1. Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignant disorder [
1]. The introduction of intensive chemotherapy, better risk stratification, and better supportive care improved the survival rate in children with ALL and today over 90% of them can be successfully treated [
2,
3,
4,
5].
However, more intensive treatment is associated with an increased prevalence of toxicities, which are found to be a significant cause of non-cancer morbidity and mortality in this group of patients [
6,
7].
Acute pancreatitis (AP) developing during chemotherapy for ALL may be a severe and fatal complication. It is widely known toxicity and several reports indicate that AP in patients treated for leukemia occurs with an incidence of 2-10% [
8,
9].
It is mainly but not only associated with asparaginase (ASP) administration which is one of the key components of multiagent chemotherapy used in ALL [
9,
10]. AP development delays subsequent courses of chemotherapy and is the most common reason for ASP discontinuation, thus increasing the potential risk of leukemia relapse in children with ALL [
9,
11,
12,
13]. It is suggested that parenchymal toxicity from chemotherapy is a causative factor of AP development [
14,
15]. However, the pathogenesis of AP in ALL and long-term outcome still need to be thoroughly investigated. Moreover, the AP prediction in routine clinical practice in children with ALL is also not feasible [
6,
8,
10,
14,
15].
This study aimed to identify the risk factors for acute pancreatitis, its characteristics, and the impact on outcome in Polish children treated for ALL using two consecutive protocols, ALL IC BFM 2002 and 2009.
2. Materials and Methods
2.1. Patients Selection
Between 2005 and 2015, 2303 children (1003 girls and 1300 boys, aged 1-18; median 5.1) were diagnosed and received intensive chemotherapy according to ALL IC BFM 2002 and 2009 protocols for de novo ALL in all 16 centers of Polish Pediatric Leukemia/Lymphoma Study Group (PPLLSG). All these children were included in the study group. Treatment according to ALL IC BFM 2002 and ALL IC BFM 2009 protocols for standard-, intermediate- and high-risk groups is summarized in Supplementary
Table 1 and
Table 2. A native formulation of E. coli l-asparaginase was used front-line in both protocols at a dose of 10 000 U/m2. The frequency of ASP administration was different according to the risk group stratification. The most intensive chemotherapy was introduced in high-risk group patients. Features that continue to be used in high-risk stratification included: poor prednisone response (PPR), ≥ 5% marrow blasts on day 33, flow cytometric minimal residual disease detection (FC-MRD) on day 15 > 10% (only for ALL IC BFM 2009 protocol), and the presence of t(4;11) or t(9;22). In the case of hypersensitivity to ASP, the drug was substituted by Erwinia chrysanthemi asparaginase.
The Atlanta criteria modified for children by the INSPPIRE group were applied to diagnose AP [
16,
17]. AP was recognized when at least two out of three criteria were fulfilled:
1. abdominal symptoms suggestive of acute pancreatitis,
2. serum amylase or lipase levels three times above the upper limit of normal,
3. imaging findings: ultrasonography (USG), computer tomography (CT), or magnetic resonance imaging (MRI) suggestive of acute pancreatitis.
The study group was subdivided into patients with at least one episode of acute pancreatitis during antileukemic treatment (Group 1) and those who did not develop AP following treatment for leukemia (Group 2).
2.2. Statistical Analysis
Data were expressed and described as absolute numbers, percentages, and mean ± SD. The continuous variables between groups were compared with the use of two-tailed Mann-Whitney. The logistic regression model was applied to identify risk factors for acute pancreatitis development, and multivariate regression analysis was performed to identify multiple independent risk factors. Fisher’s exact test was used to compare unpaired, nominal variables. Kaplan-Meier method and the log-rank test were used for survival analysis.
P values of ≤ 0.05 were considered significant. Statistical analyses were performed using the “Statistica” (StatSoft, ver. 13) software.
