Submitted:
22 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
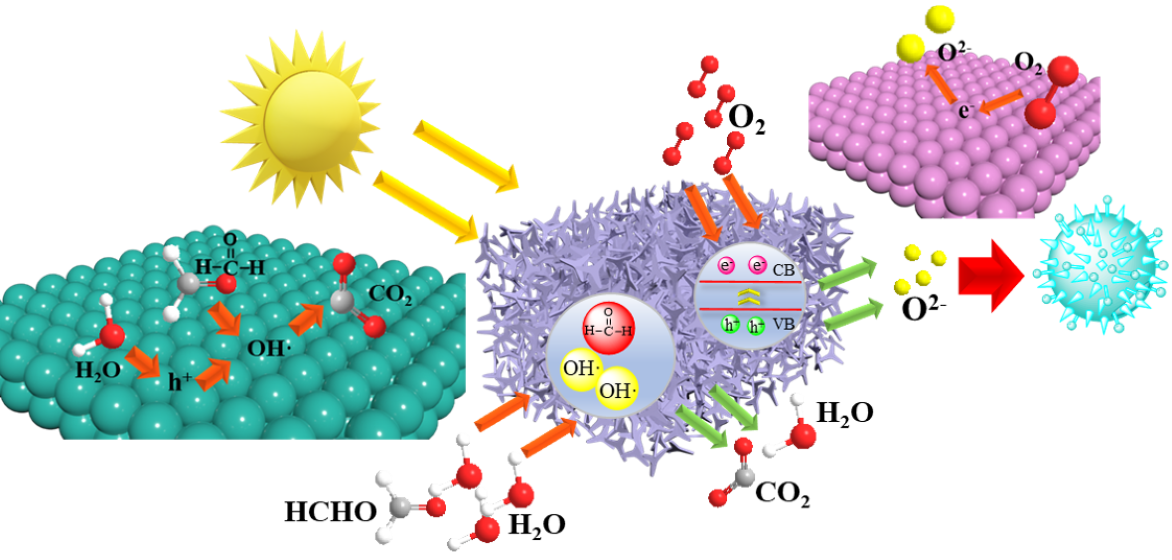
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Experiment Methods
2.3. Photocatalysis Experiment
2.4. Materials Characterization
3. Results
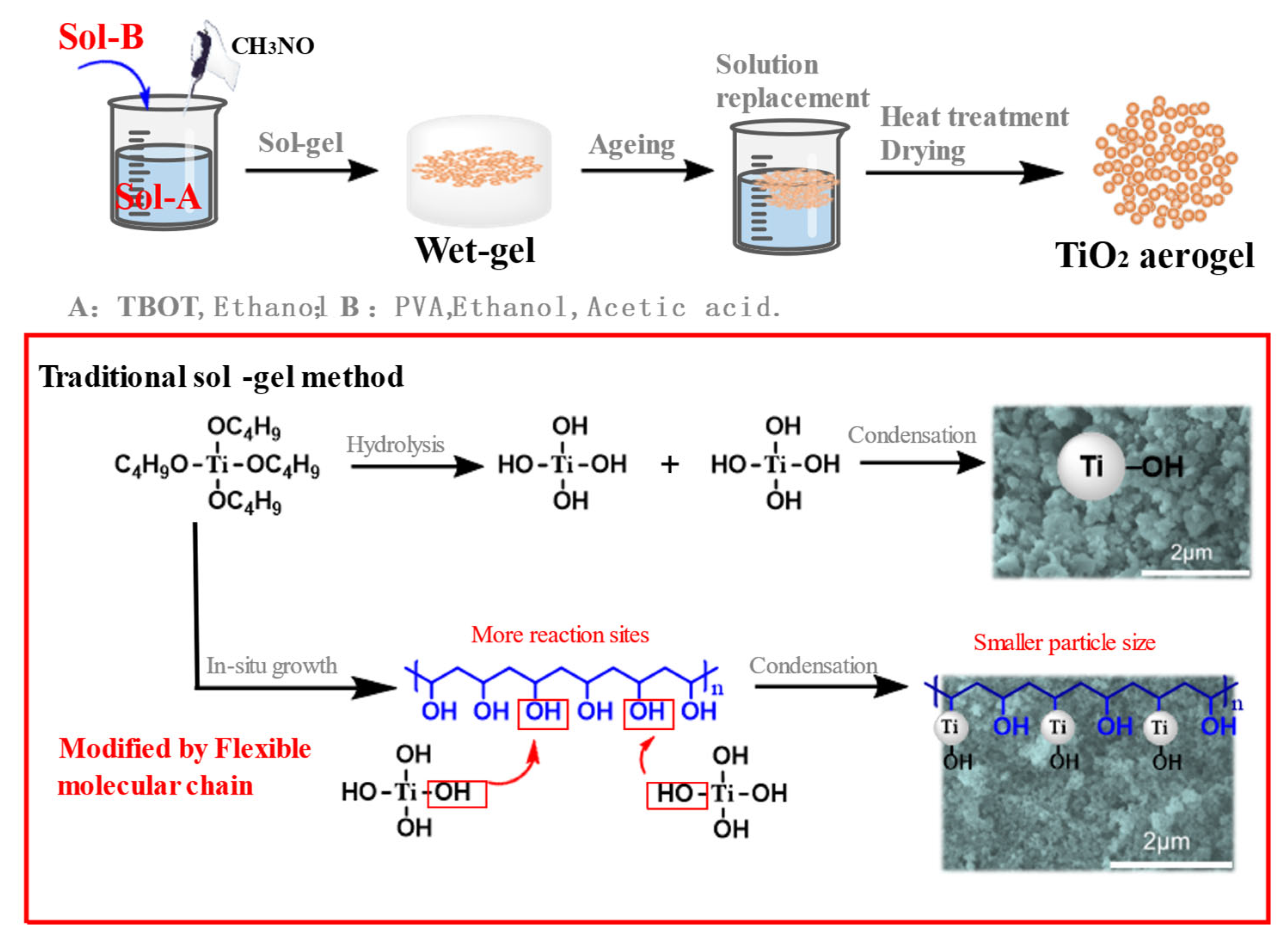
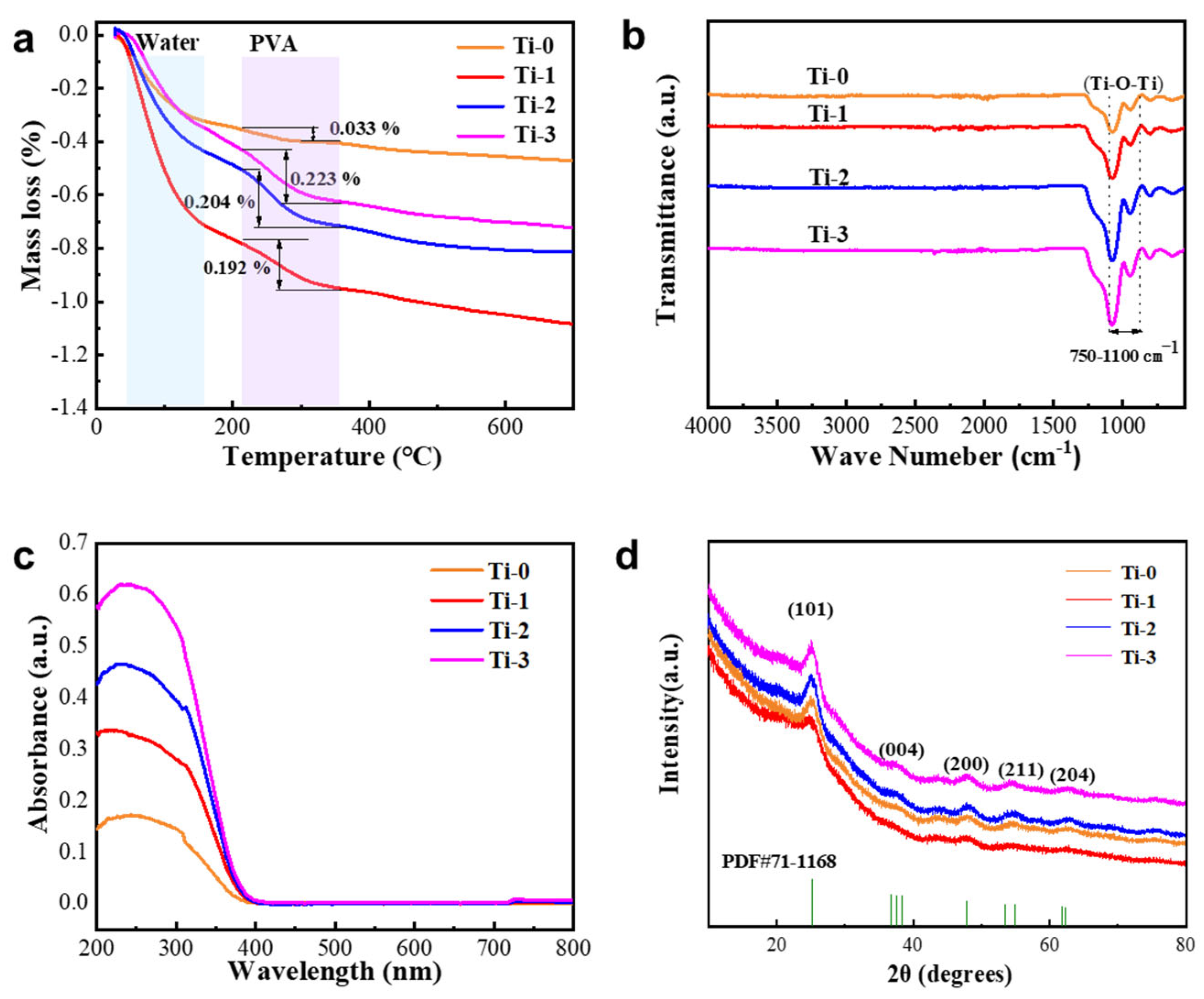
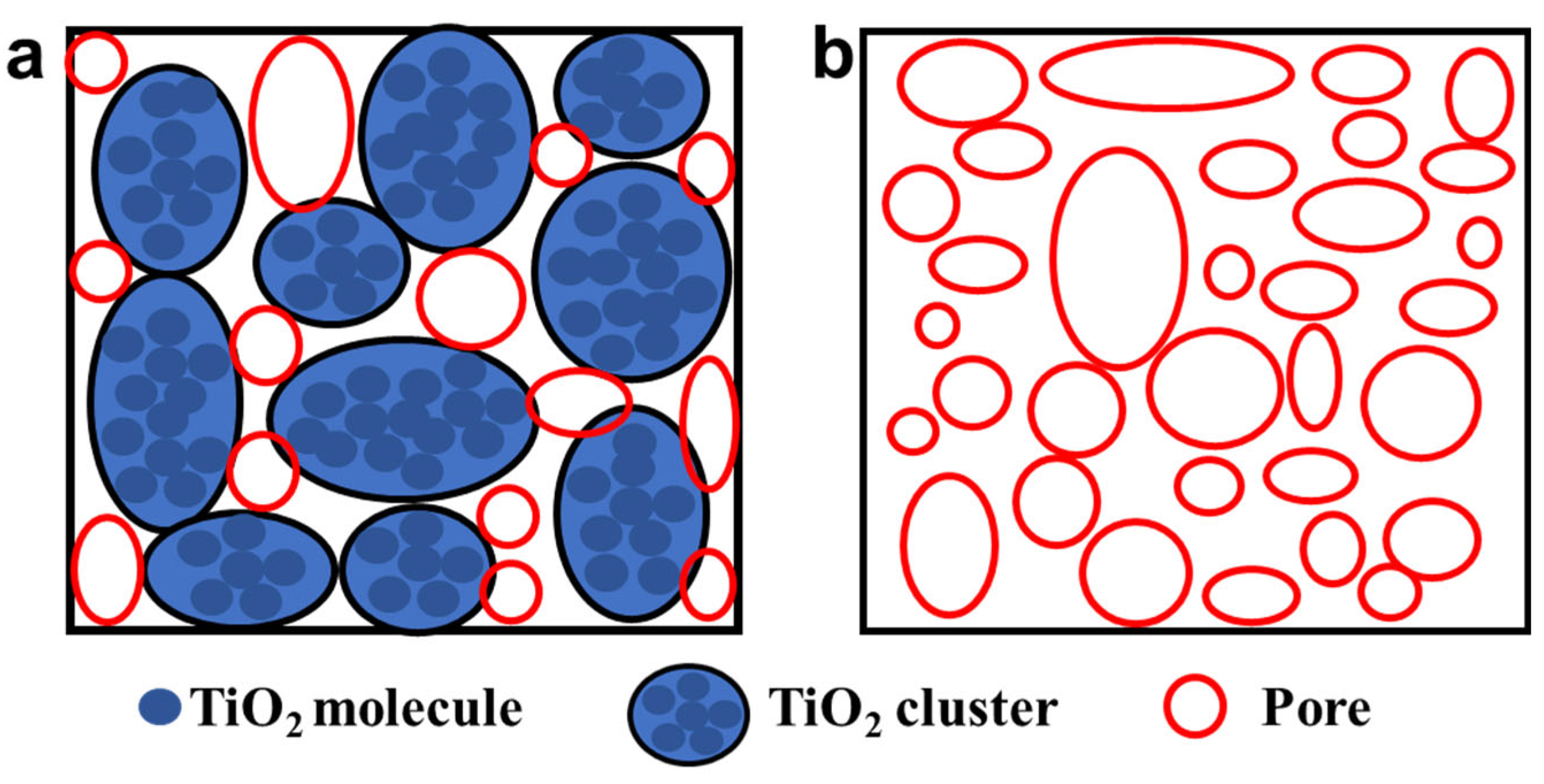
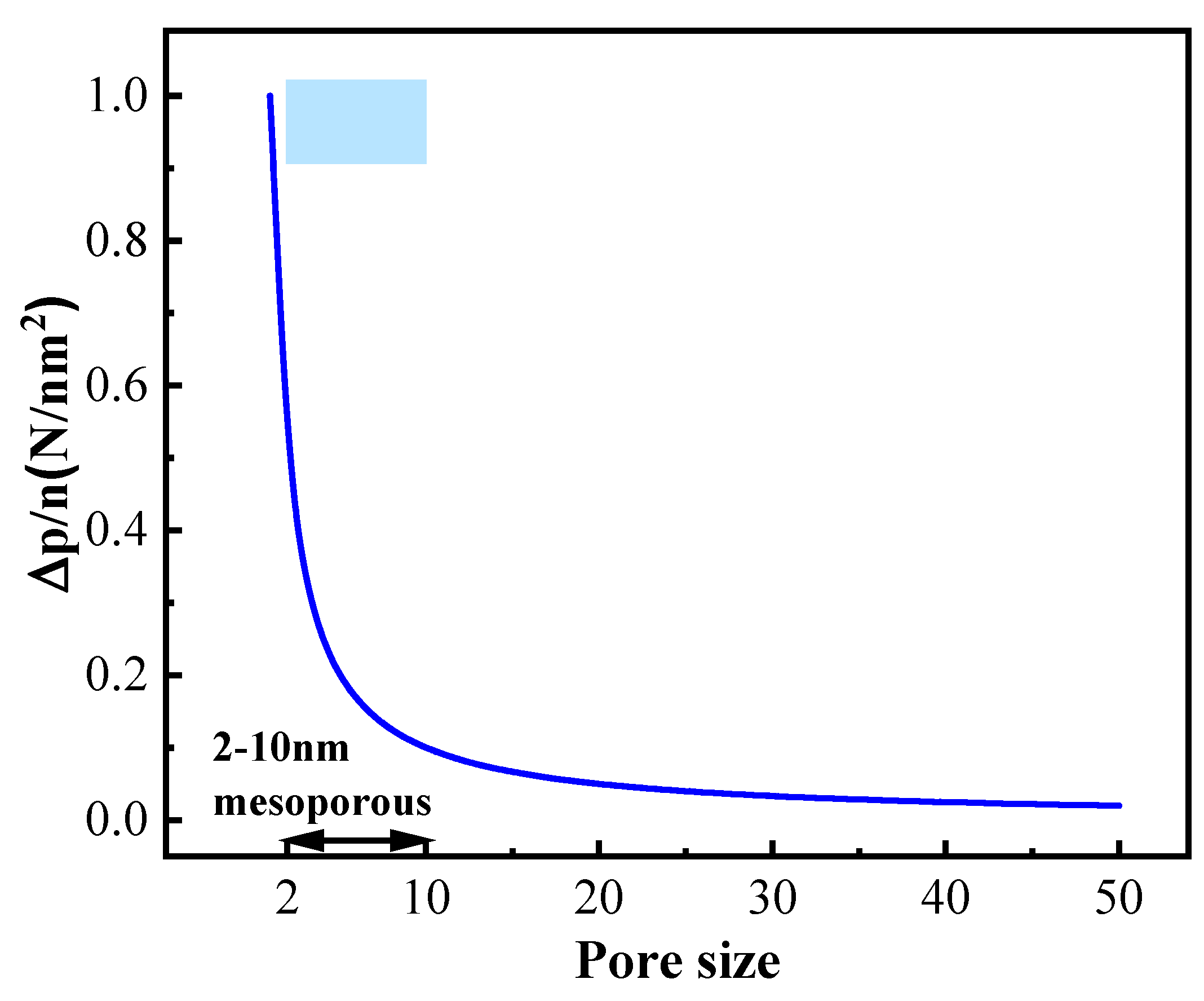
3.1. Structure and Composition Characterization of TiO2 Aerogel
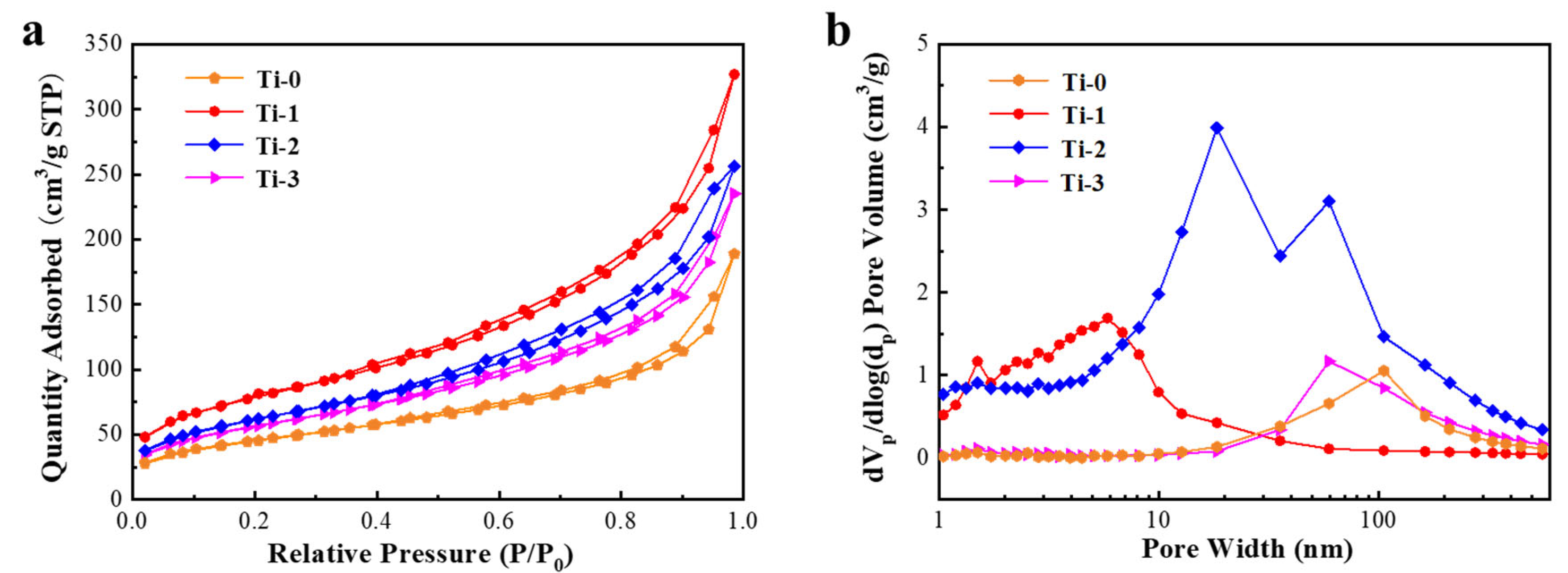
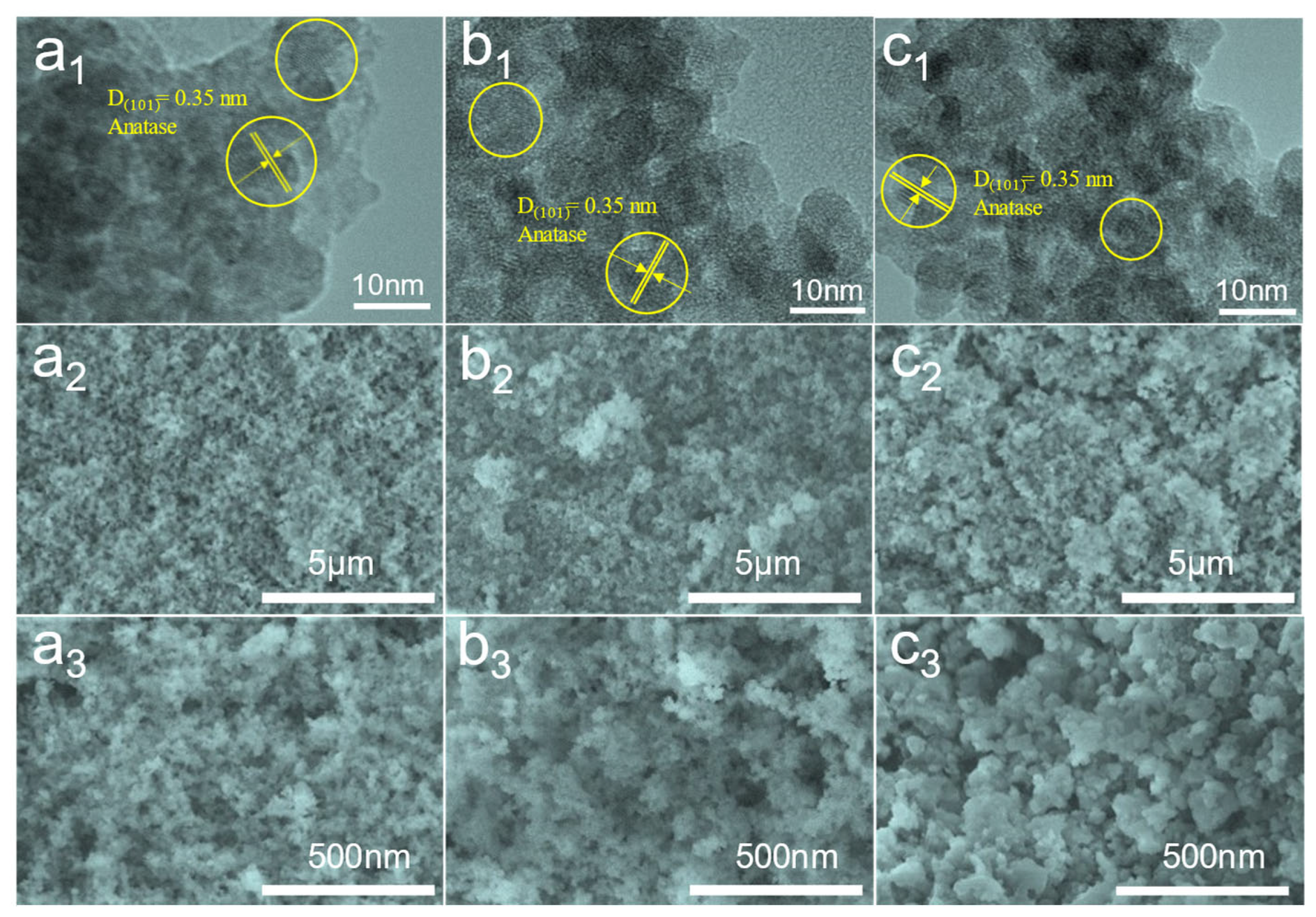
3.2. Morphology Characterization of TiO2 Aerogels
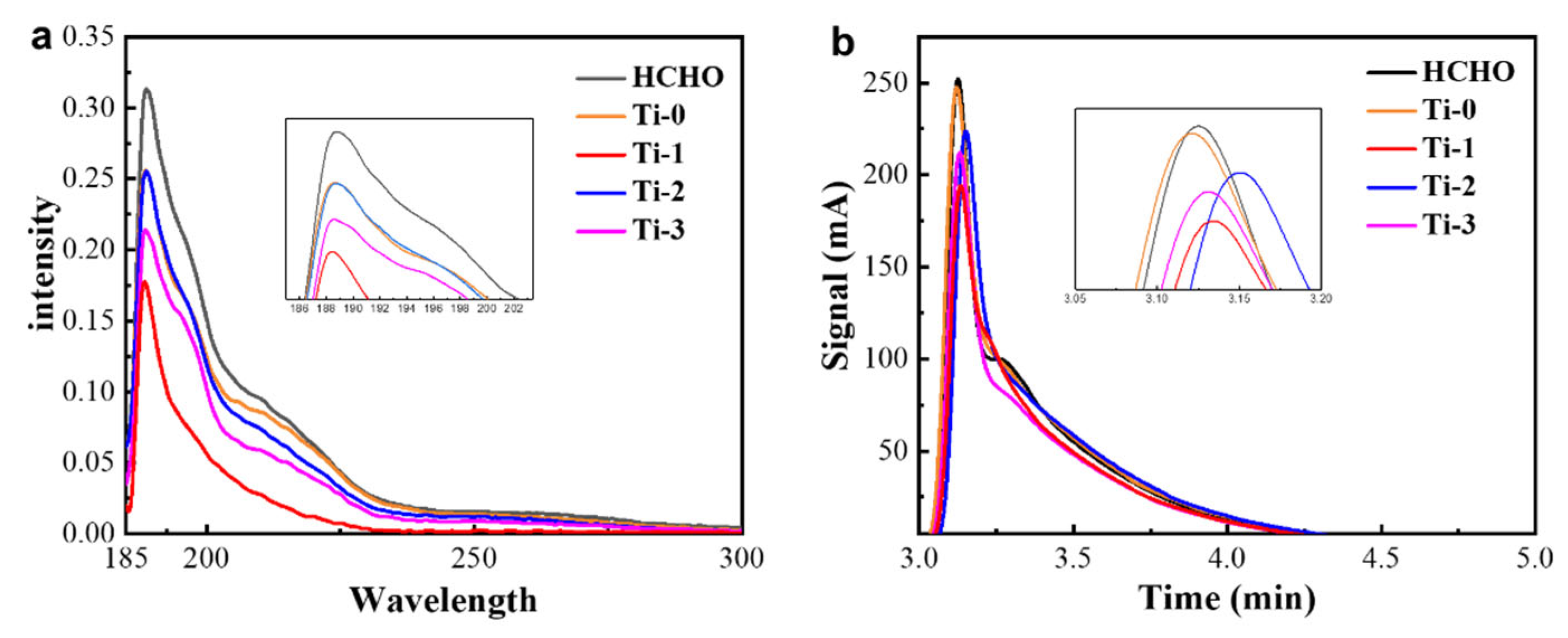
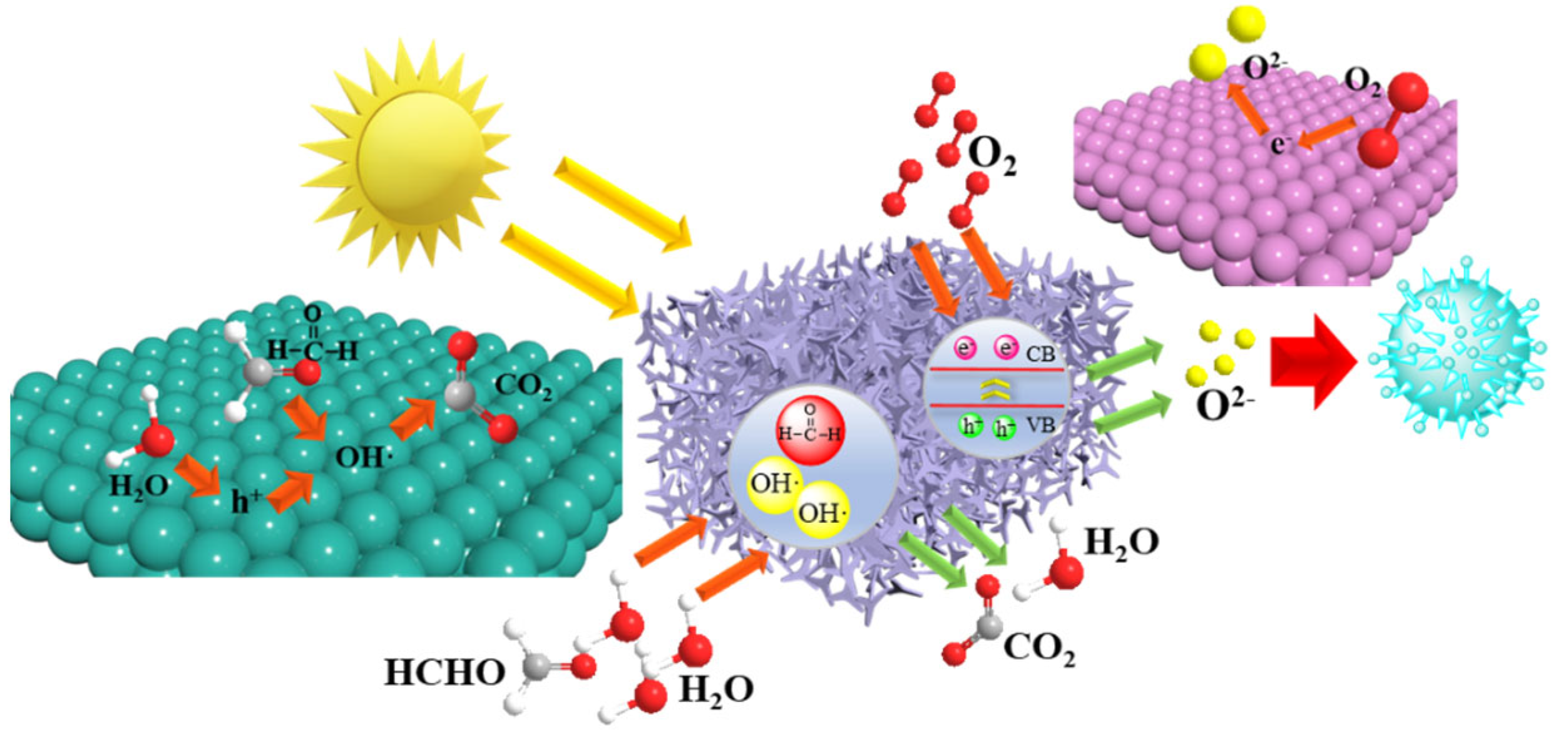
3.3. Characterization of Photocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, S.L.; Yeung, K.L.; Yue, P.L. Preparation of freestanding and crack-free titania–silica aerogels and their performance for gas phase, photocatalytic oxidation of VOCs. Applied Catalysis B Environmental 2006, 68, 99–108. [Google Scholar] [CrossRef]
- Li, X.W.; Li, H.W.; Huang, Y.; Cao, J.J.; Huang, T.T.; Li, R.; Zhang, Q.; Lee, S.C.; Ho, W.K. Exploring the photocatalytic conversion mechanism of gaseous formaldehyde degradation on TiO2-x-OV surface. Journal of Hazardous Materials 2022, 424, 127217. [Google Scholar] [CrossRef]
- Song, M.T.; Wu, Y.H.; Du, C.F.; Su, Y.G. S-scheme bismuth vanadate and carbon nitride integrating with dual-functional bismuth nanoparticles toward co-efficiently removal formaldehyde under full spectrum light. Journal of Colloid and Interface Science 2021, 588, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.S.; Zheng, J.X.; Yan, L.H.; Zhang, X.X. Sol-gel preparation of self-cleaning SiO2-TiO2/SiO2-TiO2 double-layer antireflective coating for solar glass. Results in Physics 2018, 8, 532–536. [Google Scholar] [CrossRef]
- Yang, L.X.; Guo, J.W.; Yang, T.Q.; Guo, C.; Zhang, S.Q.; Luo, S.L.; Dai, W.L.; Li, B.; Luo, X.B.; Li, Y. Self-assembly Cu2O nanowire arrays on Cu mesh: A solid-state, highly-efficient, and stable photocatalyst for toluene degradation under sunlight. Journal of Hazardous Materials 2021, 402, 123741. [Google Scholar] [CrossRef]
- Duan, C.M.; Meng, M.W.; Huang, H.; Wang, H.; Ding, H.; Zhang, Q. Adsorptivity and kinetics for low concentration of gaseous formaldehyde on bamboo-based activated carbon loaded with ammonium acetate particles. Environmental Research 2023, 222, 115364. [Google Scholar] [CrossRef] [PubMed]
