Submitted:
24 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. First Works on the Origin of Life
2.2. Ante-Cell Structures
2.3. The AL RNA Ring as an Ancestor of the Ribosome?
- i)
- if the ring form has to play a reactive role with respect to the amino-acids in order to facilitate their polymerization (as a “marriage agency”) taking advantage of the affinities between amino-acids and the codons and anticodons of their specific synonymy classes in the genetic code [40], the ring has to be short. If the ring has to survive in a stable hairpin form with minimal free energy, it does present a self-complementarity between its two half-parts.
- ii)
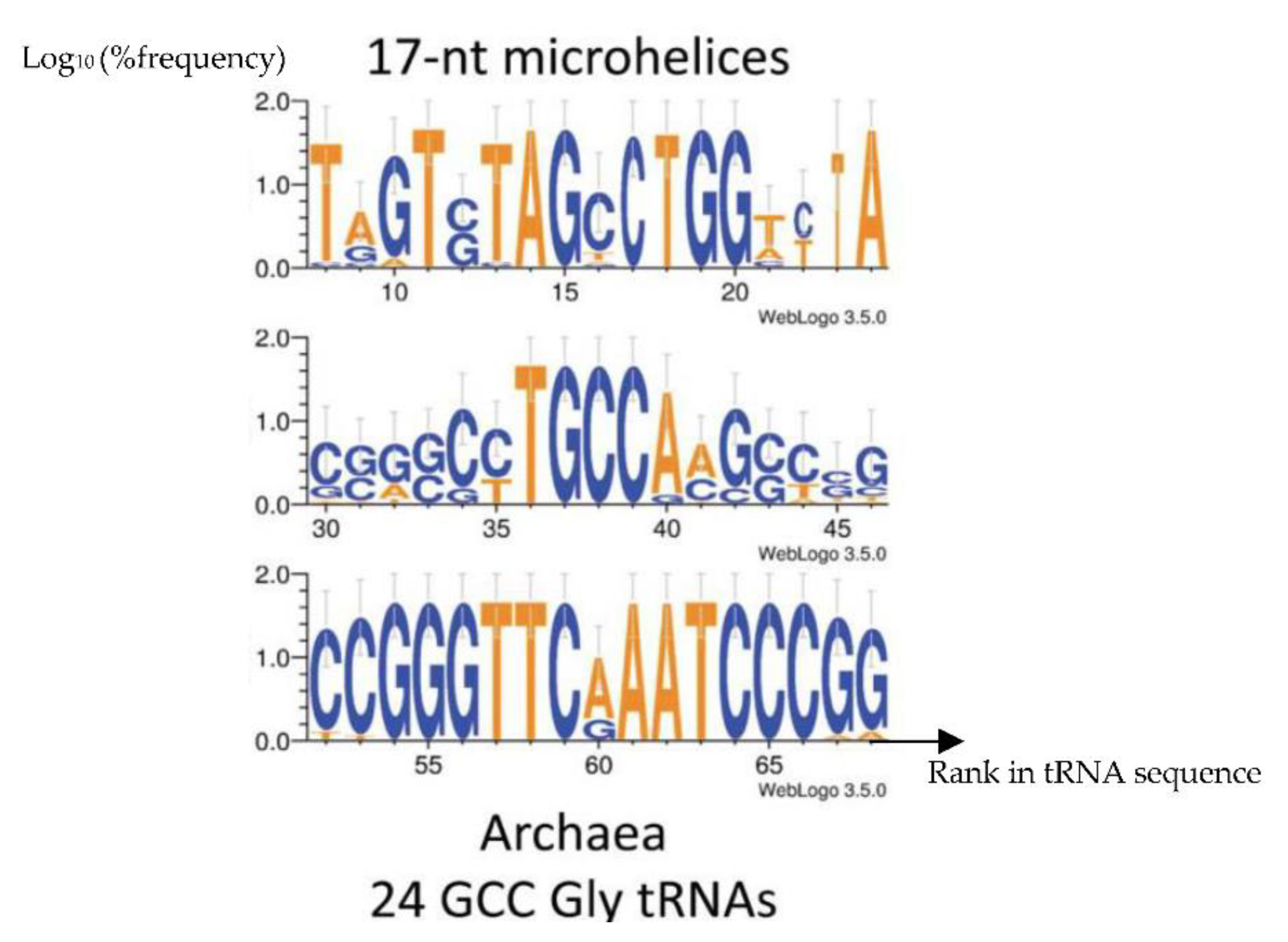
- Among the rings satisfying the principle “to be as short as possible and containing at least one codon of each amino-acid”, there is no solution for a length below 22 nucleotides. For the length 22, 29520 among 176 1011 solutions contain only one repeated codon AUN, N being G for 52% of the solutions,
- iii)
- from these 25 rings, 19 encompass both the start and stop codon UGA,
- iv)
- through calculation of several distances (e.g., circular Hamming distance, permutation distance and edit distance), the ring AL (Figure 2 left) exhibits a minimum average distance as compared to the others. Only this sequence is thus acting as barycenter of the set of the 18 others.
