Submitted:
23 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
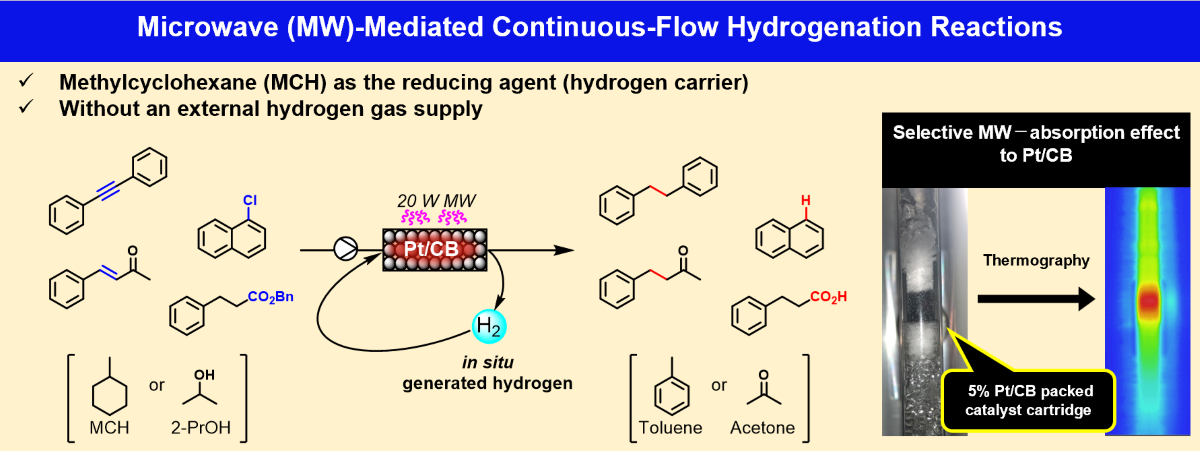
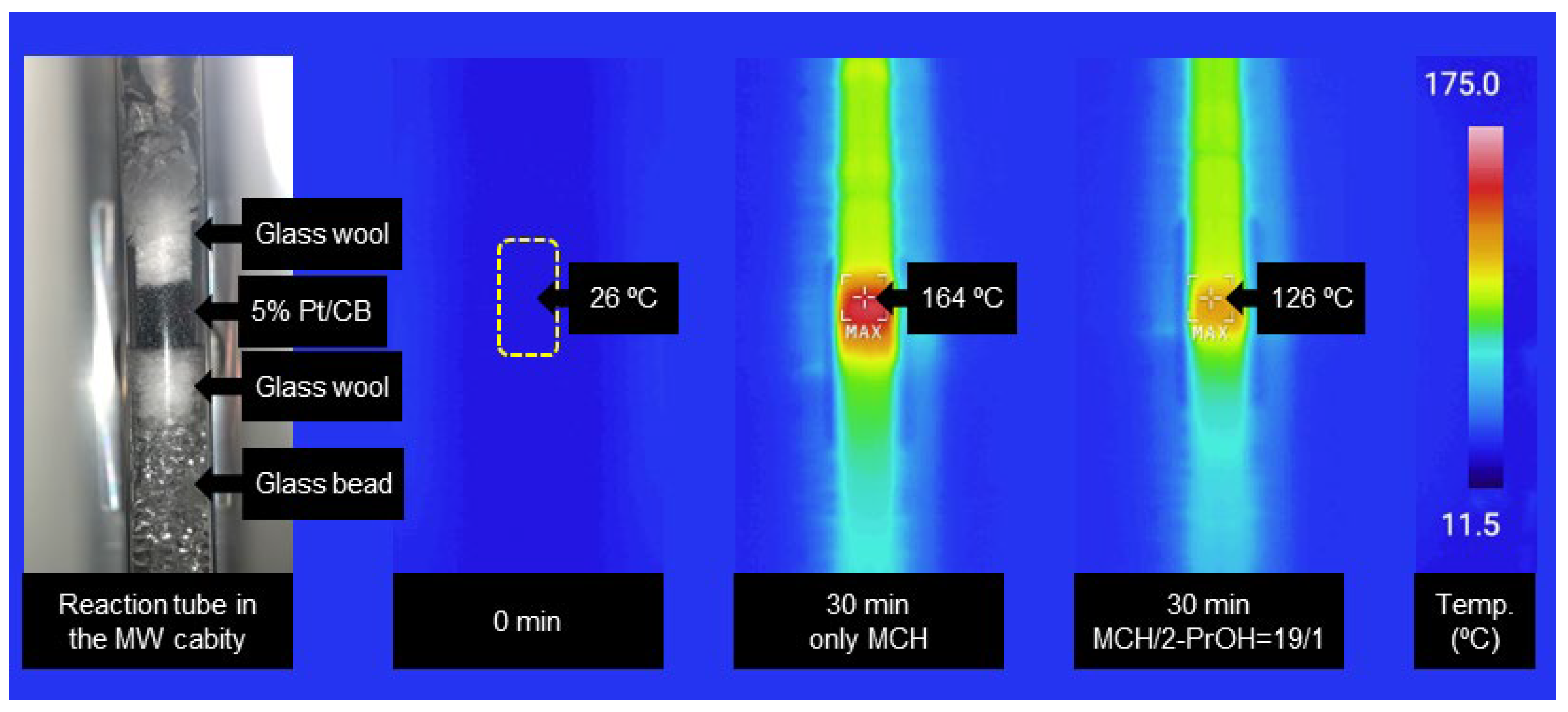
Keywords:
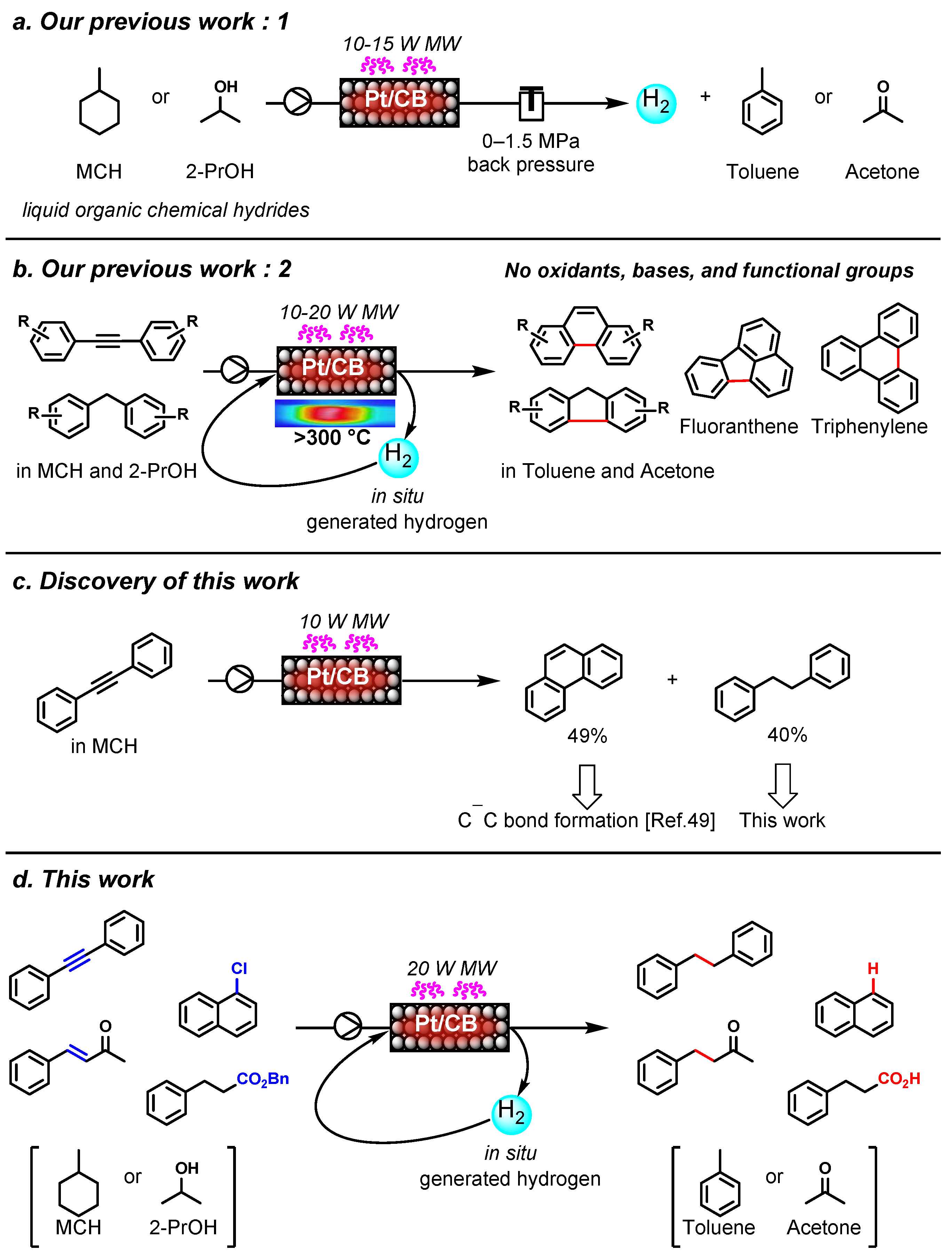
1. Introduction
2. Results and Discussion
2.1. Effect of Platinum-Group Metal Catalyts
2.2. Effect of Temperature in the Catalyst Cartridge and Concentration of the Substrate Solution
2.2. Effect of 2-PrOH as a Co-Solvent
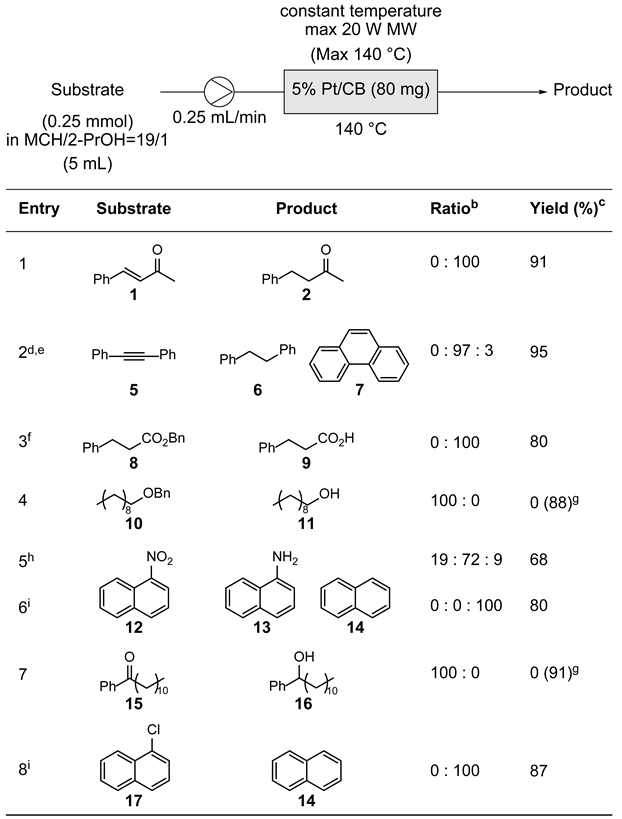
2.3. Scope of Applicable Substrates
3. Materials and Methods
3.1. Materials
3.2. General Procedure for Hydrogenations (Table 4)
3.3. Spectroscopic Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Dutta, S. Review and Outlook of Hydrogen Production through Catalytic Processes. Energy Fuels 2024, 38, 4, 2601–2629. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Zhao, Z.; Zhang, P.; Zhang, Y.; Liu, J.; Ma, S.; Cheng, P.; Chen, Y.; Zhang, Z. Green synthesis of olefin-linked covalent organic frameworks for hydrogen fuel cell applications. Nat. Commun. 2021, 12, 1982. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; Huo, S.; Brandan, N.P.; Yin, Y.; Guiver, M. D. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Stavila, V.; Snider, J.L.; Witman, M.; Bowden, M.E.; Brooks, K.; Tran, B.L.; Autrey, T. Challenges to developing materials for the transport and storage of hydrogen. Nat. Chem. 2022, 14, 1214–1223. [Google Scholar] [CrossRef]
- Chen, L.; Song, Z.; Zhang, S.; Chang, C.; Chuang, Y.; Peng, X.; Dun, C.; Urban, J.J.; Guo, J.; Chen, J.; Prendergast, D.; Salmeron, M.; Somorjai, G.A.; Su, J. Ternary NiMo-Bi liquid alloy catalyst for efficient hydrogen production from methane pyrolysis. Science 2023, 381, 857–861. [Google Scholar] [CrossRef]
- Liu, R.; He, G.; Wang, X.; Mallapragada, D.; Zhao, H.; Shao-Horn, Y.; Jiang, B. A cross-scale framework for evaluating flexibility values of battery and fuel cell electric vehicles. Nat. Commun. 2024, 15, 280. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem. Rev. 2020, 120, 9583–9674. [Google Scholar] [CrossRef]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 1–38. [Google Scholar]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility. A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Song, S.; Lin, H.; Sherman, P.; Yang, X.; Nielsen, C.P.; Chen, X.; McElroy, M.B. Production of hydrogen from offshore wind in China and cost-competitive supply to Japan. Nat. Commun. 2021, 12, 6953. [Google Scholar] [CrossRef]
- Hodges, A.; Hoang, A.L.; Tsekouras, G.; Wagner, K.; Lee, C.; Swiegers, G.F.; Wallace, G.G. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 2022, 13, 1304. [Google Scholar] [CrossRef]
- Al Ghafri, S.Z.; Munro, S.; Cardella, U.; Funke, T.; Notardonato, W.; Trusler, J.M.; Leachman, J.; Span, R.; Kamiya, S.; Pearce, G. Hydrogen liquefaction: A review of the fundamental physics, engineering practice and future opportunities. Energy Environ. Sci. 2022, 15, 2690–2731. [Google Scholar] [CrossRef]
- Kang, D.; Yun, S.; Kim, B.-k. Review of the Liquid Hydrogen Storage Tank and Insulation System for the High-Power Locomotive. Energies 2022, 15, 4357. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Zavabeti, A.; Chen, K.; Guo, Y.; Hu, G.; Fan, X.; Li, G.K. ; Hydrogen production from the air. Nat. Commun. 2022, 13, 5046. [Google Scholar] [CrossRef]
- Ghorbani, B.; Zendehboudi, S.; Saady, N.M.C.; Duan, X.; Albayati, T.M. Strategies To Improve the Performance of Hydrogen Storage Systems by Liquefaction Methods: A Comprehensive Review. ACS Omega 2023, 8, 18358–18399. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs) – techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Oh, J.; Bathula, H.B.; Park, J.H.; Suh, Y. A sustainable mesoporous palladium-alumina catalyst for efficient hydrogen release from N-heterocyclic liquid organic hydrogen carriers. Commun. Chem. 2019, 2, 68. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent developments of nanocatalyzed liquid-phase hydrogen generation. Chem. Soc. Rev. 2021, 50, 3437. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, H.; Oh, J.