Significance Statement
Observations of far fewer overwintering monarch butterflies, alongside apparent rebounds during the summer breeding season, have led to heated debate whether monarchs are truly endangered. We used ~2600 citizen scientist observations of monarch “roosts” – mass aggregations of fall-migrating monarchs – to assess whether they are struggling to reach Mexico. Positive effects of a warming and greening flyway were overwhelmed by unexplained declines in roost size of up to 80%, increasing all along the path of their arduous southern migration. This suggests that to save monarchs, we should focus on avoiding well-meaning efforts such as planting non-native milkweeds that foster parasites, sicken monarchs, and disrupt migration.
Main Text
Monarch butterflies have become a “flagship species” for insect conservation in North America, in large part because of their distinctive coloration and spectacular yearly migrations (1). In eastern North America, monarchs migrate each spring from overwintering colonies in Mexico, north to summer breeding grounds in the U.S. and Canada, over several generations, before returning to Mexico in the fall in one generation (2). It seems clear that monarch abundance has been in steep decline at the overwintering sites (3-8), and these declines have driven efforts by government and non-government agencies in the U.S. and Canada to move monarchs to endangered status (9-11). Yet, other monitoring datasets from the summer breeding range suggest little to no change in monarch population size (12, 13), supported by recent genomic analyses finding no evidence for a recent genetic bottleneck that would be consistent with population decline (14). Puzzlingly then, data from the summer therefore suggest no need for protected status for the species, in turn leading to great confusion and heated debate about what needs to be done, if anything, to “save” monarch butterflies (15).
One possible way to resolve the conundrum of fewer winter monarchs alongside relatively stable summer populations, could be substantial losses during the annual fall migration (16). Unfortunately, the magnitude of monarch fall migratory losses has been difficult to directly assess. A prior study attempted to do so using annual estimates of return-rates of tagged monarchs over time (17), though that study was challenged due to unstandardized search efforts for tagged monarch at winter colonies and questions about statistical approaches (18). Another effort demonstrated that greater plant drought stress along the migration route, likely correlated with nectar availability, correlated with fewer monarchs reaching overwintering sites in Mexico (19). While quite compelling, in that study monarch population size during fall migration could not be directly measured.
Migrating monarchs do not fly at night, and so each evening during the 2-month journey, they settle in trees or shrubs, where they remain until at least the next morning (20). These roosts can be composed of dozens to thousands of monarchs (see Fig. S1), and the size of monarch roosts, especially when considered across the entire fall flyway, may serve as a useful index of the overall size of the migratory generation that travels to Mexico. It remains unclear why monarchs roost sizes are so variable, but anecdotal reports suggest that southerly winds or rain that retard progress might encourage larger roosts (21). Of course, in the absence of a clear understanding of the underlying drivers of roosting behavior, it is difficult to correlate roost size with underlying population size.
Here, we utilized a unique, and previously untapped resource to infer monarch population size during the fall migration: reports of migratory roost sizes to the “Journey North” public science program (22). Since 2006, Journey North has encouraged citizen scientists to report the date, location and size of monarch roosts that they observe. This dataset now includes > 5000 observations of roost sizes across the eastern fall migration route, from southern Canada into Mexico (Fig. S2). Our objective was two-fold: 1) characterize spatiotemporal changes in monarch roost size across the fall migration route and 2) test whether these trends are underlain by recent changes in weather (e.g., temperature, rainfall, wind speed and direction) and landscape (e.g., NDVI, a commonly-used proxy for nectar resources along the flyway) (19, 23).
Results
We compared a suite of models via model selection to test the following hypotheses: 1) monarch roosts are experiencing directional change over time, 2) roost changes vary spatially, and 3) spatiotemporal changes in roost size are underlain by variability in weather and landscape factors. Of the 39 models compared, a few key takeaways emerged. First, including a year effect greatly improved model fit (
Table 1). Second, a spatially varying year effect was preferred to a fixed year effect; that is, temporal trends varied across the flyway. And third, including covariates improved model fit and did not affect the spatiotemporal patterns found in the year-only models (Figure S3), indicating that the spatiotemporal roost trends reported here cannot be explained by the weather and landscape variables alone. We used a model averaging approach to summarize the models that outperformed the year-only model. For models lacking a given covariate, we used zero-centered posteriors (with a S.D. = 1) as a substitute, producing conservative estimates of covariate effects.