- Belaissaoui, B.; Le Moullec, Y.; Favre, E. Energy efficiency of a hybrid membrane/condensation process for VOC (Volatile Organic Compounds) recovery from air: A generic approach. Energy 2016, 95, 291–302. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Qin, J.C.; Guo, Q.K.; Zhou, H.L.; Jin, W.Q. Microporous polyimide VOC-rejective membrane for the separation of nitrogen/VOC mixture. Journal of Hazardous Materials 2021, 402, 123817. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsu, Y.C.; Lin, C.C.; Tai, C.Y.D.; Liu, H.S. Volatile organic compounds absorption in a cross-flow rotating packed bed. Environmental Science & Technology 2008, 42, 2631–2636. [Google Scholar]
- Shah, R.K.; Thonon, B.; Benforado, D.M. Opportunities for heat exchanger applications in environmental systems. Applied Thermal Engineering 2000, 20, 631–650. [Google Scholar] [CrossRef]
- Nian, H.J.; Meng, Q.C.; Zhang, W.; Chen, L.M. Overexpression of the formaldehyde dehydrogenase gene from brevibacillus brevis to enhance formaldehyde tolerance and detoxification of tobacco. Applied Biochemistry and Biotechnology 2013, 169, 170–180. [Google Scholar] [CrossRef]
- Mohamed, E.F.; Awad, G.; Ibrahim, A.S.S. Biodegradation of formaldehyde gas pollutant by a novel immobilized haloalkaliphilic Salipaludibacillus agaradhaerens strain NRC-R isolated from hypersaline soda lakes. Results in Engineering 2023, 19, 101374. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, Y.S.; Shin, J.W.; Kim, K.H.; Kukkar, D.; Tsang, Y.F. Potential applications of graphene-based nanomaterials as adsorbent for removal of volatile organic compounds. Environment International 2020, 135, 105356. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhu, T.L.; Sun, Y.F.; Yan, X. The roles of various plasma species in the plasma and plasma-catalytic removal of low-concentration HCHO in air. Hazard Mater 2011, 196, 380–385. [Google Scholar] [CrossRef]
- Wang, F.P.; Li, W.; Zhang, W.M.; Ye, R.R.; Tan, X.H. Facile fabrication of the Ag nanoparticles decorated graphitic carbon nitride photocatalyst film for indoor air purification under visible light. Building and environment 2022, 222, 109402. [Google Scholar] [CrossRef]
- Kwon, D.W.; Seo, P.W.; Kim, G.J.; Hong, S.C. Characteristics of the HCHO oxidation reaction over Pt/TiO2 catalysts at room temperature: The effect of relative humidity on catalytic activity. Applied Catalysis B: Environmental 2015, 163, 436–443. [Google Scholar] [CrossRef]
