Submitted:
23 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Participants
2.2. Procedures for Inclusion and Exclusion
2.3. Sample Characteristics
2.4. Non-Invasive Brain Stimulation Protocol (NIBS)
2.5. Physical Exercise Protocols (PMED)
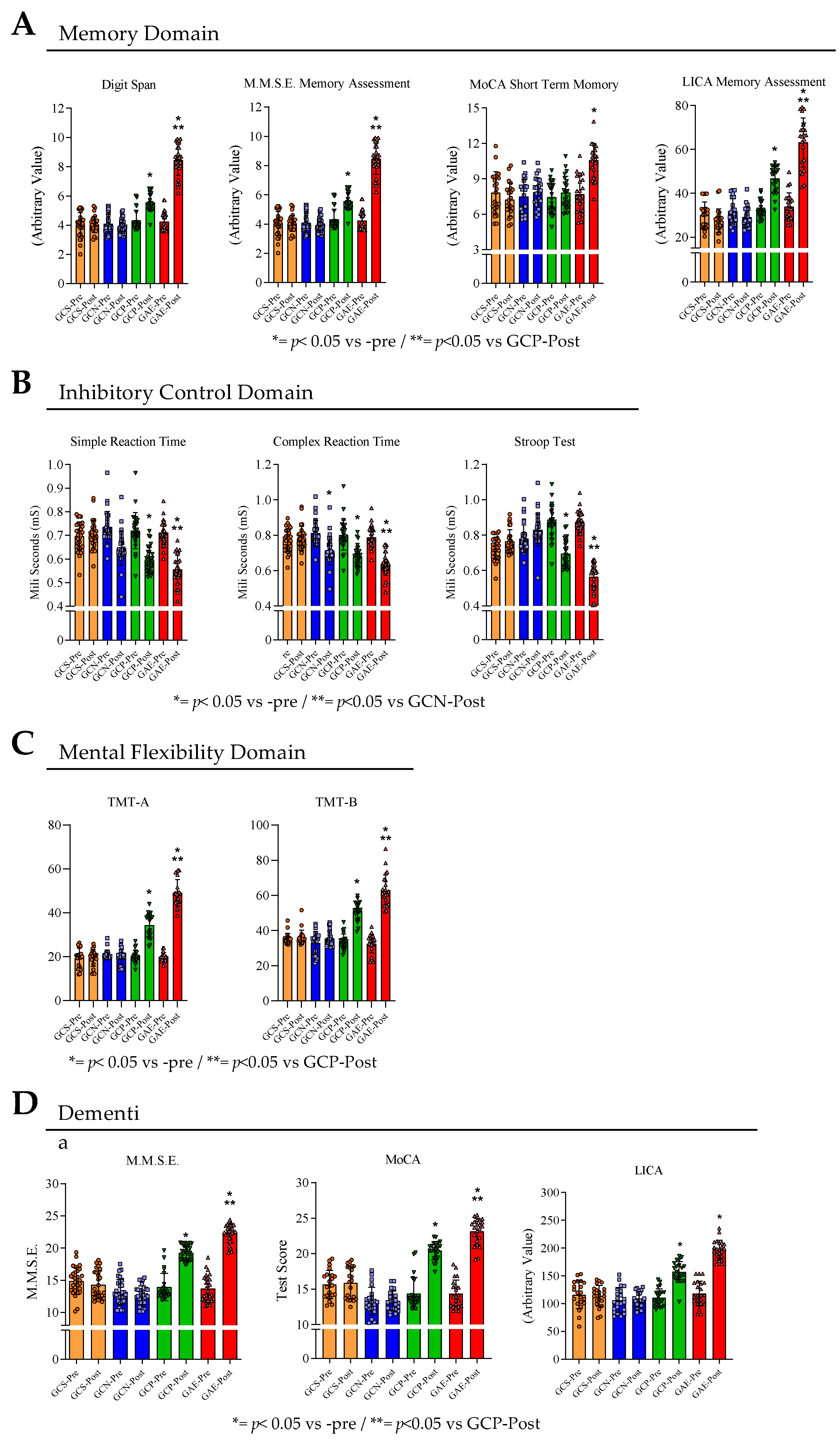
2.6. Cognitive and Dementia Symptoms Assessments
2.6.1. Memory
2.6.1.1. Digit Span
2.6.1.2. Short Term Memory Part of the Montreal Cognitive Assessment (MoCA)
2.6.2. Inhibitory Control
2.6.2.1. Simple and Complex Reaction Time
2.6.2.2. Stroop Test
2.6.3. Mental Flexibility
2.6.3.1. Trail Making Test to Assess the Mental Flexibility
2.7. Cognitive Screening and Dementia Symptoms
