1. Introduction
Protein gels are employed in various applications in food processing because proteins are considered safe and nutritious with excellent gelation properties [
1,
2,
3,
4]. Some foods, such as dairy desserts and jellies, are made by gelation. The gelation of proteins involves two main processes, including protein denaturation/unfolding and then aggregation into a self-supporting structure with a 3-dimensional network [
1,
3,
5]. The gel structure is a suitable medium for encapsulation by protecting sensitive bioactive compounds or nutraceuticals from degradation during food processing and through harsh conditions within the gastrointestinal tract [
1,
4,
6]. In this view, several attempts have been made to produce protein gels of different matrices that serve different purposes in the food industry [
2,
3,
7,
8,
9,
10].
In recent decades, ultrasound (US) technology has been used in various food applications as a green technology[
11,
12,
13,
14,
15]. Ultrasound has a shorter processing time, cost, solvent, and energy efficiency. It is environmentally friendly, with improved food quality in extractions, fruit drying, freezing and thawing, fermentation, and protein modification. The US can exert these effects through acoustic cavitation. Cavitation is generated in the medium by the collapse and explosion of microbubbles coupled with physical and chemical effects [
16,
17,
18]. These mechanical and chemical effects, together with the pressure fluctuations resulting from collapse and bubble explosions, lead to modifications in the protein structure [
12,
14,
19,
20,
21]. Changes to the structure then alter the functionality and properties of the protein. The effect of US on proteins has been demonstrated to reduce size and improve solubility, emulsifying, and gelling properties [
14,
20,
22]. Furthermore, US has been shown to enhance textural properties, gel microstructure, and rheological properties of protein gels [
23,
24,
25].
Although most ultrasonic pretreatments on proteins were conducted with a mono-frequency of 20 kHz, different US frequencies have been used to study their effect on food proteins. The effect of ultrasound frequencies between 20 and 52 kHz on soy protein extraction [
26] was assessed. Interestingly, the mono-frequency of 20 kHz was not the optimal selection. Moreover, dual-frequency ultrasonic (DFU) and tri-frequency ultrasonic (TFU) pretreatment have resulted in higher efficiency in protein enzymatic hydrolysis than mono-frequency ultrasonic (MFU) pretreatment [
20,
27]. These results confirm that different US frequencies and modes affect food proteins differently [
22].
Whey protein is one of the crucial proteins in food products. Improving the gel properties is a concern for the food industry. Ultrasound, as an innovative technology in the food industry, has been explored for its application in enhancing the gel properties of whey protein [
28,
29,
30,
31,
32,
33]. Although US has been shown to improve the gel properties, the gastrointestinal breakdown after US treatment has not been studied in detail. Studying the breakdown pattern of these US-treated proteins is expedient since the newly developed structure might affect digestion and nutrient release [
5,
34]. Our previous work showed that DFU pretreatment produced heat-induced whey protein emulsion gels with better mechanical properties and digestion than MFU [
35,
36]. However, the effect of multi-frequencies ultrasonic pretreatment on acid-induced whey protein gel’s properties and digestion was unclear.
Therefore, this work aimed to study the effect of mono, dual, and tri-frequency ultrasound pretreatments on the characterization and digestion of Glucono-delta-lactone (GDL) induced whey protein gels.
2. Materials and Methods
2.1. Materials
Whey protein Isolate (WPI) (protein content of 90.90%) was acquired from Hilmar Company (Hilmar, CA, USA). Pepsin, pancreatin, Glucono-delta-lactone (GDL), and 2,4,6-trinitrobenzene sulfonic acid (TNBS) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were analytically pure.
2.2. Ultrasound Pretreatment on Whey Protein
Whey protein solution (10% w/v dissolved in 10 mM phosphate buffer pH7.0) was pretreated with tri-frequency ultrasound device (Meibo Biotechnology Co., Ltd., Zhenjiang, China) [
37] at different ultrasound frequencies and time (MFU-20kHz for 30min, DFU-20/35kHz for 15mins and TFU-20/35/50kHz for 10 min) with pulse on and off time of 5s and 2s respectively. Dual and tri-frequency treatments were conducted in simultaneous mode. The ultrasonic power for each frequency was 100 w.
2.3. Whey Protein Gels (WPG) Preparation
Whey protein solutions were heated at 90° C for 5mins and immediately cooled to room temperature (RT). GDL (0.50%, w/w) was added, and gels were allowed to set at 25 °C. The GDL-induced gels were stored at 4° C overnight and were equilibrated at RT for an hour before use.
2.4. Rheological Properties Analysis
A rheometer (DHR-1; TA Instruments, New Castle, DE, USA) was used to measure the rheological changes during gelation of the whey protein solutions. For viscosity, the whey protein solution was assessed using 40 mm plate geometry in flow-sweep mode at 25°C with shear rate from 0.1 to 100 s–1. For small deformation analysis, the whey protein solution was preheated at 90 °C for 5mins. GDL (0.50% w/w) was added and stirred for 2 min at 200 rpm on a magnetic mixer. The mixture was immediately loaded into the cup in a concentric cylinder and covered with silicone oil to prevent evaporation. The test was done using time oscillation mode for 3 h at 25°C with a strain of 0.5% and frequency of 1.0 Hz.
2.5. Turbidity Determination
The turbidity of the whey protein solution during gelation was determined at different times using absorbance at 500nm.
2.6. Gel Solubility
Gel solubility in different chemical solvents was carried out using the method described by Zhang et al. [
38] with some modifications. Two grams of cold set whey protein gel was dissolved in 18 mL of four different solvents: (A) 50 mM sodium phosphate buffer pH 7.0,(B) solution A containing 8M Urea (C) solution A containing 0.5% sodium dodecyl sulfate (SDS) and (D) solution A containing 0.25% (w/v) 2-mercaptoethanol. The suspensions were then heated for 30 min at 80°C and cooled to room temperature, followed by centrifuging at 5000 g for 15 min. The protein content in the supernatant was estimated using the Biuret method. The solution’s dissolved protein content represented the gels’ main forces.
2.7. Gel Hardness
The hardness of the gel was measured with a texture analyzer (TA-XT Plus, Stable Microsystems, Surrey, UK). With a gel strain of 30%, a 40mm diameter aluminum cylindrical probe was applied at a pre-test, test, and post-test speed of 1mm/s. Gel hardness was calculated as the average of at least six replicates.
2.8. Scan Electron Microscopy (SEM.)
Whey protein gels (WPG) were cut into small sizes (2-5 mm) and soaked in glutaraldehyde (2.5% v/v) for 2 h. The samples were dehydrated in ethanol with serial concentrations of 50, 70, 80, 90, and 100%, then dried with a critical point drier. SEM was used to observe the microstructures of the gels after coating with gold.
2.9. Protein Distribution in Gel
WPG was centrifuged at 2000g for 10 mins. Reducing SDS-PAGE [
39]of the leached water and protein gels was run using WPI solution as a reference. Protein in the gel solid was prepared using the same procedure in 2.5, containing 0.5%(w/v) SDS and 0.25% (w/v) 2-mercaptoethanol.
2.10. Simulated Digestion of WPG
Selected WP gels and control samples were digested using the INFOGEST method described by Minekus et al.(2014) [
40] with modifications as outlined in our previous work[
35].
2.11. Free Amino Group Determination
Free amino groups were determined at different time points during gastric and intestinal static digestion with the TNBS method described by Spellman et al.(2003)[
41]. Absorbance was measured at 420nm. The free amino acid content was determined using the standard curve of L-leucine.
2.12. Statistical Analysis
At least two independent experiments were done using fresh samples prepared at different times. ANOVA was used for analyses, and the significance difference was set at p< 0.05. Figures were representations of means and standard deviations.
