Submitted:
21 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
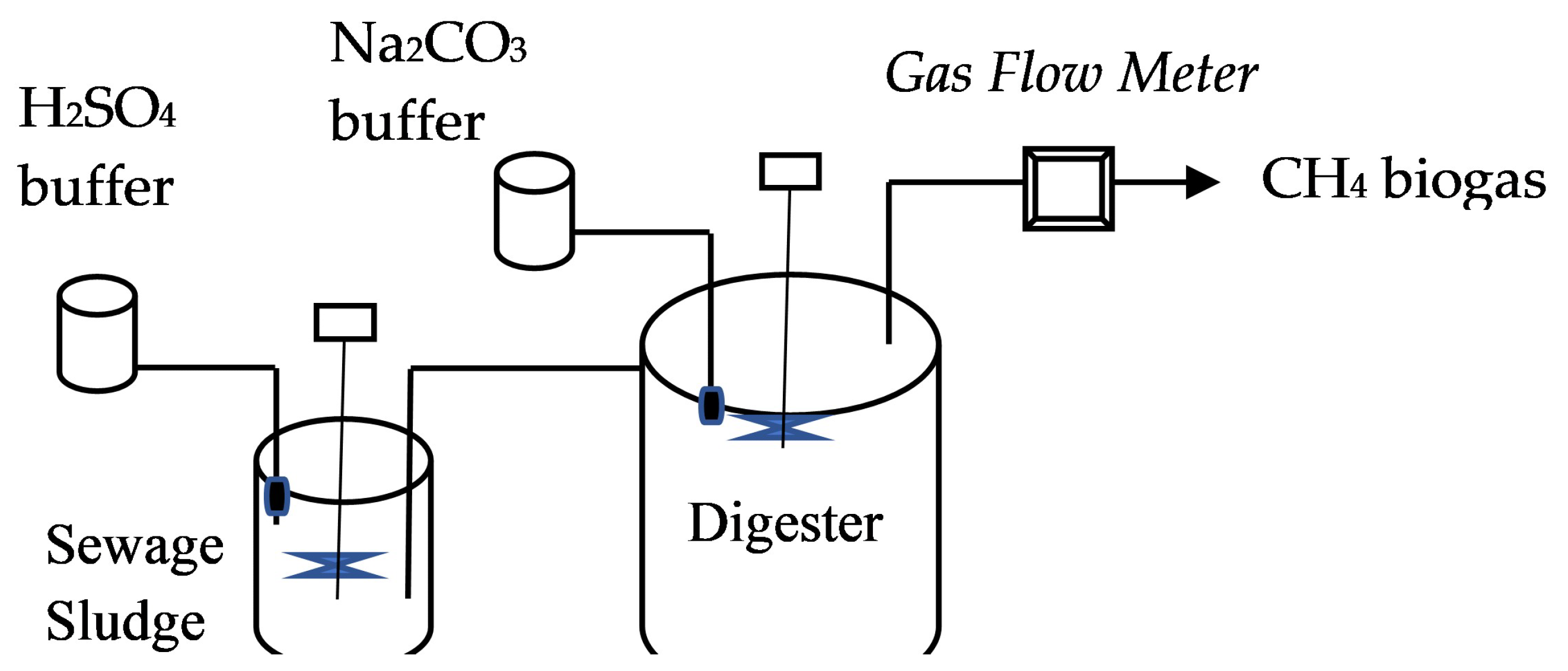
2.1. Substrate and Co-Substrate Preparation
2.2. Experimental Design
2.3. Statistical Analyses
3. Results
Effect of Sewage Sludge and Co-Substrate
- Biogas production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Romulo, H.G.J.; Jovani, T.S.; Fabio, N.P.; Cassiano, M.P.; Antonio, C.F. Biodigester location problems, its economic-environmental-social aspects and techniques: Areas yet to be explored. Energy Reports 2021, 7, 3998–4008. [Google Scholar]
- Díaz, I.; Pérez, S.; Ferrero, E.; Fdz-Polanco, M. Effect of oxygen dosing point and mixing on the microaerobic removal of hydrogen sulphide in sludge digesters. Bioresour. Technol. 2011, 102, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Biogas production: current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Oliveira, V.A.; Almeida, F.T.S.; Marotta, H.; Guiller, J.F.M.; Aparecida, C.F.R.; Fatima, D.C.; Batista, S.F. New compact biodigester model for organic waste treatment in urban residences and buildings. J. Environ. Eng. 2021, 147(2), 04020156. [Google Scholar]
- Wang, X.; Guo, M.; Koppelaar, R.H.E.M.; van Dam, K.H.; Triantafyllidis, C.P.; Shah, N. A Nexus Approach for Sustainable Urban Energy-Water-Waste Systems Planning and Operation. Environ. Sci. Technol. 2018, 52, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Fuso Nerini, F.; Tomei, J.; To, L.S.; Bisaga, I.; Parikh, P.; Black, M.; Borrion, A.; Spataru, C.; Castán Broto, V.; Anandarajah, G.; et al. Mapping synergies and trade-offs between energy and the Sustainable Development Goals. Nat. Energy 2018, 3, 10–15. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef] [PubMed]
- Loizia, P.; Neofytou, N.; Zorpas, A.A. The concept of circular economy strategy in food waste management for the optimization of energy production through anaerobic digestion. Environ. Sci. Pollut. Res. 2019, 26, 14766–14773. [Google Scholar] [CrossRef]
- Ribić, B.; Voća, N.; Ilakovac, B. Concept of sustainable waste management in the city of Zagreb: Towards the implementation of circular economy approach. J. Air Waste Manag. Assoc. 2016, 67, 241–259. [Google Scholar] [CrossRef]