3. Results
Acute pancreatitis was diagnosed in 94 out of 2303 patients (43 girls and 51 boys, aged 1-18; median 7.2) treated for ALL. The cumulative incidence of AP development was 4.08% (95%CI: 3.31-4.97), and the annual incidence of AP was 371/100 000 children with ALL. A total number of 62 out of 94 AP episodes (66%) occurred during induction protocol I, 6 (6.4%) during consolidation protocol M, 13 (13.8%) during reinduction protocol II, 12 (12.8%), and 1 (1%) during intensive protocols for high-risk group and maintenance treatment, respectively. 87 out of 94 acute pancreatitis episodes (92.6%) were associated with ASP therapy during protocols I, II, and HR. The median time for AP development was 37 days (range: 6-778 days, mean 102 days) from the onset of treatment.
Group 1 and Group 2 were not statistically different with respect to gender (p=0.74), therapeutic group distribution/ALL risk stratification (p=0.13), and treatment protocol (p=0.60). The univariate analysis showed that patients who developed AP during ALL treatment were significantly older (8.0±4.6 vs 6.7±4.6 years; p=0.004) and had T-lineage ALL (19.1% vs 11.8%; p<0.01). These data are presented in
Table 1.
In the logistic regression model, older age but not T-lineage phenotype retained independent influence on AP development (OR 1.05; 95%CI: 1.006-10.98; p=0.03 and OR 1.631; 95%CI: 0.937-2.839; p=0.08, respectively). The multivariable regression model showed that age was associated with AP development, with every year increasing risk-of-acute pancreatitis by 5.1%, and that the incidence of AP in children 7 years and older is significantly higher compared to younger (6.06%% vs. 2.7%; p<0.01; OR 0.437; 95%CI: 0.2873-0.664). These data are presented in
Table 2.
The distribution of acute pancreatitis across the age groups in the study cohort is presented in
Figure 1.
A total number of 43 acute and chronic complications of AP in 29 (30.9%) patients with AP was recorded. The most common were diabetes (10/94; 10.6%), pancreatic pseudocyst (9/94; 9.6%), acute peritonitis (7/94; 7.5%), haemorrhagic necrosis (6/94; 6.4%), pleural effusion (5/94; 5.3%), multiorgan failure (3/94; 3.25%) and ileus (2/94; 2.1%). Only 2 out of 94 patients with AP died from multiorgan failure directly caused by acute pancreatitis. These data are presented in
Table 3.
The risk of death in Group 1 was higher than in Group 2 (17/94 vs 232/2209; RR 1.7220; 95%CI: 1.1011-2.6929; p=0.02). The overall mortality related to AP in Group 1 was 2.13%; however, it constituted 2/17 (11.7%) of all deaths in Group 1. The overall mortality related to AP in the total cohort of 2303 patients was 0.087%. The probabilities of disease-free survival (p-DFS) and event-free survival (p-EFS) in Group 1 were not significantly different as compared to Group 2 (0.84 vs. 0.86, log-rank: p=0.65 and 0.75 vs. 0.80, log-rank: p=0.12, respectively). These data are presented in
Figure 2.
A total number of 22 out of 94 patients (23.4%) with AP after recovery were re-exposed to ASP during the subsequent phases of the treatment. There were no significant differences between patients re-exposed (ASP+) or not re-exposed (ASP-) to ASP in age at the time of ALL diagnosis (7.41±4.07 vs. 8.08±4.80; p=0.55), age at the time of AP diagnosis (7.17±3.73 vs. 8.35±4.89 years; p=0.32), male gender (59.1% vs. 52.1%; p=0.63) and T-lineage ALL phenotype (18.2% vs. 19.7%; p=0.84). The number of patients with the mild course of the first episode of acute pancreatitis was similar in both groups (15/22 vs. 48/71; p=1.00). These data are presented in
Table 4.