2.4. A Potential Role of AL for Ante-Cells to Join the Organic World
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nutman, A.P.; Bennet, V.C.; Friend, C.R.L.; Van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under possible primitive Earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C.; Kebler, L. On the Origin of Species by Means of Natural Selection, or, The Preservation of Favoured Races in the Struggle for life. J. Murray: London, UK, 1859.
- Dyson, F. Origins of life. Cambridge University Press: Cambridge, UK, 1999.
- Björn, L.O. Stratospheric ozone, ultraviolet radiation, and cryptogams. Biological Conservation 2007, 135, 326–333. [Google Scholar] [CrossRef]
- Khodachenko, M.L.; Lammer, H.; Lichtenegger, H.I.M.; Grießmeier, J.-M.; Holmström, M.; Ekenbäck, A. The role of intrinsic magnetic fields in planetary evolution and habitability: the planetary protection aspect. Proceedings of the International Astronomical Union 2008, 4, 283–294. [Google Scholar] [CrossRef]
- Fox, G.E. Origin and evolution of the ribosome. Cold Spring Harb. Perspect. Biol. 2010, 2, a003483. [Google Scholar] [CrossRef] [PubMed]
- Schrum, J.P.; Zhu, T.F.; Szostak, J.W. The origins of cellular life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002212. [Google Scholar] [CrossRef]
- Trevors, J.T.; Saier, M.H. Three Laws of Biology. Water, Air, and Soil Pollution 2010, 205, 87–89. [Google Scholar] [CrossRef]
- Lovett, R. A. Tidal heating shrinks the “goldilocks zone. ” Nature 2012, 485, 10601. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. (2017). Molecular Biology of the Cell. W.W. Norton & Company, New York, USA, 2017.
- Ramirez, R. A More Comprehensive Habitable Zone for Finding Life on Other Planets. Geosciences 2018, 8, 280. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A.; Atkins, J.F. RNA Worlds: New Tools for Deep Exploration; Cold Spring Harbor Laboratory Press, New York, USA, 2019.
- Kahana, A.; Schmitt-Kopplin, P.; Lancet, D. Enceladus: First Observed Primordial Soup Could Arbitrate Origin-of-Life Debate. Astrobiology 2019, 19, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Osinski, G.R.; Cockell, C.S.; Pontefract, A.; Sapers, H.M. The Role of Meteorite Impacts in the Origin of Life. Astrobiology 2020, 20, 1121–1149. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.; Loeb, A. Breakup of a long-period comet as the origin of the dinosaur extinction. Scientific Reports 2021, 11, 3803. [Google Scholar] [CrossRef] [PubMed]
- Vlachakis, D.; Chrousos, G.; Eliopoulos, E. On the origins of life: A molecular and a cellular journey driven by genentropy. International Journal of Epigenetics 2021, 1, 7. [Google Scholar] [CrossRef]
- Demongeot, J.; Thellier, M. Primitive oligomeric RNAs at the origins of life on earth. Int. J. Mol. Sci. 2023, 24, 2274. [Google Scholar] [CrossRef] [PubMed]
- Oparin, A. The origin of life, translation by Ann Synge. In: Bernal, J. D. (ed.), The origin of life. Weidenfeld & Nicolson, London, 1967, pp. 199–234.
- Blain, J.C.; Szostak, J.W. Progress towards synthetic cells. Ann. Rev. Biochem. 2014, 83, 615–640. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F.; Szostak, J.W. Protocells and RNA Self-Replication. Cold Spring Harb. Perspect. Biol. 2018, 10, a034801. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhou, L.; Giurgiu, C.; Szostak, J.W. Kinetic explanation for the sequence biases observed in the nonenzymatic copying of RNA templates. Nucleic Acid Res. 2022, 50, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J. Au sujet de quelques modèles stochastiques appliqués à la biologie. Modélisation et simulation. tel-00286222. Université Joseph-Fourier: Grenoble, 1975.
- Demongeot, J. Sur la possibilité de considérer le code génétique comme un code à enchaînement. Revue de Biomaths 1978, 62, 61–66. [Google Scholar]
- Gardes, J.; Maldivi, C.; Boisset, D.; Aubourg, T.; Demongeot, J. An unsupervised classifier for the whole genome phylogenies, the Maxwell© tool. IJMS 2023, 24, 16278. [Google Scholar] [CrossRef]
- Pennetier, G. Un débat scientifique : Pouchet et Pasteur (1858-1868). Actes du Museum d’Histoire Naturelle de Rouen. J. Girieud imprimeur, Rouen, France, 1907.
- Pasteur, L. Sur les corpuscules organisés qui existent dans l’atmosphère, examen de la doctrine des générations spontanées. Leçon professée à la Société clinique de Paris, le 19 mai 1861. Imprimerie C. Lahure, Paris, France, 1862.
- Meunier, A. La Naissance de la Terre. Dunod, Paris, France, 2014.
- GtRNAdb. Available online: http://lowelab.ucsc.edu/GtRNAdb/Lafri3/Lafri3-align.html (accessed on 23 March 2024).