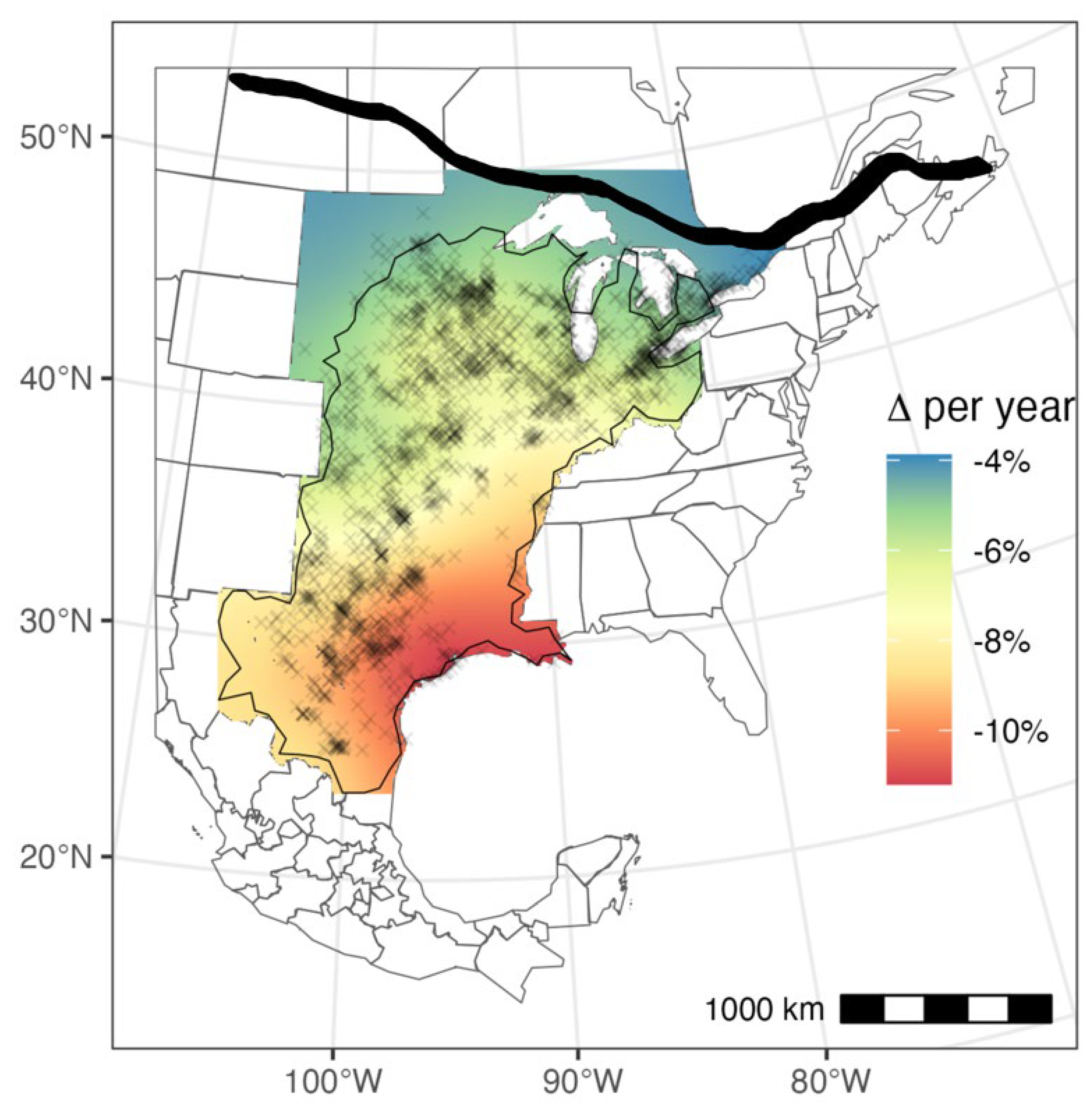
Across the flyway, we found that roost sizes were generally declining, with a median of 6.6% decline per year (95% Credible Intervals: [-11.2, -3.4]). Declines exhibited some spatial clustering, such that declines grew more severe toward the southern edge of the fall migration route (
Figure 1). However, the range parameter estimates were quite large (3190 km ± 1002 S.D.; Table S1), indicating relatively weak clustering and high synchrony in trends across the flyway. Nonetheless, information criteria ranked a spatially varying trend model higher than a fixed year effect model (
Table 1), providing support for a latitudinal gradient in roost size declines increasing in magnitude from north to south. Spatial surfaces of the median and 95% C.I. ranges for
κs,
αs, and
τs can be found in the Appendix (Figure S4).
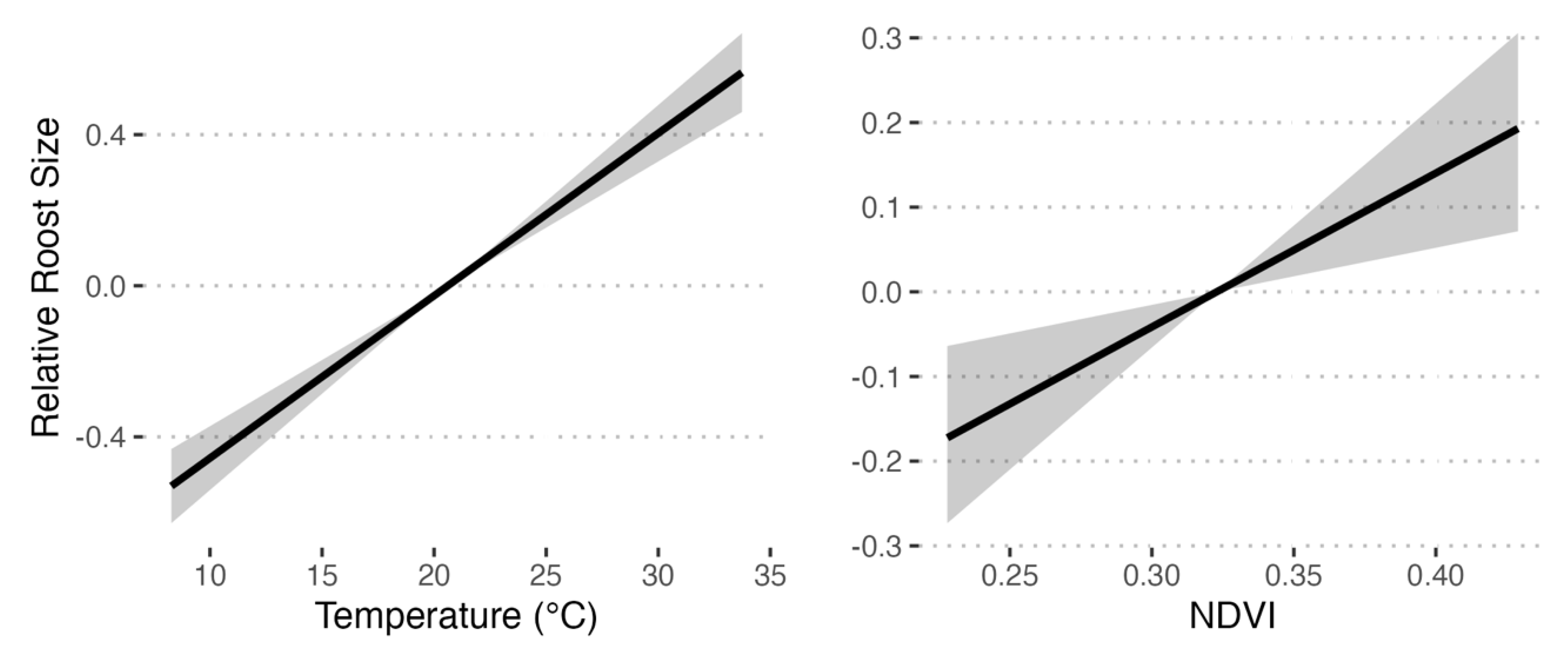
Of the models outperforming the year-only model, temperature was included in 83%, precipitation in 75%, tailwinds in 42%, NDVI in 42%, and the temperature by precipitation interaction in 25%. Averaged marginal posteriors for precipitation and tailwinds overlapped substantially with 0, whereas temperature and NDVI estimates were all or mostly greater than 0, respectively (Figure S5). Roost size tended to be larger under higher temperatures and greater NDVI values (
Figure 2). Temperatures and NDVI values were also greater in the southern and northern parts of the flyway, respectively, and each increased across the flyway (Figure S6). Moreover, despite these changes in climate and landscape features, the timing of peak monarch observations changed little over the 17-year period (Figure S6;
= 5.39,
P = 0.02; delayed 0.08 days/year).
Table 1.
Model selection results comparing the effects of temperature, precipitation, temperature*precipitation interaction, NDVI, and tailwinds on monarch roost size. Low ∆WAIC values are associated with relatively better models in terms of predictive performance and ∆WAIC values can be compared to assess relative support for the models compared. All models contained a spatially-structured intercept and an unstructured site-level intercept to account for spatial and site-level dependencies.
Table 1.
