1. Introduction
Pulmonary embolism (PE) represents a potentially life-threatening condition charac- by the obstruction of pulmonary arteries by venous embolus or in-situ thrombosis. diagnosis of PE, assessment of severity and risk of mortality is crucial for hospi- 18 therapeutic decision-making.
High-risk PE is defined by the existence of hemodynamic instability, and is usually accompanied by severe clinical parameters, elevated cardiac biomarkers and right ventricu- lar dysfunction (assessed by echocardiography or computed tomography). High-risk PE denotes a critical condition where the obstruction significantly compromises blood flow to the lungs, leading to severe complications, and ultimately, death if not promptly addressed.
Management of high-risk PE necessitates a multidisciplinary approach involving emergency physicians, cardiologists, pulmonologists, and critical care specialists. Aside from parenteral anticoagulation, immediate goals include stabilizing the patient’s hemody- namics through interventions like systemic thrombolysis, catheter-directed thrombolysis, or surgical embolectomy. Additionally, supportive measures such as oxygen therapy, fluid resuscitation, and vasopressor support may be essential to maintain adequate perfu- sion and oxygenation. Systemic thrombolysis leads to faster improvements compared to parenteral anticoagulation alone. The preferred thrombolytic agent is rtPA, followed by first-generation agents such as streptokinase or urokinase in case unavailability of rtPA.
The particularity of our study is the off-label use of Tenecteplase, a thrombolytic drug widely used in acute coronary syndrome, with results showing efficacy and tolerance comparable to other molecules.
2. Materials and Methods
This is a retrospective study conducted in our health care structure, from January 2022 to January 2023, involving 3 patients admitted for management of high-risk PE who benefited from Tenecteplase in the setting of unavailability of rtPA.
We will discuss the indications of thrombolysis in severe high-risk pulmonary em- bolism, demonstrating its effectiveness and its contribution in early pulmonary revas-cularization, as well as the efficacy and safety of Tenecteplase in the same way as other thrombolytics with a review of the literature.
