. [CrossRef]
- Rizwan, A.; Ali, I.; Jo, S.H.; Vu, T.T.; Gal, Y.S.; Kim, Y.H.; Park, S.H.; Lim, K.T. Facile Fabrication of NIR-Responsive Alginate/CMC Hydrogels Derived through IEDDA Click Chemistry for Photothermal-Photodynamic Anti-Tumor Therapy. Gels 2023, 9, 66-89. [CrossRef]
- Wang, C.; Li, J.; Sinha, S.; Peterson, A.; Grant, G.A.; Yang, F. Mimicking brain tumor-vasculature microanatomical architecture via co-culture of brain tumor and endothelial cells in 3D hydrogels. Biomaterials 2019, 202, 35-44. [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Mirzaei, H.; Asemi, Z.; Yousefi, B. DNA damage response and repair in the development and treatment of brain tumors. Eur J Pharmacol 2022, 924, 174957. [CrossRef]
- Gawade, P.M.; Shadish, J.A.; Badeau, B.A.; DeForest, C.A. Logic-Based Delivery of Site-Specifically Modified Proteins from Environmentally Responsive Hydrogel Biomaterials. Adv Mater 2019, 31, e1902462. [CrossRef]
- Wei, D.; Huang, Y.; Liang, M.; Ren, P.; Tao, Y.; Xu, L.; Zhang, T.; Ji, Z.; Zhang, Q. Polypropylene composite hernia mesh with anti-adhesion layer composed of PVA hydrogel and liposomes drug delivery system. Colloids Surf B Biointerfaces 2023, 223, 113159. [CrossRef]
- Yu, F.; Wang, Y.; Stetler, A.R.; Leak, R.K.; Hu, X.; Chen, J. Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci Ther 2022, 28, 1279-1293. [CrossRef]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv Mater 2021, 33, e2006362. [CrossRef]
- Jang, T.S.; Jung, H.D.; Pan, H.M.; Han, W.T.; Chen, S.; Song, J. 3D printing of hydrogel composite systems: Recent advances in technology for tissue engineering. Int J Bioprint 2018, 4, 126. [CrossRef]
- Chen, Q.; Passos, A.; Balabani, S.; Chivu, A.; Zhao, S.; Azevedo, H.S.; Butler, P.; Song, W. Semi-interpenetrating network hyaluronic acid microgel delivery systems in micro-flow. J Colloid Interface Sci 2018, 519, 174-185. [CrossRef]
- Estevam, B.R.; Perez, I.D.; Moraes, Â.M.; Fregolente, L.V. A review of the strategies used to produce different networks in cellulose-based hydrogels. Materials Today Chemistry 2023, 34, 101803. [CrossRef]
- Zhu, J.; Xu, H.; Hu, Q.; Yang, Y.; Ni, S.; Peng, F.; Jin, X. High stretchable and tough xylan-g-gelatin hydrogel via the synergy of chemical cross-linking and salting out for strain sensors. Int J Biol Macromol 2024, 261, 129759. [CrossRef]
- Kozicki, M.; Stempień, Z.; Rokita, B.; Dudek, M. Sandwich-type channeled chemical hydrogels manufactured by 3D ink-jet printing under freezing conditions using a photochemical process for human cell cultures. Chemical Engineering Journal 2024, 481, 587-601. [CrossRef]
- Qin, J.; Dong, B.; Wang, W.; Cao, L. Self-regulating bioinspired supramolecular photonic hydrogels based on chemical reaction networks for monitoring activities of enzymes and biofuels. J Colloid Interface Sci 2023, 649, 344-354. [CrossRef]
- Chen, S.; Wang, Y.; Zhang, X.; Ma, J.; Wang, M. Double-crosslinked bifunctional hydrogels with encapsulated anti-cancer drug for bone tumor cell ablation and bone tissue regeneration. Colloids Surf B Biointerfaces 2022, 213, 112364. [CrossRef]
