Submitted:
21 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
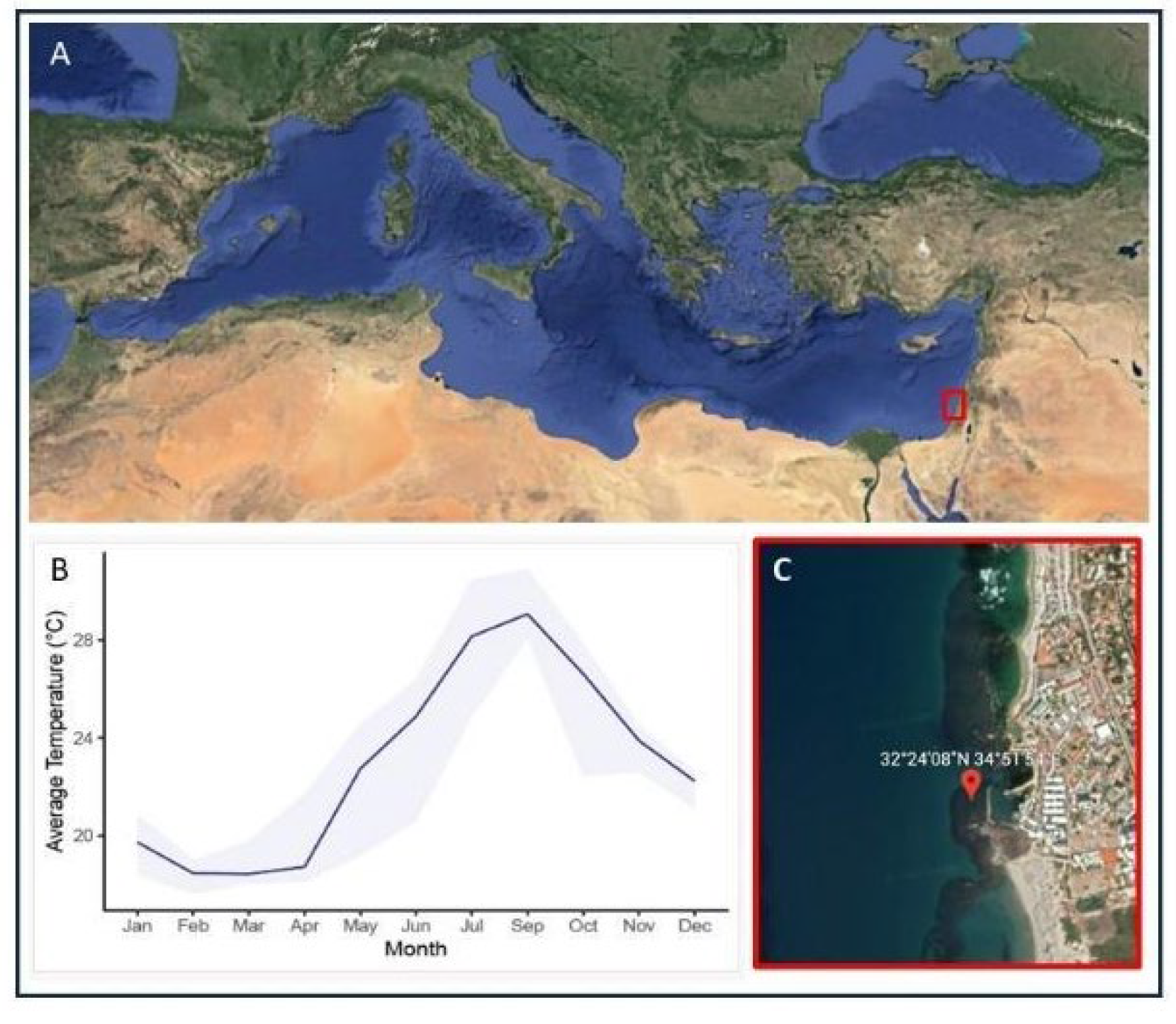
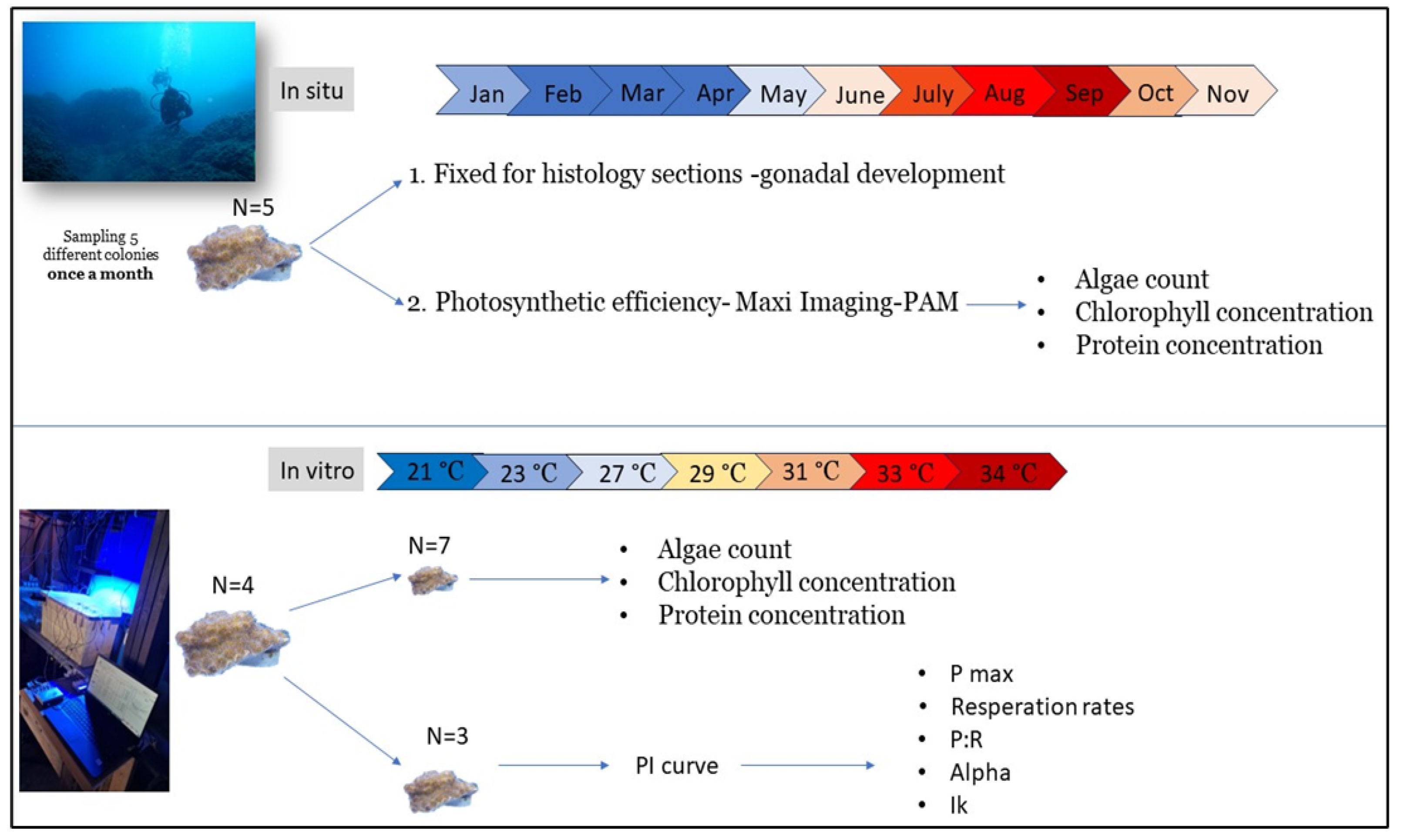
2.1. Sample Collection
2.2. Physiology
2.3. Gametogenesis
2.4. Thermal Stress Experiment
2.5. Thermal Stress Experiment Design
3. Results
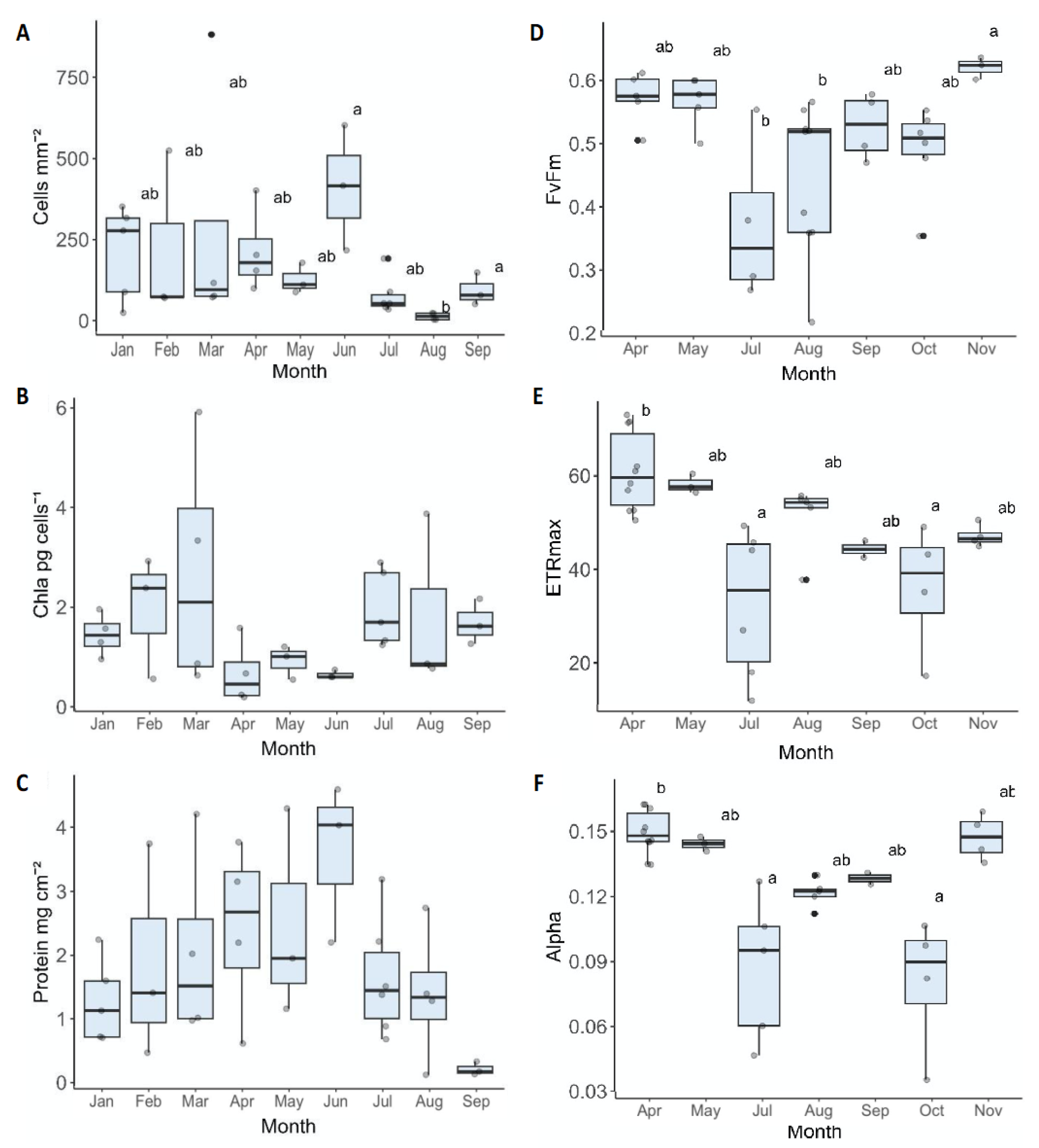
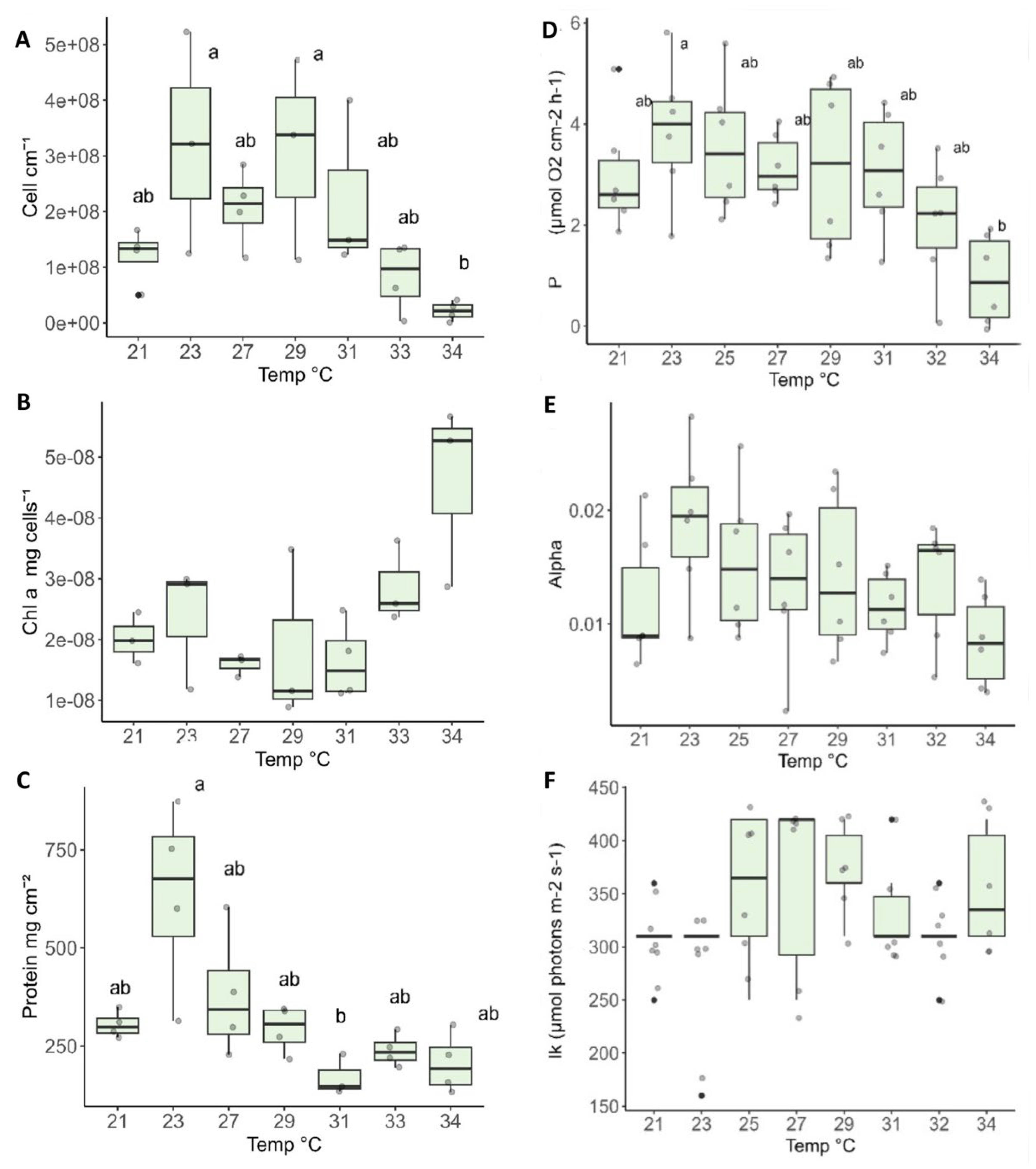
3.1. Seasonal Patterns in O. patagonica Physiology and Photosynthetic Activity
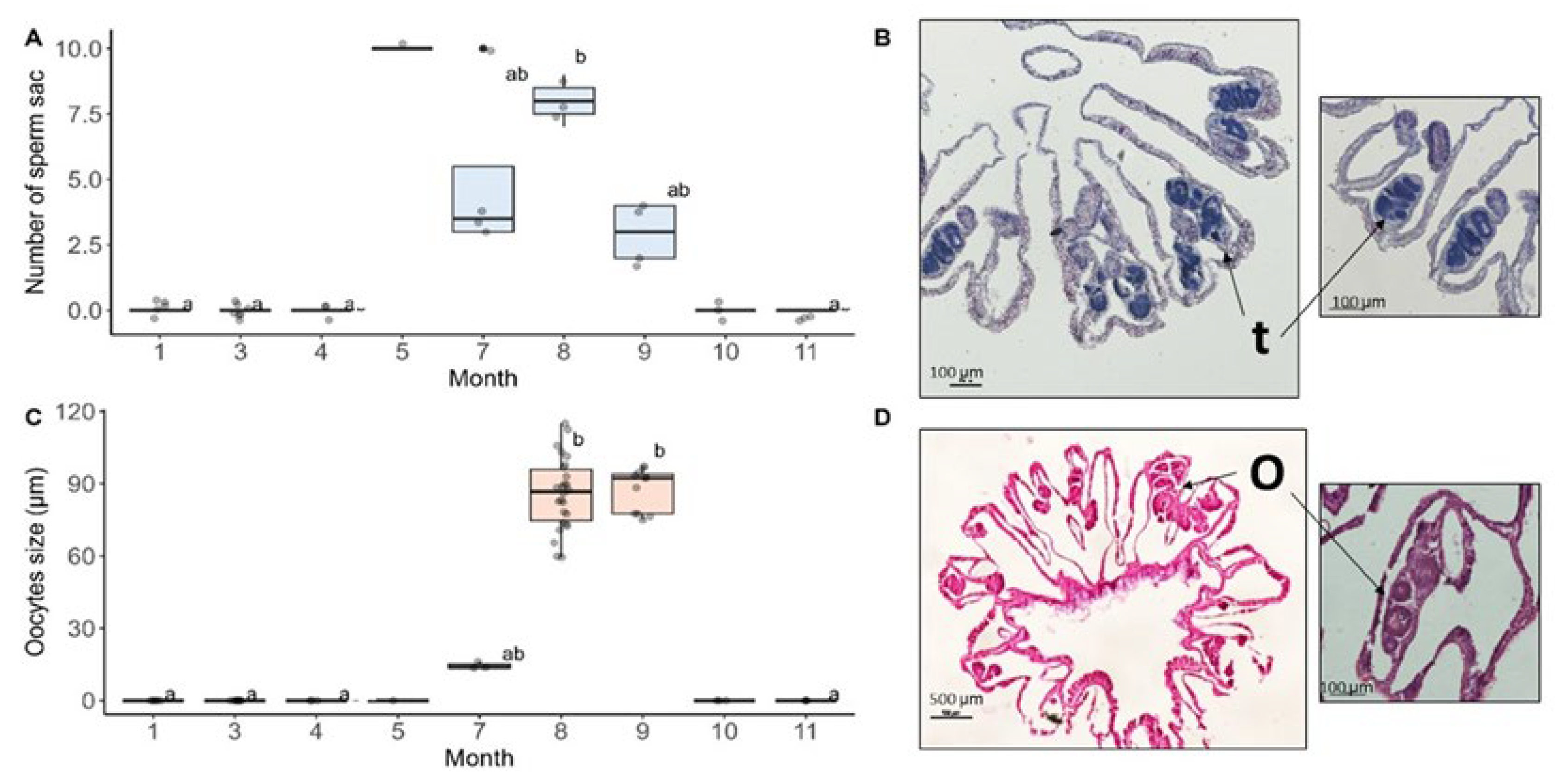
3.2. Seasonal Patterns in O. patagonica Gametogenesis
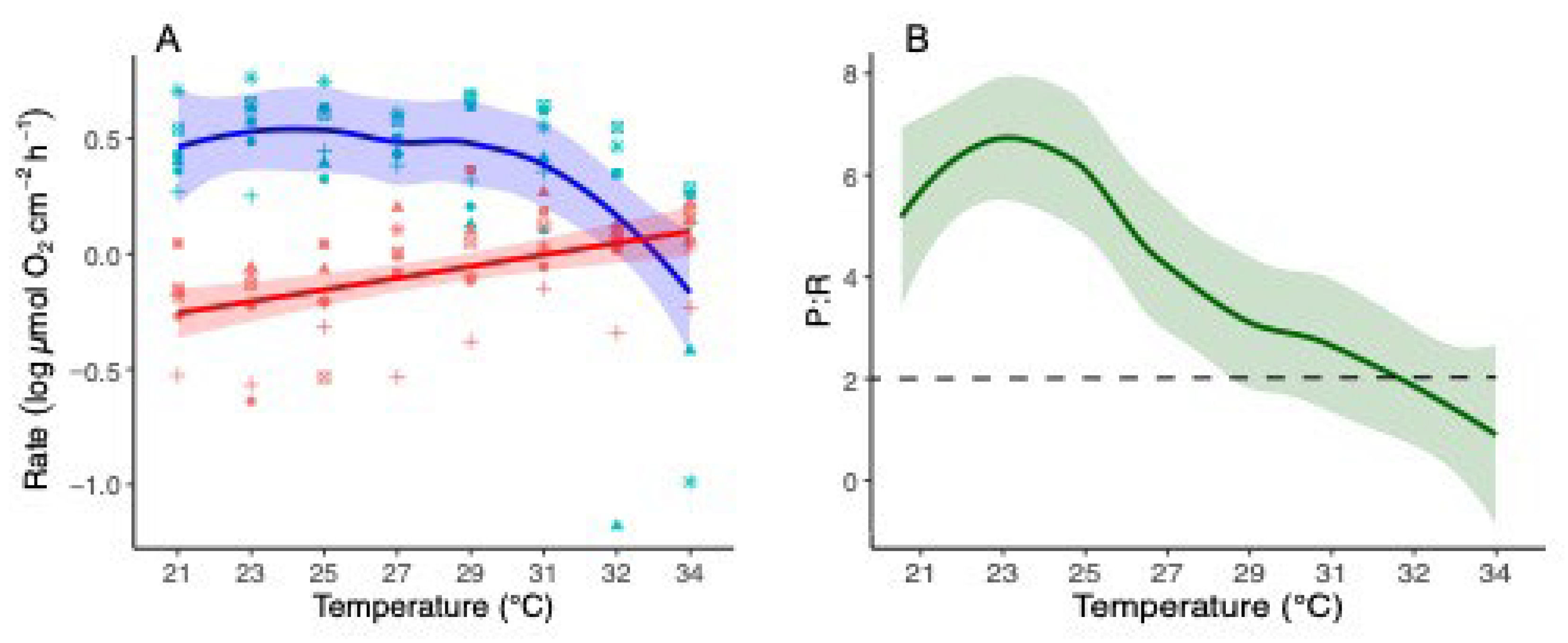
3.3. Thermal Stress Experiment: Physiology and Photosynthetic Activity
3.4. Thermal Stress Experiment: Photosynthesis Performance Parameters
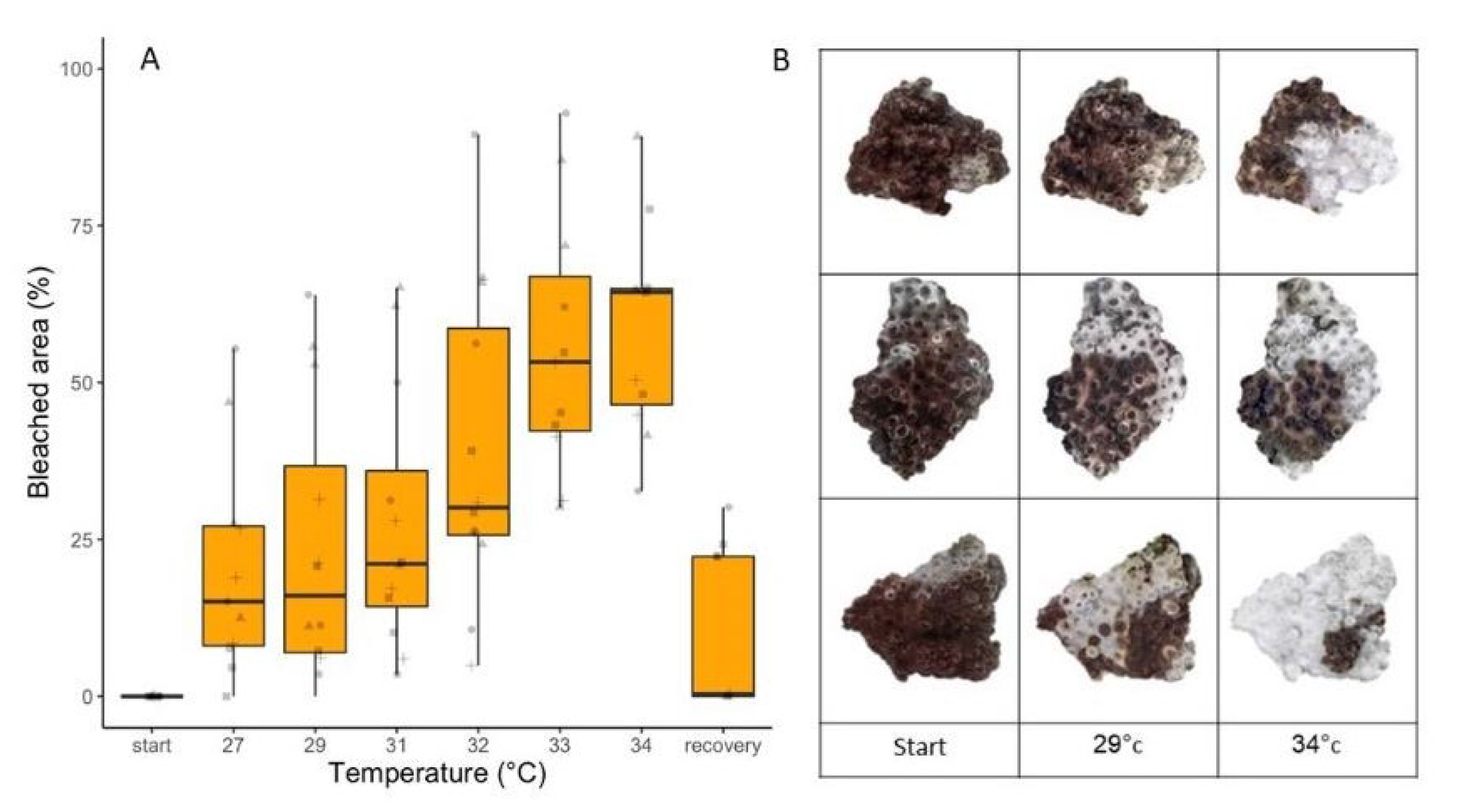
3.5. Thermal Stress Bleaching
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torda, G.; Donelson, J.M.; Aranda, M.; Barshis, D.J.; Bay, L.; Berumen, M.L.; Bourne, D.G.; Cantin, N.; Foret, S.; Matz, M.; et al. Rapid Adaptive Responses to Climate Change in Corals. Nat Clim Chang 2017, 7, 627–636. [Google Scholar] [CrossRef]
