1. Introduction
Electroencephalography stands out as a straightforward and accessible technique for capturing brain signals. It operates by detecting electrical activity in various brain regions through electrodes affixed to the scalp. Widely utilized in both research and clinical settings, EEG signals serve diverse purposes, continually expanding in their applications. Different types of sensors, such as wet and dry electrodes, exist for recording these signals. Wet electrodes, favored for their low impedance typically ranging from 200 kOhm to 5 kOhm after gel application, remain the prevalent choice. Li et al. [
1] extensively dissected the merits and drawbacks of wet and dry electrodes, highlighting the nuanced advantages and disadvantages of each type. In our work we used dry electrodes (Ag/AgCl) because opting for dry electrodes eliminates the need for gel application, simplifying EEG measurements.
2. Problem Statement and Motivation
The price range of commercial devices varies significantly, often posing a challenge for researchers due to their high cost. Nowadays, with the surge in interest surrounding neural networks and signal processing techniques, there's a growing curiosity in Brain-Computer Interface devices (BCI) across various application domains. As early as 2016, Meng et al. [
3] demonstrated the control of a robotic arm through motor imagery, utilizing neural networks to capture corresponding brain waves via electrodes. This achievement was previously exclusive to invasive methods involving implanted microelectrode matrices for direct brain signal measurement [
4]. Invasive techniques involve electrodes making direct contact with the cerebral cortex to capture brain waves locally. Consequently, such methods are cumbersome, expensive, hazardous, and demand highly skilled personnel alongside specialized equipment [
5,
6,
7]. Thus, while invasive approaches have shown promise, there's a growing focus on low-cost EEG signal recording devices in this area. Typically, EEG signal readers comprise a microcontroller (processor), a power board, and transmission adapters, the latter often contributing significantly to costs. Hence, this article concentrates on addressing this issue via a special shiel for Arduino Uno R4 Wi-Fi. Arduino (
https://www.arduino.cc/ ) is a popular board for studying electronics, with our shield we extend the opportunity of the Arduino board to measure biosignals as EEG, EMG EKG.
3. Review of Related Work
Today, there exists an array of devices utilized for recording EEG signals. Gunawan et al. [
8] utilized a low-cost device to capture EEG signals for various classification tasks, while Ashby et al. [
9] employed the same device to classify mental visual tasks. Seneviratna et al. [
10] introduced a device transmitting data to a computer via Bluetooth from seven EEG channels and one audio channel. The wireless EEG device outlined by Nithin et al. [
11] shares similar limitations. Chapman et al. [
12] presented a novel BCI, yet technical details such as component specifications or circuit board diagrams were absent from the paper. Numerous studies have explored employing RaspberryPI for EEG signal reading [
20]. For instance, Dillon [
13] utilized an ADC (mcp3008) to measure EEG signals for brain injury diagnosis. Ukveris et al. [
14], in a study closely related to this article, developed a screen for EEG measurement using Atmega2560 with Arduino. This article addresses introducing a new ardEEG device, a comprehensive self-contained BCI based on Arduino.