Model selection results comparing the effects of temperature, precipitation, temperature*precipitation interaction, NDVI, and tailwinds on monarch roost size. Low ∆WAIC values are associated with relatively better models in terms of predictive performance and ∆WAIC values can be compared to assess relative support for the models compared. All models contained a spatially-structured intercept and an unstructured site-level intercept to account for spatial and site-level dependencies.
| Model |
Year included? |
WAIC |
∆WAIC |
Weight |
| temp + NDVI + year (SVC) |
yes |
36600.03 |
0 |
0.575 |
| rain + temp + year (SVC) |
yes |
36603.23 |
3.2 |
0.116 |
| temp + year (SVC) |
yes |
36603.31 |
3.28 |
0.111 |
| wind + rain + temp + NDVI + year (SVC) |
yes |
36603.44 |
3.41 |
0.104 |
| wind + temp + year (SVC) |
yes |
36605.22 |
5.19 |
0.043 |
| rain*temp + year (SVC) |
yes |
36606.77 |
6.74 |
0.02 |
| rain + temp + NDVI + year (SVC) |
yes |
36607.41 |
7.38 |
0.014 |
| wind + rain + temp + year (SVC) |
yes |
36608.26 |
8.23 |
0.009 |
| rain*temp + wind + year (SVC) |
yes |
36609.79 |
9.76 |
0.004 |
| rain*temp + NDVI + year (SVC) |
yes |
36613.32 |
13.29 |
0.001 |
| rain + NDVI + year (SVC) |
yes |
36613.91 |
13.88 |
0.001 |
| wind + rain + year (SVC) |
yes |
36614.58 |
14.55 |
0 |
| year (SVC) |
yes |
36615.62 |
15.59 |
0 |
| rain*temp + wind + NDVI + year (SVC) |
yes |
36615.76 |
15.73 |
0 |
| wind + year (SVC) |
yes |
36618.12 |
18.09 |
0 |
| NDVI + year (SVC) |
yes |
36618.85 |
18.82 |
0 |
| wind + NDVI + year (SVC) |
yes |
36619.6 |
19.57 |
0 |
| rain + year (SVC) |
yes |
36621.2 |
21.17 |
0 |
| year (fixed) |
yes |
36622.62 |
22.59 |
0 |
| wind + rain + NDVI + year (SVC) |
yes |
36625.01 |
24.98 |
0 |
| rain*temp + NDVI |
no |
36652.71 |
52.68 |
0 |
| wind + rain + temp + NDVI |
no |
36657.42 |
57.39 |
0 |
| temp + NDVI |
no |
36657.55 |
57.52 |
0 |
| rain + temp + NDVI |
no |
36657.97 |
57.94 |
0 |
| wind + NDVI |
no |
36659.41 |
59.38 |
0 |
| rain*temp + wind + NDVI |
no |
36661.1 |
61.07 |
0 |
| NDVI |
no |
36662.23 |
62.2 |
0 |
| wind + rain + NDVI |
no |
36664.19 |
64.16 |
0 |
| rain + NDVI |
no |
36664.41 |
64.38 |
0 |
| temp |
no |
36668.88 |
68.85 |
0 |
| rain*temp + wind |
no |
36671.2 |
71.17 |
0 |
| wind + rain |
no |
36671.29 |
71.26 |
0 |
| wind |
no |
36671.56 |
71.53 |
0 |
| wind + temp |
no |
36672.35 |
72.32 |
0 |
| rain + temp |
no |
36673.57 |
73.54 |
0 |
| wind + rain + temp |
no |
36680.35 |
80.32 |
0 |
| intercept |
no |
36680.36 |
80.33 |
0 |
| rain*temp |
no |
36681.61 |
81.58 |
0 |
| rain |
no |
36682.89 |
82.86 |
0 |
Figure 1.