- Sabia, P.; Romeo, F.; de Joannon, M.; Cavaliere, A. VOC destruction by water diluted hydrogen mild combustion. Chemosphere 2007, 68, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Miao, G.; Pi, Y.H.; Xia, Q.B.; Wu, J.L.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chemical Engineering Journal 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, H.L.; Pan, W.G.; Mu, X.T.; Qiu, K.N.; Ma, J.C.; Zhao, Y.T.; Song, J.; Zhang, Z.Y. Research progress of a composite metal oxide catalyst for VOC degradation. Environmental Science & Technology 2022, 56, 9220–9236. [Google Scholar]
- Liang, W.J.; Li, J.; Jin, Y.Q. Photo-catalytic degradation of gaseous formaldehyde by TiO2/UV, Ag/TiO2/UV and Ce/TiO2/UV. Building and Environment 2012, 51, 345–350. [Google Scholar] [CrossRef]
- Ding, J.Y.; Yang, Y.J.; Liu, J.; Wang, Z. Catalytic reaction mechanism of formaldehyde oxidation by oxygen species over Pt/TiO2 catalyst. Chemosphere 2020, 248, 125980. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation - A review. Journal of Environmental Management 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. Journal of Environmental Chemical Engineering 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Rao, Z.P.; Lu, G.H.; Chen, L.; Mahmood, A.; Shi, G.S.; Tang, Z.X.; Xie, X.F.; Sun, J. Photocatalytic oxidation mechanism of Gas-Phase VOCs: Unveiling the role of holes, ·OH and ·O2-. Chemical Engineering Journal 2022, 430, 132766. [Google Scholar] [CrossRef]
- Backus, E.H.G.; Hosseinpour, S.; Ramanan, C.; Sun, S.M.; Schlegel, S.J.; Zelenka, M.; Jia, X.Y.; Gebhard, M.; Devi, A.; Wang, H.I.; Bonn, M. Ultrafast surface-specific spectroscopy of water at a photoexcited TiO2 model water-splitting photocatalyst. Angewandte Chemie-International Edition 2024, 63, 2312123. [Google Scholar] [CrossRef]
- Li, J.Y.; Cui, W.; Chen, P. .; Dong, X.A.; Chu, Y.H.; Sheng, J.P.; Zhang, Y.X.; Wang, Z.M.; Dong, F. Unraveling the mechanism of binary channel reactions in photocatalytic formaldehyde decomposition for promoted mineralization. Applied Catalysis B-Environmental 2020, 260, 118130. [Google Scholar] [CrossRef]
- Chen, H.H.; Nanayakkara, C.E.; Grassian, V.H. Grassian. Titanium dioxide photocatalysis in atmospheric chemistry. Chemical Reviews 2012, 112, 5919–5948. [Google Scholar] [CrossRef]