3. Results
3.1. Effect of Ultrasonic Frequency Mode on the Viscosity of Whey Protein Solution
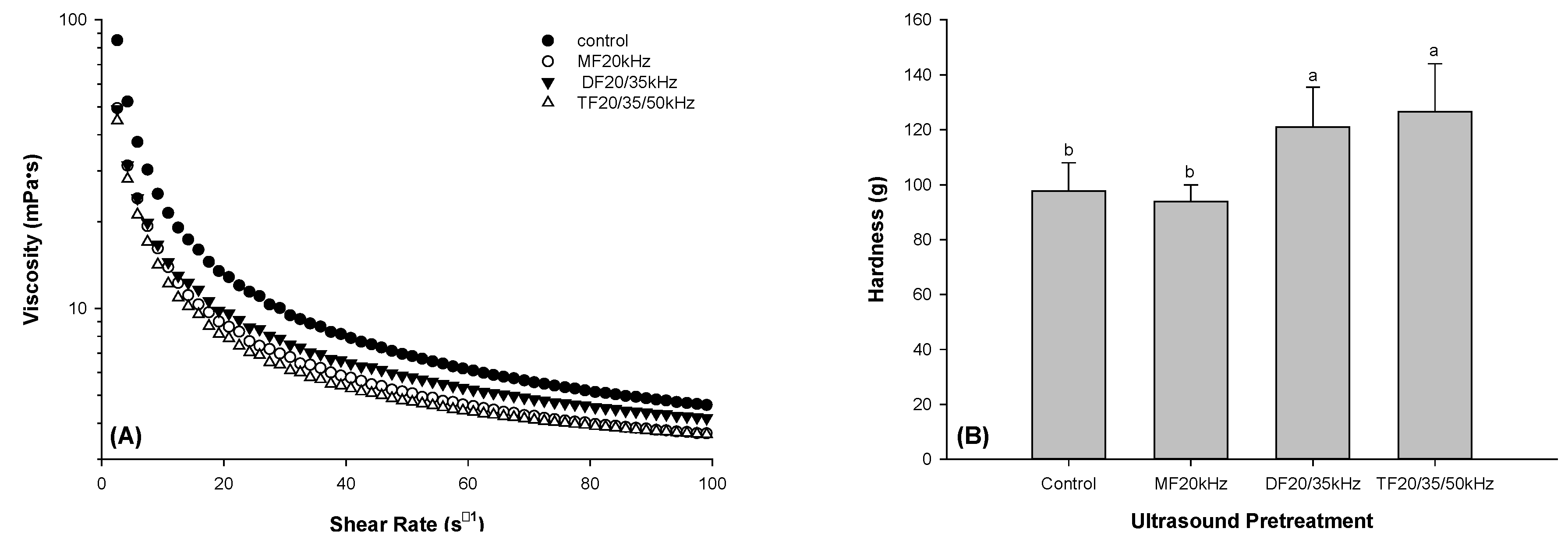
Ultrasound is known to decrease the protein viscosity by reducing the size of protein particles [
42]. The reduction in particle size could be due to the unfolding and breakage of intermolecular hydrophobic bonds in the protein structure as an effect of acoustic cavitation. Our findings confirmed that multi-frequency US treatment decreased the viscosity of whey protein solutions compared to control, as shown in
Figure 1A. Whey protein solutions with TFU treatment showed a more thinning behavior than those with DFU pretreatment. However, with increasing shear, TFU and MFU showed a further decline in viscosity compared to DFU. It was unsurprising because DFU of 20 and 35 kHz might lower the acoustic cavitation effect. The higher thinning effect in TFU could be due to its maximizing acoustic cavitation effect of the three frequencies on the whey solution. Although TFU treatment exhibited similar thinning behavior to MFU treatment, it could be more efficient for spending less ultrasonic time.
3.2. Hardness of WPG
Figure 1B is a graphical representation of the hardness of GDL-induced WPG. It is evident from the graph that DFU and TFU pretreatment improved the hardness of gels. Compared with the control, the hardness of DFU and TFU pretreated WPG increased 23.8% and 29.4%, respectively. Our results agreed with existing literature [
28] using MFU treatment. US improvement in hardness might be due to US-induced protein unfolding, increasing the availability of more protein bonds to strengthen the gel [
43]. This was confirmed by previous studies on US-pretreated whey protein emulsion gel [
36,
44]. DFU-pretreated whey protein showed more protein unfolding than MFU, resulting in more rigid gels.
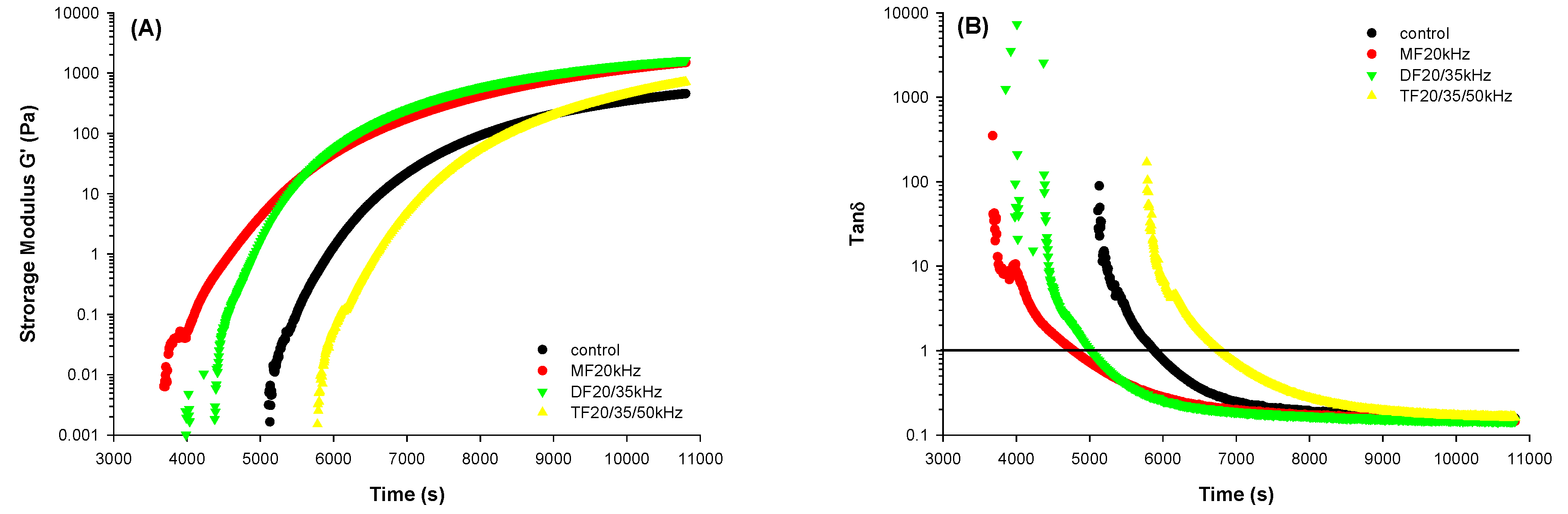
3.3. Rheological Properteis of WPG
Storage modulus (G’), which is a measure of gel rigidity, and tan delta (δ), which shows the viscoelastic property of gels, were studied (
Figure 2). US treatments resulted in an improvement in the storage modulus of gels. This finding is in agreement with existing literature [
28,
31], where the US caused significant improvement in the gel rigidity. DFU treatment gave a more rigid gel than MFU, which is in line with the findings of Cheng et al. (2019) [
44], where DFU improved the rigidity (higher G’) of whey protein emulsion gels. This effect has been attributed to enhanced unwinding in the protein structure induced by the US, as it increases the availability of free proteins involved in gelation, bridging, and incorporation into strengthening the gel structure[
36,
44].