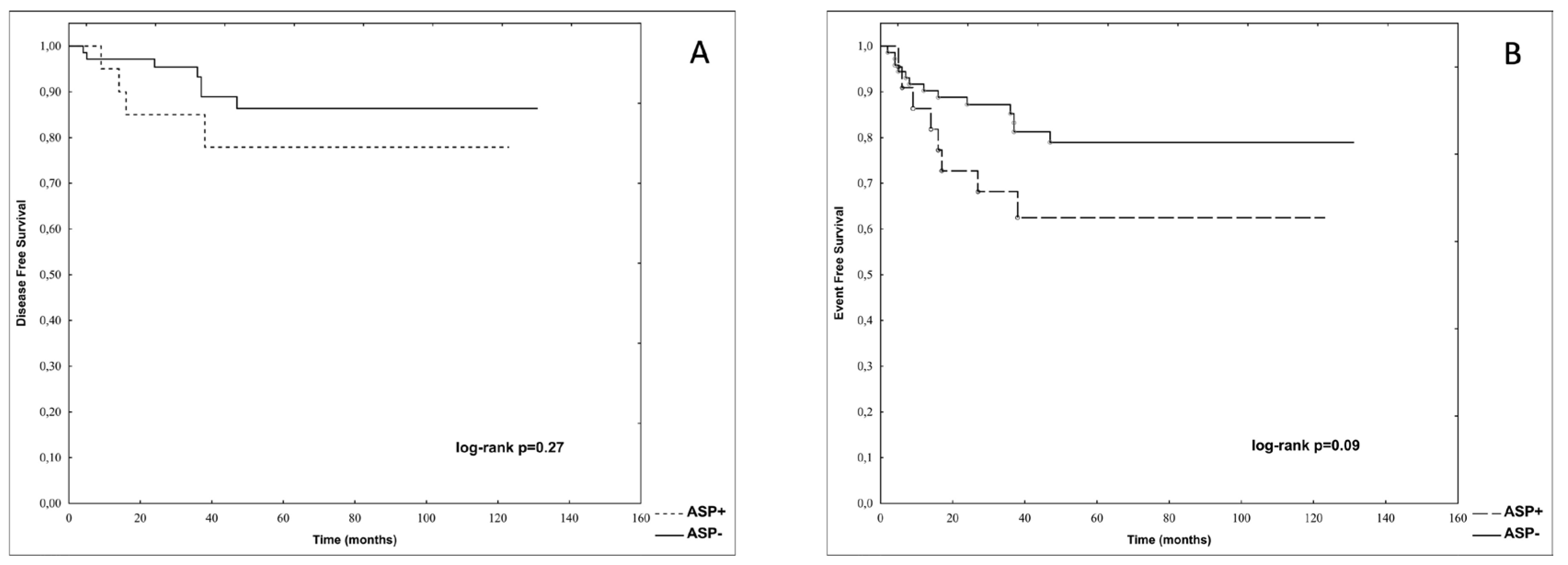
Only one out of 22 re-exposed to ASP patients (4.5%) developed a second episode of AP. There were no significant differences in p-DFS and p-EFS between patients re-exposed (ASP+) and not re-exposed to asparaginase (ASP-) after the first episode of AP (0.78 vs. 0.86, log-rank p=0.27 and 0.63 vs. 0.79, log-rank p=0.09 respectively). These data are presented in
Figure 3.
4. Discussion
Acute pancreatitis is a severe and relatively common complication of anti-leukemic treatment in children [
8,
9,
13,
18]. It is mostly, but not always, associated with asparaginase administration, an essential component of intensive therapy for acute lymphoblastic leukemia [
9,
11,
13,
15,
18]. The incidence of AP following ALL treatment reported by several studies varies with a range from 1.5 to even 18% [
14,
18,
19,
20,
21]. In the present study, the total incidence of AP in children treated for ALL was 4.08%, and asparaginase-associated AP constituted even 92.5% of them. Thus, the incidence of asparaginase-associated pancreatitis in the studied group was 3.7%. It is in contrasts to the study of Rank et al. who found that cumulative incidence of first-time asparaginase associated AP in patients with ALL was 8.3% (95% CI, 7.0 to 9.9). It is almost 2.3 times higher than in our study population [
19]. Such relatively high incidence of AP reported by Rank et al. may be explained by the fact that Rank’s study group comprised not only children but also adults. However, it is also worth to mention that contrary results were reported by Liu et al. and Samarasinghe et al. They also studied a cohort of children and young adults with ALL and found that the risk of AP development was only 2.3% and 1.5% respectively [
20,
21]. The relatively low incidence of AP in our study group may be explained that l-asparaginase was used as a standard formulation of asparaginase in the study. Alvarez et al. confirmed that using pegylated asparaginase (PEG-ASP), the drug with longer half-life, is associated with a significant increase in the frequency of pancreatitis compared to the classic formulation of l-asparaginase (18% vs. 1.9%) probably due to prolonged asparagine elimination [
11].