-E.; Park, B.Y. Recent Advances in Homogeneous/Heterogeneous Catalytic Hydrogenation and Dehydrogenation for Potential Liquid Organic Hydrogen Carrier (LOHC) Systems. Catalysts 2021, 11, 1497. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Zheng, J. Beyond Hydrogen Storage: Metal Hydrides for Catalysis. ACS Catal. 2024, 14, 3139–3157. [Google Scholar] [CrossRef]
- Rampai, M. M.; Mtshali, C.B.; Seroka, N.S.; Khotseng, L. Hydrogen production, storage, and transportation: recent advances. RSC Adv. 2024, 14, 6699–6718. [Google Scholar] [CrossRef]
- Obara, S. Energy and exergy flows of a hydrogen supply chain with truck transportation of ammonia or methyl cyclohexane. Energy 2019, 174, 848–860. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Azizb, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Al-ShaikhAli, A.H.; Jedidi, A.; Anjum, D.H.; Cavallo, L.; Takanabe, K. Kinetics on NiZn Bimetallic Catalysts for Hydrogen Evolution via Selective Dehydrogenation of Methylcyclohexane to Toluene. ACS Catalysis 2017, 7, 1592–1600. [Google Scholar] [CrossRef]
- Takise, K.; Sato, A.; Ogo, S.; Seo, J.G.; I, K.; Kado, S.; Sekine, Y. Low-temperature selective catalytic dehydrogenation of methylcyclohexane by surface protonics. RSC Adv. 2019, 9, 27743–27748. [Google Scholar] [CrossRef]
- Hamayun, M.H.; Maafa, I.M.; Hussain, M.; Aslam, R. Simulation Study to Investigate the Effects of Operational Conditions on Methylcyclohexane Dehydrogenation for Hydrogen Production. Energies 2020, 13, 206. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Lin, L.; Zhu, Q.; Wang, G.; Guo, H. Study of the carbon cycle of a hydrogen supply system over a supported Pt catalyst: Methylcyclohexane–toluene–hydrogen cycle. Catal. Sci. Technol. 2020, 10, 1171–1181. [Google Scholar] [CrossRef]
- Kojima, H.; Matsuda, T.; Kano, K.; Tsujimura, T. Methylcyclohexane production under fluctuating hydrogen flow rate conditions. Int. J. Hydrogen Energy 2021, 46, 9433–9442. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, F.; Ma, H.; Yuan, X.; Wang, Y.; Zhang, J. A Review of Catalysts for Methylcyclohexane Dehydrogenation. Top Catal. 2021, 64, 509–520. [Google Scholar] [CrossRef]