- Bioinf. Available online: http://trna.bioinf.uni-leipzig.de/DataOutput/Result (accessed on 23 March 2024).
- tRNAviz. Available online: http://trna.ucsc.edu/tRNAviz/ (accessed on 23 March 2024).
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/nucleotide?cmd=search (accessed on 23 March 2024).
- Demongeot, J.; Besson, J. Code génétique et codes à enchaînement I. C. R. Acad. Sc. III 1983, 296, 807–810. [Google Scholar]
- Demongeot, J.; Moreira, A. A circular RNA at the origin of life. J. Theor. Biol. 2007, 249, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Norris, V. Emergence of a "Cyclosome" in a primitive network capable of building "infinite" proteins. Life 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Moreira, A.; Seligmann, H. Negative CG dinucleotide bias: An explanation based on feedback loops between Arginine codon assignments and theoretical minimal RNA rings. Bioessays 2021, 43, 2000071. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Thellier, M. Primitive oligomeric RNAs at the origins of life on Earth. IJMS 2023, 24, 2274. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Kim, Y.; Sanjay, A.; Burton, Z.F. tRNA evolution from the proto-tRNA minihelix world. Transcription 2016, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Mishra, A.K.; Hashem, A.; Abd Allah, E.F.; Khan,A.L.; Al-Harrasi, A. Construction of anti-codon table of the plant kingdom and evolution of tRNA selenocysteine (tRNASec). BMC Genomics 2020, 21, 804.
- Moghadam, S.A.; Preto, J.; Klobukowski, M.; Tuszynski, J.A. Testing amino acid-codon affinity hypothesis using molecular docking. Biosystems 2020, 198, 104251. [Google Scholar] [CrossRef]