Nonetheless, WPG with TFU treatment had a lower G’ than DFU and MFU. The reason might be that WPG with TFU treatment exhibited a later gelling time according to tanδ < 1 (
Figure 2B). The US is known to reduce the gelling time for gels [
31]. However, our findings from this study contrasted this for TFU samples, as control samples gelled faster than TFU treatments (
Figure 2B). It might be related to the different changes in protein structure. The gelling time affected the final G’. It was not surprising because the time sweep interval was set at a limited time and could only represent the initial step of gel formation. Extending the time might lead to higher G’. The hardness result could be evident because G’ was positively related to the hardness of gels [
28,
44].
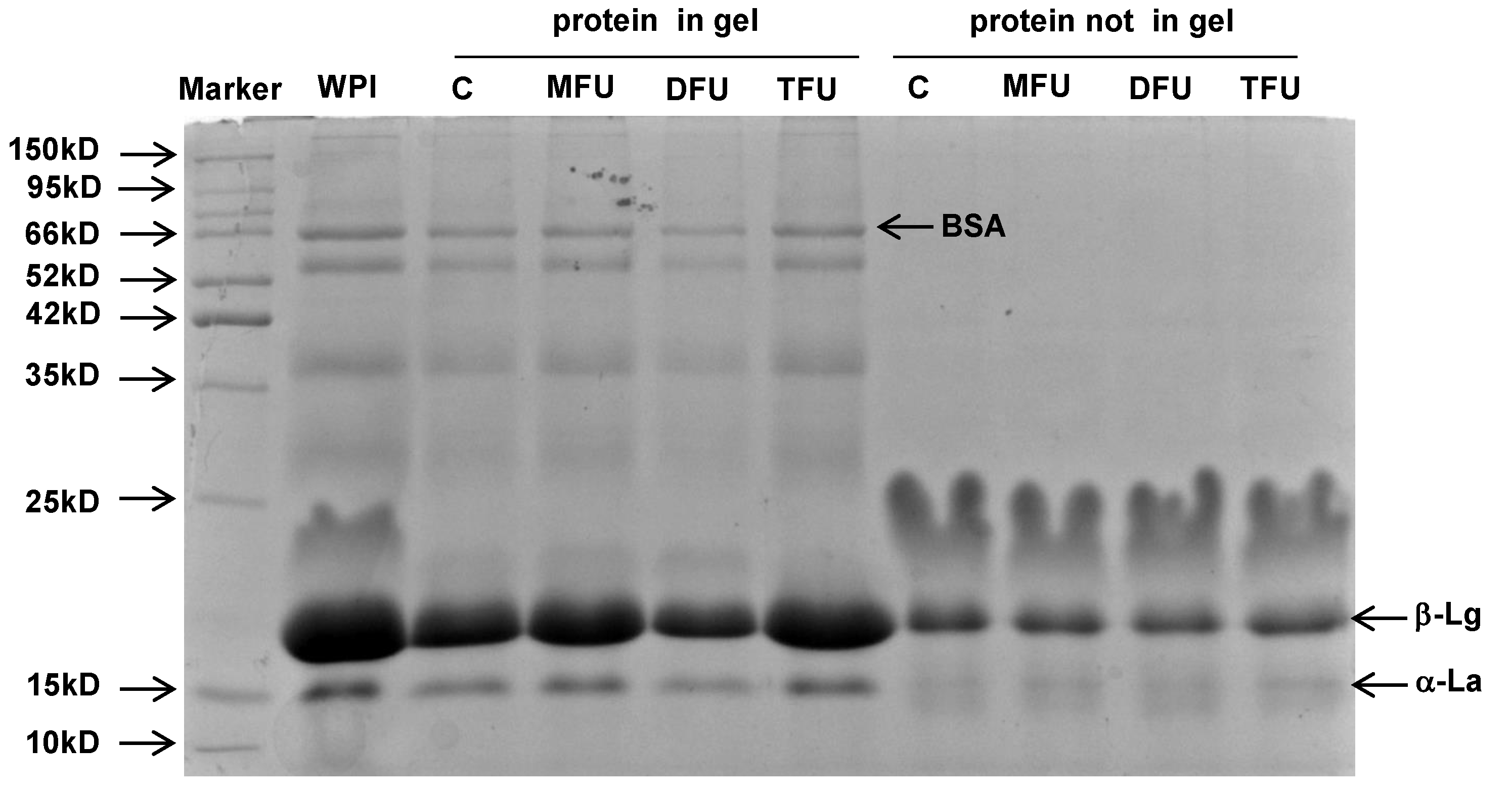
3.4. Protein Distribution in Whey Protein Gel
To identify the proteins that were involved in gel formation, SDS-PAGE was carried out on US and control GDL-WPG after centrifugation (
Figure 3). The results indicated that β-lactoglobulin was the main active protein in structuring the gel network regardless of treatment. BSA and α-lactalbumin being present in the gel of all samples indicated that they were responsible for strengthening and filling up the gel matrix. This finding demonstrated that the US had no effect on the primary structure of proteins.
3.5. Turbidity and Chemical Forces
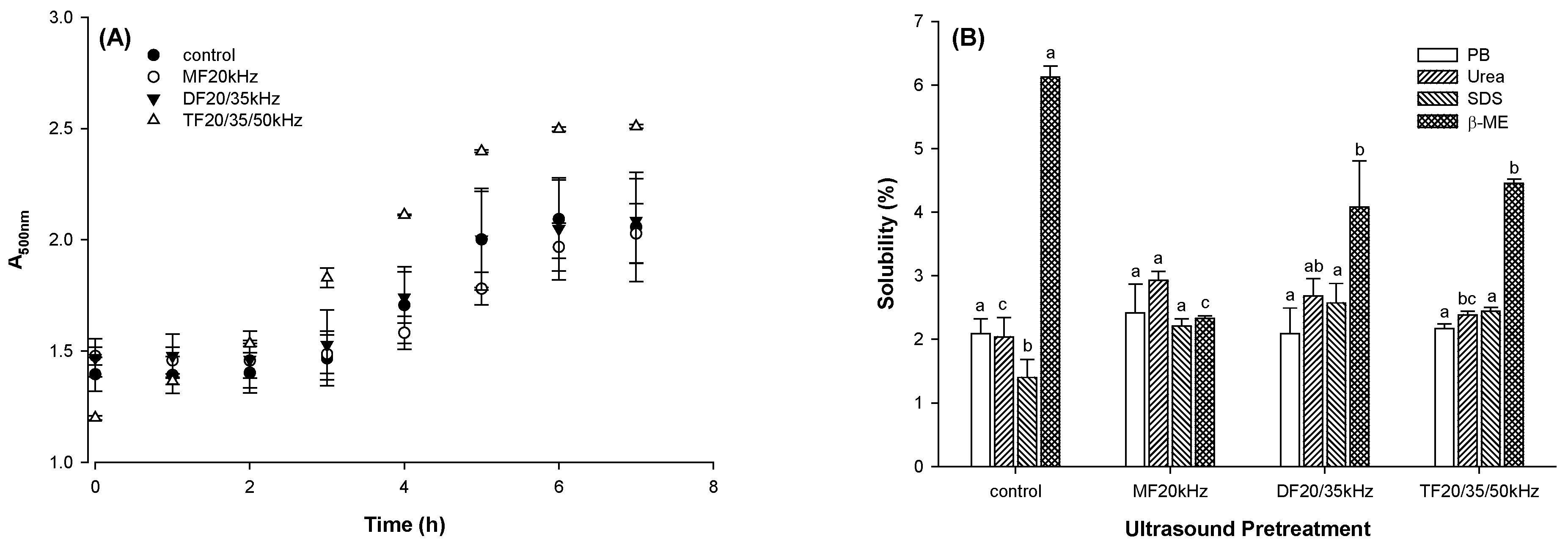
3.5.1. Turbidity
The turbidity of the diluted protein solution was used to indicate the kinetic of protein aggregation during gel formation, as shown in
Figure 4A. The kinetic model could fit a sinusoidal curve, indicating three stages of gel formation. From the onset of gel formation to 2 hours, there were little changes in turbidity for control, MFU, and DFU. Beyond this, the turbidity of TFU increased with time. It is worth noting that the turbidity of DFU was similar to that of control. Although TFU was less turbid from the onset of gel formation, this treatment produced the most turbid gel at the end of the 8 hours. It suggested that TFU pretreatment led to more and larger fine protein aggregates. This consisted of the gel hardness results, which TFU resulted in more rigid gels. Because those protein aggregates from TFU samples could serve as sub-units and support the compact particle gel network. The extended exponential growth stage of TFU-treated samples suggested they took longer to form a gel network, agreeing with the above rheological results that TFU-treated samples had longer gelation time.