- Ye, H.L.; Liu, S.X.; Zhang, C.; Cai, Y.Q.; Shi, Y.F. Dehydrogenation of methylcyclohexane over Pt-based catalysts supported on functional granular activated carbon. RSC Adv. 2021, 11, 29287–29297. [Google Scholar] [CrossRef]
- Chen, L.; Verma, P.; Hou, K.; Qi, Z.; Zhang, S.; Liu, Y.; Guo, J.; Stavila, V.; Allendorf, M.D.; Zheng, L.; Salmeron, M.; Prendergast, D.; Somorjai, G.A.; Su, J. Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst. Nat. Commun. 2022, 13, 1092. [Google Scholar] [CrossRef]
- Miyamura, H.; Suzuki, A.; Zhu, Z.; Kobayashi, S. Hydrogen Generation from Organic Hydrides under Continuous-Flow Conditions Using Polymethylphenylsilane-Aluminum Immobilized Platinum Catalyst. Chem. Asian J. 2022, 17, e202200569. [Google Scholar] [CrossRef]
- Kappe, C. O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Kappe, C. O. Microwave dielectric heating in synthetic organic chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A. S. Microwave-assisted hydrothermal treatments for biomass valorization: a critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hot spots. Part V. Mechanisms of hot-spot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. Applied Catalysis A: General. 2013, 460–461, 52–60. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Mitani, T.; Serpone, N. Control of microwave-generated hot spots. 6. Generation of hot spots in dispersed catalyst particulates and factors that affect catalyzed organic syntheses in heterogeneous media. Ind. Eng. Chem. Res. 2014, 53, 14941–14947. [Google Scholar] [CrossRef]
- Días-Ortiz, Á.; Prieto, P.; de la Hoz, A. A critical overview on the effect of microwave irradiation in organic synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Al-Raggad, M.; Shareef, N. Production of activated carbon derived from agricultural by-products via microwave-induced chemical activation: a review. Carbon Lett. 2021, 31, 957–971. [Google Scholar] [CrossRef]