- Lee, M.T. Biophysical characterization of peptide–membrane interactions. Advances in Physics 2018, 3, 1. [Google Scholar] [CrossRef]
- Fiore, M.; Strazewski, P. Prebiotic Lipidic Amphiphiles and Condensing Agents on the Early Earth. Life 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Laczano, A.; Oro, J.; Miller, S.L. Primitive Earth environments: organic syntheses and the origin and early evolution of life. Precambrian Research 1983, 20, 259–282. [Google Scholar]
- Jordan, S.F.; Nee, E.; Lane, N. Isoprenoids enhance the stability of fatty acid membranes at the emergece of life potentially leading to an early lipid divide. Interface Focus 2019, 9, 20190067. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.R.; Todd, Z.R.; Wogan, N.; Black, R.A.; Keller, S.L.; Catling, D.C. Plausible Sources of Membrane-Forming Fatty Acids on the Early Earth: A Review of the Literature and an Estimation of Amounts. ACS Earth and Space Chemistry 2023, 7, 11–27. [Google Scholar] [CrossRef]
- Kraft, M.L.; Weber, P.K.; Longo, M.L.; Hutcheon, I.D.; Boxwer, S.G. Phase separation of lipid membranes analyzed with high-resolution secondary ion mass spectrometry. Science 2006, 313, 1948–1951. [Google Scholar] [CrossRef] [PubMed]
- Raine, D.J.; Norris, V. Lipid domain boundaries as prebiotic catalysts of peptide bond formation. J. Theor. Biol. 2007, 246, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. The Role of Lipid Membranes in Life's Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Dalai, P.; Sahai, N. Mineral-Lipid Interactions in the Origins of Life. Trends Biochem Sci. 2019, 44, 331–341. [Google Scholar] [CrossRef]