- Langer, S.G.; Gabris, C.; Einfalt, D.; Wemheuer, B.; Kazda, M.; Bengelsdorf, F.R. Different response of bacteria, archaea and fungi to process parameters in nine full-scale anaerobic digesters. Microb. Biotechnol. 2016, 12, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Seiple, T.E.; Coleman, A.M.; Skaggs, R.L. Municipal wastewater sludge as a sustainable bioresource in the United States. J. Environ. Manag. 2017, 197, 673–680. [Google Scholar] [CrossRef] [PubMed]
- SEMARNAT. Informe del Medio Ambiente. Sistema Nacional de Información Ambiental y de Recursos Naturales 2018. https://apps1.semarnat.gob.mx:8443/dgeia/informe18/tema/cap6.html.
- Kiselev, A.; Magaril, E.; Magaril, R.; Panepinto, D.; Ravina, M.; Zanetti, M.C. Towards Circular Economy: Evaluation of Sewage Sludge Biogas Solutions. Resources 2019, 8, 91. [Google Scholar] [CrossRef]
- Mattioli, A.; Gatti, G.; Mattuzzi, G.; Cecchi, F.; Bolzonella, D. Co-digestion of the organic fraction of municipal solid waste and sludge improves the energy balance of wastewater treatment plants: Rovereto case study. Renew. Energy 2017, 113, 980–988. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Campos-Garcia, S.; Zavala-Loria, J.; Canedo-Lopez, Y. Hydrological aspects of the lagoons of Atasta and Pom, Mexico, Tropical and Subtropical Agroecosystems 2009, 10,63-74.
- Salgado, L.D.; Marques, A.E.M.L.; Kramer, R.D.; de Oliveira, F.G.; Moretto, S.L.; de Lima, B.A.; Prodocimo, M.M.; Cestari, M.M.; de Azebedo, J.C.R.; de Assis, H.C.S. Integrated assessment of sediment contaminant levels and biological responses in sentinel fish species Atherinella brasiliensis from a sub-tropical estuary in south Atlantic. Chemosphere 2018, 219, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana NOM-021-RECNAT-2000, que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreo y análisis. Diario Oficial de La Federación 2002.
- Norma Mexicana. NMX-AA-034-SCFI-2015. Análisis de Agua - Medición de Sólidos y Sales Disueltas en Aguas Naturales, Residuales y Residuales Tratadas - Método de Prueba. Diario Oficial de La Federación 2015. https://www.gob.mx/cms/uploads/attachment/file/166146/nmx-aa-034-scfi-2015.pdf.