- Mumby, P.J.; Steneck, R.S. Coral Reef Management and Conservation in Light of Rapidly Evolving Ecological Paradigms. Trends Ecol Evol 2008, 23, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Putnam, H.M.; Barott, K.L.; Ainsworth, T.D.; Gates, R.D. The Vulnerability and Resilience of Reef-Building Corals. Current Biology 2017, 27, R528–R540. [Google Scholar] [CrossRef]
- Muscatine, L.; Falkowski, P.G; Porter, J.W; Dubinsky, Z. Fate of Potosynthetic Fixed Carbon in Light and Shade-Adapted Colonies of The Symbiotic Coral Stylophora Pistillata. Proceedings of the Royal Society of London Series B. Biological Sciences 1984, 222, 181–202. [Google Scholar] [CrossRef]
- Hoogenboom, M.; Rodolfo-Metalpa, R.; Ferrier-Pagès, C. Co-Variation between Autotrophy and Heterotrophy in the Mediterranean Coral Cladocora Caespitosa. Journal of Experimental Biology 2010, 213, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L. V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and More Frequent Marine Heatwaves over the Past Century. Nat Commun 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.L.; Mass, T.; Stolarski, J.; Von Euw, S.; van de Schootbrugge, B.; Falkowski, P.G. How Corals Made Rocks through the Ages. Glob Chang Biol 2020, 26, 31–53. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Benthuysen, J.A.; Darmaraki, S.; Donat, M.G.; Hobday, A.J.; Holbrook, N.J.; Schlegel, R.W.; Gupta, A. Marine Heatwaves. Sen Annual Review of Marine Science 2021. [Google Scholar]