The time of AP occurrence was also analyzed in our study. We found that the median time to AP development was 37 days, meaning that at least 50% of AP episodes occurred after the eight doses of ASP planned for the induction phase (
Supplementary Materials no 1).
Analysis several potential risk factors for AP development, including gender, therapeutic group, treatment protocol, and leukemia immunophenotype showed that older age is the only independent risk factor for acute pancreatitis development in children with ALL and that the rate of AP in children older than six years was significantly higher compared to youngers. It is partially in line with data published by Kearney et al. They confirmed that children with ALL older than ten years are at increased risk of developing AP when compared with younger children [
22]. Similar observations were reported by others [
21]. However, it is also worth mentioning that Raja et al. found that AP development in children with ALL in the NOPHO ALL2008 protocol was not significantly associated with the patient’s age [
9].
Several studies confirm the relation of AP with ALL risk stratification. Samarasinghe et al. found that patients with high-risk ALL had a higher frequency of AP than standard-risk patients. The authors speculated that these were associated with cumulative dose of ASP since patients with high-risk ALL received higher doses of ASP [
21]. Liu et al. also confirmed that a higher cumulative dose of ASP was an independent risk factor for AP development [
20]. It is in line with the observation of Raja et al. In this study, the high-risk patients with ALL treated according to the NOPHO ALL2008 protocol received a lower cumulative dose of ASP. In this group of patients the rate of AP was also lower [
9]. On the contrary, our results did not confirm a higher frequency of AP in high-risk ALL, although patients stratified to a high-risk group received a higher cumulative dose of ASP in ALL IC BFM 2002 and 2009 protocols [
Supplementary Materials no 2].
Since using asparaginase in treating ALL improves event-free survival, its early discontinuation caused by AP or other toxicities may be associated with worse outcomes [
23,
24,
25]. Although asparaginase was discontinued in 76.6% of patients with AP in our study, both disease- and event-free survivals were comparable to patients who received remaining doses of ASP during subsequent therapy phases. It may be explained by the fact that at least 50% of all patients with AP received all eight doses of ASP planned for the induction. With remaining chemotherapeutic agents, these are probably efficient enough to achieve and maintain complete remission. It may be in line with data published by Dos Santos et al. They found that children with ALL who received less than ten doses of ASP may be at an increased risk of treatment failure only if they were in a high-risk group. However, this study did not show statistical significance for the number of asparaginase doses in children with ALL who were at standard or medium risk [
25]. These and our results may indicate that occurrence of acute pancreatitis in children with ALL is not a risk factor for leukemia relapse.
Acute pancreatitis in children may lead to severe complications, including systemic inflammatory response syndrome, multiorgan failure, shock, acute respiratory distress syndrome, severe sepsis, pancreatic necrosis, renal failure, and others [
9,
11]. Immunosuppressed cancer children are supposed to be at risk of severe course of acute pancreatitis with a high mortality rate. Although there was relatively significant morbidity in our cohort (10.6% developed diabetes mellitus, 9.6% developed pseudocyst, 5.3% pleural effusion, and 3.2% multiorgan failure), the overall mortality related to AP was 2.13% for the group of patients with AP, and only 0.089% for total cohort of children with ALL. It is in line with data published by Rank et al. [
19]. However, it should also be noted that AP related mortality reported by Wang et al. was almost three times higher (5.71%) compared to our cohort [
18].