3. Results
3.1. Case 1:
A 65 years old woman, with a background of menopause cholecystectomy 17 years ago, presented with dry cough, chest pain and resting dyspnea (stage 4 of the NYHA classification), treated with symptomatic medications one week with no improvement. The clinical examination on admission found a polypneic patient at 28 c/min, oxygen saturation at 90% on room air, a heart rate at 100bpm, a blood pressure at 100/60 mmHg and exaggerated S2 sound on auscultation of the pulmonary focus.
The chest X-ray was normal. ECG showed sinus tachycardia, left deviated axis, S1Q3 pattern, an incomplete right bundle branch block, ST-segment depression in inferior and lateral leads, as well ST-elevation in aVR. Labs results showed elevated cardiac biomarkers (BNP and troponin). A TTE was performed, showing moderate dilatation of the right cham- bers, with severe right ventricular dysfunction, paradoxical motion of the interventricular septum and pulmonary hypertension (estimated SPAP at 70mmHg.)
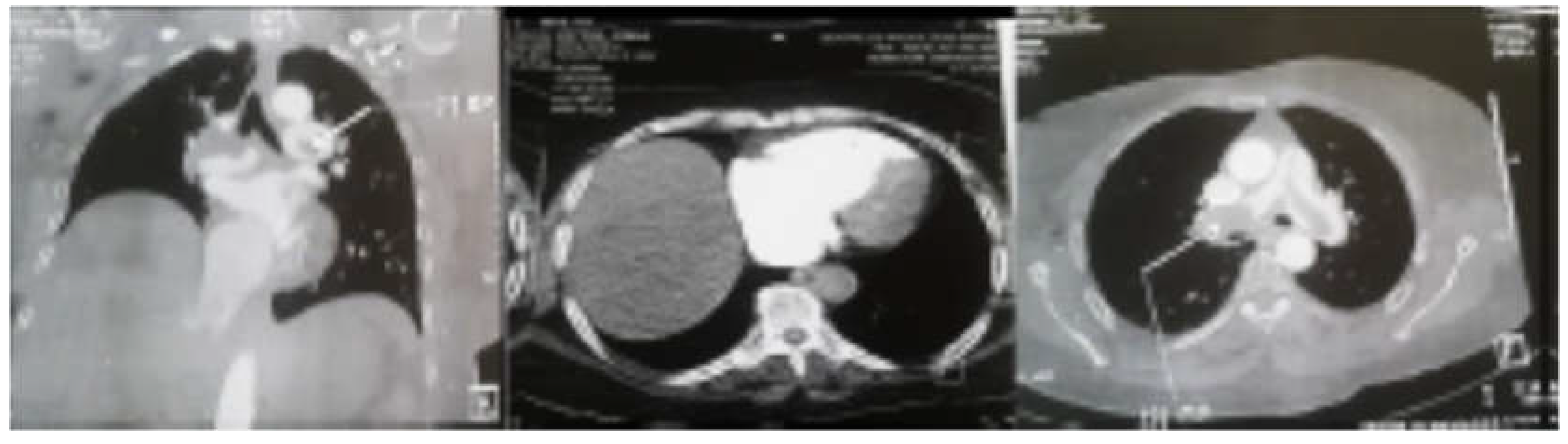
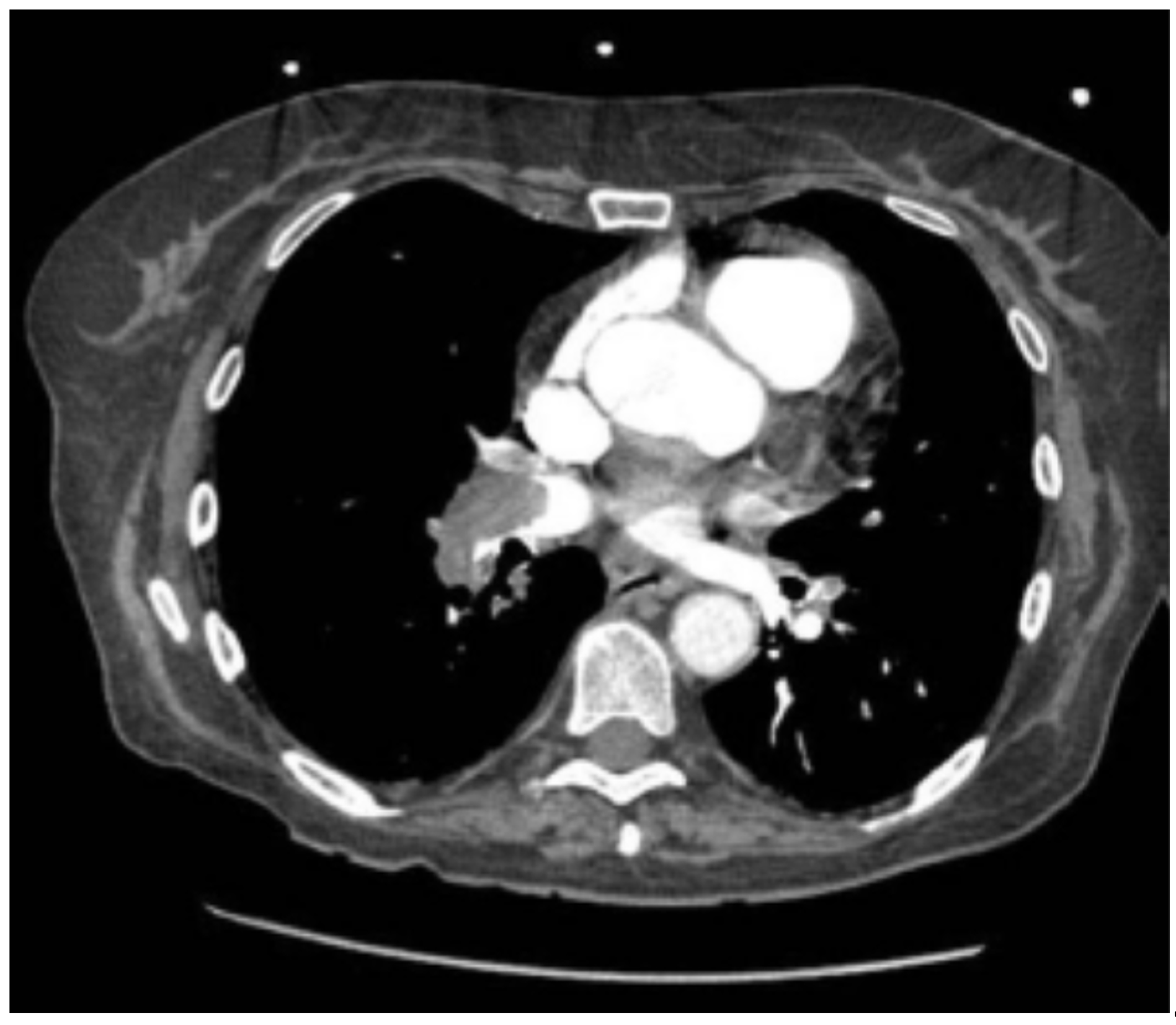
Thoracic CT angiography revealed massive bilateral pulmonary embolism with right chamber involvement and signs of right heart failure. (
Figure 1)
UFH was initiated. 2 hours after her admission in the intensive care unit, the patient showed prolonged hypotension. Response to fluid expansion was negative. Systemic thrombolysis was indicated, and Tenecteplase was administrated in one dose of 0.5mg/kg. 40 minutes later, the hemodynamic improvement was significant, blood pressure raised at 120/80, oxygen saturation at 100%, and without hemorrhagic complications.
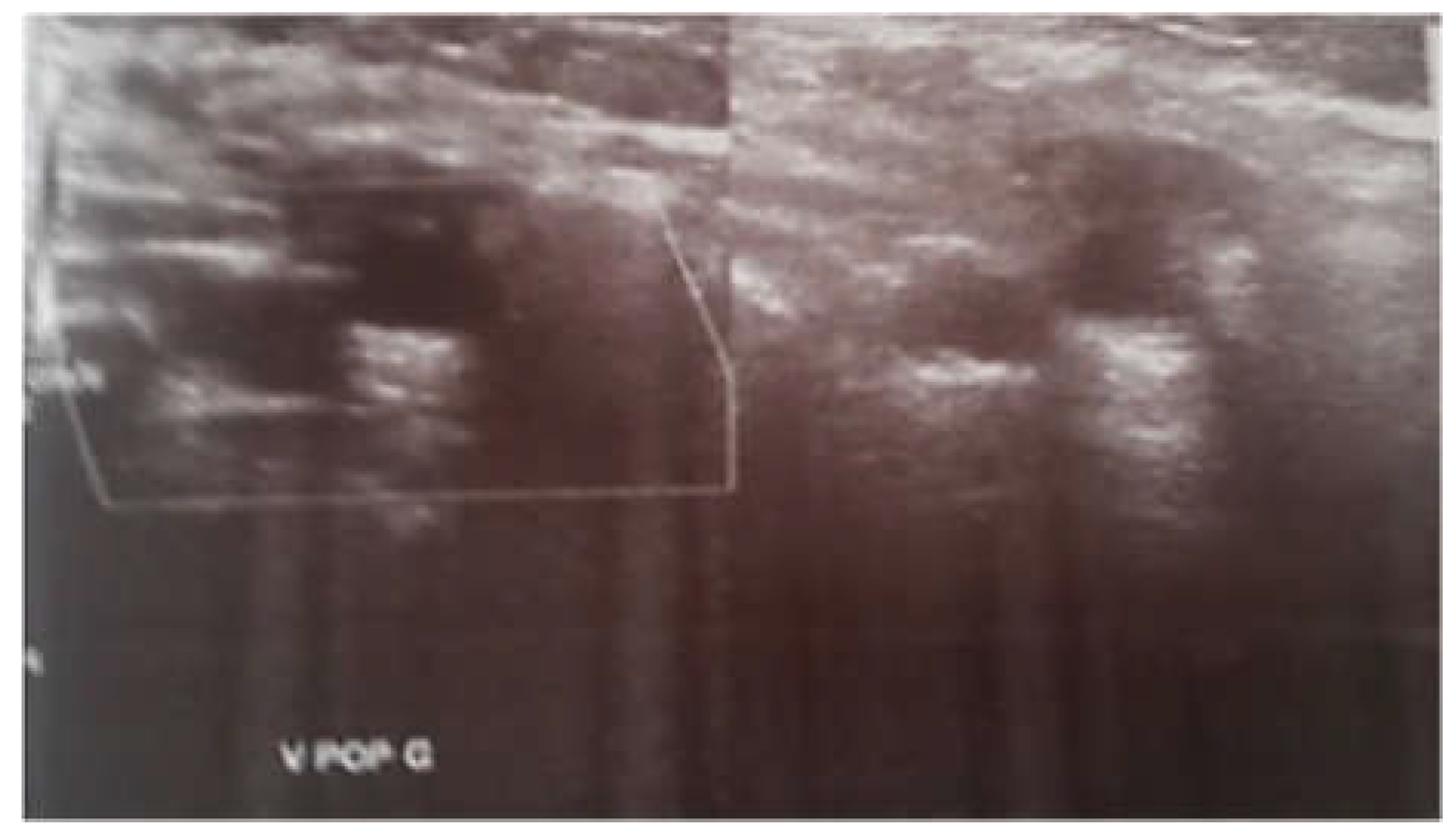
The etiologic workup revealed a left femoral and popliteal deep vein thrombosis. (
Figure 2) The rest of the etiological workup was unremarkable. The patient was discharged later on VKA. On follow-up consultation after 1 month, the patient was asymptomatic with regression of dyspnea and improvement in RV function on cardiac ultrasound.