- Oakley, C.A.; Davy, S.K. Cell Biology of Coral Bleaching. Coral Bleaching. Ecological Studies 2018, 233, 189–211. [CrossRef]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate Change and Coral Reef Bleaching: An Ecological Assessment of Long-Term Impacts, Recovery Trends and Future Outlook. Estuar Coast Shelf Sci 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Mar Freshw Res 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Rodolfo-Metalpa, R.; Hoogenboom, M.O.; Rottier, C.; Ramos-Esplá, A.; Baker, A.C.; Fine, M.; Ferrier-Pagès, C. Thermally Tolerant Corals Have Limited Capacity to Acclimatize to Future Warming. Glob Chang Biol 2014, 20, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global Warming and Recurrent Mass Bleaching of Corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Zibrowius, H. Oculina Patagonica Scléractiniaire Hermatypique Introduit en Méditerranée. Helgolander Marine Research Wiss 1974, 26, 153–173. [Google Scholar] [CrossRef]
- Fine, M.; Loya, Y. The Coral Oculina Patagonica: A New Immigrant to the Mediterranean Coast of Israel. Isr J Zool 1995, 41. [Google Scholar] [CrossRef]
- Fine, M.; Zibrowius, H.; Loya, Y. Oculina Patagonica: A Non-Lessepsian Scleractinian Coral Invading the Mediterranean Sea. Mar Biol 2001, 138, 1195–1203. [Google Scholar] [CrossRef]
- Serrano, E.; Ribes, M.; Coma, R. Recurrent Partial Mortality Events in Winter Shape the Dynamics of the Zooxanthellate Coral Oculina Patagonica at High Latitude in the Mediterranean. Coral Reefs 2017, 36, 27–38. [Google Scholar] [CrossRef]
- Bongaerts, P.; Ridgway, T.; Sampayo, E.M.; Hoegh-Guldberg, O. Assessing the “deep Reef Refugia” Hypothesis: Focus on Caribbean Reefs. Coral Reefs 2010, 29, 309–327. [Google Scholar] [CrossRef]
- Semmler, R.F.; Hoot, W.C.; Reaka, M.L. Are Mesophotic Coral Ecosystems Distinct Communities and Can They Serve as Refugia for Shallow Reefs? Coral Reefs 2017, 36, 433–444. [Google Scholar] [CrossRef]
- Shlesinger, T.; Grinblat, M.; Rapuano, H.; Amit, T.; Loya, Y. Can Mesophotic Reefs Replenish Shallow Reefs? Reduced Coral Reproductive Performance Casts a Doubt. Ecology 2018, 99, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Bellworthy, J.; Ferrier-Pagès, C.; Mass, T. Selection of Mesophotic Habitats by Oculina Patagonica in the Eastern Mediterranean Sea Following Global Warming. Sci Rep 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Zaquin, T.; Zaslansky, P.; Pinkas, I.; Mass, T. Simulating