3.5.2. Chemical Forces
The chemical forces between protein molecules supported protein aggregates and gel networks. A solubility test was used to determine the possible forces stabilizing the gels in four different solvents (
Figure 4B). Compared with the control, US generally increased gel solubility in urea and SDS (p < 0.05), indicating that US increased the number of hydrogen bonds and hydrophobic interactions in WPG. While US decreased gel solubility in
β-ME (p < 0.05), indicating the US decreased the content of disulfide linkages. US treatment might have resulted in disulfide bond cleavages and sulphydryl group oxidation [
28,
45], reducing the total number of disulfide bonds available for gel formation when compared to control. Disrupting disulfide bonds might improve the molecular flexibility. It could increase the hydrogen bond and hydrophobic interactions between protein molecules. Hydrogen bonds and hydrophobic interactions are short-range forces. An increase in those chemical forces might lead to a shortened molecular distance. That could result in a compact gel network and strengthening gel texture.
For US pretreated gels, MFU gels exhibited higher solubility in Urea than TFU gels(p < 0.05), showing that hydrogen bond content in MFU gels was higher than that in TFU gels. In contrast, disulfide content in TFU gels was higher than in MFU gels according to the solubility in
β-ME (p < 0.05). MFU, DFU, and TFU gels had little difference in hydrophobic bonds supporting the gel structure. The reason might be that MFU, DFU, and TFU treatment resulted in little difference in the whey protein tertiary structure, as revealed by intrinsic fluorescence [
35].
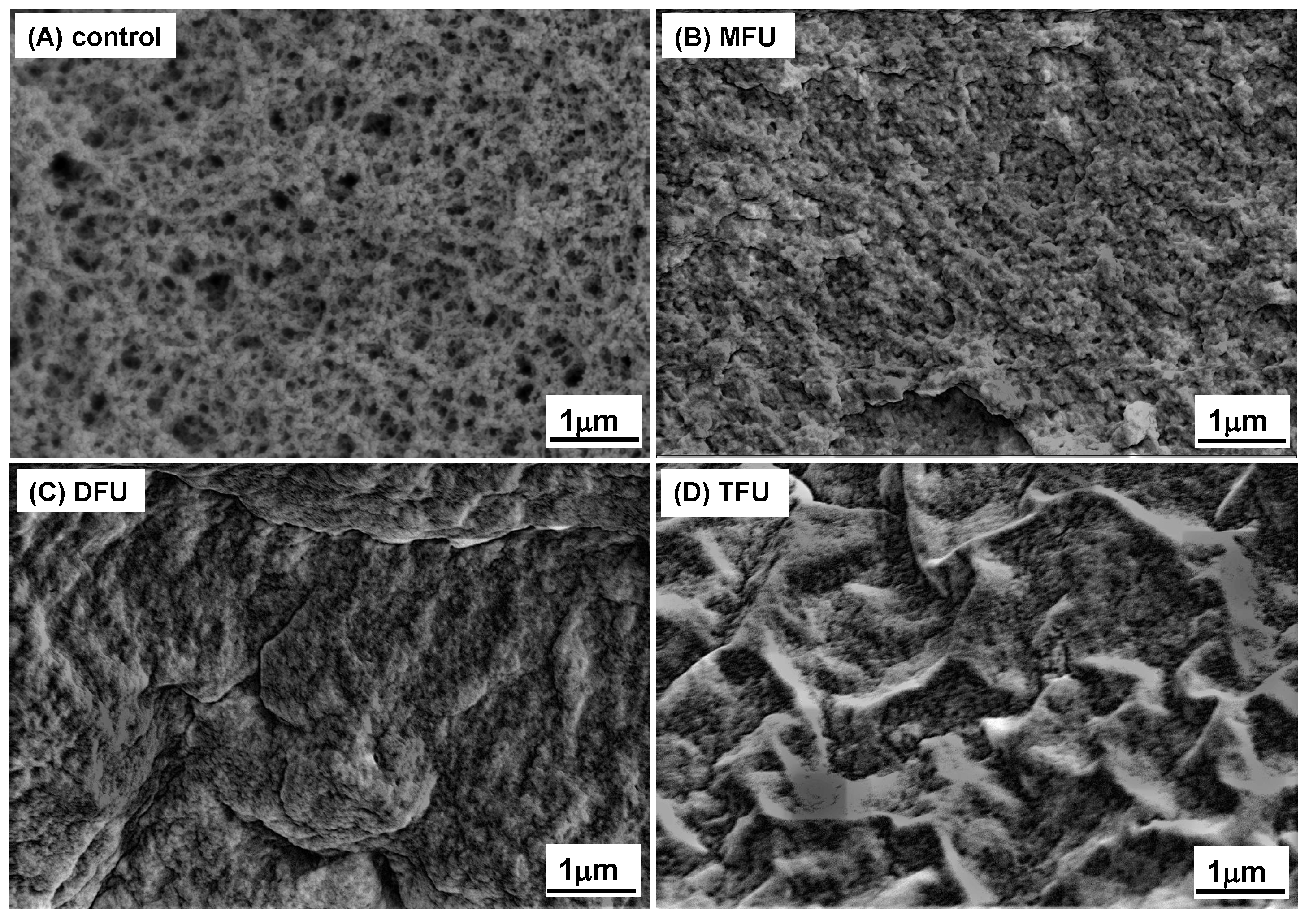
3.6. Microstructure of GDL-Induced Whey Protein Gel
SEM was used to study the gel
s’ microstructure, as shown in
Figure 5. Our findings showed that the US could modify the microstructure of whey protein gel, as previously reported [
45,
46]. It was demonstrated that MFU, DFU, and TFU treatment produced compact gel microstructures and smaller pore sizes relative to the control, which exhibited larger pore sizes. The compactness of gels is known to influence the hardness because a compact structure could resist deformation. DFU and TFU produced a more uniform homogenous gel network and dense particle cross-linking. It agreed with the discussion on chemical forces above. This finding explained the hardness results. TFU and DFU gave better results, with control samples yielding the softest gels. The increased compactness after US treatment could be due to unfolded protein structure [
36,
44], increased hydrogen bonds, and hydrophobic interactions in gel formation (
Figure 4B). The higher short-range forces could increase intermolecular attraction by shortening the intermolecular distance, resulting in tiny pore sizes.Moreover, the US-induced microstructure changes might have resulted from the type of interactions involved in gel formation and different aggregation rates [
47].