3.2. Case 2:
A 26-year-old obese patient, with a history of clinical depression on antidepressants, was admitted for syncope and dyspnea (Stage III of NYHA classification)
The physical examination was unremarkable and the labs check-up was normal. The electrocardiogram showed a sinus tachycardia at 105 bpm. A transthoracic ultrasound showed signs of right ventricular pressure overload and pulmonary hypertension.
A thoracic CT angiography revealed massive bilateral proximal pulmonary embolism, which urged hospitalization in intensive care and the initiation of UFH therapy. (
Figure 3)
The follow-up was marked by clinical worsening and obstructive shock signs (persis- tent hypotension at 90/50 mm Hg, sweating, neurological deterioration, distended jugular vein).
Thrombolysis was indicated for high-risk PE, and Tenecteplase was administrated at a dose of 0.5 mg/kg. Clinical improvement was remarkable after 1 hour, and patient was continued on UFH without the need for vasoactive drugs.
Later thrombophilia screening returned positive. The patient was referred to internal medicine for further treatment and anticoagulation.
3.3. Case 3:
A 56 years old woman, with a history of type 2 diabetes, hypertension, obesity, and menopause, admitted to the emergency room for acute chest pain associated with acute dyspnea (stage 3 of the NYHA classification). Clinical examination on admission was unremarkable.
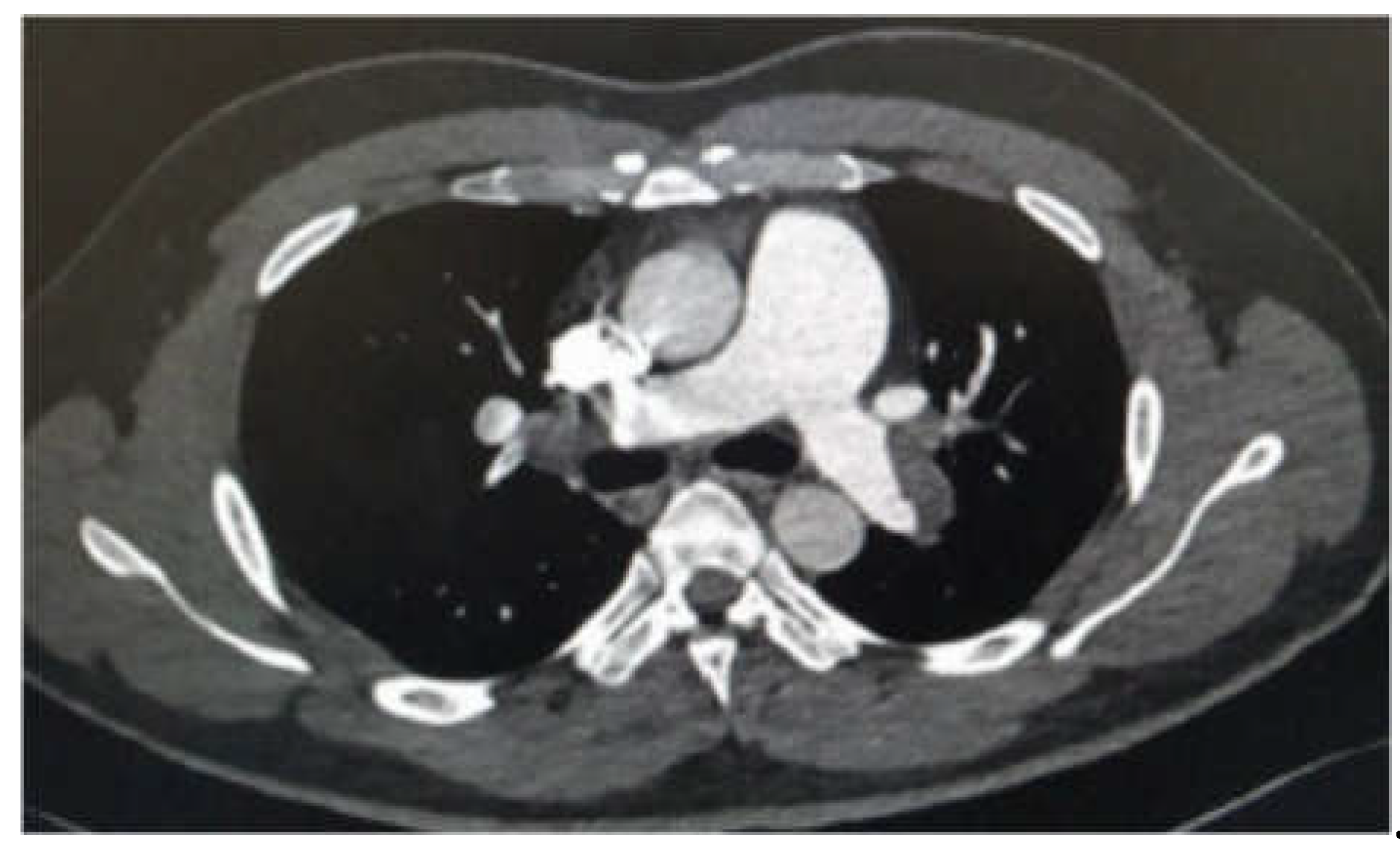
Chest X-ray was normal, and the Electrocardiogram showed sinus tachycardia at 110 bpm with inverted negative T waves in the anteroseptal and inferior precordial leads. Labs results showed D-dimer level at 1700 ng/ml and negative troponin. A thoracic CT angiography was performed showing massive bilateral proximal pulmonary embolism with indirect signs of severity (dilatation of the right ventricle). (
Figure 4).
Curative anticoagulation with Unfractionated heparin (UFH) was started. A transtho- racic ultrasound (TTE) showed a dilated right ventricle with impaired systolic function and increased estimated pulmonary systolic pressure (67mmHg). The follow-up was marked by the sudden onset of respiratory distress with obstructive shock signs (persistent hypoten- sion at 79/53mmhg, sweating, oliguria). Systemic thrombolysis was indicated, and after elimination of contraindications, the patient benefited from a monodose of Tenecteplase at a dose of 0.5 mg/kg. After 15 minutes.
The patient’s clinical condition has improved, with a blood pressure of 110/60 mmHg, a heart rate of 101 beats/min, a respiratory rate of 23/min, an oxygen saturation of 95%. Patient was judged out of obstructive shock, and no bleeding adverse events were noted. Patient was continued on UFH with VKA relay. An etiological workup (Doppler ultrasound of the lower limbs, tumor markers, CT scan) came back negative. The patient was referred to internal medicine consultation for further investigations.