Bleaching: Long-Term Adaptation to the Dark Reveals Phenotypic Plasticity of the Mediterranean Sea Coral Oculina Patagonica. Front Mar Sci 2019, 6. [Google Scholar] [CrossRef]
- Brooke, S.; Young, C.M. Embryogenesis and Larval Biology of the Ahermatypic Scleractinian Oculina Varicosa. Mar Biol 2005, 146, 665–675. [Google Scholar] [CrossRef]
- Ozer, T.; Gertman, I.; Kress, N.; Silverman, J.; Herut, B. Interannual Thermohaline (1979–2014) and Nutrient (2002–2014) Dynamics in the Levantine Surface and Intermediate Water Masses, SE Mediterranean Sea. Glob Planet Change 2017, 151, 60–67. [Google Scholar] [CrossRef]
- Lin, C.H.; Nozawa, Y. The Influence of Seawater Temperature on the Timing of Coral Spawning. Coral Reefs 2023, 42, 417–426. [Google Scholar] [CrossRef]
- Shlesinger, T.; Loya, Y. Breakdown in Spawning Synchrony: A Silent Threat to Coral Persistence. Science (1979) 2019, 365, 1002–1007. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R.; Larkum, A.W.D.; Schreiber, U. In Situ Underwater Measurements of Photosynthetic Activity of Coral Zooxanthellae and Other Reef-Dwelling Dinoflagellate Endosymbionts, Marine Ecology Progress Series 1999, 180, 139-147. [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a, b, c, and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents, Photosynthetica 2008, 46, 115–126. [CrossRef]