3.7. Digestion of GDL-Induced Whey Protein Gel
Our results showed that the US affected the microstructure of gels. Moreover, the structure of food plays an essential role in digestion, according to Dupont et al. (2018) [
34]. With this idea, simulated digestion of WPG samples was carried out for oral and gastrointestinal phases.
3.7.1. Physical Digestion of W.P.G.
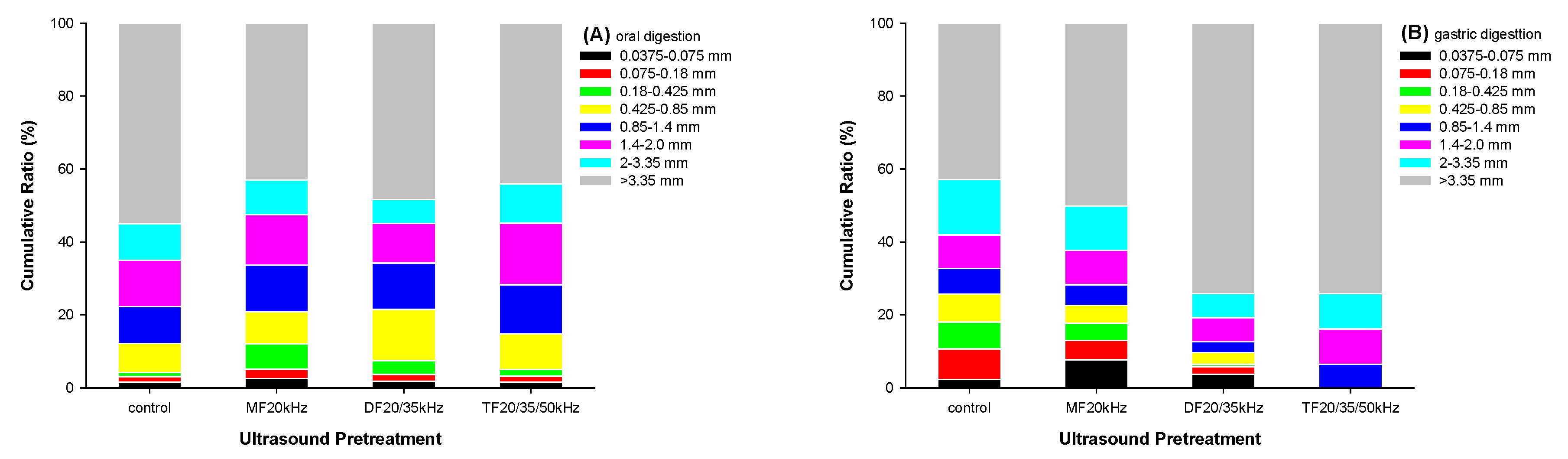
Physical digestion of WPG during oral and gastric phases was investigated by sieving and estimated with a particle size distribution, as shown in
Figure 6. After simulated mastication, US-pretreated WPG samples produced more large fragments (> 2.0 mm) while fewer small fragments (< 0.425 mm) than the control (
Figure 6A). It agrees with other research findings [
48,
49], where softer gels produced larger fragments than more rigid gels.
After 2 hours of gastric digestion in the presence of pepsin, the weight percentage of DFU and TFU gastric fragments lower than 3.35 mm decreased by 48.1% and 52.5%, respectively, compared with oral fragments. By contrast, the weight percentage of the control sample gastric fragments higher than 3.35 mm decreased by 23.9%. It suggested that large boluses in the hard gel could resist pepsin digestion. The reason might be the difference in gel microstructure. The softer control sample had a loose structure in the gel network with a large pore size. Pepsin was easy to diffuse inside the gel network and contact with whey the protein aggregates. It could degrade the large fragments in the boluses by attacking the protein aggregate interior and hydrolyzing the whey protein, fragmentizing the large boluses into small fragments.
On the contrary, the harder DFU and TFU WPG samples had a dense gel network with tiny pore sizes. The low porosity of the gel network might disturb pepsin diffusing into the protein aggregate interior, resulting in pepsin partitioning at the surface of protein aggregates [
35]. It hindered the large boluses from enzymatic degradation, remaining the oral digesta in large size. On the other hand, US treatment could improve the digestion of whey protein [
35,
36], and fragments in small sizes decreased fast in US samples. Since the gastric chyme not exceeding 1.4 mm was subjected to the intestinal phase, little solid particles existed in the intestinal digesta. The existence of large fragments in gastric chyme could delay gastric emptying. It might affect the digestion characteristic of whey protein.
3.4.2. Chemical Digestion of WPG
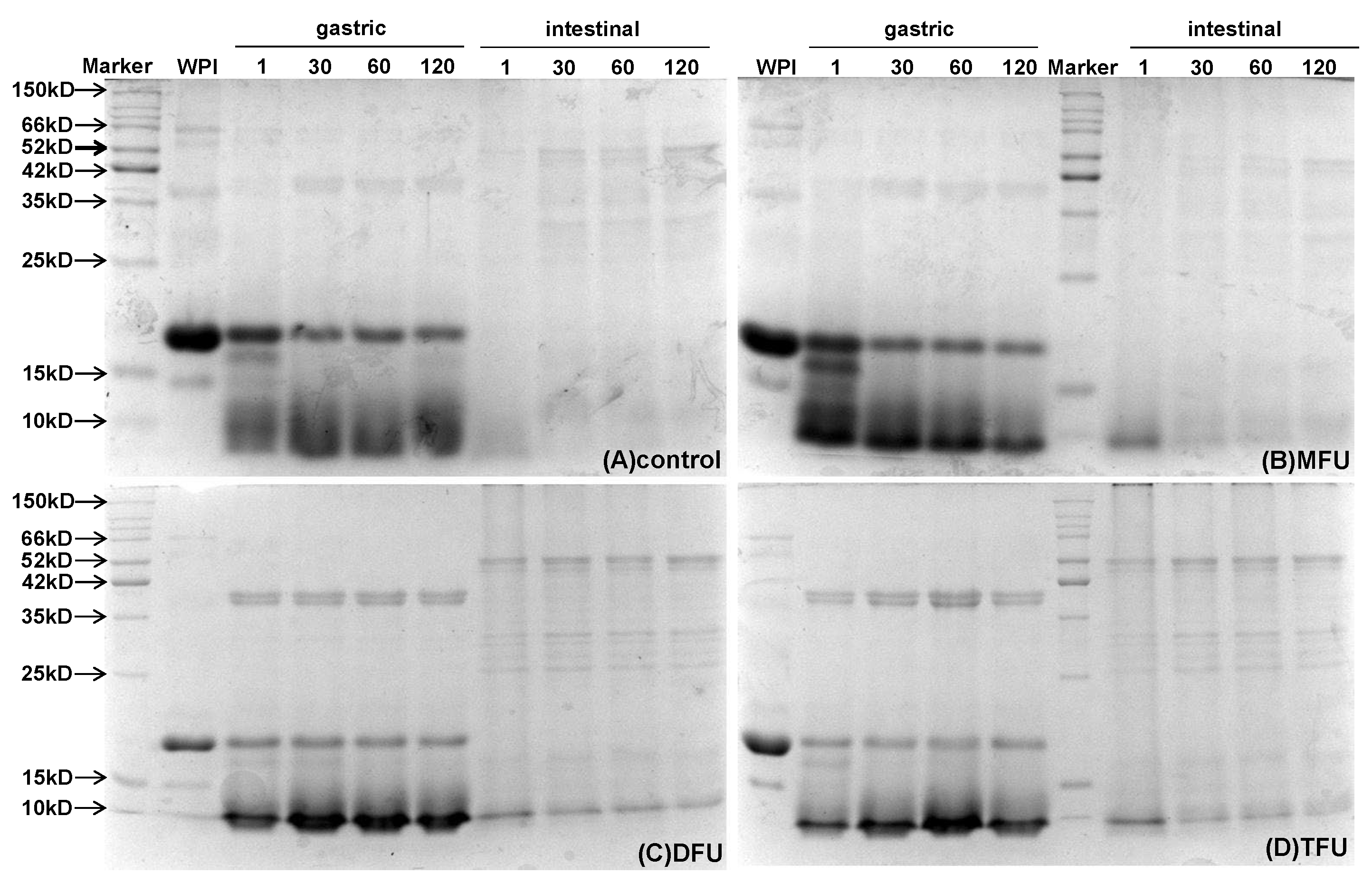
SDS-PAGE was used to monitor the progress of WPG digestion at different times (
Figure 7). Bands corresponding to β-lactoglobulin were persistent throughout the gastric digestion (2 h), indicating the resistance of gel structure to gastric digestion for all treatments. By contrast, the band of α-lactalbumin disappeared quickly when gastric digestion started. The bands of DFU and TFU were fainter than MFU with the progression of gastric digestion. DFU and TFU samples displayed light bands of β-lactoglobulin. It might be due to the resistance of gel structure and sensitive US-treated whey protein to pepsin hydrolysis. Those were in agreement with the results for particle size distribution. There were, however, no bands right from the onset of intestinal digestion, as shown in all samples. This pattern concurs with the literature that whey protein is a ‘fast protein’ hydrolyzed faster in the intestine and resistant to gastric digestion [
48]. Thus, the US affected gastric digestion by its effect on gel structure but not intestinal digestion.