- Li, P.; Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. NIR- and pH-responsive injectable nanocomposite alginate-graft-dopamine hydrogel for melanoma suppression and wound repair. Carbohydr Polym 2023, 314, 120899. [CrossRef]
- He, W.; Chen, K.; Gao, W.; Duan, R.; Li, Z.; Li, B.; Xia, J.; Zhao, Y.; Liu, W.; Zhou, H.; et al. A sequential physical and chemical dual crosslinked multifunctional hydrogel with enhanced mechanical and osteogenic effects for vascularized bone tissue regeneration. Materials & Design 2024, 237, 112563. [CrossRef]
- Song, Y.; Liu, C.; Xu, X.; Ren, L.; Zhou, X.; Xu, H.; Zhao, L.; Xin, J.; Wang, S.; Wang, Z. Chitosan-based multifunctional hydrogel with bio-adhesion and antioxidant properties for efficient wound hemostasis. Colloids Surf B Biointerfaces 2024, 234, 113697. [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem Rev 2021, 121, 4309-4372. [CrossRef]
- Muresan, P.; McCrorie, P.; Smith, F.; Vasey, C.; Taresco, V.; Scurr, D.J.; Kern, S.; Smith, S.; Gershkovich, P.; Rahman, R.; et al. Development of nanoparticle loaded microneedles for drug delivery to a brain tumour resection site. Eur J Pharm Biopharm 2023, 182, 53-61. [CrossRef]
- Dai, R.; Gao, Y.; Sun, Y.; Shi, K.; Gao, G.; Zhang, H. Ionic conductive amylopectin hydrogels for biocompatible and anti-freezing wearable sensors. European Polymer Journal 2023, 200, 112496. [CrossRef]
- Indrakumar, S.; Panicker, A.T.; Parasuram, S.; Joshi, A.; Kumar Dash, T.; Mishra, V.; Tandon, B.; Chatterjee, K. 3D-printed ultra-stretchable silk fibroin-based biocompatible hydrogels. Bioprinting 2023, 36, 1133-1149. [CrossRef]
- Moura, D.; Rohringer, S.; Ferreira, H.P.; Pereira, A.T.; Barrias, C.C.; Magalhaes, F.D.; Bergmeister, H.; Goncalves, I.C. Long-term in vivo degradation and biocompatibility of degradable pHEMA hydrogels containing graphene oxide. Acta Biomater 2024, 173, 351-364. [CrossRef]
- Lessmann, T.; Jones, S.A.; Voigt, T.; Weisbrod, S.; Kracker, O.; Winter, S.; Zuniga, L.A.; Stark, S.; Bisek, N.; Sprogoe, K. Degradable Hydrogel for Sustained Localized Delivery of Anti-Tumor Drugs. J Pharm Sci 2023, 112, 2843-2852. [CrossRef]
- Wang, H.; Chen, X.; Gong, C.; Bu, Y.; Wu, T.; Yan, H.; Lin, Q. Intelligent response organo-montmorillonite/Fe3+-alginate/poly (N-isopropylacrylamide) interpenetrating network composite hydrogels for controlled release of water-insoluble pesticides. Applied Clay Science 2024, 251, 107302. [CrossRef]
- Li, Y.; Zhang, L.; Song, Z.; Li, F.; Xie, D. Intelligent temperature-pH dual responsive nanocellulose hydrogels and the application of drug release towards 5-fluorouracil. Int J Biol Macromol 2022, 223, 11-16. [CrossRef]
- Cai, Y.; Xin, L.; Sun, P.; Li, H.; Liu, C.; Fang, L. Temperature-sensitive multifunctional intelligent responsive hydrogel based on carboxymethyl agarose and N-isopropylacrylamide: Controlled drug release and accelerated wound healing. Carbohydr Polym 2023, 322, 121327. [CrossRef]