- Rinkevich, B.; Loya, Y. The Reproduction of the Red Sea Coral Stylophora Pistillata. I. Gonads and Planulae. Mar Ecol Prog Ser 1979, 133–144. [Google Scholar] [CrossRef]
- Jones, A.M.; Berkelmans, R. Tradeoffs to Thermal Acclimation: Energetics and Reproduction of a Reef Coral with Heat Tolerant Symbiodinium Type-D. J Mar Biol 2011, 1–12. [Google Scholar] [CrossRef]
- Leinbach, S.E.; Speare, K.E.; Rossin, A.M.; Holstein, D.M.; Strader, M.E. Energetic and Reproductive Costs of Coral Recovery in Divergent Bleaching Responses. Sci Rep 2021, 11. [Google Scholar] [CrossRef]
- Levitan, D.R. The Relationship between Egg Size and Fertilization Success in Broadcast-Spawning Marine Invertebrates. Integrative and Comparative Biology 2006, 46, 298–311. [Google Scholar] [CrossRef]
- Michalek-Wagner, K.; Willis, B.L. Impacts of Bleaching on the Soft Coral Lobophytum Compactum. II. Biochemical Changes in Adults and Their Eggs, Coral Reefs 2001, 19, 240–246. [CrossRef]
- Armoza-Zvuloni, R.; Segal, R.; Kramarsky-Winter, E.; Loya, Y. Repeated Bleaching Events May Result in High Tolerance and Notable Gametogenesis in Stony Corals: Oculina Patagonica as a Model. Mar Ecol Prog Ser 2011, 426, 149–159. [Google Scholar] [CrossRef]