Map of monarch roost size trends with blue regions indicating stable roost patterns over time and red regions indicating relatively severe annual decline rates. Roost observation locations are marked with black X’s and regions where trends are significantly differed from zero (95% C.I.) are demarcated by the black polygon. Thick black line represents the estimated start of the monarch journey south for the northernmost summer populations.
Figure 1.
Map of monarch roost size trends with blue regions indicating stable roost patterns over time and red regions indicating relatively severe annual decline rates. Roost observation locations are marked with black X’s and regions where trends are significantly differed from zero (95% C.I.) are demarcated by the black polygon. Thick black line represents the estimated start of the monarch journey south for the northernmost summer populations.
Figure 2.
Predicted effects of temperature and NDVI on relative monarch roost size using weighted means of the marginal posterior distributions for each covariate. Weights were determined by relative model weight using WAIC information criteria. All models performing better than the year-only
model (
Table 1) were averaged. Predictions were then obtained by sampling the mean marginal posterior distributions of each covariate 5000 times and summarizing the median count across the range of covariate values (black line) ± 50% Credible Intervals (gray shaded region).
Figure 2.
Predicted effects of temperature and NDVI on relative monarch roost size using weighted means of the marginal posterior distributions for each covariate. Weights were determined by relative model weight using WAIC information criteria. All models performing better than the year-only
model (
Table 1) were averaged. Predictions were then obtained by sampling the mean marginal posterior distributions of each covariate 5000 times and summarizing the median count across the range of covariate values (black line) ± 50% Credible Intervals (gray shaded region).
Discussion
Using 17 years of citizen-reported sightings of migratory roosts and their estimated sizes, along with analyses of landscape characteristics and climate data, our study provides the most detailed and comprehensive picture to date of the health of the monarch fall migration in eastern North America. Our analysis shows that there may be two opposing trends operating simultaneously. On one hand, consistent with climate change extending summer conditions into the fall, the flyway is becoming warmer and greener over time, and these conditions correlated with larger monarch roost sizes (Fig. 2). This suggests that climate change might generally be benefiting monarch migration by creating milder flight conditions and enhancing nectar availability along the flyway (but see 24). However, once controlling for these increasingly beneficial weather conditions, our analyses revealed severe roost size declines through time that increased in magnitude from north to south (
Figure 1). That is, while roost sizes were declining throughout the flyway, these declines grew increasingly severe further along the migration route. This latitudinal gradient in roost size declines would be consistent with increasing mortality during migration and/or monarchs increasingly abandoning migration as they move south. This apparent disruption of migration might be the missing puzzle piece that explains relatively stable summer populations in the Midwest (12) and declining overwintering populations in Mexico (3).
An obvious limitation of our study is that roost size is determined, at least in part, by butterfly behavior, and does not necessarily correlate with monarch population size. For example, observation of 50% smaller roost size would not indicate overall population decline if there are twice as many roosting sites established. However, our analyses suggest that this scenario may be unlikely. Roost size was positively correlated with warmer and greener conditions (Fig. 2), and these same conditions were generally increasing during the years sampled by Journey North (Fig. S6). So, weather conditions were generally encouraging the formation of larger, rather than smaller, roosts. This is consistent with previous work linking inter-annual variation in NDVI in the southern portion of the midwestern flyway to variation in abundance in the overwintering colonies (19). Likewise, Diffendorfer et al. (25) found that land use, climate, and milkweed and nectar availability are relatively stable or actually improving over the last 20-30 years along the flyway in Texas and Mexico. A second possibility is that climate change is shifting roost formation outside of times when citizen-scientist observers would be expecting to see them, leading to increasing misalignment between when people are looking for and recording roosts, and when roosts are actually being formed. Here again our results suggest this explanation is unlikely, as the timing of peak roost observations appears relatively unchanged during the 17-year course of the data (Fig. S7).
Then what is driving the clear, dramatic declines in roost size seen in these data? We can only speculate, but the literature suggests at least four possibilities. First, prevalence of the monarch parasite Ophryocystis elektroscirrha (“OE”) has increased ca. 10-fold in North America since the early 2000s (26). Parasite infection reduces monarch wing strength and flight capacity, contributing to migratory dropout (27, 28). Second, concern about monarch decline has led to the widespread planting of non-native milkweeds such as Asclepias curassavica and Calotropis gigantea by homeowners and even land managers (29, 30). These host plants maintain vegetative growth later into the fall than native milkweeds, and this can pull monarchs out of their migratory phase (31). Also, monarch caterpillars feeding on tropical milkweeds tend to develop stunted wings (32) or accelerated metabolic rates (33) that limit later migratory ability. The longer leaf period of non-native milkweeds also allows accumulation of OE spores (34, 35). Third, well-meaning, concerned individuals are raising and releasing thousands of captive-reared monarch each year, which, compared to wild monarchs, are weaker (36), have reduced navigational ability (37-39), and thus exhibit reduced survival during migration (40). When these captive reared butterflies mate with wild monarchs, they may be diluting genes associated with effective migratory ability (37). Finally, year-round resident populations of monarchs appear to be increasingly common and growing along the western and southern edges of the species range (29). Monarch migration leads to a natural culling of monarchs weakened by OE infection or other flaws, and abandonment of migration by these year-round residents is associated with dramatically higher OE infection levels (34, 35), smaller wing and body sizes (41), and weakened dispersal abilities (42). Interbreeding between migratory and non-migratory monarchs, in turn, likely dilutes genes associated with migratory ability (37). Thus, one or all of these factors could be leading to increasingly higher mortality during travel, behavioral abandonment of migration, or both.