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Yen, H.-W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Li, F.; Feng, Y.; Ren, G.; Han, X. Evaluation of two statistical methods for optimizing the feeding composition in anaerobic co-digestion: Mixture design and central composite design. Bioresour. Technol. 2013, 131, 172–178. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Dokianakis, S.N.; Stamatelatou, K.; Zafiri, C.; Kornaros, M. Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 2009, 248, 891–906. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process. Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Chan, P.C.; Lu, Q.; de Toledo, R.A.; Gu, J.-D.; Shim, H. Improved anaerobic co-digestion of food waste and domestic wastewater by copper supplementation – Microbial community change and enhanced effluent quality. Sci. Total. Environ. 2019, 670, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Aslanzadeh, S.; Johansson, F.; Taherzadeh, M.J. Experimental and economical evaluation of a novel biogas digester. Energy Convers. Manag. 2013, 74, 183–191. [Google Scholar] [CrossRef]
- Nwaigwe, K.N.; Enweremadu, C.C. Comparative análisis of a locally developed biogas digester using selected substrates. In Vol. 1 of proc., ASME 2016 10th Int. Con fon Energy Sustainability and 14th Int. Conf. on Fuel Cell Science. Engineering and Technology. New York. ASTM.
- Walker, M.; Theaker, H.; Yaman, R.; Poggio, D.; Nimmo, W.; Bywater, A.; Blanch, G.; Pourkashanian, M. Assessment of micro-scale anaerobic digestion for management of urban organic waste: A case study in London, UK. Waste Manag. 2017, 61, 258–268. [Google Scholar] [CrossRef]
| Reactor | Clue | Treatment |
|---|---|---|
| Substrate (control) | SLB50 | Biological sludge (Efficiency 50%) |
| Substrate (control) | SLB90 | Biological sludge (Efficiency 90%) |
| Co-substrate 1 | CEV50 | SLB50 + cattle manure (CEV) |