2.7.1. Mini Mental State Exam (M.M.S.E.)
2.7.2. Montreal Cognitive Assessment (MoCA)
2.7.3. Literacy Independent Cognitive Assessment (LICA)
2.8. Brain Wave Patterns Assessment
2.9. Study Type and Research Ethics
2.10. Statistical Procedures
3. Results
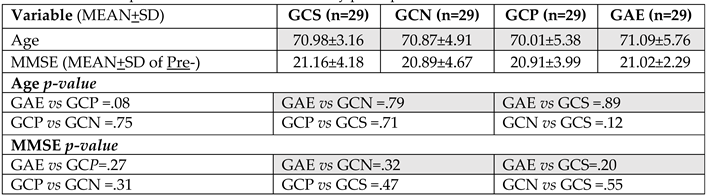
3.1. The Group Displayed a Homogenous Distribution of Age, Marital Status, and Formal Instruction
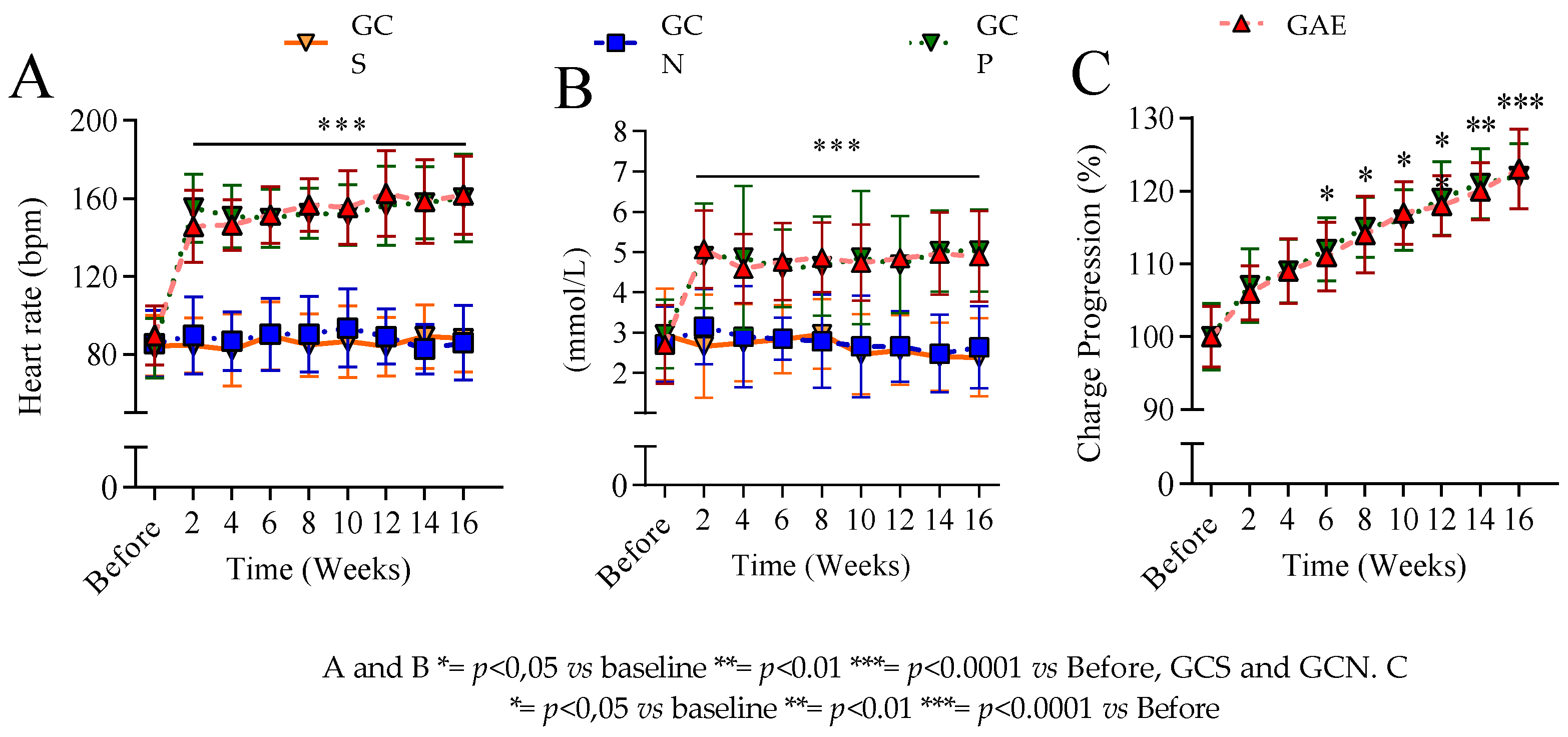
3.2. The Exercise Control Showed no Difference Between the Training of the Groups
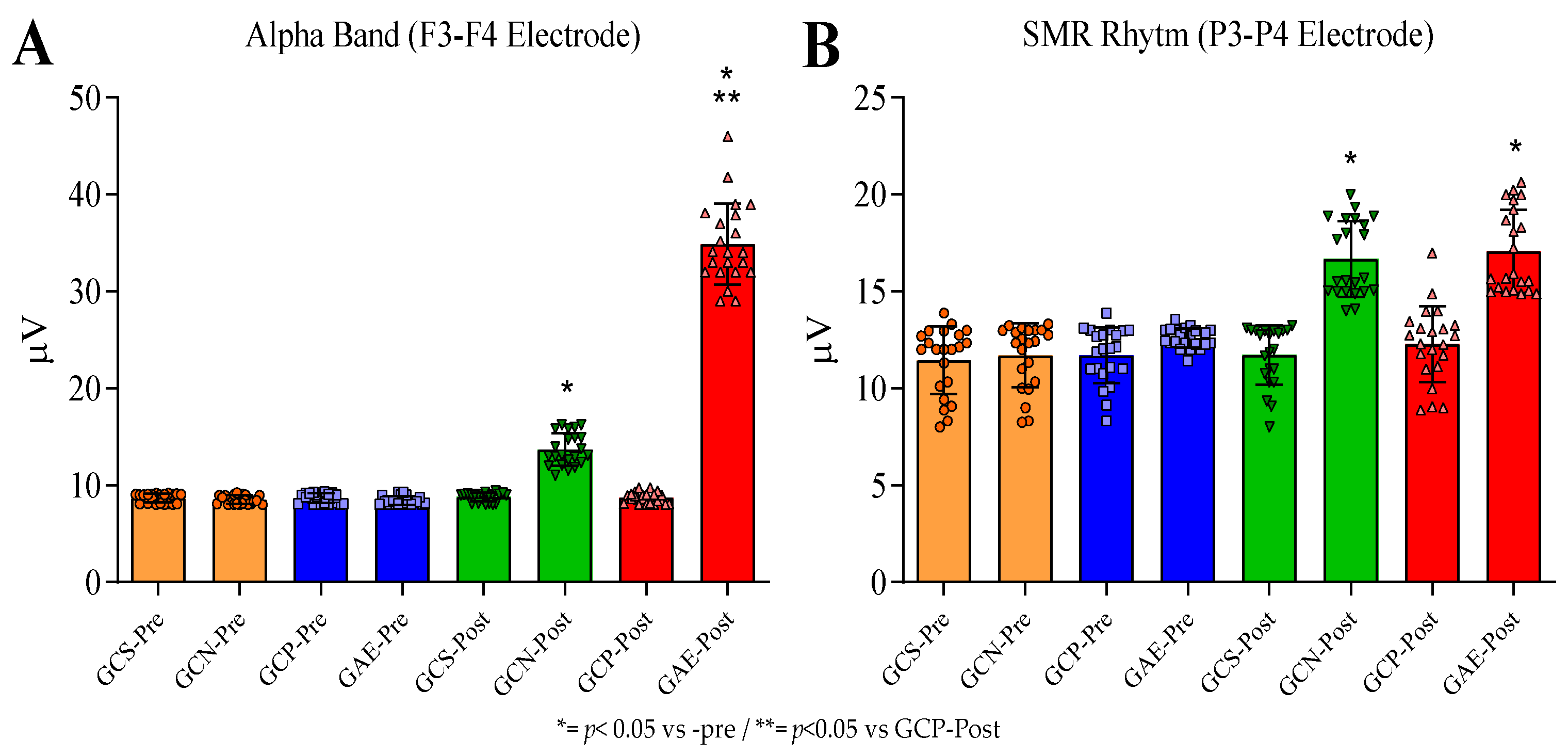
3.3. The NIBS, and PMED Interventions Both Enhanced the Alpha Brain Wave and SMR Rhythm in the Pre-Frontal Area
4. Discussion
5. Conclusions
Acknowledgements
Funding
References
- Raskin J, Cummings J, Hardy J, Schuh K, Dean R. Neurobiology of Alzheimer’s Disease: Integrated Molecular, Physiological, Anatomical, Biomarker, and Cognitive Dimensions. Curr Alzheimer Res. 2015. [CrossRef]
- Curcio C-L, Wu YY, Vafaei A, Barbosa JF de S, Guerra R, Guralnik J; et al. A Regression Tree for Identifying Risk Factors for Fear of Falling: The International Mobility in Aging Study (IMIAS). The Journals of Gerontology: Series A. 2019. [CrossRef]
- Lee J, Kim Y, Liu T, Hwang YJ, Hyeon SJ, Im H; et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell. 2018. [CrossRef]
- Kumar A, Tsao JW. Alzheimer Disease: REVUE. StatPearls. 2018.