One-fourth of the studied patients (22/94; 23.4%) with AP were re-exposed to ASP after recovery during the subsequent phases of treatment, of whom only one (1/22; 4.5%) developed a second episode of AP. These data contrast with observations of Raja et al., who found a 17% risk of a second episode of AP in patients rechallenged with ASP. [
9]. A higher incidence of AP after re-exposure to ASP was also reported by Rank et al. [
19]. They found that as many as 44% of rechallenged ASP patients developed a second episode of AP. The higher rate of a second episode of AP in the study of Rank et al. might be partially explained by the fact that they used PEG-ASP formulation in their study [
19]. The reintroduction of ASP after the resolution of AP in children with ALL was also studied by Kearney et al. [
22]. They found that 63% of children re-exposed to ASP developed a second episode of AP. It is worth mentioning that in all of these studies the course of a second AP was in most of patients mild [
9,
19,
22].
Although ASP was withdrawn in 76.6% of patients after the first episode of AP, there were no significant differences in disease-free and event-free survival in these patients compared to those re-exposed to ASP. It is in line with the results of Samarasinghe et al., who found that ASP withdrawal due to AP did not affect event-free and overall survival in children and young adults with ALL [
21]. It should also be underlined that it contrasts with data published by Silverman et al., who found lower event-free survival in childhood ALL after discontinuation of ASP due to its toxicity [
24]. Considering all these data, the question if whether discontinuation of ASP after the first episode of AP may influence event-free or disease-free survival remains unanswered.
The main strength of this study is its multicenter design, which included the enrollment of more than 2000 uniformly treated consecutively diagnosed children with acute lymphoblastic leukemia. To our knowledge, this is one of the biggest studies comprising such a large group (2303) of children with ALL. It makes us believe that the results are reliable.
The limitations include the retrospective study design and the sample size of re-exposed ASP patients, which limits definitive conclusions regarding disease-free and event-free survival in this group of ALL patients. Due to the small sample size (only one patient developed a second episode of AP), it was impossible to analyze potential risk factors for a second AP development.
5. Conclusions
In summary, we demonstrated that the incidence of acute pancreatitis in children treated for acute lymphoblastic leukemia is relatively low and is related to the patient’s age.
We also demonstrated that the development of AP during chemotherapy and the truncation of AP doesn’t impact disease-free and event-free survival.
We also found that the recurrence of AP in rechallenged patients and overall mortality related to AP in children with ALL is low.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary Table 1: Treatment for standard-, intermediate- and high-risk groups according to ALL IC BFM 2002 protocol.; Supplementary Table 2: Treatment for standard-, intermediate- and high-risk groups according to ALL IC BFM 2009 protocol.
Author Contributions
Conceptualization, T.O., A.M.; methodology, T.O., A.M., T.U.; software, T.O., A.M., K.S.; validation, T.O., A.M., K.S., T.U.; formal analysis, K.S.; investigation, M.S-B., A.K., K.K., J.O-L., J.K., J.Z., T.S., N. I-J., E. A-D., K.A., A. S-B., W.B., M.C., J.W., K.D., W.M., B.Z-S., M.K-R., M.S-Ż., J.S., A.K., T.O., A.M.; resources, A.M., T.U.; data curation, A.M., T.O.; writing—original draft preparation, T.O., A.M., T.U.; writing—review and editing, M.S-B., A.K., K.K., J.O-L., J.K., J.Z., T.S., N. I-J., E. A-D., M.M., K.A., A. S-B., W.B., M.C., J.W., K.D., W.M., B.Z-S., M.K-R., M.S-Ż., J.S., A.K., T.U.; visualization, T.O., A.M.; supervision, T.O., T.U.; project administration, T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived since the character of the study was noninterventional, observational and retrospective.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010, 36, 277–85. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; Goulden, N.; Wade, R.; Mitchell, C.; Hancock, J.; Hough, R.; Rowntree, C.; Richards, S. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2013, 14, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Pei, D.; Campana, D.; Cheng, C.; Sandlund, J.T.; Bowman, W.P.; Hudson, M.M.; Ribeiro, R.C.; Raimondi, S.C.; Jeha, S.; Howard, S.C.; Bhojwani, D.; Inaba, H.; Rubnitz, J.E.; Metzger, M.L.; Gruber, T.A.; Coustan-Smith, E.; Downing, J.R.; Leung, W.H.; Relling, M.V.; Evans, W.E. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014. [CrossRef] [PubMed]