- Wang, W.J.; Lin, F.W.; An, T.C.; Qiu, S.X.; Yu, H.D.; Yan, B.B.; Chen, G.Y.; Hou, L.A. Photocatalytic mineralization of indoor VOC mixtures over unique ternary TiO2/C/MnO2 with high adsorption selectivity[J]. Chemical Engineering Journal 2021, 425, 131678. [Google Scholar] [CrossRef]
- Huang, H.B.; Ye, X.G.; Huang, H.L.; Zhang, L.; Leung, D.Y.C. Mechanistic study on formaldehyde removal over Pd/TiO2 catalysts: oxygen transfer and role of water vapor. Chemical Engineering Journal 2013, 230, 73–79. [Google Scholar] [CrossRef]
- Li, Y.B.; Chen, X.Y.; Wang, C.Y.; Zhang, C.B.; He, H. Sodium enhances Ir/TiO2 activity for catalytic oxidation of formaldehyde at ambient temperature. ACS Catalysis 2018, 8, 11377–11385. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, K.; Guo, X.L.; Shenb, G.D.; Xiang, J.Y. Synthesis and characterization of N-doped TiO2 loaded onto activated carbon fiber with enhanced visible-light photocatalytic activity. New Journal of Chemistry 2014, 38, 6139–6146. [Google Scholar] [CrossRef]
- Cheng, X.W.; Yu, X.J.; Xing, Z.P.; Wan, F.; Yang, G. Enhanced photocatalytic activity of nitrogen doped TiO2 anatase nano-particle under simulated sunlight irradiation. Energy Procedia 2012, 16, 598–605. [Google Scholar] [CrossRef]
- Liu, W.X.; Jiang, P.; Shao, W.N.; Zhang, J.; Cao, W.B. A novel approach for the synthesis of visible-light-active nanocrystalline N-doped TiO2 photocatalytic hydrosol. Solid State Sciences 2014, 33, 45–48. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (Pbca, Z=8). Journal of Raman Spectroscopy 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Yue, X.; Xiang, J.H.; Chen, J.Y.; Li, H.X.; Qiu, Y.S.; Yu, X.B. High surface area, high catalytic activity titanium dioxide aerogels prepared by solvothermal crystallization. Journal of Materials Science & Technology 2020, 47, 223–230. [Google Scholar]
- Cao, S.L.; Yeung, K.L.; Kwan, J.K.C.; To, P.M.T.; Yu, S.C.T. An investigation of the performance of catalytic aerogel filters. Applied Catalysis B-Environmental 2009, 86, 127–136. [Google Scholar] [CrossRef]
- Chen, W.; Sui, L.X.; Long, X.; Liao, J.X.; Wang, S.Z.; Wei, X.B. Anatase TiO2 aerogel with high specific surface areas and porous network structures for ultra-fast response hydrogen sensor. International Journal of Hydrogen Energy 2024, 50, 973–991. [Google Scholar] [CrossRef]
- Li, F.Z.; Gao, S.; Niu, Y.T.; Song, J.B.; Zhao, W.X.; Niederberger, M.; Cheng, W. Large-scale synthesis of macroscopic layered inorganic-organic hybrid nanobelt aerogel monoliths with multifunctionality. Cell Reports Physical Science 2022, 3, 101079. [Google Scholar] [CrossRef]