- Kochhann R, Wilson MA. Introduction: Special issue on neuropsychology of aging. Psychol Neurosci. 2019. [CrossRef]
- Lilamand M, Raynaud-Simon A. Prevalence and consequences of frailty. Pratiques en Nutrition. 2018;14: 13–14. [CrossRef]
- Elliott E, Atlas R, Lange A, Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3Kinase signalling mechanism. European Journal of Neuroscience. 2005. [CrossRef]
- Hausner L, Frölich L. Antidementia Drug Therapy of Alzheimer’s Dementia: Status 2018 and Outlook. Deutsche Medizinische Wochenschrift. 2019. [CrossRef]
- Van Slegtenhorst M, Lewis J, Hutton M. The molecular genetics of the tauopathies. Experimental Gerontology. 2000. [CrossRef]
- Belarbi K, Burnouf S, Fernandez-Gomez FJ, Laurent C, Lestavel S, Figeac M; et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis. 2011;43: 486–494. [CrossRef]
- Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S; et al. Alzheimer’s disease. The Lancet. 2016. [CrossRef]
- Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early-stage Alzheimer’s disease. Journal of Alzheimer’s Disease. 2018;61: 1323–1332. [CrossRef]
- Cruceanu VD, Rotarescu VS. Alpha brainwave entrainment as a cognitive performance activator. Cognition, Brain, Behavior. 2013.
- Reichert JL, Kober SE, Neuper C, Wood G. Resting-state sensorimotor rhythm (SMR) power predicts the ability to up-regulate SMR in an EEG-instrumental conditioning paradigm. Clinical Neurophysiology. 2015. [CrossRef]
- Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clinical Interventions in Aging. 2018. [CrossRef]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis. 2013;57: 47–55. [CrossRef]
- Bernardo T, Marques-Aleixo I, Beleza J, Oliveira P, Ascensão A, Magalhães J. PHYSICAL EXERCISE AND BRAIN MITOCHONDRIAL FITNESS : THE POSSIBLE ROLE AGAINST ALZHEIMER ’ S DISEASE. Brain Pathology. 2016;26: 648–663.