| Co-substrate 1 | CEV90 | SLB90 +cattle manure (CEV) |
| Co-substrate 2 | CAV50 | SLB50 +recovered vegetable oil (CAV) |
| Co-substrate 2 | CAV90 | SLB90 + recovered vegetable oil (CAV) |
| Control factors | Units |
|---|---|
| pH | 6-8 |
| Mixture (sludge: water) | 1:1 |
| Co-substrate | 25% |
| Agitation | 120 rpm |
| Hydraulic holding time | 30 days |
| Initial C/N | ~20 |
| Temperature | 20-30 °C |
| Parameter | Units | SLB90 | SLB50 | CEV |
|---|---|---|---|---|
| pH | - | 7.04± 0.084 | 7.30± 0.014 | 7.4± 0.091 |
| ST | g L-1 | 35.58± 0.056 | 44.91± 0.056 | 156± 0.02 |
| SV | g L-1 | 24.24± 0.339 | 11.56± 0.346 | 32.5± 0.02 |
| C.O | % | 18.82± 0.542 | 8.22± 0.433 | 18.99± 0.051 |
| M.O | % | 32.46± 0.362 | 14.17± 0.544 | 32.74± 0.089 |
| N.T | % | 20.11± 0.829 | 11.46± 1.965 | 1.11± 0.0003 |
| P.T | g kg-1 | 1.76± 0.016 | 1.74± 0.001 | 10.97± 0.001 |
| C/N | - | 3.080 | 0.558 | 17.01 |
| Parameter | Units | CEV90 | CEV50 | CAV90 | CAV50 |
|---|---|---|---|---|---|
| pH | - | 7.04± 0.084 | 7.30± 0.014 | 7.4± 0.091 | 7.4± 0.091 |
| ST | g L-1 | 27.85± 0.026 | 26.46± 0.056 | 22.87± 0.02 | 56.21± 0.02 |
| SV | g L-1 | 11.56± 0.029 | 23.15± 0.034 | 21.81± 0.02 | 45.16± 0.02 |
| C.O | % | 23.39± 0.054 | 17.56± 0.423 | 18.55± 0.051 | 12.46± 0.051 |
| M.O | % | 40.34± 0.32 | 30.28± 0.544 | 31.99± 0.089 | 21.48± 0.089 |
| N.T | % | 2.52± 0.082 | 18.12± 0.465 | 4.74± 0.06 | 21.48± 0.06 |
| C/N | - | 23.16 | 6.26 | 6.16 | 0.58 |
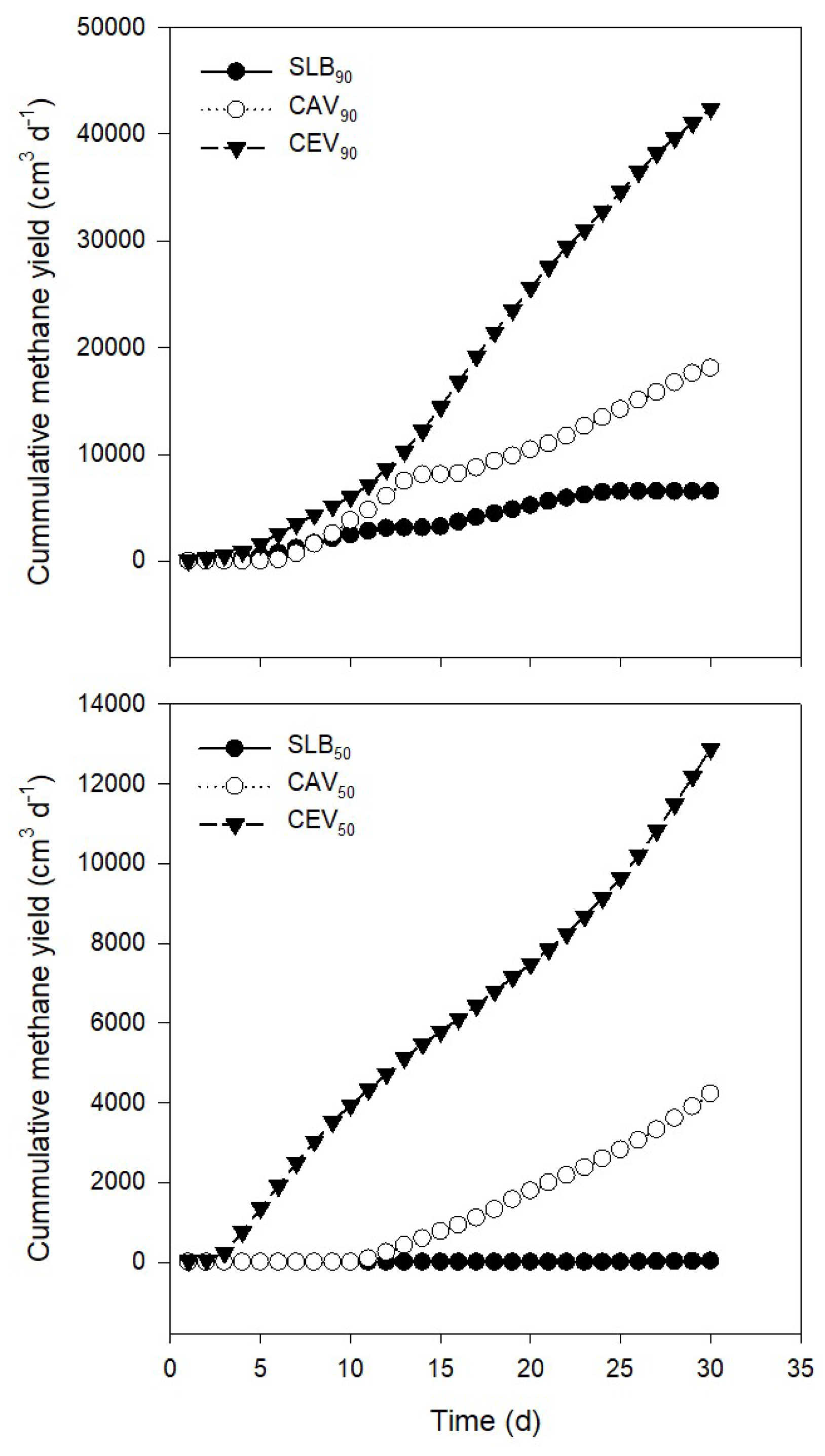
| Day | SLB90 | CAV90 | CEV90 | SLB50 | CAV50 | CEV50 |
|---|---|---|---|---|---|---|
| 1 | 9.10 | 66.96 | 26.94 | |||
| 2 | 13.94 | 223.72 | 26.87 | |||
| 3 | 65.52 | 239.90 | 175.42 | |||
| 4 | 186.01 | 375.6 | 534.33 | |||
| 5 | 177.22 | 701.76 | 589.48 | |||
| 6 | 307 | 141.02 | 971.66 | 566.08 | ||
| 7 | 455.58 | 574.89 | 925.34 | 571.01 | ||
| 8 | 466.47 | 865.53 | 838.56 | 547.66 | ||
| 9 | 409.05 | 945.45 | 820.22 | 481.64 | ||