- Zhou, T.P.; Xu, Y.T.; Zhen, Y.; Wu, K.J.; Ding, H.H.; Wang, L.J.; Tai, X.L.; Cai, X.R.; Zhang, X.; Xia, T.P.; Zhu, J.F.; Chu, W.S.; Ni, Y.; Xie, Y.; Wu, C.Z. Layered inorganic silicate aerogel pillared by nanoclusters for high temperature thermal insulation. Advanced Materials 2023, 35, 2306135. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.N.; Shao, G.N.; Jeon, S.J.; Imran, S.M.; Sarawade, P.B.; Kim, H.T. Sol–gel synthesis of sodium silicate and titanium oxychloride based TiO2-SiO2 aerogels and their photocatalytic property under UV irradiation. Chemical Engineering Journal 2013, 231, 502–511. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Malfait, W.J.; Jeong, E.H.; Fischer, B.; Zhang, Y.C.; Xu, H.X.; Angelica, E.; Risen, W.M.; Suggs, W.; Koebel, M.M. Facile one-pot synthesis of mechanically robust biopolymer silica nanocomposite aerogel by cogelation of silicic acid with chitosan in aqueous media. Acs Sustainable Chemistry & Engineering 2016, 4, 5674–5683. [Google Scholar]
- Oschatz, M.; Nickel, W.; Thommes, M.; Cychosz, K.A.; Leistner, M.; Adam, M.; Mondin, G.; Strubel, P.; Borchardt, L.; Kaskel, S. Evolution of porosity in carbide-derived carbon aerogels. Journal of Materials Chemistry A 2014, 2, 18472–18479. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: a review. Environmental Science and Pollution Research 2019, 26, 3262–3291. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, G.Q.; Liu, Z.W.; Zhang, X.T. Polyimide aerogel fibers with superior flame resistance, strength, hydrophobicity, and flexibility made via a universal sol-gel confined transition strategy. Acs Nano 2021, 15, 4759–4768. [Google Scholar] [CrossRef] [PubMed]
- Prokic-Vidojevic, D.; Glisic, S.B.; Krstic, J.B.; Orlovic, A.M. Aerogel Re/Pd-TiO2 SiO2 and Co/Mo-Al2O3 SiO2 catalysts for hydrodesulphurisation of dibenzothiophene and 4,6-dimethyldibenzothiophene. Catalysis Today 2021, 378, 10–23. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Malfait, W.J.; Sadeghpour, A.; Girao, A.V.; Silva, R.F.; Duraes, L. Influence of 1D and 2D carbon nanostructures in silica-based aerogels. Carbon 2021, 180, 146–162. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Developing dual nano/macroporous starch bioaerogels via emulsion templating and supercritical carbon dioxide drying. Carbohydrate Polymers 2022, 292, 119607. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.T.P.N.X.; Giang, N.T.H.; Huong, L.M.; Thinh, D.B.; Dat, N.M.; Trinh, D.N.; Hai, N.D.; Oanh, D.T.Y.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Hydrothermal synthesis of titanium dioxide/graphene aerogel for photodegradation of methylene blue in aqueous solution. Journal of Science-Advanced Materials and Devices 2022, 7, 100433. [Google Scholar] [CrossRef]