The factors triggering roost-forming behavior during fall migration have been a subject of some speculation (20, 43, 44) but relatively little study. A reasonable assumption is that these aggregations protect monarchs from predators and insulates them from rainy and/or cold weather, similar to the perceived value of forming mass overwintering aggregations in Mexico. That is, roosts form to allow monarchs to escape from ecological and meteorological threats. Our findings suggest that the largest roosts, in contrast, are formed during relatively benign warm weather, and in landscapes where green plants were relatively abundant. So, roosts might instead represent stopover locations serving as refueling sites. Indeed, we found that roosts were larger in sites with higher NDVI values, which is often used as a proxy for the availability of nectar resources along the flyway (19, 23).
Debate over the conservation status of monarch butterflies has largely focused on how to weigh two very different sets of population trends, with clear declines at overwintering sites in Mexico (3, 4, 8) but possible relative stability in the summer breeding range (despite clear summer declines in regions where use of the herbicide glyphosate is particularly high, for example) (12). This disparity in population trends for winter versus summer monarchs has led to confusion and controversy over the conservation status of monarchs, perhaps best exemplified by the recent listing, and then delisting, of monarchs as “endangered” by IUCN (45). Our findings suggest that steep declines among fall-migration monarchs might bring these seemingly irreconcilable trends into alignment (see also 7, 16, 46). Indeed, migrations of many other animals have been diminishing in the face of rapid global change (47). Our analyses of roost size data collected by citizen scientists and analyzed here, suggest that the original IUCN listing of an “endangered biological phenomenon” (48), but not necessarily an endangered species overall, may best fit monarch butterflies in North America. This idea was later reinforced by the late monarch researcher, Lincoln Brower (49). In fact, Dr. Brower once said that “the (potential) loss of the monarch migration would be like losing the Mona Lisa – both are cherished symbols of inspiration and wonderment” (50). It is perhaps unfortunate that well-meaning efforts to “save” monarchs by planting non-native milkweeds and releasing captive-reared butterflies might be contributing to the loss of the migration phenomenon. Instead, conservation efforts might best focus upon eliminating non-native milkweeds and ending captive rearing, and turning great effort to enhancing nectar resources along the southern flyway.
Materials and Methods
Roost Dataset. Since the 1990s, The Journey North program has provided an online platform for people to submit observations of events relevant to monarchs and other migratory animals, which are logged with date and site-specific GPS information and mapped in real time (
https://maps.journeynorth.org). Since 2003, the program has asked volunteers to provide a count of the number of monarch butterflies in each roost. The roost database consisted of 4984 records of roosts between 2003 and 2023. However, records were relatively sparse until 2007 (n = 52 total). So, to identify spatiotemporal patterns in roost size trends, we used data from 2007-2023 in our models to ensure adequate sample size and spatial coverage of the Midwest flyway (Figure S2). In some cases, multiple observations were recorded for a given location within a year. Because our goal was to characterize changes in peak roost size, we only used maximum roost size values recorded for a particular locale for a given year. We also excluded observations below 25.5° latitude where the distinction between migrating and overwintering roosts is unclear. A roost here is defined as an observation of at least five monarchs. Lastly, we only included observations recorded within the following Midwest flyway states: Nuevo León, Coahuila, and Tamaulipas in Mexico; Texas, Oklahoma, Kansas, Missouri, Arkansas, Louisiana, Iowa, Michigan, Minnesota, Ohio, Indiana, South Dakota, North Dakota, and Wisconsin in the United States; and Ontario in Canada. This resulted in a total of 2670 roost observations from 2007-2023, with a median of 134 unique site observations per year (min: 47 obs/yr, max: 384 obs/yr).
Modeling Spatiotemporal Variation in Roost Size and Determining Drivers. Our first objective was to assess directional changes in fall roost size through time and whether these changes varied spatially. If monarchs experience similar mortality or migratory behavior each year, then we would expect relatively stable roost size trends across the flyway. If mortality during migration is increasing or if migratory behavior is becoming increasingly disrupted over time, then we would expect roost size declines across the flyway. Moreover, these declines should become more pronounced from north to south if threats to migration have a cumulative effect on roost size during the journey. Using the analytical approach outlined in Meehan et al. (51), we modeled monarch roost size counts as a random variable from a negative binomial distribution with a spatially varying coefficient (SVC) model. The expected count has a log-linear predictor:
log(λst)=κs + αs + τsYearst
where the natural log of expected count, log(λst), at site s during year t, is modeled with a zero-centered, normally distributed intercept per site, κs, a spatially varying intercept, αs, and a spatially varying linear effect of year, τs. We used a penalized complexity prior (52) for κs, set such that the probability of the standard deviation associated with the random effect exceeding 1 is 0.01. The spatially structured effects are modeled as Gaussian random fields with Matérn covariance functions with range and variance parameters, and we used penalized complexity priors (52), with the probability of the range exceeding 500 km set to 0.5, and the probability of the standard deviation of the range exceeding 1 set 0.5 (53). Following trend model analysis, we used the modeling mesh to obtain an interpolated raster of posterior medians and 95% C.I. for κs, αs, and τs that can be mapped to visualize spatial variation in site effects, relative roost size, and relative roost size trends.