4. Discussion
4.1. Pulmonary Embolism in a Nutshell:
Acute pulmonary embolism (PE) can be defined as the sudden obstruction of part of the pulmonary arterial vasculature, which is usually due to embolization of thrombus from the deep veins within the lower limbs. It may also be caused by air, fat, or amniotic fluid. Massive pulmonary embolism represents the obstruction of the pulmonary arterial tree that overreaches 50% of the cross-sectional area, causing acute and severe cardiopulmonary failure from right ventricular overload [
1]. ‘Massive’ is a radiological connotation, and should not be confounded with risk stratification of PE. Pulmonary embolism is the third most common cardiovascular disorder behind myocardial infarction and embolic ischemic stroke [
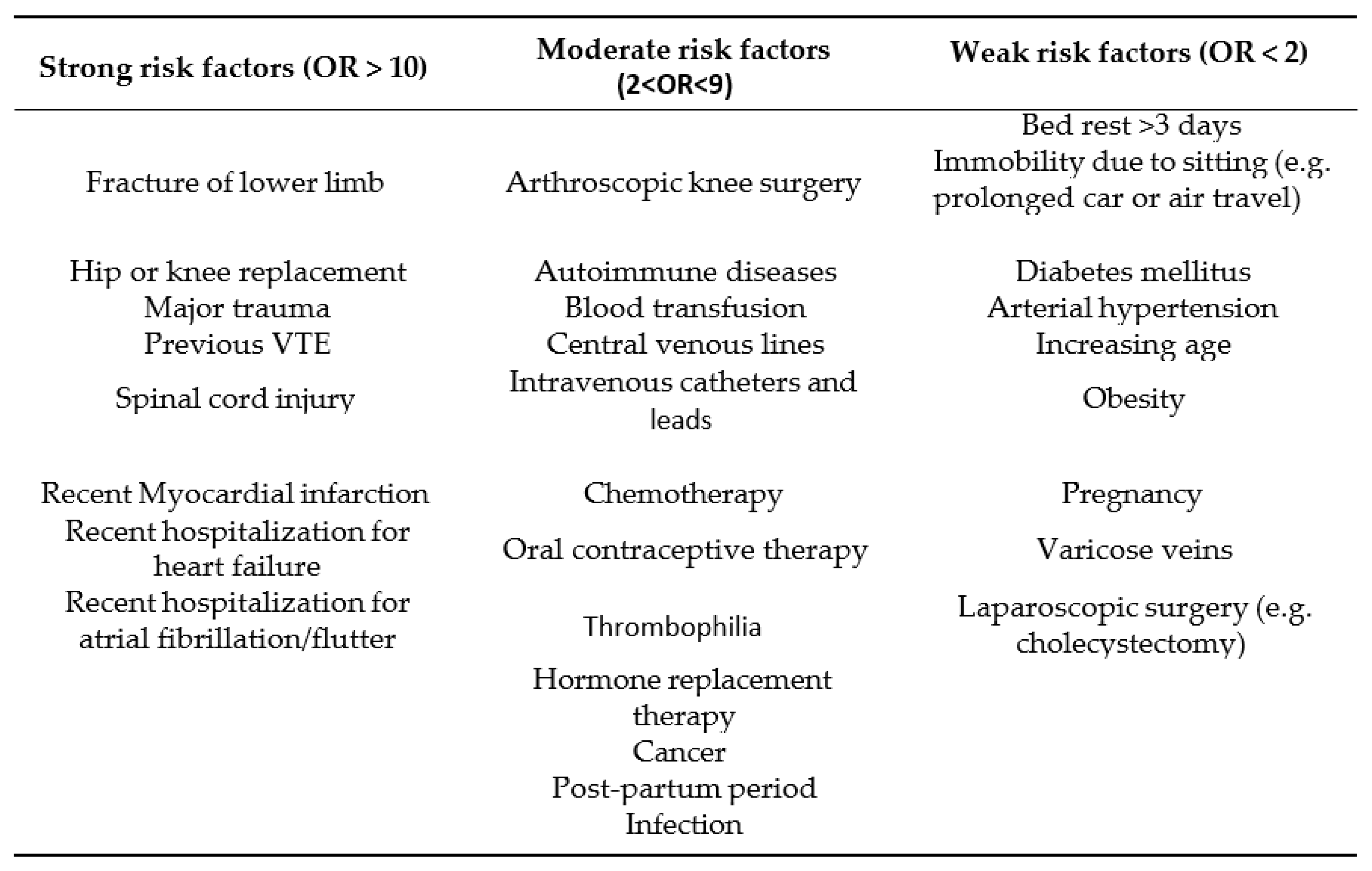
2]. Several risk factors for development of PE are proven to predispose for this disease [
3]. (
Table 1)
According to the 2019 European Society of Cardiology guidelines for pulmonary 121 embolism [
3], diagnosis is based, after assessment of pre-test clinical probability, on non-invasive imaging tests, mainly pulmonary angiography (CTPA), as well as D-dimer testing. After establishment of diagnosis, assessment of severity and early death is essential to determine the appropriate therapeutic approach.
Risk stratification is based on four prognostic major elements : Clinical parameters (assessed by the Pulmonary Embolism Severity Index (
Table 2)) [
5], Right ventricle dys- function (on ultrasound of CTPA), elevated cardiac biomarkers (troponin essentially, BNP), and the existence of hemodynamic instability [
2].
4.2. High-Risk Pulmonary Embolism: Diagnosis
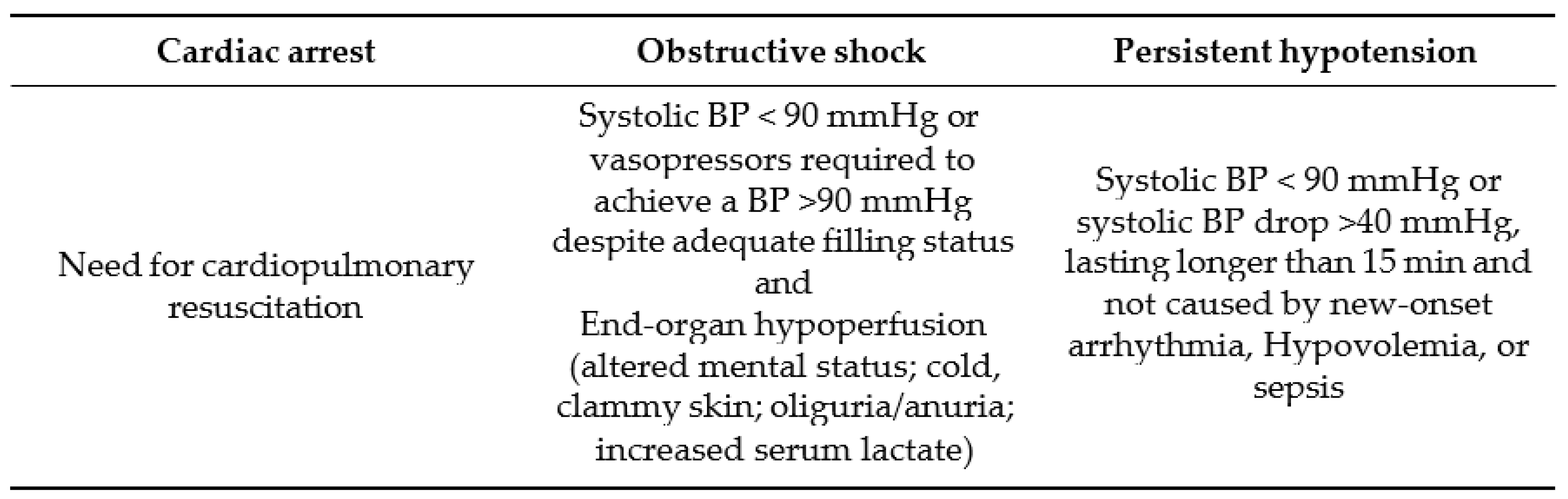
High-risk pulmonary embolism is defined by the existence of hemodynamic instability, which could present as either a cardiac arrest, obstructive shock of persistent hypotension at times (
Table 3). Its incidence is increase worldwide, arising up to 10/100,000 population globally [
4].