- Chen, W.; Gutmann, B.; Kappe, C. O. Characterization of microwave-induced electric discharge phenomena in metal–solvent mixtures. ChemistryOpen 2012, 1, 39–48. [Google Scholar] [CrossRef]
- Horikoshi, S.; Arai, Y.; Ahmad, I.; DeCamillis, C.; Hicks, K.; Schauer, B.; Serpone, N. Application of Variable Frequency Microwaves in Microwave-Assisted Chemistry: Relevance and Suppression of Arc Discharges on Conductive Catalysts. Catalysts 2020, 10, 777. [Google Scholar] [CrossRef]
- 47. Ichikawa,T.; Matsuo, T.; Tachikawa, T.; Yamada, T.; Yoshimura, T.; Yoshimura, M.; Takagi, Y.; Sawama, Y.; Sugiyama, J.; Monguchi, Y.; Sajiki, H. Microwave-Mediated Site-Selective Heating of Spherical-Carbon-Bead-Supported Platinum for the Continuous, Efficient Catalytic Dehydrogenative Aromatization of Saturated Cyclic Hydrocarbons. ACS Sustainable Chem. Eng. 2019; 7, 3052–3061. [CrossRef]
- Ichikawa, T.; Matsuo, T.; Tachikawa, T.; Teranishi, W.; Yamada, T.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Microwave-Mediated Continuous Hydrogen Abstraction Reaction from 2-PrOH Catalyzed by Platinum on Carbon Bead. Catalysts 2019, 9, 655. [Google Scholar] [CrossRef]
- Yamada, T.; Teranishi, W.; Sakurada, N.; Otri, S.; Abe, Y.; Matsuo, T.; Morii, Y.; Yoshimura, M.; Yoshimura, T.; Ikawa, T.; Sajiki, H. Microwave-assisted C–C bond formation of diarylacetylenes and aromatic hydrocarbons on carbon beads under continuous-flow conditions. Commun. Chem. 2023, 6, 78. [Google Scholar] [CrossRef]
- Colbon, P.; Ruan, J.; Purdie, M.; Mulholland, K.; Xiao, J. Double Arylation of Allyl Alcohol via a One-Pot Heck Arylation–Isomerization–Acylation Cascade. Org. Lett. 2011, 13, 5456–5459. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ida, T.; Maejima, T.; Yanase, T.; Sawama, Y.; Sasai, Y.; Kondo, S.; Sajiki, S. Palladium on Carbon-Catalyzed Gentle and Quantitative Combustion of Hydrogen at Room Temperature. Adv. Synth. Catal 2014, 356, 313–318. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, C.; Bai, S.; Liu, X.; Wu, Q.; Wang, J.; Jiang, C.; Qi, X. Visible-Light-Induced Nickel-Catalyzed Cross-Coupling with Alkylzirconocenes from Unactivated Alkenes. Chem 2020, 6, 675–688. [Google Scholar] [CrossRef]