Determining Weather and Landscape Attributes that Influence Roost Size. Our second objective was to explain spatiotemporal patterns in roost size trends. We predicted that headwinds (southerly winds) would increase roost sizes by making migration more difficult, and wind speed might magnify this effect when winds come from the south. In contrast, winds from the north might reduce roost size by facilitating migration, and this might be exacerbated when northerly winds are stronger. Because monarch flight is restricted by cold temperatures and rainfall, temperature and precipitation were included as main effects and allowed to interact. That is, we anticipated that rain and cold might interact synergistically to increase roost size. The normalized difference vegetation index (NDVI) has been used as a proxy for the availability of nectar resources along the flyway (19). It also likely reflects variation in plant productivity due to variability in cumulative rainfall. Thus, we predicted that higher NDVI values reflect higher quality stopover conditions for monarchs.
Weather data associated with the roost locations were compiled using the Visual Crossing weather API (
https://www.visualcrossing.com/), which provides access to historic weather dailies using distance-based weighted means of available weather station data within a 50 km radius of the queried coordinates. Wind direction ranges from 0-360° and describes the direction of the wind source, with 0° and 180° indicating winds coming from the north and south, respectively. We combined wind direction and windspeed into a simple tailwind metric using the following equation: tailwind = speed*cos(θ), where θ is the direction of the wind source in radians (54). We assumed that wind coming from the north (i.e., 0°) was best for facilitating migration. We extracted and averaged weather data 7 days prior to the day of each roost observation (including the observation day). Same-day NDVI data were obtained from the NOAA Climate Data Record database using 0.05° resolution (55).
We then compared a suite of models we deemed plausible using the widely applicable information criteria (WAIC), which approximates out of sample prediction error (56). Lower WAIC values indicate lower error, and these models are considered relatively better fit after penalizing the models for parameter count to avoid overfitting. All models compared contained both an unstructured, site-level random effect (κs) and a spatially-structured random effect (αs). Covariates were combined using all possible additive combinations, with temperature and precipitation being allowed to interact These covariate-only models were compared to both year-only models, as well as covariate models containing year effects. The idea here is that if the spatiotemporal patterns were being driven by a combination of covariates, then the covariate-only models should perform better than, or as well as, the year-only models. However, if covariates and year-effect models perform best, then this would suggest that the covariates explain different axes of roost size variation. It is also particularly important to include both year and covariates in the same model because our model selection procedure does not include an exhaustive comparison of hypothetical roost size drivers. For instance, parasite infection is thought to be disrupting monarch migration (26), but we do not have comprehensive parasite load data to include in this modeling effort. Therefore, we may find spurious relationships between roost size and covariates if we do not include year as a stand-in variable for unknown sources of roost size variation. For covariates, we used default priors for covariates (log gamma distribution with shape parameter = 1 and a scale parameter = 0.00005).
To summarize the models compared, we averaged models that performed better than the year-only models (see
Table 1). To do this, we first calculated ∆WAIC values for each model (i.e., differences in WAIC between a model and the top model) to obtain the relative likelihood of each model using the following equation:
. The relative likelihoods were then divided by the sum of the relative likelihood to obtain model weights. These weights were used to compute a weighted average of the marginal posterior distributions of each covariate estimate, using zero-centered, normally distributed posteriors with a S.D. = 1 in place for the marginal posterior distribution when a model did not contain the covariate. This zero-centered approach biases the distributions towards 0 (i.e., shrinkage) to produce conservative covariate estimates.
All models were analyzed within a Bayesian framework using the R-INLA package and related wrapper functions using inlabru in R (51, 57-59).
Author Contributions
A.K.D., J.R.C., and W.E.S. designed and performed research; J.R.C. and W.E.S. analyzed data; A.K.D., J.R.C., and W.E.S. wrote the paper.
Data, Materials and Software Availability
Acknowledgments
We thank Nancy Sheehan for providing access to the data from the Journey North program, and for reviewing an early manuscript. We also thank the thousands of volunteers and community members who submitted observations of monarch roosts to the Journey North program over the years, which has allowed this project to advance scientific understanding of the monarch migration and will help to tailor conservation actions. We also are grateful to former Journey North director, Elizabeth Howard, and other members of the Journey North staff, who have worked tirelessly to organize and maintain the sightings databases. Finally, we are grateful to Tim Meehan, for providing expertise on statistical analyses of the roost data.
Competing Interest Statement
The authors declare no competing interest.
References
- K. M. Gustafsson, A. A. Agrawal, B. V. Lewenstein, S. A. Wolf, The monarch butterfly through time and space: the social construction of an icon. Bioscience Online Early -. (2015). [CrossRef]
- L. P. Brower, Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857-1995. Journal of the Lepidopterists' Society 49, 304-385 (1995).
- W. E. Thogmartin et al., Monarch butterfly population decline in North America: identifying the threatening processes. R. Soc. Open Sci. 4, 16 (2017). [CrossRef]
- L. P. Brower et al., Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect. Conserv. Divers. 5, 95-100 (2012).