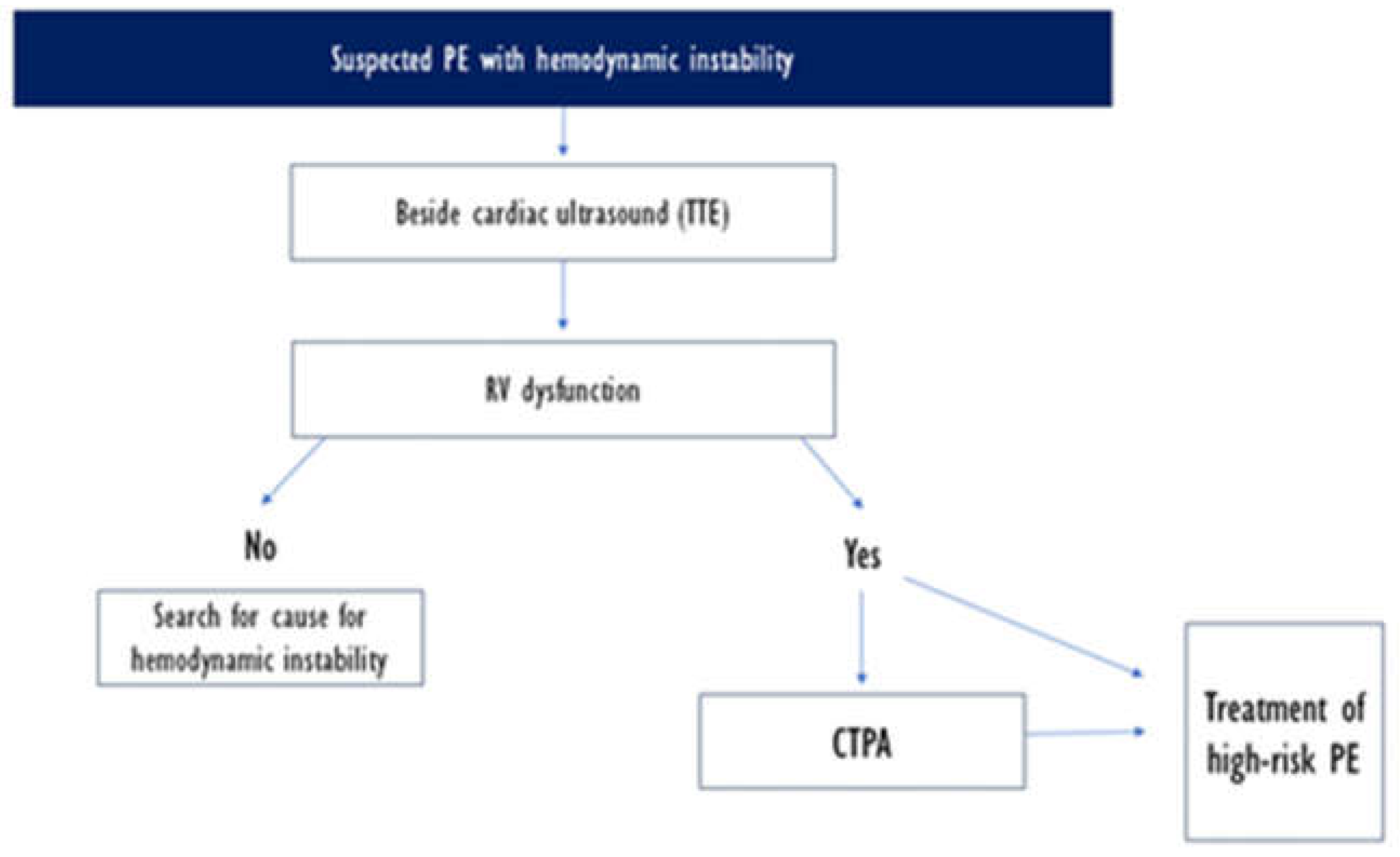
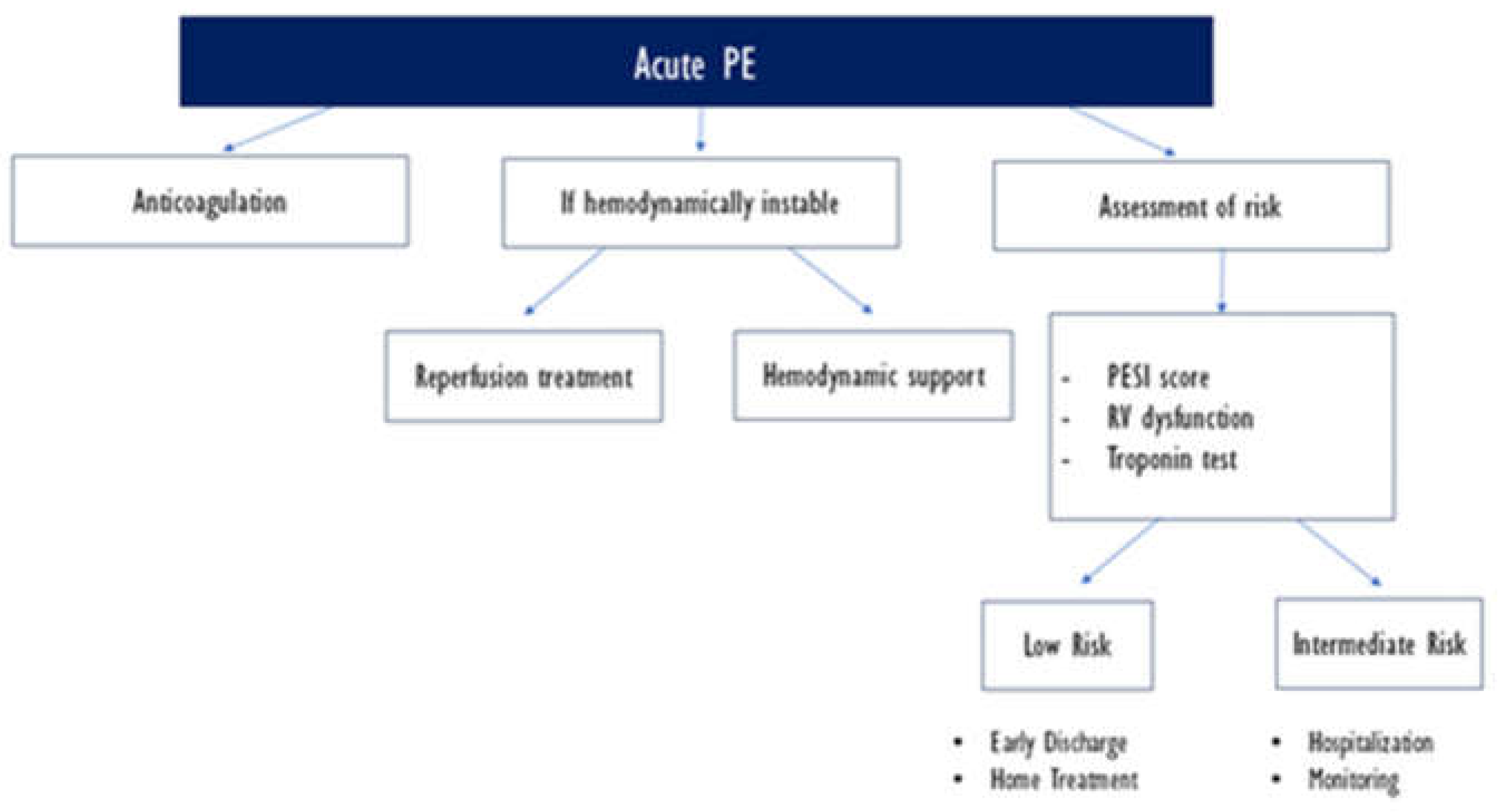
This clinical setting necessitates an urgent diagnostic and management approach. 2019 ESC guidelines suggest an algorithm for rapid diagnosis, starting with systematic bedside ultrasound echocardiography and CTPA should be performed if right ventricle dysfunction s suspected. RV dysfunction on TTE should urge the treatment of high-risk PE even in settings of unavailability of C TPA.
Differential diagnosis includes cardiac tamponade, acute coronary syndrome, aortic dissection, acute valvular dysfunction, and hypovolemia, but the clinical probability is usually high in PE [
3].
Figure 6.
2019 ESC guidelines on treatment of PE [
3].
Figure 6.
2019 ESC guidelines on treatment of PE [
3].
4.3. Therapeutic Approach in High-Risk PE:
The 2019 ESC guidelines highlight the initial therapeutic strategy in high-risk severe PE [