- Yue, H.; Guo, L.; Liu, X.; Rueping, M. Nickel-Catalyzed Synthesis of Primary Aryl and Heteroaryl Amines via C–O Bond Cleavage. Org. Lett. 2017, 19, 1788–1791. [Google Scholar] [CrossRef]
- Yamada, T.; Ogawa, A.; Masuda, H.; Teranishi, W.; Fujii, A.; Park, K.; Ashikari, Y.; Tomiyasu, N.; Ichikawa, T.; Miyamoto, R.; Bai, H.; Matsuyama, K.; Nagaki, A.; Sajiki, H. Pd catalysts supported on dual-pore monolithic silica beads for chemoselective hydrogenation under batch and flow reaction conditions. Catal. Sci. Technol. 2020, 10, 6359–6367. [Google Scholar] [CrossRef]
- Hu, P.; Tan, M.; Cheng, L.; Zhao, H.; Feng, R.; Gu, W.; Han, W. Bio-inspired iron-catalyzed oxidation of alkylarenes enables late-stage oxidation of complex methylarenes to arylaldehydes. Nat. Commun. 2019, 10, 2425. [Google Scholar] [CrossRef]
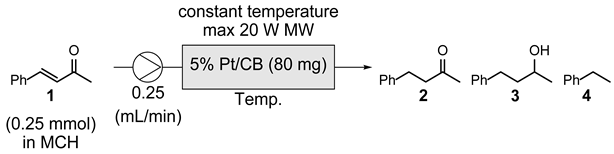
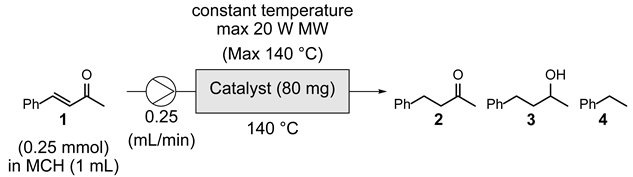 | ||
|---|---|---|
| Entry | Catalyst | Product ratioa (1 : 2 : 3 : 4) |
| 1 | 5% Pt/CB | 0 : 87 : 0 : 13 |
| 2 | 1% Pt/CB | 0 : 57 : 0 : 43 |
| 3 | 5% Pd/CB | 94 : trace : 0 : 6 |
| 4 | 5% Rh/CB | 95 : 0 : 0 : 5 |
| 5 | CB | 95 : 0 : 0 : 3 |
 | |||
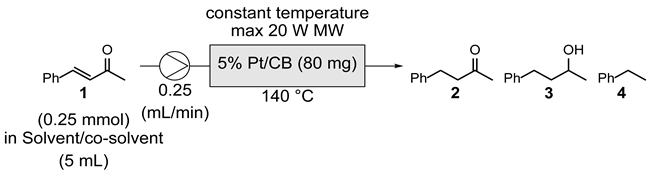
|---|---|---|---|
| Entry | Temp. (°C) | Concentration (M) | Product ratioa (1 : 2 : 3 : 4) |
| 1 | 150 | 0.25 | 0 : 72 : 0 : 28 |
| 2 | 140 | 0.25 | 0 : 87 : 0 : 13 |
| 3 | 140 | 0.05 | 0 : 92 : 0 : 8 |
| 4 | 130 | 0.05 | 0 : 89 : 0 : 11 |
| 5 | 120 | 0.05 | 0 : 82 : 0 : 18 |
 | ||
|---|---|---|
| Entry | Solvent/co-solvent | Product ratioa (1 : 2 : 3 : 4) |
| 1 | MCH/- | 0 : 92 : 0 : 8 |
| 2 | MCH/2-PrOH = 4/1 | 5 : 94 : 0 : 1 |
| 3 | MCH/2-PrOH = 19/1 | 0 : 98 (91)b : 0 : 2 |
| 4 | MCH/2-PrOH = 99/1 | 0 : 95 : 0 : 5 |
| 5 | 2-PrOH/- | 52 : 48 : 0 : trace |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