- Lesser, M.P.; Weis, V.M.; Patterson, M.R.; Jokiel, P.L. Effects of Morphology and Water Motion on Carbon Delivery and Productivity in the Reef Coral, Pocillopora Damicornis (Linnaeus): Diffusion Barriers, Inorganic Carbon Limitation, and Biochemical Plasticity. J Exp Mar Biol Ecol 1994, 178, 153–179. [Google Scholar] [CrossRef]
- Lesser, M.P. Using Energetic Budgets to Assess the Effects of Environmental Stress on Corals: Are We Measuring the Right Things? Coral Reefs 2013, 32, 25–33. [Google Scholar] [CrossRef]
- Yakovleva, I.; Bhagooli, R.; Takemura, A.; Hidaka, M. Differential Susceptibility to Oxidative Stress of Two Scleractinian Corals: Antioxidant Functioning of Mycosporine-Glycine. Comp Biochem Physiol B Biochem Mol Biol 2004, 139, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Stochaj, W.R.; Tapley, D.W.; Shick, J.M. Bleaching in Coral Reef Anthozoans: Effects of Irradiance, Ultraviolet Radiation, and Temperature on the Activities of Protective Enzymes against Active Oxygen, Coral Reefs 1990, 8, 225–232. [CrossRef]
- Hoegh-Guldber, O.; Jones, R.J. Photoinhibition and Photoprotection in Symbiotic Dinoflagellates from Reef-Building Corals, Marine Ecology Progress Series 1999, 183, 73-86. [CrossRef]