3]. The work-up requires appropriate and rapid therapeutic management, with initial conditioning of the patient in intensive care units (hemodynamic monitoring and venous access, oxygen therapy / mechanical ventilation, volume expansion) and vasoactive drugs in certain situations (inotropes such as norepinephrine) [
6].
Anticoagulation is initiated in all risk types of PE, prioritizing UFH over LMWH or other parenteral or oral anticoagulation regimens.
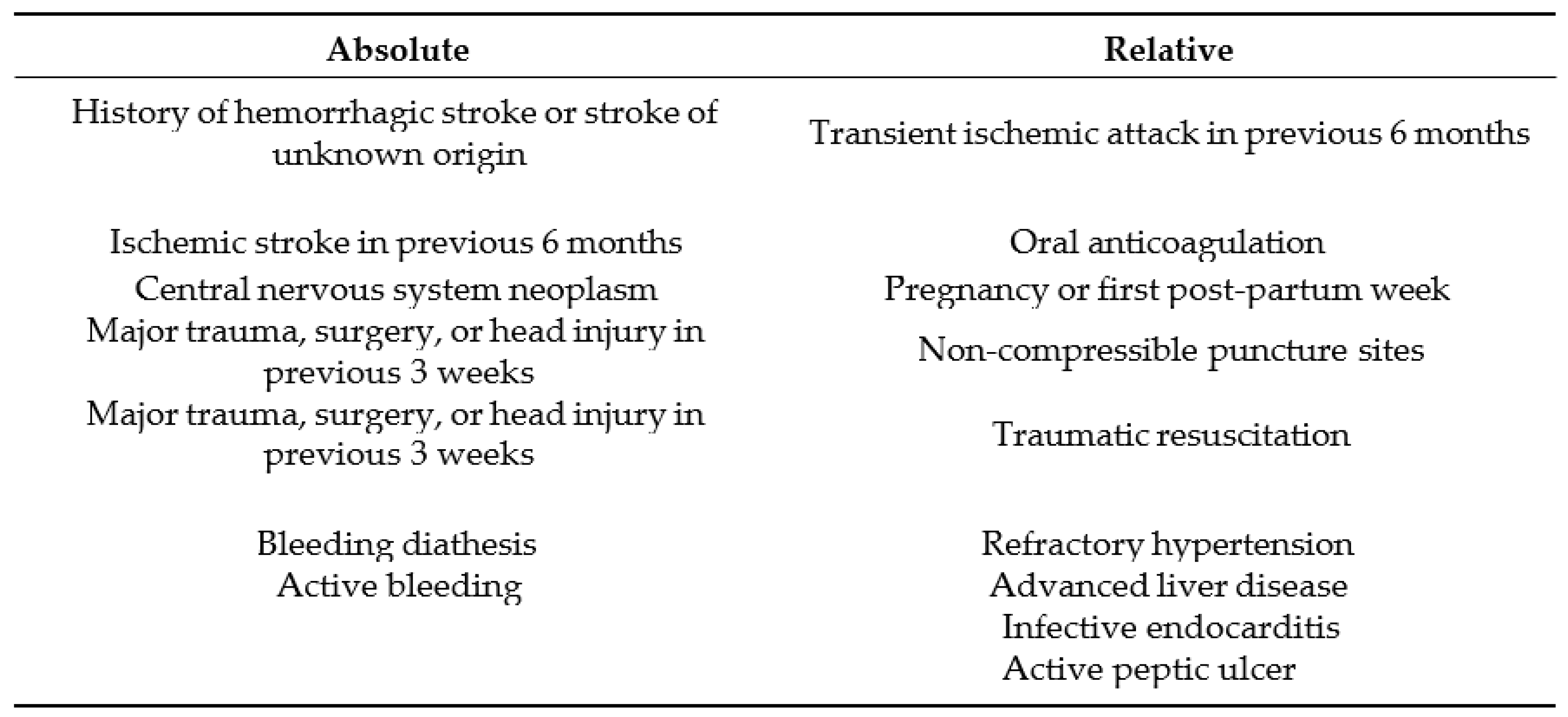
Aside from systemic thrombolysis, which will be tackled in a section later, surgical pulmonary embolectomy is recommended for patients with high-risk PE, in whom throm- bolysis is contraindicated or has failed. Moreover, the use of thrombolysis "in situ" using an ultrasound catheter should be an alternative treatment in case of failure or contraindication to systemic thrombolysis in the case of severe pulmonary embolism according to European guidelines [
3].