- Liu, J.; Yu, J.; Xu, C.; Li, B.; Liu, L.; Lu, C.; Fan, Y. One-pot and one-step preparation of "living" cellulose nanofiber hydrogel with active double-bond via chemical vapor deposition. Int J Biol Macromol 2023, 245, 125415. [CrossRef]
- Yang, Z.; Yu, S.; Chen, H.; Guo, X.; Cai, P.; Meng, H. One-step electrogelation of pectin hydrogels as a simpler alternative for antibacterial 3D printing. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 654, 1229964. [CrossRef]
- Liu, Y.; Zhu, Y.; Mu, B.; Zong, L.; Wang, X.; Wang, A. One-step green construction of granular composite hydrogels for ammonia nitrogen recovery from wastewater for crop growth promotion. Environmental Technology & Innovation 2024, 33, 103465,. [CrossRef]
- Li, X.; Gong, N.; Tian, F.; Zhang, S.; Zhang, Y.; Wang, Y.; Qing, G.; Wang, Y.; Li, F.; Xu, Y.; et al. Suppression of cytokine release syndrome during CAR-T-cell therapy via a subcutaneously injected interleukin-6-adsorbing hydrogel. Nat Biomed Eng 2023, 7, 1129-1141. [CrossRef]
- Zhang, J.; Wei, X.; Liu, W.; Wang, Y.; Kahkoska, A.R.; Zhou, X.; Zheng, H.; Zhang, W.; Sheng, T.; Zhang, Y.; et al. Week-long norm glycaemia in diabetic mice and minipigs via a subcutaneous dose of a glucose-responsive insulin complex. Nat Biomed Eng 2023, 1557-1584. [CrossRef]
- Tondera, C.; Wieduwild, R.; Röder, E.; Werner, C.; Zhang, Y.; Pietzsch, J. In Vivo Examination of an Injectable Hydrogel System Crosslinked by Peptide-Oligosaccharide Interaction in Immunocompetent Nude Mice. Advanced Functional Materials 2017, 27, 113-127. [CrossRef]
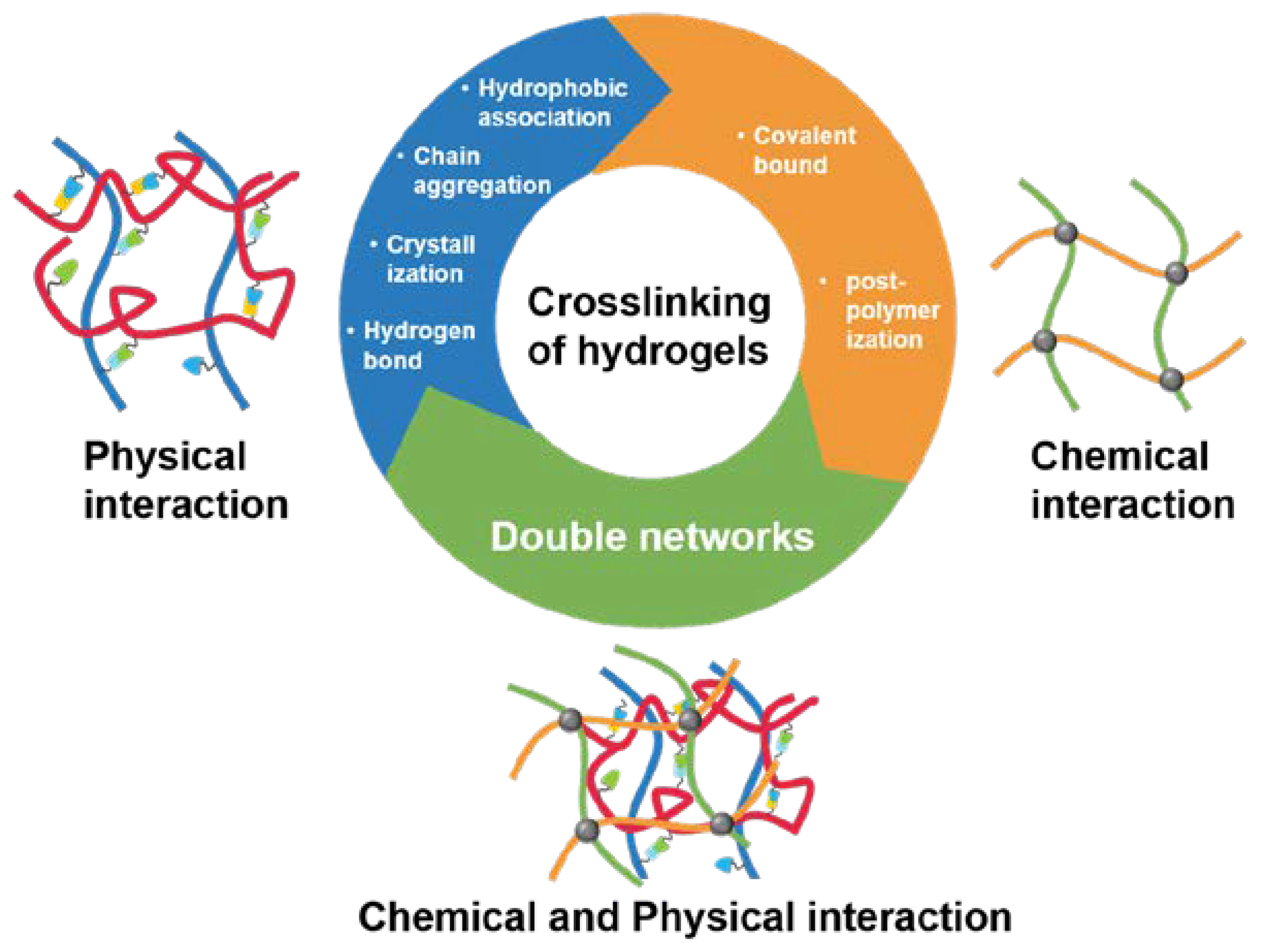
| Drug delivery way | Hydrogel material | Feature | Application |
|---|---|---|---|
| Injection | Composite nanohydrogels containing drug-loaded micelles and wFIONs | Injectable heat-responsive system | Operative brain tumor therapy using injectable hydrogel nanocomposites |
| Poly (ethylene glycol)-based hydrogel crosslinked by thiol-Michael addition reaction | Chemical and physical modalities were synergistically employed for therapeutic intervention | Injectable sulfhydryl Michael addition hydrogel for glioblastoma therapy | |
| The gelato consists of 9-fluorenylmethoxycarbonyl Phe and Phe-Phe-dihydroxyphenylalanine | Benign biodegradability and drug release properties | Tumor-killing immunity is stimulated after surgical resection of GBM to reduce its recurrence | |
| Spray | Pectin with nanocrystals coated with polylactic acid and polyethylene glycol (NCPPs)-loaded etoposide and olaparib | Drugs are delivered using a spray device | Bioadhesive spray hydrogels containing etoposide and olaparib polymer-coated nanoparticles |
| Implantation | Temozolomide+Erastin@liposome-cyclic RGD +gelatin methacrylamide | The orthotopic implantation procedure elicits ferroptosis and impedes tumor recurrence | The platform of implantable hydrogels inhibits the recurrence of GBM by inducing ferroptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