- A. K. Davis, L. Dyer, Long-term trends in eastern North American monarch butterflies: a collection of studies focusing on spring, summer, and fall dynamics. Annals of the Entomological Society of America 108, 661-663 (2015). [CrossRef]
- L. A. Dyer, M. L. Forister, Wherefore and whither the modeler: understanding the population dynamics of monarchs will require integrative and quantitative techniques. Annals of the Entomological Society of America 109, 172-175 (2016). [CrossRef]
- H. Inamine, S. P. Ellner, J. P. Springer, A. A. Agrawal, Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125, 1081-1091 (2016). [CrossRef]
- J. Pleasants, W. E. Thogmartin, K. S. Oberhauser, O. R. Taylor, C. Stenoien, A comparison of summer, fall and winter estimates of monarch population size before and after milkweed eradication from crop fields in North America. Insect. Conserv. Divers. (2023). [CrossRef]
- USFWS, United States Fish and Wildlife Service Website - Assessing the status of the monarch butterfly https://www.fws.gov/savethemonarch/SSA.html. (2020).
- COSEWIC (2016) COSEWIC assessment and status report on the monarch Danaus plexippus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 59 pp. https://publications.gc.ca/collections/collection_2022/eccc/En14-475-2016-eng.pdf.
- IUCN, Status assessment of the migratory monarch butterfly, listed as Vulnerable, under criteria A2b. IUCN Red List of Threatened Species Website: https://www.iucnredlist.org/species/194052138/246096271.
- M. S. Crossley et al., Opposing global change drivers counterbalance trends in breeding North American monarch butterflies. Global Change Biology 28, 4726-4735 (2022). [CrossRef]
- L. Ries, D. J. Taron, E. Rendon-Salinas, The disconnect between summer and winter monarch trends for the eastern migratory population: possible links to differing drivers. Annals of the Entomological Society of America 108, 691-699 (2015). [CrossRef]
- J. H. Boyle et al., Temporal matches between monarch butterfly and milkweed population changes over the past 25,000 years. Current Biology 33, 3702-+ (2023). [CrossRef]
- E. Pennisi, Are monarchs endangered? Scientists debate as United States mulls protection. Science AAAS. https://www.science.org/content/article/are-monarchs-endangered-scientists-debate-united-states-mulls-protection.
- A. A. Agrawal, H. Inamine, Mechanisms behind the monarch's decline. Science 360, 1294-1296 (2018).
- O. R. Taylor et al., Evaluating the migration mortality hypothesis using monarch tagging data. Frontiers in Ecology and Evolution 7 Aug, 2020 (2020). [CrossRef]
- J. A. Fordyce, C. C. Nice, M. L. Forister, Commentary: evaluating the migration mortality hypothesis using monarch tagging data. Frontiers in Ecology and Evolution 8 (2020). [CrossRef]
- S. P. Saunders et al., Multiscale seasonal factors drive the size of winter monarch colonies. Proc. Natl. Acad. Sci. U. S. A. 116, 8609-8614 (2019). [CrossRef]
- A. K. Davis, N. P. Nibbelink, E. Howard, Identifying large- and small-scale habitat characteristics of monarch butterfly migratory roost sites with citizen-science observations. International Journal of Zoology Volume 2012, 9 pages (2012). [CrossRef]
- K. Davis, M. S. Garland, "Stopover ecology of monarchs in coastal Virginia: using ornithological methods to study monarch migration" in The monarch butterfly. Biology and Conservation, K. Oberhauser, M. Solensky, Eds. (Cornell University Press, Ithaca, NY, 2004), pp. 89-96.
- N. Sheehan, L. Weber-Grullon, Journey North – Monarch Butterfly and Milkweed observations by volunteer community scientists across Central and North America (1996-2020) ver 1. Environmental Data Initiative. [CrossRef]
- E. R. Zylstra, Ries. L., Neupane, N., Saunders, S.P., Ramírez, M.I., Rendón-Salinas, E., Oberhauser, K.S., Farr, M.T., and Zipkin, E.F. , Changes in climate drive recent monarch butterfly dynamics. Nat. Ecol. Evol. (2021). [CrossRef]
- A. F. Parlin et al., The cost of movement: assessing energy expenditure in a long-distant ectothermic migrant under climate change. J. Exp. Biol. 226 (2023). [CrossRef]
- J. E. Diffendorfer et al., Changes in landscape and climate in Mexico and Texas reveal small effects on migratory habitat of monarch butterflies (<i>Danaus plexippus</i>). Scientific Reports 14 (2024).