An appropriate environment should be provided for those alternatives, furthermore they must be the subject of a multidisciplinary decision and carried out by experienced teams.
4.3.1. Systemic Thrombolysis: Current Guidelines
Primary reperfusion treatment, in most cases systemic thrombolysis, is the treatment of choice for patients with high-risk PE. Thrombolytic therapy is recommended as standard, first-line treatment in patients with massive PE, unless contraindicated [
7], (as shown in
Table 4). It leads to rapid lysis of clot and faster improvements in pulmonary obstruction, PAP, and PVR in patients with PE, compared with heparin alone; a reduction in RV dilation is also noticed on echocardiography [
8] [? ].
The major gain of fibrinolysis is perceived when treatment is initiated within 48 h of symptoms debut, but it can still be useful in patients who have had symptoms for 6-14 days [
10,
11]. Clinical instability’s persistence and unchanged RV dysfunction on echocardiography judge the failure of thrombolysis after 36h [
12].
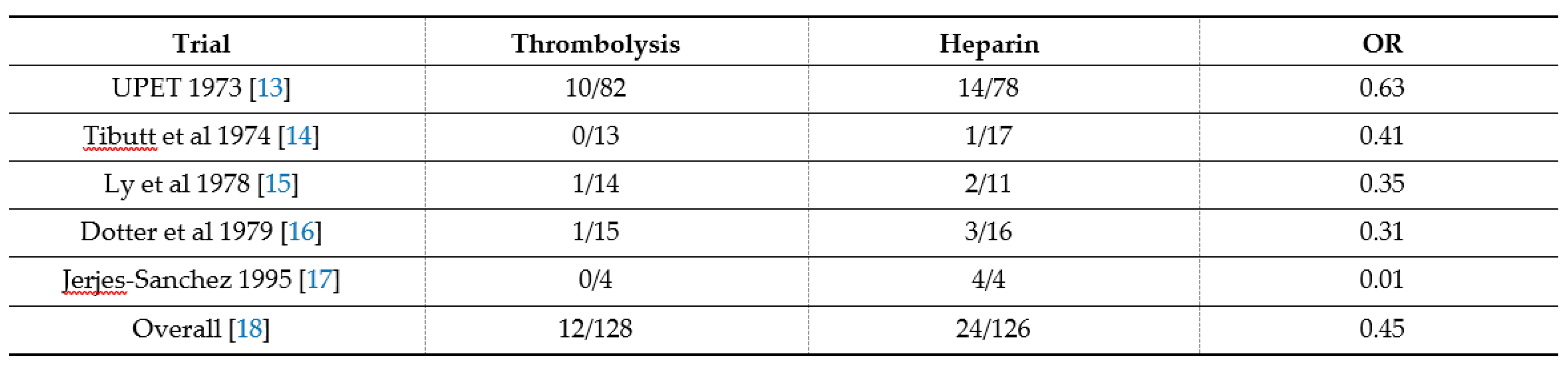
In two meta-analysis overviews of randomized controlled trials (presented in
Table 5), 171 that included patients with high-risk/severe PE, fibrinolysis reduced the risk of death or 172 recurrent PE by 55% [
13,
14,
15,
16,
17,
18,
19].
Concerning bleeding adverse events, major bleeding complications occurred in 9.1-9,9% of fibrinolysis-treated and in 6.1% of heparin-treated patients, and with only 1,7% of intracranial hemorrhage. It is to be noted that major bleeding is observed in both settings with fibrinolysis plus heparin and with heparin alone in high-risk PE.
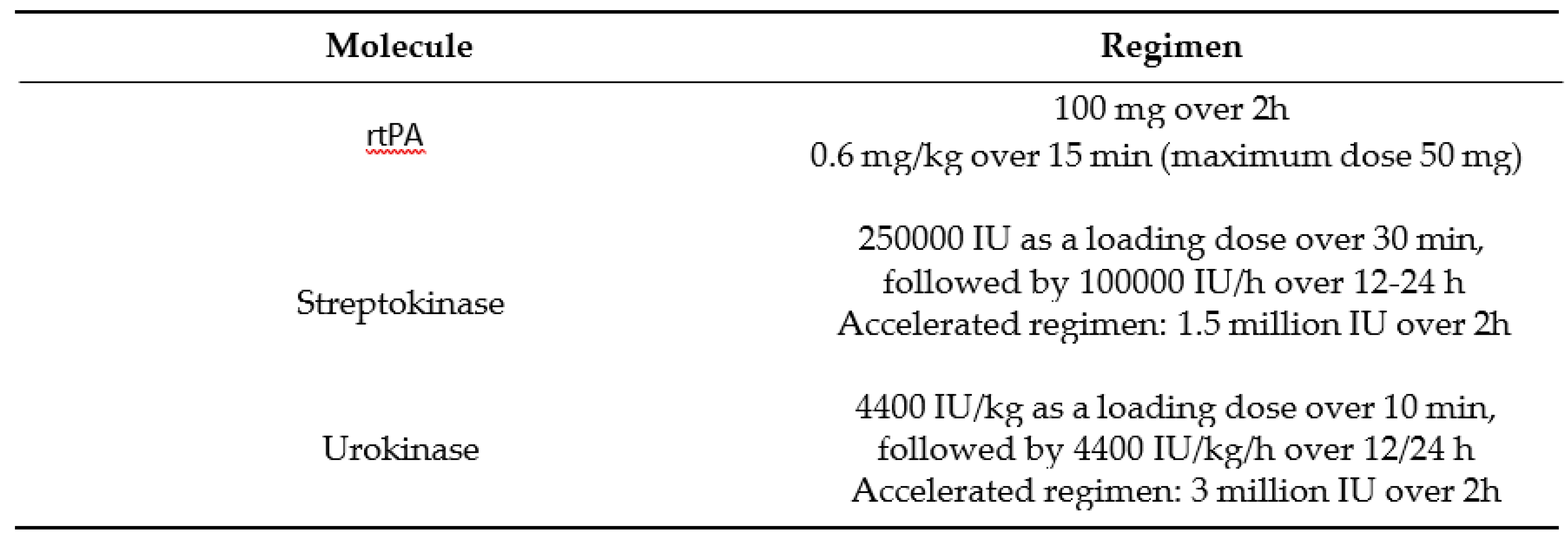
Thrombolytic agents approved by the 2010 European guidelines are presented in Table 63.The preferred fibrinolytic agent is rtPA (Alteplase) with 100-mg continuous 2- hour infusion [
20], followed by first-generation agents with reduced guidelines preference, narrowing their indication in the setting of unavailability of rtPA.