- Zawitkowska, J.; Lejman, M.; Romiszewski, M.; Matysiak, M.; Ćwiklińska, M.; Balwierz, W.; Owoc-Lempach, J.; Kazanowska, B.; Derwich, K.; Wachowiak, J.; Niedźwiecki, M.; Adamkiewicz-Drożyńska, E.; Trelińska, J.; Młynarski, W.; Kołtan, A.; Wysocki, M.; Tomaszewska, R.; Szczepański, T.; Płonowski, M.; Krawczuk-Rybak, M.; Urbańska-Rakus, J.; Machnik, K.; Ociepa, T.; Urasiński, T.; Mizia-Malarz, A.; Sobol-Milejska, G.; Karolczyk, G.; Kowalczyk, J. Results of two consecutive treatment protocols in Polish children with acute lymphoblastic leukemia. Sci Rep. 2020, 10, 20168. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.W.; Devidas, M.; Wang, C.; Mattano, L.A.; Friedmann, A.M.; Buckley, P.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; Kadan-Lottick, N.; Loh, M.L.; Matloub, Y.H.; Marshall, D.T.; Stork, L.C.; Raetz, E.A.; Wood, B.; Hunger, S.P.; Carroll, W.L.; Winick, N.J. Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children's Oncology Group Trial AALL0331. J Clin Oncol. 2020, 38, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, N.S.; Keegan, T.H.M.; Li, Q.C.; Chung, J.H.; Rosenthal, J.L.; Winestone, L.E.; Muffly, L.; Malogolowkin, M.H.; Alvarez, E.M. Treatment-related toxicities associated with hospitalization in children, adolescents, and young adults with acute lymphoblastic leukemia: Population level analysis. Leuk Lymphoma. [CrossRef]
- O'Connor, D.; Bate, J.; Wade, R.; Clack, R.; Dhir, S.; Hough, R.; Vora, A.; Goulden, N.; Samarasinghe, S. Infection-related mortality in children with acute lymphoblastic leukemia: An analysis of infectious deaths on UKALL2003. Blood 2014, 124, 1056–61. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.A. , Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. 2012 Oct;159(1):18-27. [CrossRef] [PubMed]
- Raja RA, Schmiegelow K, Albertsen BK, Prunsild K, Zeller B, Vaitkeviciene G, Abrahamsson J, Heyman M, Taskinen M, Harila-Saari A, Kanerva J, Frandsen TL; Nordic Society of Paediatric Haematology and Oncology (NOPHO) group. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the NOPHO ALL2008 protocol. Br J Haematol. 2014 Apr;165(1):126-33. [CrossRef]
- Hijiya, N.; van der Sluis, I.M. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016, 57, 748–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez, O.A.; Zimmerman, G. Pegaspargase-induced pancreatitis. Med Pediatr Oncol. 2000, 34, 200–5. [Google Scholar] [CrossRef] [PubMed]
- Knoderer, H.M.; Robarge, J.; Flockhart, D.A. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2007, 49, 634–9. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.; Hernandez, C.; Tejada, F.N.H.; Kawedia, J.; Rytting, M.; Cuglievan, B. Asparaginase-Associated Pancreatitis in Pediatric Patients with Acute Lymphoblastic Leukemia: Current Perspectives. Paediatr Drugs. 2021, 23, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Morin, C.E.; Wheeler, C.A.; Guo, Y.; Li, Y.; Jeha, S.; Inaba, H.; Pui, C.H.; Karol, S.E.; McCarville, M.B. Ultrasound has limited diagnostic utility in children with acute lymphoblastic leukemia developing pancreatitis. Pediatr Blood Cancer. 2021, 68, e28730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolthers, B.O.; Frandsen, T.L.; Baruchel, A.; Attarbaschi, A.; Barzilai, S.; Colombini, A.; Escherich, G.; Grell, K.; Inaba, H.; Kovacs, G.; Liang, D.C.; Mateos, M.; Mondelaers, V.; Möricke, A.; Ociepa, T.; Samarasinghe, S.; Silverman, L.B.; van der Sluis, I.M.; Stanulla, M.; Vrooman, L.M.; Yano, M.; Zapotocka, E.; Schmiegelow, K.; Ponte di Legno Toxicity Working Group. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: An observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017, 18, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Morinville, V.D.; Husain, S.Z.; Bai, H.; et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr.