- Wu, Q.S.; Sun, H. Preparation and properties of porous ceramics from nickel slag by aerogel gelcasting. Ceramics International 2022, 48, 33058–33065. [Google Scholar] [CrossRef]
- Coles, M.P.; Lugmair, C.G.; Terry, K.W.; Tilley, T.D. Titania-silica materials from the molecular precursor Ti[OSi(OtBu)3]4:: Selective epoxidation catalysts. Chemistry of Materials 1999, 12, 122–131. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, M.R.; Lv, J.B.; He, X.; Chen, M.H.; Tan, W.; Yang, W.J.; Zeng, K.; Hu, J.H.; Yang, G. Biomineralization-inspired confined-space fabrication of polyimide aerogels. Acs Applied Materials & Interfaces 2024, 16, 2763–2773. [Google Scholar]
- Yang, X.; Ma, J.J.; Ling, J.; Li, N.; Wang, D.; Yue, F.; Xu, S.M. Cellulose acetate-based SiO2/TiO2 hybrid microsphere composite aerogel films for water-in-oil emulsion separation. Applied Surface Science 2018, 435, 609–616. [Google Scholar] [CrossRef]
- Jia, H.J.; Liu, S.; Mao, Z.Y.; Wang, D.J. Preparation and properties of the Al2O3-SiO2 aerogel/alumina framework composite. Ceramics International 2021, 47, 1466–1471. [Google Scholar] [CrossRef]
- Giang, N.T.H.; Tan, N.N.; Huong, L.; Hai, N.D.; Thinh, N.T.; Phuc, N.T.; Dat, N.M.; Phong, M.T.; Hieu, N.H. Photocatalytic degradation of crystal violet on titanium dioxide/graphene aerogel doped sulfur. Journal of Molecular Structure 2023, 1271, 134031. [Google Scholar] [CrossRef]
- Abdullah, H.B.; Irmawati, R.; Ismail, I.; Yusof, N.A. Utilization of waste engine oil for carbon nanotube aerogel production using floating catalyst chemical vapor deposition. Journal of Cleaner Production 2020, 261, 121188. [Google Scholar] [CrossRef]
- Ye, X.L.; Chen, Z.F.; Ai, S.F.; Hou, B.; Zhang, J.X.; Zhou, Q.B.; Wang, F.; Liu, H.Z.; Cui, S. Microstructure characterization and thermal performance of reticulated SiC skeleton reinforced silica aerogel composites. Composites Part B-Engineering 2019, 177, 107409. [Google Scholar] [CrossRef]
- Zhou, X.P.; Wang, Y.F.; Xiao, L.J.; Zhang, M.Y.; Su, Z.A.; Huang, Q.Z. Preparing carbon black aerogel quickly by chemical vapor deposition. Composites Communications 2023, 37, 101460. [Google Scholar] [CrossRef]