- Belaya I, Ivanova M, Sorvari A, Ilicic M, Loppi S, Koivisto H; et al. Astrocyte remodeling in the beneficial effects of long-term voluntary exercise in Alzheimer’s disease. J Neuroinflammation. 2020;17: 1–19. [CrossRef]
- Chow ZS, Moreland AT, Macpherson H, Teo WP. The Central Mechanisms of Resistance Training and Its Effects on Cognitive Function. Sports Medicine. 2021;51: 2483–2506. [CrossRef]
- Xu Y, Qiu Z, Zhu J, Liu J, Wu J, Tao J; et al. The modulation effect of non-invasive brain stimulation on cognitive function in patients with mild cognitive impairment: A systematic review and meta-analysis of randomized controlled trials 11 Medical and Health Sciences 1103 Clinical Sciences 11 Medica. BMC Neurosci. 2019. [CrossRef]
- Calomeni MR, Furtado da Silva V, Velasques BB, Feijó OG, Bittencourt JM, Ribeiro de Souza e Silva AP. Modulatory Effect of Association of Brain Stimulation by Light and Binaural Beats in Specific Brain Waves. Clinical Practice & Epidemiology in Mental Health. 2017;13: 134–144. [CrossRef]
- Brabenec L, Klobusiakova P, Barton M, Mekyska J, Galaz Z, Zvoncak V; et al. Non-invasive stimulation of the auditory feedback area for improved articulation in Parkinson’s disease. Parkinsonism Relat Disord. 2019. [CrossRef]
- Luber B, Deng Z De. Application of non-invasive brain stimulation in psychophysiology. Handbook of Psychophysiology, Fourth Edition. 2016. [CrossRef]
- Dinkelbach L, Brambilla M, Manenti R, Brem AK. Non-invasive brain stimulation in Parkinson’s disease: Exploiting crossroads of cognition and mood. Neuroscience and Biobehavioral Reviews. 2017. [CrossRef]
- Padala KP, Padala PR, Lensing SY, Dennis RA, Bopp MM, Roberson PK; et al. Home-Based Exercise Program Improves Balance and Fear of Falling in Community-Dwelling Older Adults with Mild Alzheimer’s Disease: A Pilot Study. Journal of Alzheimer’s Disease. 2017. [CrossRef]
- Bertolucci PH, Brucki SM, Campacci SR, Juliano Y. The Mini-Mental State Examination in a general population: Impact of educational status. Arq Neuropsiquiatr. 1994;52: 1–7. [CrossRef]
- Fabrício-Wehbe SCC, Schiaveto FV, Vendrusculo TRP, Haas VJ, Dantas RAS, Rodrigues RAP. Cross-cultural adaptation and validity of the “Edmonton Frail Scale - EFS” in a Brazilian elderly sample. Rev Lat Am Enfermagem. 2009. [CrossRef]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6: 734–746. [CrossRef]
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale [4]. Age and Ageing. 2006. pp. 526–529. [CrossRef]
- Reisberg D, Rappaport I, O’Shaughnessy M. Limits of working memory: The digit digit-span. J Exp Psychol Learn Mem Cogn. 1984;10: 203–221. [CrossRef]
- Wambach D, Lamar M, Swenson R, Penney DL, Kaplan E, Libon DJ. Digit Span. Encyclopedia of Clinical Neuropsychology. 2011. pp. 844–849. [CrossRef]
- Okamura H, Otani M, Shimoyama N, Fujii T. Combined Exercise and Cognitive Training System for Dementia Patients: A Randomized Controlled Trial. Dement Geriatr Cogn Disord. 2018. [CrossRef]
- Tsukamoto H, Takenaka S, Suga T, Tanaka D, Takeuchi T, Hamaoka T; et al. Effect of exercise intensity and duration on postexercise executive function. Med Sci Sports Exerc. 2017. [CrossRef]
- Souza TR, Campos PF, Almeida M, Faria VM, Silveira Chaves B, Faria WM; et al. Exercício progressivo de curtíssima duração possui potente efeito sobre a memória de trabalho, controle inibitório e motricidade fina de adultos jovens sedentários. / Progressive exercise of very short duration has a potent effect on working memory, inhib. Motricidade. 2019;15: 154–163.
- Sun F, Norman IJ, While AE. Physical activity in older people: A systematic review. BMC Public Health. 2013. [CrossRef]
- Forbes D, Thiessen EJ, Blake CM, Forbes SS, Forbes S. Exercise programs for people with dementia. Sao Paulo Medical Journal. 2014. [CrossRef]
- Blazer DG, Yaffe K, Karlawish J. Cognitive aging: A report from the Institute of Medicine. JAMA - Journal of the American Medical Association. 2015. [CrossRef]
- Tam A, Luedke AC, Walsh JJ, Fernandez-Ruiz J, Garcia A. Effects of reaction time variability and age on brain activity during Stroop task performance. Brain Imaging Behav. 2015. [CrossRef]
- Lord SR, Delbaere K, Sturnieks DL. Aging. Handbook of Clinical Neurology. 2018. [CrossRef]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. Journals of Gerontology - Series B Psychological Sciences and Social Sciences. 2002. [CrossRef]
- Jasper H. The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 1958.