- A. Majewska, A. K. Davis, S. Altizer, J. C. de Roode, Parasite dynamics in North American monarchs predicted by host density and seasonal migratory culling. Journal of Animal Ecology 91, 780-793 (2022). [CrossRef]
- R. A. Bartel, K. S. Oberhauser, J. C. de Roode, S. M. Altizer, Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92, 342-351 (2011). [CrossRef]
- A. Bradley, S. Altizer, Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol. Lett. 8, 290-300 (2005). [CrossRef]
- Steele, I. G. Ragonese, A. A. Majewska, Extent and impacts of winter breeding in the North American monarch butterfly. Curr. Opin. Insect Sci. 59 (2023). [CrossRef]
- B. M. Mach, W. L. Long, J. C. Daniels, A. G. Dale, Aphid infestations reduce monarch butterfly colonization, herbivory, and growth on ornamental milkweed. PLoS One 18 (2023). [CrossRef]
- A. A. Majewska, S. Altizer, Exposure to non-native tropical milkweed promotes reproductive development in migratory monarch butterflies. Insects 10, 17 (2019). [CrossRef]
- J. Soule, L. E. Decker, M. D. Hunter, Effects of diet and temperature on monarch butterfly wing morphology and flight ability. J. Insect Conserv. 24, 961-975 (2020). [CrossRef]
- V. M. Pocius et al., Impacts of larval host plant species on dispersal traits and free-flight energetics of adult butterflies. Communications Biology 5 (2022). [CrossRef]
- A. Satterfield, J. C. Maerz, S. Altizer, Loss of migratory behaviour increases infection risk for a butterfly host. Proc. R. Soc. B-Biol. Sci. 282, 9 (2015). [CrossRef]
- A. Satterfield, F. X. Villablanca, J. C. Maerz, S. Altizer, Migratory monarchs wintering in California experience low infection risk compared to monarchs breeding year-round on non-native milkweed. Integr. Comp. Biol. 56, 343-352 (2016). [CrossRef]
- A. K. Davis, F. M. Smith, A. M. Ballew, A poor substitute for the real thing: captive-reared monarch butterflies are weaker, paler and have less elongated wings than wild migrants. Biol. Lett. 16, 5 (2020). [CrossRef]
- Tenger-Trolander, Environmental and genetic effects of captivity — are there lessons for monarch butterfly conservation? Curr. Opin. Insect Sci. 59, 1-8 (2023).
- Tenger-Trolander, M. R. Kronforst, Migration behaviour of commercial monarchs reared outdoors and wild-derived monarchs reared indoors. Proc. R. Soc. B-Biol. Sci. 287, 8 (2020). [CrossRef]
- Tenger-Trolander, W. Lu, M. Noyes, M. R. Kronforst, Contemporary loss of migration in monarch butterflies. Proc. Natl. Acad. Sci. U. S. A. 116, 14671-14676 (2019). [CrossRef]
- Steffy, Trends observed in fall migrant monarch butterflies (Lepidoptera: Nymphalidae) east of the Appalachian Mountains at an inland stopover in southern Pennsylvania over an eighteen year period. Annals of the Entomological Society of America 108, 718-728 (2015). [CrossRef]
- K. Davis, Monarchs reared in winter in California are not large enough to be migrants. Comment on James et al. First population study on winter breeding monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the urban South Bay of San Francisco, California. Insects 2021, 12, 946. Insects 13 (2022).
- D. G. James, M. C. Schaefer, K. K. Easton, A. Carl, First population study on winter breeding monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the urban south bay of San Francisco, California. Insects 2021, 946 (2021).
- J. L. Tracy, T. Kantola, K. A. Baum, R. N. Coulson, Modeling fall migration pathways and spatially identifying potential migratory hazards for the eastern monarch butterfly. Landsc. Ecol. 34, 443-458 (2019). [CrossRef]
- V. K. Fyson, D. Ethier, K. Tuininga, E. D. Shapiro, C. Callaghan, Landscape factors influencing roost site selection by monarch butterflies Danaus plexippus during fall migration in Ontario, Canada. Endangered Species Research 50, 267-277 (2023). [CrossRef]
- T. D. Meehan, M. S. Crossley, Change in monarch winter abundance over the past decade: a red list perspective. Insect. Conserv. Divers. Online - (2023). [CrossRef]
- Badgett, A. K. Davis, Population trends of monarchs at a northern monitoring site: analyses of 19 years of fall migration counts at Peninsula Point, MI. Annals of the Entomological Society of America 108, 700-706 (2015). [CrossRef]
- D. S. Wilcove, M. Wikelski, Going, going, gone: Is animal migration disappearing? Plos Biology 6. (2008). [CrossRef]
- S. M. Wells, R. M. Pyle, N. M. Collins, The IUCN Invertebrate Red Data Book (IUCN, Gland, Switzerland, 1983).
- L. P. Brower, S. B. Malcolm, Animal migrations: endangered phenomena. American Zoologist 31, 265-276 (1991). [CrossRef]
- M. Jordan, K. Sullivan (2005) The waning reign of monarchs. in Washington Post.
- T. D. Meehan, E. T. Krainski, F. Lindgren, H. Rue (2023) Spatially Varying Coefficient Models with inlabru. https://inlabru-org.github.io/inlabru/articles/web/svc.html.
- D. Simpson, H. Rue, A. Riebler, T. G. Martins, S. H. Sorbye, Penalising Model Component Complexity: A Principled, Practical Approach to Constructing Priors. Statistical Science 32, 1-28 (2017). [CrossRef]
- E. Krainski et al., Advanced Spatial Modeling with Stochastic Partial Differential Equations Using R and INLA (Chapman and Hall/CRC, 2018).
- M. U. Kemp et al., Quantifying flow-assistance and implications for movement research. Journal of Theoretical Biology 308, 56-67 (2012). [CrossRef]
- E. Vermote, NOAA Climate Data Record (CDR) of AVHRR Normalized Difference Vegetation Index (NDVI), Version 5. NOAA CDR Program. [CrossRef]
- A. Gelman, J. Hwang, A. Vehtari, Understanding predictive information criteria for Bayesian models. Statistics and Computing 24, 997-1016 (2014). [CrossRef]
- F. Lindgren, H. Rue, Bayesian Spatial Modelling with R-INLA. Journal of Statistical Software 63, 1-25 (2015). [CrossRef]
- Rue et al., "Bayesian Computing with INLA: A Review" in Annual Review of Statistics and Its Application, Vol 4, S. E. Fienberg, Ed. (2017), vol. 4, pp. 395-421.
- R Core Team, R: A language and environment for statistical computing. Available at: https://www.r-project.org/ [Accessed 16 August 2019].
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).