4.4. High-Risk PE and Tenecteplase: Continuing Controversy
The European guidelines state directly that Tenecteplase, and other agents (Reteplase, desmoteplase) are not approved for use in acute PE, regardless of risk. Yet many trials and investigations have shown plausible interest of Tenecteplase in high-risk PE [
21,
22,
23,
24].
In the TIPES study published in 2009, a multicenter, randomized, double-blind, placebo-controlled study, with 58 randomized patients, which evaluated the effect of Tenecteplase on right ventricle dysfunction (RVD) assessed by echocardiography for hemo- dynamically stable patients with PE, concluded that the treatment with single bolus Tenecteplase is feasible at the same dosages used for acute myocardial infarction and is associated with reduction of RVD at 24 hours. However, this study did not raise safety concerns [
21] .
The TOPCOAT trial published in 2014, a multicenter double-blind, placebo-controlled randomized trial, evaluated the benefit of Tenecteplase in increasing the probability of a favorable composite patient-oriented outcome after sub massive PE. The trial was ter- minated prematurely, within 5 days, adverse outcomes occurred in three placebo-treated patients (death in one and intubation in two) and one tenecteplase-treated patient (fatal intracranial hemorrhage), but it concluded that the treatment of patients with sub massive pulmonary embolism using tenecteplase was associated with increased probability of a favorable composite outcome [
22] .
The PEITHO trial published in 2014, which is a randomized, double-blind trial, com-paring enecteplase plus heparin with placebo plus heparin in normotensive patients with intermediate-risk pulmonary embolism, concluded that a fibrinolytic therapy based on a single-bolus injection of the fibrinolytic agent Tenecteplase, in a weight-based dose prevented hemodynamic decompensation, but increased the risk of major hemorrhage and stroke, compared with standard anticoagulation, within the first 7 days after random-ization. This study did not allow to establish clear recommendations for the use and the authorization and marketing of Tenecteplase as a reference treatment for severe pulmonary embolism in the same way as Alteplase [
23] .
The first, largest and most comprehensive Chinese meta-analysis, published in 2022, summarizing multiple randomized controlled trials (RCTs) and cohort studies studying the efficacy and safety of Tenecteplase in PE patients, has objectified that tenecteplase could improve patient survival over 30 days without increasing major bleeding rates for patients with high-risk PE. For patients with intermediate-risk PE, Tenecteplase could prevent the disease progression and improve the clinical symptoms rapidly without affecting mortality in a short/long-term. However, tenecteplase could increase the major bleeding risk in the short term as could other thrombolytic agents [? ].
5. Conclusion
Tenecteplase was effective in our patients and without bleeding complications. While waiting for international guidelines in this regard, our observations and the trials reported in the literature suggest that thrombolysis with Tenecteplase does have an efficacy and a tolerance similar to other molecules, and should be suggested in settings of unavailability of first-line recommended agents.
Author Contributions
Conceptualization, B.E. and Z.R.; methodology, B.E.; software, H.B.; vali- dation, Z.R and A.E.; formal analysis, N.Z.; investigation, N.Z and S.M.; resources, N.Z and S.M.; data curation, H.B.; writing—original draft preparation, B.E.; writing—review and editing, H.B.; visualization, H.B.; supervision, L.H.;All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper’
Data Availability Statement
Data can be shared by reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- A. Sadeghi et al. Acute massive pulmonary embolism: role of the cardiac surgeon.Tex Heart Inst J 2005, 32, 430-433.
- M. Swaroop, A. Tarbox. Pulmonary embolism. Int J Crit Illn Inj Sci 2013, 3, 69.
- Stavros V Konstantinides, Guy Meyer, Cecilia Becattini et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society. Eur Heart J 2020, 41, 543–603.
- Paul D Stein MD , Fadi Matta MD , Mary J. Hughes DO. Hospitalizations for High-Risk Pulmonary Embolism. The American Journal of Medicine 2020.
- Jiménez D, Aujesky D, Moores L, et al. RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010, 170, 1383-9.
- A. Schellhaaß, A. Walther, S. Konstantinides, B. W. Böttiger. The Diagnosis and Treatment of Acute Pulmonary Embolism. Deutsches Ärzteblatt international 2010.
- Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for VTE: ACCP evidence-based clinical practice guidelines. Chest 2008 133, 454–545.
- Goldhaber SZ, Come PC, Lee RT et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993 341, 507-511.
- Becattini C, Agnelli G, Salvi A, et al. TBolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res 2010 125, 82-86.
- Igneri, L. A. Systemic Thrombolytic Therapy for Massive and Submassive Pulmonary Embolism. Semantic Scholar 2018.
- Daniels LB, Parker JA, Patel SR, Grodstein F, Goldhaber SZ. The Relation of duration of symptoms with response to thrombolytic therapy in pulmonary embolism. Am J Cardiol 1997 80, 184-188.
- Meneveau N, Seronde MF, Blonde MC et al. The Diagnosis and Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006 129, 1043-1050.
- The Urokinase Pulmonary Embolism Trial: a national cooperative study. Circulation. 1973 47.
- Tibbutt DA, Davies JA, Anderson JA, et al. Comparison by controlled clinical trial of streptokinase and heparin in treatment of life-threatening pulmonary embolismBMJ. 1974 1, 343–347.
- Ly B, Arnesen H, Eie H, Hol R. A controlled clinical trial of streptokinase and heparin in the treatment of major pulmonary embolism. Acta Med Scand 1978 203, 465–470.
- Dotter CT, Seamon AJ, Rosch J. Streptokinase and heparin in the treatment of pulmonary embolism: a randomized comparison. Vasc Surg 1979 13, 42-52.
- Jerjes-Sanchez C, Ramirez-Rivera A, de Lourdes Garcia M et al. Streptokinase and heparin versus heparin alone in massive pulmonary embolism: a randomized controlled trial.J Thromb Thrombolysis 1995; 2, 227–229.
- Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004 110, 744–749.
- Marti C, John G, Konstantinides S et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2015 10, 605-14.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016 149, 315–52.
- Becattini C, Agnelli G, Salvi A, et al. For the TIPES study group. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res 2010 125, 82-86.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at three months (TOPCOATf submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at three months (TOPCOAT): Multicenter double-blind, placebo-controlled randomized trial.J Thromb Haemos 2014 12, 459–68.
- Meyer G, Vicaut E, Danays T, et al. for the PEITHO Investigators. Fibrinolysis for patients with intermediate-Risk Pulmonary embolism. N Eng J Med 2014 370, 1402-11.
- Zhang, Z., Xi, L., Zhang, S., et al. Tenecteplase in Pulmonary Embolism Patiens: A Meta-Analysis and Systematic Review. Frontiers in Medicine 2022 9.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).