- Doneliene, J.; Fataraite-Urboniene, E.; Danchova, N.; Gutzov, S.; Ulbikas, J. The influence of the precursor’s nature and drying conditions on the structure, morphology, and thermal properties of TiO2 aerogels. Gels 2022, 8, 422. [Google Scholar] [CrossRef]
- Doneliene, J.; Fataraite-Urboniene, E.; Rudzikas, M.; Pakalka, S.; Danchova, N.; Ulbikas, J. Effect of precursor nature and sol-gel synthesis conditions on TiO2 aerogel's structure. Molecules 2021, 26, 5059. [Google Scholar] [CrossRef]
- Feng, Q.G.; Cai, H.D.; Lin, H.Y.; Qin, S.Y.; Liu, Z.; Ma, D.C.; Ye, Y.Y. Synthesis and structural characteristics of high surface area TiO2 aerogels by ultrasonic-assisted sol–gel method. Nanotechnology 2018, 29, 050702. [Google Scholar]
- Somjit, V.; Thinsoongnoen, P.; Sriphumrat, K.; Pimu, S.; Arayachukiat, S.; Kongpatpanich, K. Metal-organic framework aerogel for full ph range operation and trace adsorption of arsenic in water. Acs Applied Materials & Interfaces 2022, 14, 40005–40013. [Google Scholar]
- Su, X.P.; Liao, Q.; Liu, L.; Meng, R.J.; Qian, Z.Q.; Gao, H.Y.; Yao, J.M. Cu2O nanoparticle-functionalized cellulose-based aerogel as high-performance visible-light photocatalyst. Cellulose 2017, 24, 1017–1029. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Synthesis of TiO2 nanoparticles coated on cellulose nanofibers with different morphologies: Effect of the template and sol-gel parameters. Materials Science in Semiconductor Processing 2020, 109, 104927. [Google Scholar] [CrossRef]
- Konuk, O.P.; Alsuhile, A.A.A.M.; Yousefzadeh, H.; Ulker, Z.; Bozbag, S.E.; García-González, C.A.; Smirnova, I.; Erkey, C. The effect of synthesis conditions and process parameters on aerogel properties. Frontiers in Chemistry 2023, 11, 1294520. [Google Scholar] [CrossRef] [PubMed]
- Rebber, M.; Sannemüller, H.; Jaruszewski, M.; Pfannkuche, D.; Urakawa, A.; Koziej, D. Light and mass transport computations guide the fabrication of 3D-structured TiO2 and Au/TiO2 aerogel photocatalysts for efficient hydrogen production in the gas phase. Chemistry of Materials 2023, 35, 3849–3858. [Google Scholar] [CrossRef]
- Dong, G.L.; Gao, Y.B.; Chen, S.Y. Effects of different drying methods on the properties of TiO2 particles. Acta Physico-Chimica Sinica 1998, 14, 142–146. [Google Scholar]