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000. [CrossRef]
- Brueggen K, Fiala C, Berger C, Ochmann S, Babiloni C, Teipel SJ. Early changes in alpha band power and DMN BOLD activity in Alzheimer’s disease: A simultaneous resting state EEG-fMRI study. Front Aging Neurosci. 2017. [CrossRef]
- Furtado V, Calomeni MR, Alkmim R, Nunes M, Elias C, Martins GP; et al. Brain stimulation used as biofeedback in neuronal activation of the temporal lobe area in autistic children. 2016; 632–637. [CrossRef]
- Communications S, Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C; et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002. [CrossRef]
- Wedell-Neergaard A-S, Lehrskov LL, Christensen RH, Pedersen BK, Ellingsgard H, Krogh-Madsen R. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019;29: 844–855.
- Silva VF da, Silva DAS, Martins PC, Calomeni MR, Freire I de A, Militão AG; et al. Effect of physical exercise and noninvasive brain stimulation on cognition and dementia of elderly people with frailty : A randomized study. International Journal Imaging System and Technology. 2022;1: 1–12. [CrossRef]
- Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016. [CrossRef]
- Mrcpuk CC, Mrcpuk BS. Exercise – Cognition Interaction: Neuroscience Perspectives. Ment Health Phys Act. 2017;12: 1. [CrossRef]
- Silva VF da, Calomeni MR, Nunes RAM, Pimentel CE, Martins GP, Oliveira P da CA; et al. Brain stimulation used as biofeedback in neuronal activation of the temporal lobe area in autistic children. Arq Neuropsiquiatr. 2016. [CrossRef]
- Furtado da Silva V, Simões KM, de Freire IA, Cárdenas RN, O Gonçalvez LG, Borges CJ; et al. Quality of Life, Cognitive Impairment, Treatment, and Physical Exercise in Patients with Parkinson’s Disease: A Review. Journal of Exercise Physiology. 2017;20. Available: https://www.asep.org/asep/asep/JEPonlineOCTOBER_5_2017_Valentim-Silva.pdf.
- Macpherson H, Teo WP, Schneider LA, Smith AE. A life-long approach to physical activity for brain health. Front Aging Neurosci. 2017. [CrossRef]
- Chittrakul J, Siviroj P, Sungkarat S, Sapbamrer R. Multi-system physical exercise intervention for fall prevention and quality of life in pre-frail older adults: A randomized controlled trial. Int J Environ Res Public Health. 2020;17: 1–13. [CrossRef]
- Kawanishi N, Yano H, Mizokami T, Takahashi M, Oyanagi E, Suzuki K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav Immun. 2012;26: 931–941. [CrossRef]
- Boer PH, Moss SJ. Effect of continuous aerobic vs. interval training on selected anthropometrical, physiological and functional parameters of adults with Down syndrome. Journal of Intellectual Disability Research. 2016. [CrossRef]
- Braga JC, de Freitas RE, dos Santos KM, Pontes da Silva R, Mota da Silva J, Junior AL; et al. Twelve Weeks of High-Intense Interval Training Enhance the Neuromuscular and Cardiorespiratory Performance of Elderly. Open Sports Sci J. 2020;13: 42–48. [CrossRef]
- Louzada Júnior A, Mota da Silva J, Furtado da Silva V, Clodoaldo Melo Castro A, Eufrásio de Freitas R, Braga Cavalcante J; et al. Multimodal HIIT is More Efficient Than Moderate Continuous Training for Management of Body Composition, Lipid Profile and Glucose Metabolism in the Diabetic Elderly. Int J Morphol. 2020;38: 392–399.
- Silva VF da, Castro ACM, Freitas RE de, Braga JC, Santos KM dos, Albuquerque APA; et al. EIGHT WEEKS OF HIGHINTENSITY INTERVAL TRAINING IMPROVE HAEMATOLOGICAL HEALTH AND DECREASE BLOOD PRESSURE IN SLIGHTLYHYPERTENSIVE ELDERLY. 2018.
- Ringseis R, Eder K, Mooren FC, Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exercise Immunology Review. 2015. pp. 58–68.