- Subbotin, V.; Fiksel, G. Exploring the Lipid World Hypothesis: A Novel Scenario of Self-Sustained Darwinian Evolution of the Liposomes. Astrobiology 2023, 23, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Gardes, J.; Maldivi, C.; Boisset, *!!! REPLACE !!!*; Aubourg, T.; Demongeot, J. ; Demongeot, J. An unsupervised classifier for the whole genome phylogenies, the Maxwell© tool. IJMS 2023, 24, 16278. [Google Scholar] [CrossRef]
- Demongeot, J.; Gardes, J.; Maldivi, C.; Boisset, D.; Boufama, K; Touzouti, I. Genomic phylogeny by Maxwell®, a new classifier based on Burrows-Wheeler transform. Computation 2023, 11, 158. [Google Scholar] [CrossRef]
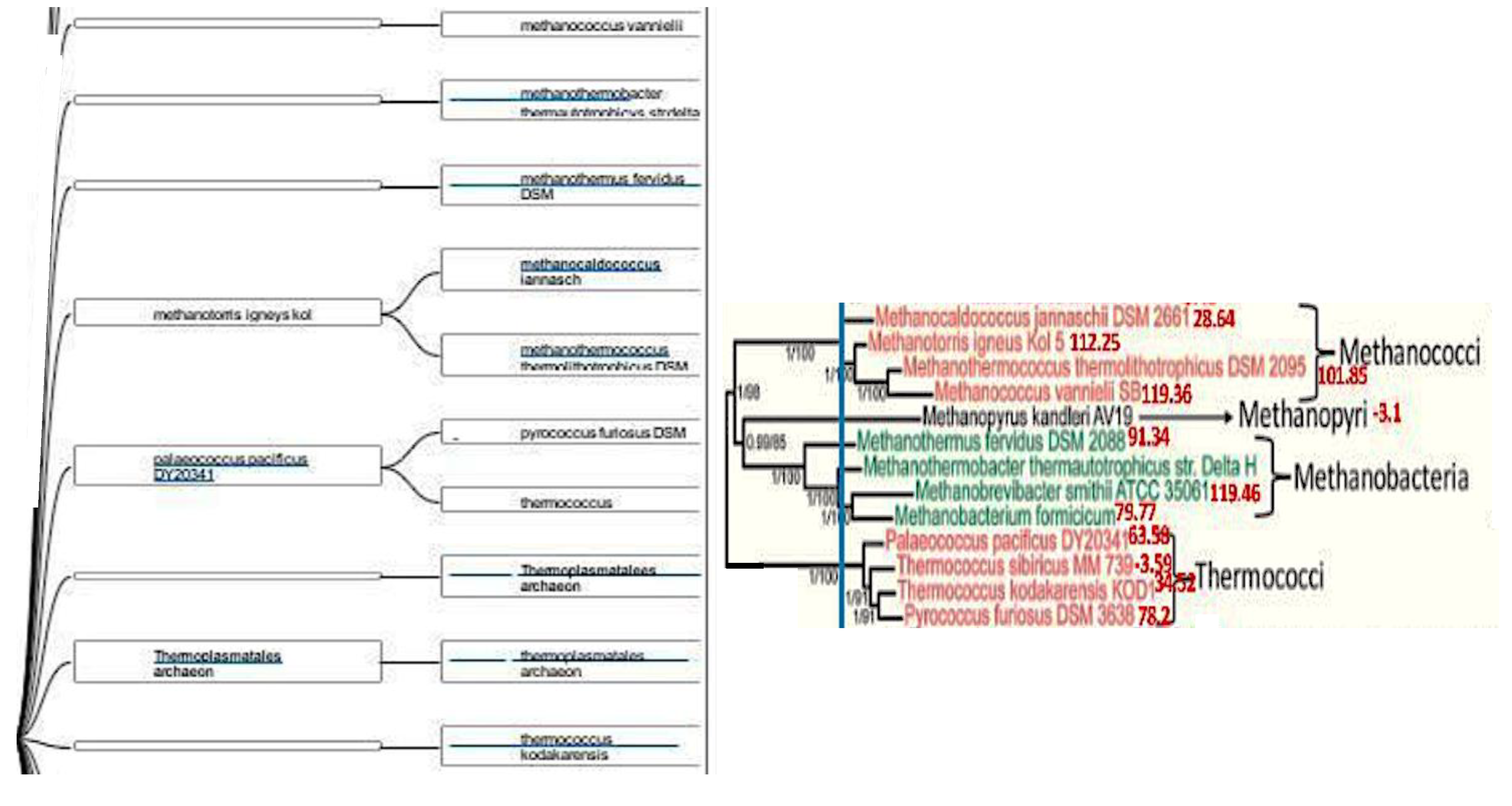
- Adam, P.; Borrel, G.; Brochier-Armanet, C. The growing tree of Archaea: new perspectives on their diversity, evolution and ecology. ISME J. 2017, 11, 2407–2425. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Gogarten, J.P.; Lajoie, J.; Makkay, A.M.; Papke, R.T. Characterizing the DNA Methyltransferases of Haloferax volcanii via Bioinformatics, Gene Deletion, and SMRT Sequencing. Genes 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Mardanov, A.V; Ravin, N.V.; Svetlitchnyi, V.A.; Beletsky, A.V.; Miroshnichenko, M.L; Bonch-Osmolovskaya, E.A.; Skryabin, K.G. Metabolic versatility and indigenous origin of the archaeon Thermococcus sibiricus, isolated from a siberian oil reservoir, as revealed by genome analysis. Appl. Environ. Microbiol. 2009, 75, 4580–4588. [Google Scholar] [CrossRef]
- Gorlas, A.; Koonin, E.V.; Bienvenu, N.; Prieur, D.; Geslin, C. TPV1, the first virus isolated from the hyperthermophilic genus Thermococcus. Environ. Microbiol. 2012, 14, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, N.; Smole, Z.; Krisko, A. Proteomic properties reveal phyloecological clusters of Archaea. PLoS One 2012, 7, e48231. [Google Scholar] [CrossRef] [PubMed]