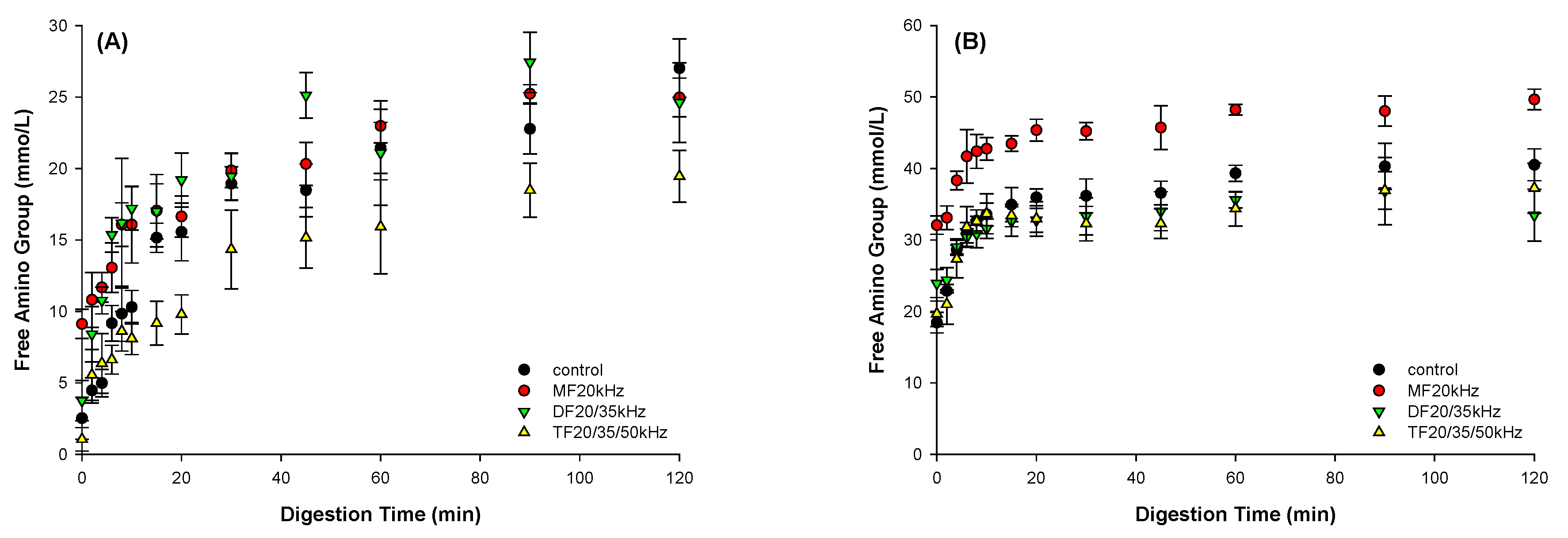
The hydrolysis of MFU and DFU released more amino groups than TFU throughout gastric digestion, as shown in
Figure 8A, with the available amino group content ranging from 27.0 mmol/L (Control) to 19.4 mmol/L (TFU). This result indicated that the US influenced the degree of amino acid bioavailability. Increasing the frequency delayed gastric release of the amino group. This could be due to the inhomogeneous microstructure of the TFU gels, which minimizes pepsin diffusion in the gel matrix. It is in line with Guo et al. (2017) [
50], where gels with more rigid structures showed limited protein hydrolysis and a lower amino group release from the gel matrix. The structure of MFU best facilitated the release of amino groups compared to other treatments, including control during the intestinal phase (
Figure 8B). It might be due to its coarse microstructure. The results show that MFU facilitated digestion with high amino group release compared to control, while TFU delayed gastric digestion.
4. Conclusions
This study showed that multi-frequency US could improve whey protein gels’ textural and rheological properties and digestion. DFU and TFU gave better gel properties on hardness and microstructure than MFU. Regarding digestion, the release of the amino group was higher in MFU-treated WPG due to its softer texture and coarse microstructure. Findings from this study showed that using the multi-frequency US as a food processing technique has implications for developing innovative protein gel products for nutrient delivery. It is recommended that the digestion pattern and kinetics of foods treated with US be investigated. As the static digestion model was used in this study, the physical fragment of WPG solid during the gastric phase was not considered. The dynamic digestion model will be used in further research to get more knowledge on the role of multi-frequency US.