- Liu, J.X.; Bai, L.N.; Shi, F.; Hu, Z.Q.; Jiang, Y.Y.; Zhao, T. Pore structure characterization of TiO2-SiO2 composite aerogel prepared via ambient pressure drying by sol pre-modification process. Advanced Materials Research 2012, 534, 101–105. [Google Scholar] [CrossRef]
- Moussaoui, R.; Elghniji, K.; ben Mosbah, M.; Elaloui, E.; Moussaoui, Y. Sol-gel synthesis of highly TiO2 aerogel photocatalyst via high temperature supercritical drying. Journal of Saudi Chemical Society 2017, 21, 751–760. [Google Scholar] [CrossRef]
- Ghosal, S.; Baumann, T.F.; King, J.S.; Kucheyev, S.O.; Wang, Y.M.; Worsley, M.A.; Biener, J.; Bent, S.F.; Hamza, A.V. Controlling atomic layer deposition of TiO2 in aerogels through surface functionalization. Journal of Immunology 2009, 183, 6619–6628. [Google Scholar] [CrossRef]
- Xia, Y.; Man, J.W.; Wu, X.D.; Huang, S.T.; Lu, A.Q.; Shen, X.D.; Cui, S.; Chen, X.B.; Fu, G.T. Oxygen-vacancy-assisted construction of Ce–TiO2 aerogel for efficiently boosting photocatalytic CO2 reduction without any sacrifice agent. Ceramics International 2023, 49, 6100–6112. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shen, J.; Wang, W.Q.; Zou, L.P.; Lian, Y.; Zhang, Z.H. Silica-titania composite aerogel photocatalysts by chemical liquid deposition of titania onto nanoporous silica scaffolds. Acs Appl Mater Interfaces 2015, 7, 5400–5409. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Li, G.; Chen, Q.; Wang, F. Facile green synthesis of degraded-PVA coated TiO2 nanoparticles with enhanced photocatalytic activity under visible light. Journal of Physics and Chemistry of Solids 2019, 129, 92–98. [Google Scholar] [CrossRef]
- Li, L.S.; Yang, G.D.; Lyu, J.; Sheng, Z.Z.; Ma, F.G.; Zhang, X.T. Folk arts-inspired twice-coagulated configuration-editable tough aerogels enabled by transformable gel precursors. Nature Communications 2024, 14, 8450. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.T.; Zhu, C.Y.; Feng, X.L.; Wali, Q.; Fan, W.; Liu, T.X. Polyimide Aerogel Fibers with Controllable Porous Microstructure for Super-Thermal Insulation Under Extreme Environments. Advanced Fiber Materials 2022, 4, 1118–1128. [Google Scholar] [CrossRef]
- Rasalingam, S.; Wu, C.M.; Koodali, R.T. Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: implications for photocatalytic degradation of rhodamine B. Acs Appl Mater Interfaces 2015, 7, 4368–4380. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review. Chemical Engineering Journal 2017, 334, 2408–2439. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