- Gavette, J.V.; Stoop, M.; Hud, N.V.; Krishnamurthy, R. RNA-DNA Chimeras in the Context of an RNA World Transition to an RNA/DNA World. Angew. Chem. Int. Ed. Engl. 2016, 55, 13204–13209. [Google Scholar] [CrossRef] [PubMed]
- Todd, Z.R.; Cohen, Z.R.; Catling, D.C.; Keller, S.L.; Black, R.A. Growth of Prebiotically Plausible Fatty Acid Vesicles Proceeds in the Presence of Prebiotic Amino Acids, Dipeptides, Sugars, and Nucleic Acid Components. Langmuir 2022, 38, 15106–15112. [Google Scholar] [CrossRef] [PubMed]
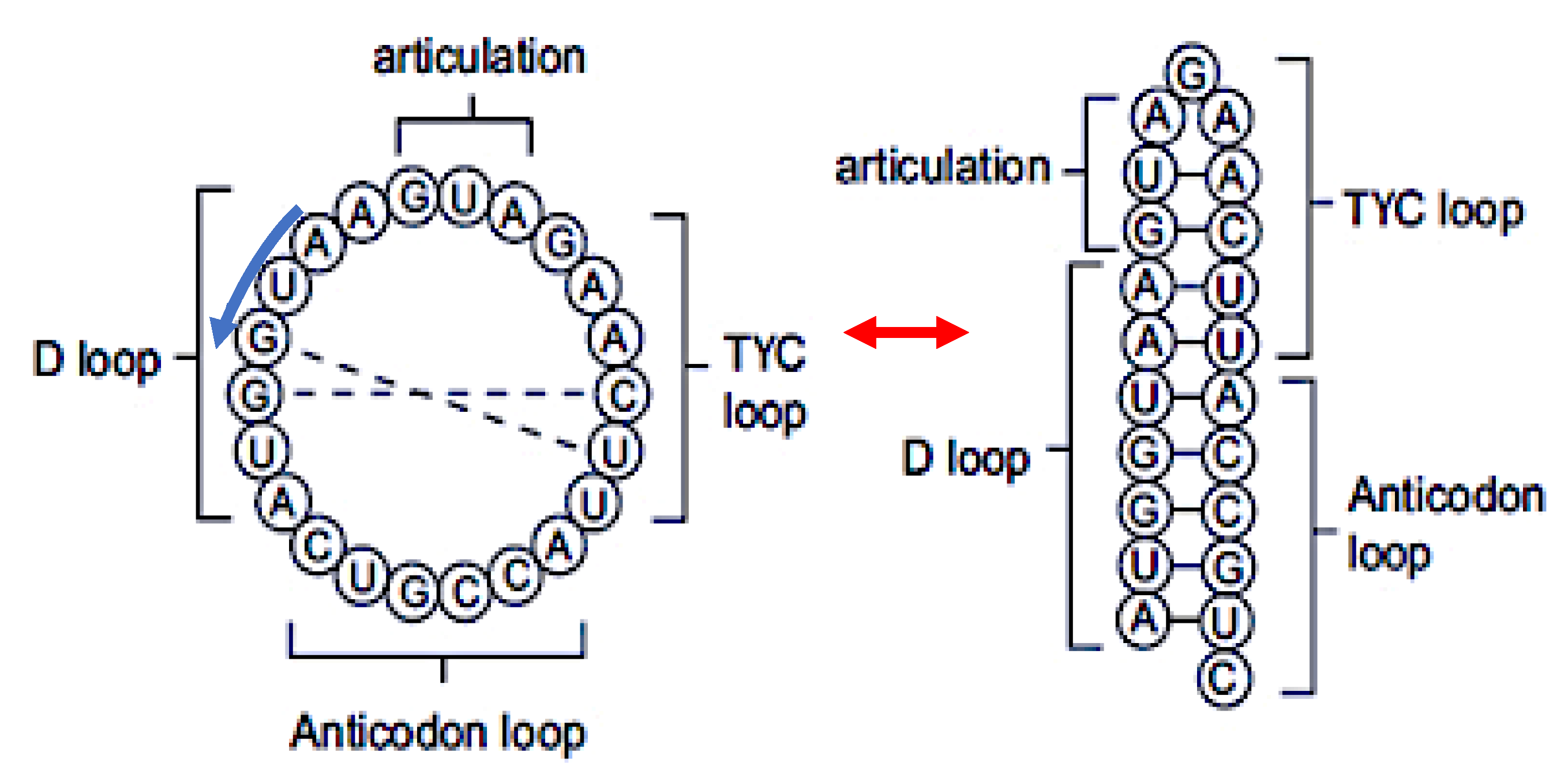
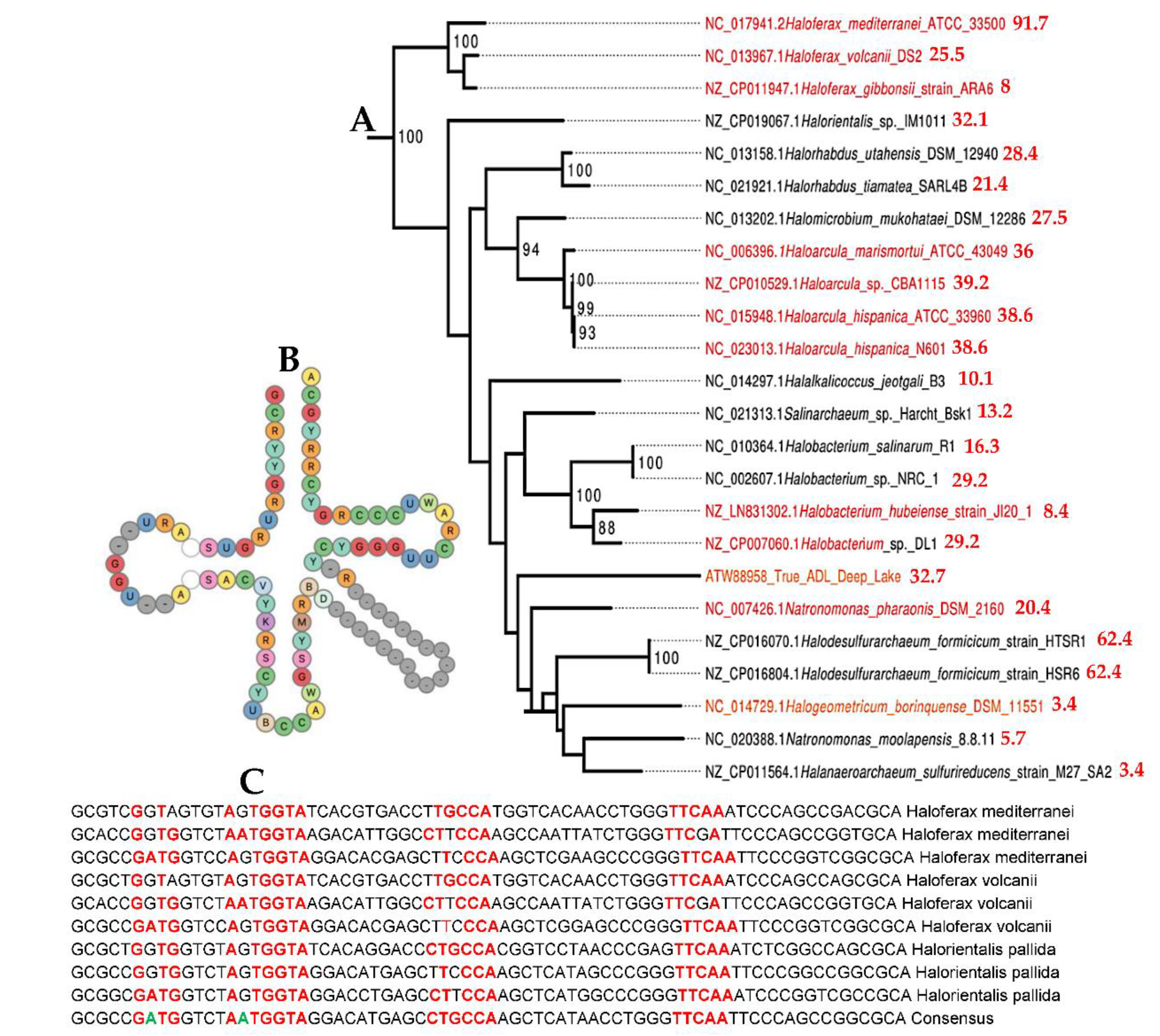
| Species | Articulation D-loop Anticodon-loop Ty-loop |
| Methanococcus maripaludis | GCGGCTTTGATGTAG ACTGGTATCATACGGCCCTGCCACGGCCGACACCCGGGTTCAAATCCCGGAGGCCGCA |
| Methanococcus vannielii | GCGGCTTTGATGTAG ACTGGTATCATACGGCCCTGCCACGGCCGACACCCGGGTTCAAATCCCGGAGGCCGCA |
| Methanococcus voltae | GCGGCCTTGATGTAG TGGTATCATACGGCCCTGCCACGGCCGATACCCGGGTTCAAATCCCGGAGGCCGCA |
| Methanocaldococcus jannaschii | GCGGCCTTGGTGTAG CCTGGTAACACACGGGCCTGCCACGCCCGGACCCCGGGTTCAAATCCCGGAGGCCGCA |
| Halorhabdus utahensis | GCGACGGTGGTGTAGTGGTATCACAGGACCCTGCCACGGTCCTAACCCGAGTTCAAATCTCGGCCGTCGCA |
| Petromyzon marinus (lamprey) | GCATCGGTGGTTCAGTGGTAGAAATCTCGCCTGCCACGCGGGAGGCCCGGGTTCAATTCCCGGCCGATGCA |
| Danio rerio (zebrafish) | ACATTGGTGGTTCAGTGGTAGATTTCTCGCCTGCCACGTGGGAGGCCCGGGTTCAATTCCCGGCCAATGCA |
| Strongylocentrotus purpuratus | GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTGCCACGCGGGGGACCCGGGTTCAATTCCCGGCCAATGCA |
| Loxodonta africana (elephant) | GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTGCCACGTGGGAGGCCTGGGTTCAATTCCCAGCCAGTTCT |
| Callithrix jacchus (marmoset) | GCATGGGTGGTTCAGTGGTAGAATTCTCGCCTGCCACGCGGGAGTCCTGGGTTCAATCCCCGGCCCACGCA |