- Uc, A.; Sohail, Z.H. Pancreatitis in Children. Gastroenterology 2019, 156, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, W.G.; Yang, L.H.; Kuang, W.Y.; Huang, L.B.; Chen, H.Q.; Wang, L.N.; Zhou, D.H.; Liao, N. Clinical summary of pediatric acute lymphoblastic leukemia patients complicated with asparaginase-associated pancreatitis in SCCLG-ALL-2016 protocol. Hematology. 2023, 28, 2171723. [Google Scholar] [CrossRef] [PubMed]
- Rank, C.U.; Wolthers, B.O.; Grell, K.; Albertsen, B.K.; Frandsen, T.L.; Overgaard, U.M.; Toft, N.; Nielsen, O.J.; Wehner, P.S.; Harila-Saari, A.; Heyman, M.M.; Malmros, J.; Abrahamsson, J.; Norén-Nyström, U.; Tomaszewska-Toporska, B.; Lund, B.; Jarvis, K.B.; Quist-Paulsen, P.; Vaitkevičienė, G.E.; Griškevičius, L.; Taskinen, M.; Wartiovaara-Kautto, U.; Lepik, K.; Punab, M.; Jónsson, Ó.G.; Schmiegelow, K. Asparaginase-Associated Pancreatitis in Acute Lymphoblastic Leukemia: Results From the NOPHO ALL2008 Treatment of Patients 1-45 Years of Age. J Clin Oncol. 2020, 38, 145–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Yang, W.; Devidas, M.; Cheng, C.; Pei, D.; Smith, C.; Carroll, W.L.; Raetz, E.A.; Bowman, W.P.; Larsen, E.C.; Maloney, K.W.; Martin, P.L.; Mattano LAJr Winick, N.J.; Mardis, E.R.; Fulton, R.S.; Bhojwani, D.; Howard, S.C.; Jeha, S.; Pui, C.H.; Hunger, S.P.; Evans, W.E.; Loh, M.L.; Relling, M.V. Clinical and Genetic Risk Factors for Acute Pancreatitis in Patients With Acute Lymphoblastic Leukemia. J Clin Oncol. 2016, 34, 2133–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samarasinghe, S.; Dhir, S.; Slack, J.; Iyer, P.; Wade, R.; Clack, R.; Vora, A.; Goulden, N. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013, 162, 710–3. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.L.; Dahlberg, S.E.; Levy, D.E.; Voss, S.D.; Sallan, S.E.; Silverman, L.B. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2009, 53, 162–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valtis, Y.K.; Flamand, Y.; Shimony, S.; Place, A.E.; Silverman, L.B.; Vrooman, L.M.; Brunner, A.M.; Sallan, S.E.; Wadleigh, M.; Stone, R.M.; DeAngelo, D.J.; Luskin, M.R. Treatment completion, asparaginase completion, and oncologic outcomes among children, adolescents and young adults with acute lymphoblastic leukemia treated with DFCI Consortium Protocols. Leukemia. [CrossRef] [PubMed]
- Silverman, L.B.; Gelber, R.D.; Dalton, V.K.; Asselin, B.L.; Barr, R.D.; Clavell, L.A.; Hurwitz, C.A.; Moghrabi, A.; Samson, Y.; Schorin, M.A.; Arkin, S.; Declerck, L.; Cohen, H.J.; Sallan, S.E. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol 91-01. Blood 2001, 97, 1211–8. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.C.; Dos Santos, J.M.B.; da Costa Lima, E.; Land, M.G.P. L-asparaginase doses number as a prognostic factor in childhood acute lymphoblastic leukemia: A survival analysis study. Cancer Rep (Hoboken). 2022, 5, e1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).