- Reinbothe, S.; Reinbothe, C. Evolution of Chlorophyll Biosynthesis-The Challenge to Survive photooxidation. Minireview 1996, 86. [Google Scholar] [CrossRef]
- Kushmarol, A.; Rosenberg2, E.; Fine1, M.; Haim2, Y. Ben; Loyal, Y. Effect of Temperature on Bleaching of the Coral Oculina Patagonica by Vibrio AK-1. Marine Ecology Progress Series 1998, 17, 131–137. [Google Scholar] [CrossRef]
- Shenkar, N.; Fine, M.; Kramarsky-Winter, E.; Loya, Y. Population Dynamics of Zooxanthellae during a Bacterial Bleaching Event. Coral Reefs 2006, 25, 223–227. [Google Scholar] [CrossRef]
- Coles, S.L.; Jokiel, P.L. Effects of Temperature on Photosynthesis and Respiration in Hermatypic Corals. Mar. Biol 1977, 43, 209–216. [Google Scholar] [CrossRef]
- Silbiger, N.J.; Goodbody-Gringley, G.; Bruno, J.F.; Putnam, H.M. Comparative Thermal Performance of the Reef-Building Coral Orbicella Franksi at Its Latitudinal Range Limits. Mar Biol 2019, 166. [Google Scholar] [CrossRef]
- Gould, K.; Bruno, J.F.; Ju, R.; Goodbody-Gringley, G. Upper-Mesophotic and Shallow Reef Corals Exhibit Similar Thermal Tolerance, Sensitivity and Optima. Coral Reefs 2021, 40, 907–920. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