| Arabidopsis thaliana | GCACCAGTGGTCTAGTGGTAGAATAGTACCCTGCCACGGTACAGACCCGGGTTCAATTCCCGGCTGGTGCA |
| Medicago truncatula | GCACCAGTGGTCTAGTGGTAGAATAGTACCCTGCCACGCTACAGACCCGGGTTCAATTCCTGGCTGGTGCA |
| Species | Articulation D-loop Anticodon-loop Ty-loop |
| Methanococcus maripaludis | GCGGCTTTGATGTAG ACTGGTATCATACGGCCCTGCCACGGCCGACACCCGGGTTCAAATCCCGGAGGCCGCA |
| Methanococcus vannielii | GCGGCTTTGATGTAG ACTGGTATCATACGGCCCTGCCACGGCCGACACCCGGGTTCAAATCCCGGAGGCCGCA |
| Methanococcus voltae | GCGGCCTTGATGTAG TGGTATCATACGGCCCTGCCACGGCCGATACCCGGGTTCAAATCCCGGAGGCCGCA |
| Methanocaldococcus jannaschii | GCGGCCTTGGTGTAG CCTGGTAACACACGGGCCTGCCACGCCCGGACCCCGGGTTCAAATCCCGGAGGCCGCA |
| Halorhabdus utahensis | GCGACGGTGGTGTAGTGGTATCACAGGACCCTGCCACGGTCCTAACCCGAGTTCAAATCTCGGCCGTCGCA |
| Petromyzon marinus (lamprey) | GCATCGGTGGTTCAGTGGTAGAAATCTCGCCTGCCACGCGGGAGGCCCGGGTTCAATTCCCGGCCGATGCA |
| Danio rerio (zebrafish) | ACATTGGTGGTTCAGTGGTAGATTTCTCGCCTGCCACGTGGGAGGCCCGGGTTCAATTCCCGGCCAATGCA |
| Strongylocentrotus purpuratus | GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTGCCACGCGGGGGACCCGGGTTCAATTCCCGGCCAATGCA |
| Loxodonta africana (elephant) | GCATTGGTGGTTCAGTGGTAGAATTCTCGCCTGCCACGTGGGAGGCCTGGGTTCAATTCCCAGCCAGTTCT |
| Callithrix jacchus (marmoset) | GCATGGGTGGTTCAGTGGTAGAATTCTCGCCTGCCACGCGGGAGTCCTGGGTTCAATCCCCGGCCCACGCA |
| Arabidopsis thaliana | GCACCAGTGGTCTAGTGGTAGAATAGTACCCTGCCACGGTACAGACCCGGGTTCAATTCCCGGCTGGTGCA |
| Medicago truncatula | GCACCAGTGGTCTAGTGGTAGAATAGTACCCTGCCACGCTACAGACCCGGGTTCAATTCCTGGCTGGTGCA |
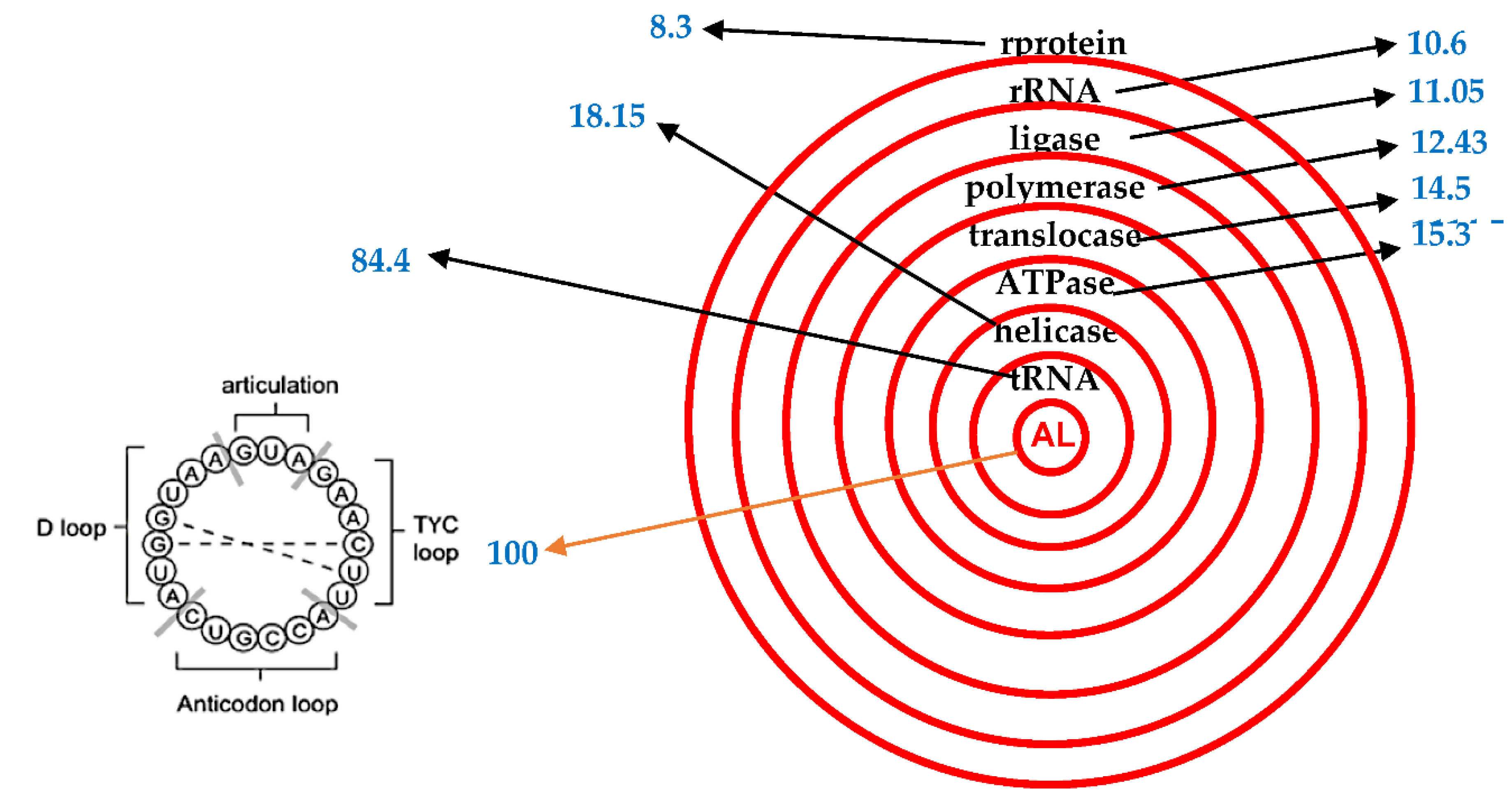
| Molecule | Species | PpAL=2PAL/s | no N ne (se) pAL | Mean PpAL |
|---|---|---|---|---|
| rprotein L18 | HS | 7.4 | 13 552 4.9 (2.2) 3.7s | 8.3 |
| SC | 10 | 16 557 4.9 (2.2) 5s | ||