Supplementary Materials
none
Author Contributions
Yu Cheng: Conceptualization, Methodology, Investigation, Visualization, Writing – review & editing, Supervision, Funding acquisition; Xiaolong Shi: Validation, Formal analysis, Data curation, Writing – original draft. Georgina Benewaa Yeboah: Investigation, Data curation, Writing – original draft; Lihong Chen: Writing – review & editing; Juan Wu: Conceptualization, Writing – review & editing, Supervision. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (GrantsNo. 32072349), the Young Backbone Teachers Program of Jiangsu University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
Data available on request from the authors.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cao, Y.P.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Hebishy, E.; Du, H.; Brito-Oliveira, T.C.; Pinho, S.C.; Miao, S. Saltiness perception in gel-based food systems (gels and emulsion-filled gels). Crit. Rev. Food Sci. Nutr. 2023, 18. [Google Scholar] [CrossRef]
- Nascimento, L.G.L.; Odelli, D.; de Carvalho, A.F.; Martins, E.; Delaplace, G.; Peixoto, P.P.D.; Silva, N.F.N.; Casanova, F. Combination of Milk and Plant Proteins to Develop Novel Food Systems: What Are the Limits? Foods 2023, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, S.; Kasapis, S.; Dokouhaki, M. Diffusional characteristics of food protein-based materials as nutraceutical delivery systems: A review. Trends Food Sci. Technol. 2022, 122, 201–210. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Alvi, T.; Biswas, A.; Shityakov, S.; Gusinskaia, T.; Lavrentev, F.; Dutta, K.; Khan, M.K.I.; Stephen, J.; Radhakrishnan, M. Food gels: principles, interaction mechanisms and its microstructure. Crit. Rev. Food Sci. Nutr. 2023, 63, 12530–12551. [Google Scholar] [CrossRef] [PubMed]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, C.; Zhao, J.; Guo, F.; Qin, J.; Wang, Y. Hyaluronic acid modulates techno-functional and digestion properties of heat-induced ginkgo seed protein isolate gel. Food Bioscience 2024, 59, 104204. [Google Scholar] [CrossRef]
- Cheng, Y.; Ye, A.Q.; Singh, H. Characterizations of emulsion gel formed with the mixture of whey and soy protein and its protein digestion under in vitro gastric conditions. Curr. Res. Food Sci. 2024, 8, 10. [Google Scholar] [CrossRef]
- Grasberger, K.; Hammershoj, M.; Corredig, M. Lupin protein-stabilized oil droplets contribute to structuring whey protein emulsion-filled gels. Food Res. Int. 2024, 178, 10. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ge, H.F.; Zhao, J.; Liu, C.Q.; Wang, Y.S. L-Theanine Improves the Gelation of Ginkgo Seed Proteins at Different pH Levels. Gels 2024, 10, 14. [Google Scholar] [CrossRef]
- Chen, W.Q.; Ding, Y.H.; Zhao, Y.M.; Ma, H.L. Strategies to improve the emulsification properties of rice proteins as a promising source of plant-based emulsifiers: An updated mini-review. Food Bioscience 2023, 53, 8. [Google Scholar] [CrossRef]
- Kang, D.C.; Zhang, W.A.; Lorenzo, J.M.; Chen, X. Structural and functional modification of food proteins by high power ultrasound and its application in meat processing. Crit. Rev. Food Sci. Nutr. 2021, 61, 1914–1933. [Google Scholar] [CrossRef] [PubMed]
- Pinton, M.B.; dos Santos, B.A.; Lorenzo, J.P.M.; Cichoski, A.J.P.; Boeira, C.P.; Campagnol, P.C.B. Green technologies as a strategy to reduce NaCl and phosphate in meat products: an overview. Curr. Opin. Food Sci. 2021, 40, 1–5. [Google Scholar] [CrossRef]
- Taha, A.; Mehany, T.; Pandiselvam, R.; Siddiqui, S.A.; Mir, N.A.; Malik, M.A.; Sujayasree, O.J.; Alamuru, K.C.; Khanashyam, A.C.; Casanova, F.; et al. Sonoprocessing: mechanisms and recent applications of power ultrasound in food. Crit. Rev. Food Sci. Nutr. 2022, 39. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.G.; Tiliwa, E.S.; Yan, W.Q.; Azam, S.M.R.; Wei, B.X.; Zhou, C.S.; Ma, H.L.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2022, 152, 19. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Ashokkumar, M. Theory of Sonochemistry. Top. Curr. Chem. 2016, 374, 28. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Oliyer, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef]
- Yusof, N.S.M.; Anandan, S.; Sivashanmugam, P.; Flores, E.M.M.; Ashokkumar, M. A correlation between cavitation bubble temperature, sonoluminescence and interfacial chemistry - A minireview. Ultrason. Sonochem. 2022, 85, 10. [Google Scholar] [CrossRef]
- Chen, H.L.; Xu, B.G.; Zhou, C.S.; Yagoub, A.A.; Cai, Z.; Yu, X.J. Multi-frequency ultrasound-assisted dialysis modulates the self-assembly of alcohol-free zein-sodium caseinate to encapsulate curcumin and fabricate composite nanoparticles. Food Hydrocolloids 2022, 122, 14. [Google Scholar] [CrossRef]
- Dabbour, M.; Jiang, H.; Mintah, B.K.; Wahia, H.; He, R.H. Ultrasonic-assisted protein extraction from sunflower meal: Kinetic modeling, functional, and structural traits. Innov. Food Sci. Emerg. Technol. 2021, 74, 10. [Google Scholar] [CrossRef]
- Qayum, A.; Rashid, A.; Liang, Q.F.; Wu, Y.; Cheng, Y.; Kang, L.X.; Liu, Y.X.; Zhou, C.W.; Hussain, M.; Ren, X.F.; et al. Ultrasonic and homogenization: An overview of the preparation of an edible protein-polysaccharide complex emulsion. Compr. Rev. Food. Sci. Food Saf. 2023, 22, 4242–4281. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Ma, H.L.; Wang, Y.Y. Recent advances in modified food proteins by high intensity ultrasound for enhancing functionality: Potential mechanisms, combination with other methods, equipment innovations and future directions. Ultrason. Sonochem. 2022, 85, 12. [Google Scholar] [CrossRef] [PubMed]
- Glover, Z.J.; Gregersen, S.B.; Wiking, L.; Hammershoj, M.; Simonsen, A.C. Microstructural changes in acid milk gels due to temperature-controlled high-intensity ultrasound treatment: Quantification by analysis of super-resolution microscopy images. Int. J. Dairy Technol. 2022, 75, 321–328. [Google Scholar] [CrossRef]
- Bangar, S.P.; Esua, O.J.; Sharma, N.; Thirumdas, R. Ultrasound-assisted modification of gelation properties of proteins: A review. J. Texture Stud. 2022, 53, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gu, C.; Wei, R.; Luan, Y.; Liu, R.; Ge, Q.; Yu, H.; Wu, M. Enhanced gelling properties of myofibrillar protein by ultrasound-assisted thermal-induced gelation process: Give an insight into the mechanism. Ultrason. Sonochem. 2023, 94, 106349. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Ma, H.L.; Wang, K.; Azam, S.M.R.; Wang, Y.Y.; Zhou, J.; Qu, W.J. Ultrasound frequency effect on soybean protein: Acoustic field simulation, extraction rate and structure. LWT-Food Sci. Technol. 2021, 145, 10. [Google Scholar] [CrossRef]
- Xu, B.G.; Azam, S.M.R.; Feng, M.; Wu, B.G.; Yan, W.Q.; Zhou, C.S.; Ma, H.L. Application of multi-frequency power ultrasound in selected food processing using large-scale reactors: A review. Ultrason. Sonochem. 2021, 81, 15. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, C.H.; Guo, M.R. Effects of high intensity ultrasound on acid-induced gelation properties of whey protein gel. Ultrason. Sonochem. 2017, 39, 810–815. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalin, A.S. New approach for yoghurt and ice cream production: High-intensity ultrasound. Trends Food Sci. Technol. 2019, 86, 392–398. [Google Scholar] [CrossRef]
- Shi, R.J.; Li, T.; Wang, K.L.; He, Y.T.; Fu, R.X.; Yu, R.; Zhao, P.P.; Oh, K.C.; Jiang, Z.M.; Hou, J.C. Investigation of the consequences of ultrasound on the physicochemical, emulsification, and gelatinization characteristics of citric acid-treated whey protein isolate. J. Dairy Sci. 2021, 104, 10628–10639. [Google Scholar] [CrossRef]
- Gregersen, S.B.; Wiking, L.; Hammershoj, M. Acceleration of acid gel formation by high intensity ultrasound is linked to whey protein denaturation and formation of functional milk fat globule-protein complexes. J. Food Eng. 2019, 254, 17–24. [Google Scholar] [CrossRef]