- Baburamani AA, Patkee PA, Arichi T, Rutherford MA. New approaches to studying early brain development in Down syndrome. Developmental Medicine and Child Neurology. 2019. [CrossRef]
- Birba A, Ibáñez A, Sedeño L, Ferrari J, García AM, Zimerman M. Non-invasive brain stimulation: A new strategy in mild cognitive impairment? Frontiers in Aging Neuroscience. 2017. [CrossRef]
- Calverley TA, Ogoh S, Marley CJ, Steggall M, Marchi N, Brassard P; et al. HIITing the brain with exercise: Mechanisms, consequences and practical recommendations. Journal of Physiology. 2020;598: 2513–2530. [CrossRef]
- Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance Training and Executive Functions: A 12-Month Randomised Controlled Trial. Arch Intern Med. 2010;170: 170–178. [CrossRef]
- Tsai CL, Pan CY, Chen FC, Wang CH, Chou FY. Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp Physiol. 2016. [CrossRef]
- de Greeff JW, Bosker RJ, Oosterlaan J, Visscher C, Hartman E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. Journal of Science and Medicine in Sport. 2018. [CrossRef]
- Kelling NJ, Corso GM. The effect of spatial working memory capacity on ball flight perception. Journal of Human Sport and Exercise. 2018. [CrossRef]
- Crush EA, Loprinzi PD. Dose-Response Effects of Exercise Duration and Recovery on Cognitive Functioning. Percept Mot Skills. 2017. [CrossRef]
- Diamond A. Executive Functions. Annu Rev Psychol. 2013;64: 135–68. [CrossRef]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci. 1998. [CrossRef]
- Lima RF, Da Silva VF, De Oliveira GL, De Oliveira TAP, Filho JF, Mendonça JGR; et al. Practicing karate may improves executive functions of 8-11-year-old schoolchildren. Journal of Physical Education and Sport. 2017;17. [CrossRef]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010. [CrossRef]
- Calomeni MR, Rocha JAMDS, da Silva APR, Ribeiro LHB, Marques L, Siza MAF; et al. Brain stimulation used as biofeedback training for recovery of motor functions deteriorated by stroke. Arq Neuropsiquiatr. 2013. [CrossRef]
- Tsuchimoto S, Shibusawa S, Mizuguchi N, Kato K, Ebata H, Liu M; et al. Resting-State Fluctuations of EEG Sensorimotor Rhythm Reflect BOLD Activities in the Pericentral Areas: A Simultaneous EEG-fMRI Study. Front Hum Neurosci. 2017. [CrossRef]
- Moraes H, Ferreira C, Deslandes A, Cagy M, Pompeu F, Ribeiro P; et al. Beta and alpha electroencephalographic activity changes after acute exercise. Arq Neuropsiquiatr. 2007;65: 637–641.
- Chung JW, Ofori E, Misra G, Hess CW, Vaillancourt DE. Beta-band activity and connectivity in sensorimotor and parietal cortex are important for accurate motor performance. Neuroimage. 2017. [CrossRef]
- Fahimi G, Tabatabaei SM, Fahimi E, Rajebi H. Index of theta/alpha ratio of the quantitative electroencephalogram in Alzheimer’s disease: A case-control study. Acta Med Iran. 2017.
- Silva VF da, Matsuura C. Efeitos da prática regular de atividade física sobre o estado cognitivo e a prevenção de quedas em idosos. Fitness & Performance Journal. 2002. [CrossRef]
- O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews. 2014. [CrossRef]
- Hallett M. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007. [CrossRef]
- Lourenco M V., Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019. [CrossRef]
- Tsai CL, Chen FC, Pan CY, Wang CH, Huang TH, Chen TC. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014. [CrossRef]
| Variables | GCS | GCN | GCP | GAE | |
|---|---|---|---|---|---|
| Number of individuals in each group | |||||
| Age (Years) | 65-70 71-75 76 > |
18 08 03 |
19 06 04 |
18 05 06 |
20 05 04 |
| Sex | Men Women |
16 13 |
15 14 |
18 11 |
16 13 |
| Marital Status | Divorced Married Widowed |
14 14 01 |
12 15 02 |
16 10 03 |
15 13 01 |
| Educational Level | High School complete> High School incomplete > College complete > |
19 07 03 |
18 10 01 |
17 08 04 |
17 09 03 |
| Social Income | Minimum Wage or < 1-2 Minimum wage(s) 2-4 Minimum Wages 5 > minimum wage |
09 11 03 06 |
07 10 05 07 |
08 07 06 08 |
07 09 08 05 |
| Duration of the disease | 1 year or less 1 year or more 2 years > |
- - - |
15 07 07 |
13 08 08 |
14 09 06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