| Mv | 6 | 12 581 5.1 (2.26) 3s | ||
| Mmi | 9.8 | 16 578 5 (2.25) 4.9s | ||
| rRNA 5S | HS | 2 | 2 117 1 (1) 1s | 10.6 |
| SC | 2 | 2 117 1 (1) 1s | ||
| Mv | 26.2 | 14 112 0.9 (1) 13.1s | ||
| Mmi | 12.2 | 7 111 0.98 (0.99) 6.1s | ||
| Gly-tRNA ligase | HS | 8.8 | 36 2015 17.7 (4.2) 4.4s | 11.05 |
| SC | 13 | 45 2000 17.6 (4.2) 6.5s | ||
| Mv | 11 | 37 1751 15.4 (3.9) 5.5s | ||
| Mmi | 11.4 | 37 1721 15.1 (3.9) 5.7s | ||
| DNA polymerase | HS | 5.8 | 21 1286 11.3 (3.36) 2.9s | 12.43 |
| SC | 9.5 | 36 1895 16.6 (4) 4.75s | ||
| Mv | 16.2 | 59 2472 21.7 (4.6) 8.1s | ||
| Mmi | 18.2 | 30 749 6.6 (2.6) 9.1s | ||
| Translocase | HS | 5.4 | 42 3178 27.9 (5.3) 2.7s | 14.5 |
| SC | 26.8 | 130 4856 42.7 (6.53) 13.4s | ||
| Mv | 19.6 | 76 2969 26 (5.1) 9.8s | ||
| Mmi | 6 | 22 1325 11.6 (3.4) 3s | ||
| ATPase | HS | 7 | 29 1755 15.4 (3.9) 3.5s | 15.3 |
| SC | 12.8 | 42 1850 16.26 (4) 6.4s | ||
| Mv | 13.6 | 21 608 5.34 (2.3) 6.8s | ||
| Mmi | 27.8 | 97 2978 26.2 (5.1) 13.9s | ||
| Helicase | HS | 12.6 | 48 2255 19.8 (4.45) 6.3s | 18.15 |
| SC | 26.6 | 95 3029 26.6 (5.16) 13.3s | ||
| Mv | 15.2 | 57 2465 21.7 (4.65) 7.6s | ||
| Mmi | 18.2 | 60 2236 19.6 (4.4) 9.1s | ||
| tRNA-Gly | HS | 81 | 18 22 0.19 (0.44) 40.5s | 84.4 |
| SC | 76.4 | 17 22 0.19 (0.44) 38.2s | ||
| Mv | 85.5 | 19 22 0.19 (0.44) 42.8s | ||
| Mmi | 94.6 | 21 22 0.19 (0.44) 47.3s | ||
| AL | 100 | 22 22 0.19 (0.44) 50s | 100 |
| tRNA database | http://lowelab.ucsc.edu/GtRNAdb/Lafri3/Lafri3-align.html http://trna.bioinf.uni-leipzig.de/DataOutput/Result |
| Secondary structure | http://trna.ucsc.edu/tRNAviz/ |
| Gene sequence | https://www.ncbi.nlm.nih.gov/nucleotide?cmd=search |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