- Tomczynska-Mleko, M.; Nishinari, K.; Mleko, S.; Terpilowski, K.; Pérez-Huertas, S. Cold gelation of whey protein isolate with sugars in an ultrasound environment. Food Hydrocolloids 2023, 139, 9. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yan, M.; Xue, S.Q.; Zhang, T.H.; Shen, X. Influence of ultrasound and enzymatic cross-linking on freeze-thaw stability and release properties of whey protein isolate hydrogel. J. Dairy Sci. 2022, 105, 7253–7265. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Le Feunteun, S.; Marze, S.; Souchon, I. Structuring food to control its disintegration in the gastrointestinal tract and optimize nutrient bioavailability. Innov. Food Sci. Emerg. Technol. 2018, 46, 83–90. [Google Scholar] [CrossRef]
- Cheng, Y.; Yeboah, G.B.; Guo, X.Y.; Donkor, P.O.; Wu, J. Gelling Characteristics of Emulsions Prepared with Modified Whey Protein by Multiple-Frequency Divergent Ultrasound at Different Ultrasonic Power and Frequency Mode. Polymers 2022, 14, 15. [Google Scholar] [CrossRef]
- Cheng, Y.; Donkor, P.O.; Yeboah, G.B.; Ayim, I.; Wu, J.; Ma, H.L. Modulating the in vitro digestion of heat-set whey protein emulsion gels via gelling properties modification with sequential ultrasound pretreatment. LWT-Food Sci. Technol. 2021, 149, 10. [Google Scholar] [CrossRef]
- Mao, C.; Wu, J.; Zhang, X.; Ma, F.; Cheng, Y. Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature. Foods 2020, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Cheng, Y. Mechanical Properties, Microstructure, and In Vitro Digestion of Transglutaminase-Crosslinked Whey Protein and Potato Protein Hydrolysate Composite Gels. Foods 2023, 12, 2040. [Google Scholar] [CrossRef]
- You, J.; Liu, C.; Zhao, J.; Guo, F.; Wang, Y. pH dominates the formation of ginkgo seed protein and whey protein composite gels. Food Bioscience 2024, 60, 104245. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food – an international consensus. Food & Function 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. International Dairy Journal 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Liang, Z.Q.; Zhang, C.Y.; Hao, S.Q.; Han, H.Y.; Du, P.; Li, A.L.; Shao, H.; Li, C.; Liu, L.B. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT-Food Sci. Technol. 2021, 152, 9. [Google Scholar] [CrossRef]
- Ramı́rez-Suárez, J.C.; Xiong, Y.L. Effect of transglutaminase-induced cross-linking on gelation of myofibrillar/soy protein mixtures. Meat Science 2003, 65, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Donkor, P.O.; Ren, X.F.; Wu, J.; Agyemang, K.; Ayim, I.; Ma, H.L. Effect of ultrasound pretreatment with mono-frequency and simultaneous dual frequency on the mechanical properties and microstructure of whey protein emulsion gels. Food Hydrocolloids 2019, 89, 434–442. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Xu, X.; Qin, L.; Wu, C.; Du, M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019, 121, 247–256. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Razavi, S.M.A.; Varidi, M. Sequential ultrasound and transglutaminase treatments improve functional, rheological, and textural properties of whey protein concentrate. Innov. Food Sci. Emerg. Technol. 2017, 43, 207–215. [Google Scholar] [CrossRef]
- Kharlamova, A.; Nicolai, T.; Chassenieux, C. Heat-induced gelation of mixtures of casein micelles with whey protein aggregates. Food Hydrocolloids 2019, 92, 198–207. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. Effect of gel structure on the gastric digestion of whey protein emulsion gels. Soft Matter 2014, 10, 1214–1223. [Google Scholar] [CrossRef]
- Luo, N.; Ye, A.; Wolber, F.M.; Singh, H. In-mouth breakdown behaviour and sensory perception of emulsion gels containing active or inactive filler particles loaded with capsaicinoids. Food Hydrocolloids 2020, 108, 106076. [Google Scholar] [CrossRef]
- Guo, Q.; Bellissimo, N.; Rousseau, D. Role of gel structure in controlling in vitro intestinal lipid digestion in whey protein emulsion gels. Food Hydrocolloids 2017, 69, 264–272. [Google Scholar] [CrossRef]
Figure 1.
The viscosity of whey protein solution (A) and hardness of whey protein gel (B) Pretreated with mono, dual, and tri-frequency (MF, DF, TF) ultrasound.
Figure 1.
The viscosity of whey protein solution (A) and hardness of whey protein gel (B) Pretreated with mono, dual, and tri-frequency (MF, DF, TF) ultrasound.
Figure 2.
Storage modulus G’ (A) and tan δ (B) of whey protein solution pretreated with mono, dual, and tri-frequency (MF, DF, TF) ultrasound at different frequencies during time sweep.
Figure 2.
Storage modulus G’ (A) and tan δ (B) of whey protein solution pretreated with mono, dual, and tri-frequency (MF, DF, TF) ultrasound at different frequencies during time sweep.
Figure 3.
SDS-PAGE of protein distribution in whey protein gels with different ultrasound pretreatment (C: control without ultrasound, MFU: mono-frequency ultrasound, DFU: dual-frequency ultrasound, TFU: tri-frequency ultrasound).
Figure 3.
SDS-PAGE of protein distribution in whey protein gels with different ultrasound pretreatment (C: control without ultrasound, MFU: mono-frequency ultrasound, DFU: dual-frequency ultrasound, TFU: tri-frequency ultrasound).
Figure 4.
Turbidity of whey protein solution during gelation (A) and protein solubility of whey protein gel pretreated with mono, dual, and triple frequency (MF, DF, TF) ultrasound in different chemical solvents (B) (The symbols a-c indicated the significant difference of US pretreatment).
Figure 4.
Turbidity of whey protein solution during gelation (A) and protein solubility of whey protein gel pretreated with mono, dual, and triple frequency (MF, DF, TF) ultrasound in different chemical solvents (B) (The symbols a-c indicated the significant difference of US pretreatment).
Figure 5.
SEM images of whey protein gel prepared with different ultrasound pretreatment (control: without ultrasound, MFU: mono-frequency ultrasound, DFU: dual-frequency, TFU: tri-frequency ultrasound).
Figure 5.
SEM images of whey protein gel prepared with different ultrasound pretreatment (control: without ultrasound, MFU: mono-frequency ultrasound, DFU: dual-frequency, TFU: tri-frequency ultrasound).
Figure 6.
Particle size distributions in cumulative weight percentage of oral (A) and gastric (B) digesta from whey protein gel pretreated with mono, dual, and triple frequency (MF, DF, TF) ultrasound.
Figure 6.
Particle size distributions in cumulative weight percentage of oral (A) and gastric (B) digesta from whey protein gel pretreated with mono, dual, and triple frequency (MF, DF, TF) ultrasound.
Figure 7.
SDS-PAGE of liquid digesta extracted at different time (1, 30, 60, 120 min) of whey protein gel prepared with different ultrasound pretreatment during gastric and intestinal digestion (control: without ultrasound, MFU: mono-frequency ultrasound, DFU: dual-frequency ultrasound, TFU: tri-frequency ultrasound).
Figure 7.
SDS-PAGE of liquid digesta extracted at different time (1, 30, 60, 120 min) of whey protein gel prepared with different ultrasound pretreatment during gastric and intestinal digestion (control: without ultrasound, MFU: mono-frequency ultrasound, DFU: dual-frequency ultrasound, TFU: tri-frequency ultrasound).
Figure 8.
Effect of mono, dual, and triple frequency (MF, DF, TF) ultrasound pretreatment on free amino group content in the liquid extracted from WPG gastric (A) and intestinal (B) digesta.
Figure 8.
Effect of mono, dual, and triple frequency (MF, DF, TF) ultrasound pretreatment on free amino group content in the liquid extracted from WPG gastric (A) and intestinal (B) digesta.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).