| 10 | 360.55 | 1266.76 | 870.57 | 420.98 | ||
| 11 | 350.26 | 969.79 | 1097.08 | 88.01 | 390.24 | |
| 12 | 365.31 | 1305.41 | 1497.52 | 155.81 | 396.11 | |
| 13 | 344.65 | 1410.62 | 1670.73 | 174.07 | 399.07 | |
| 14 | 390.62 | 621.79 | 1909.77 | 175.55 | 343.15 | |
| 15 | 413.67 | 619.52 | 2232.19 | 172.69 | 317.50 | |
| 16 | 435.88 | 599.02 | 2325.74 | 165.21 | 321.53 | |
| 17 | 428.84 | 523.44 | 2425.05 | 173.94 | 326.26 | |
| 18 | 388.91 | 636.09 | 2255.23 | 214.02 | 364.84 | |
| 19 | 342 | 498.14 | 2066.54 | 250.32 | 348.62 | |
| 20 | 379.19 | 569.66 | 2097.6 | 219.91 | 333.18 | |
| 21 | 384.21 | 540.48 | 1944.67 | 196.62 | 362.03 | |
| 22 | 315.9 | 726.28 | 1873.15 | 187.69 | 403.05 | |
| 23 | 296.19 | 929.47 | 1609.2 | 198.19 | 430.84 | |
| 24 | 234.04 | 784.51 | 1742.88 | 223.74 | 463.14 | |
| 25 | 88.07 | 782.4 | 1836.52 | 1.062 | 222.53 | 498.18 |
| 26 | 0.74 | 865.63 | 1853.66 | 2.843 | 235.04 | 569.71 |
| 27 | - | 742.56 | 1747.72 | 4.549 | 269.82 | 626.36 |
| 28 | - | 893.47 | 1427.95 | 5.628 | 283.32 | 654.67 |
| 29 | - | 881.76 | 1416.91 | 6.229 | 291.48 | 699.17 |
| 30 | - | 489.50 | 1358.4 | 5.68 | 330.54 | 693.39 |
| ∑CH4 | 7599.82a | 19183.19b | 42422.8c | 25.99a | 4228.5a | 12881.45ab |
| Media | 303.99 | 767.32 | 1414.09 | 4.33 | 211.42 | 429.38 |
| SD | 149.39 | 344.37 | 676.62 | 2.00 | 55.53 | 167.04 |
| Digestion | SLB90 | CAV90 | CEV90 | [28] | [29] | [30] | [5] |
|---|---|---|---|---|---|---|---|
| Predominant substrate |
Waste activated sludge | waste edible oil |
Waste activated sludge |
Municipal solid waste | Vegetable waste |
Food waste |
Food waste |
| Inoculum |
- | Waste activated sludge | Cow manure |
Cow manure |
Cow manure |
Cattle slurry | Bovine manure |
| Biogas yield (m3 d-1) |
7.59x10-3 | 19.18x10-3 | 42.42 x10-3 | 9.3x10-3 56.95x10-3 |
0.005 x 10-3 | 3.16 | 11.83 x 10-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