4. Technical Realizations
The leading role in EEG task measurements among analog-digital converters (ADCs) can be placed on the ADS1299 block produced by Texas Instruments. With over a decade in the market, it has consistently been regarded as one of the top units available. What sets this ADC apart from its competitors is its built-in multiplexer. Usman et al. [
15] and Rakhmatulin et al. [
16] extensively reviewed the capabilities of this ADC and the features of the ADS1299 multiplexer, a discussion that is beyond the scope of this article. Prior to testing, impedance was verified using the ADS1299 function following the methodology outlined in the article [
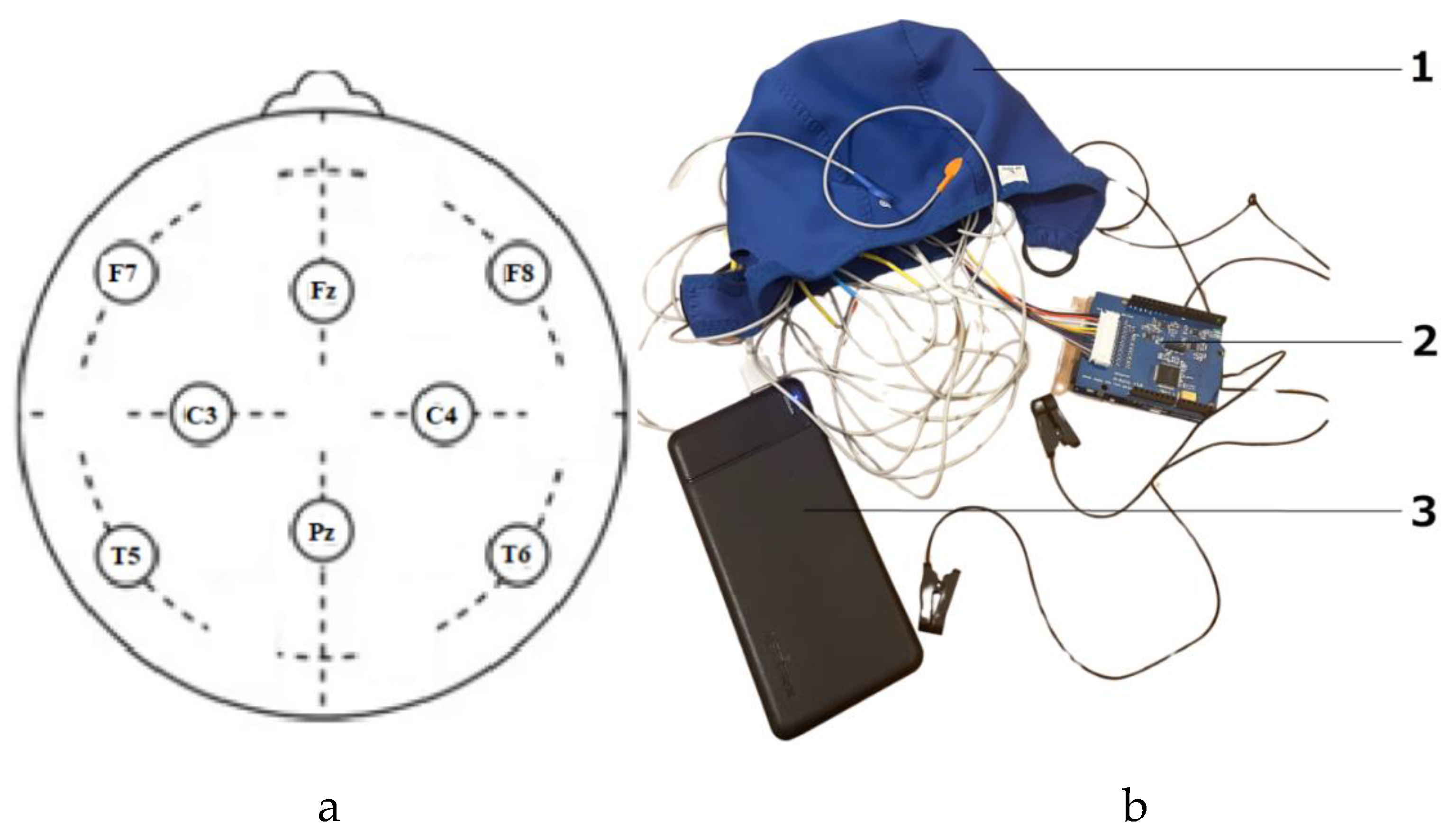
17]. We positioned 8 Ag/AgCl dry electrodes according to the International Electrode Placement System "10-20", selecting the following locations: F7, Fz, F8, C3, C4, T5, Pz, and T6. The general view of ardEEG is presented in
Figure 1.
During the test, the device remained disconnected from the network. We employed a power bank for the purpose of security and to prevent any potential network disruptions. The device was exclusively tested using 5V batteries (and must be tested and used only from 5V batteries). A general view of the in the assembly device configuration and the arrangement of the electrodes is shown in
Figure 2.
5. Test Device
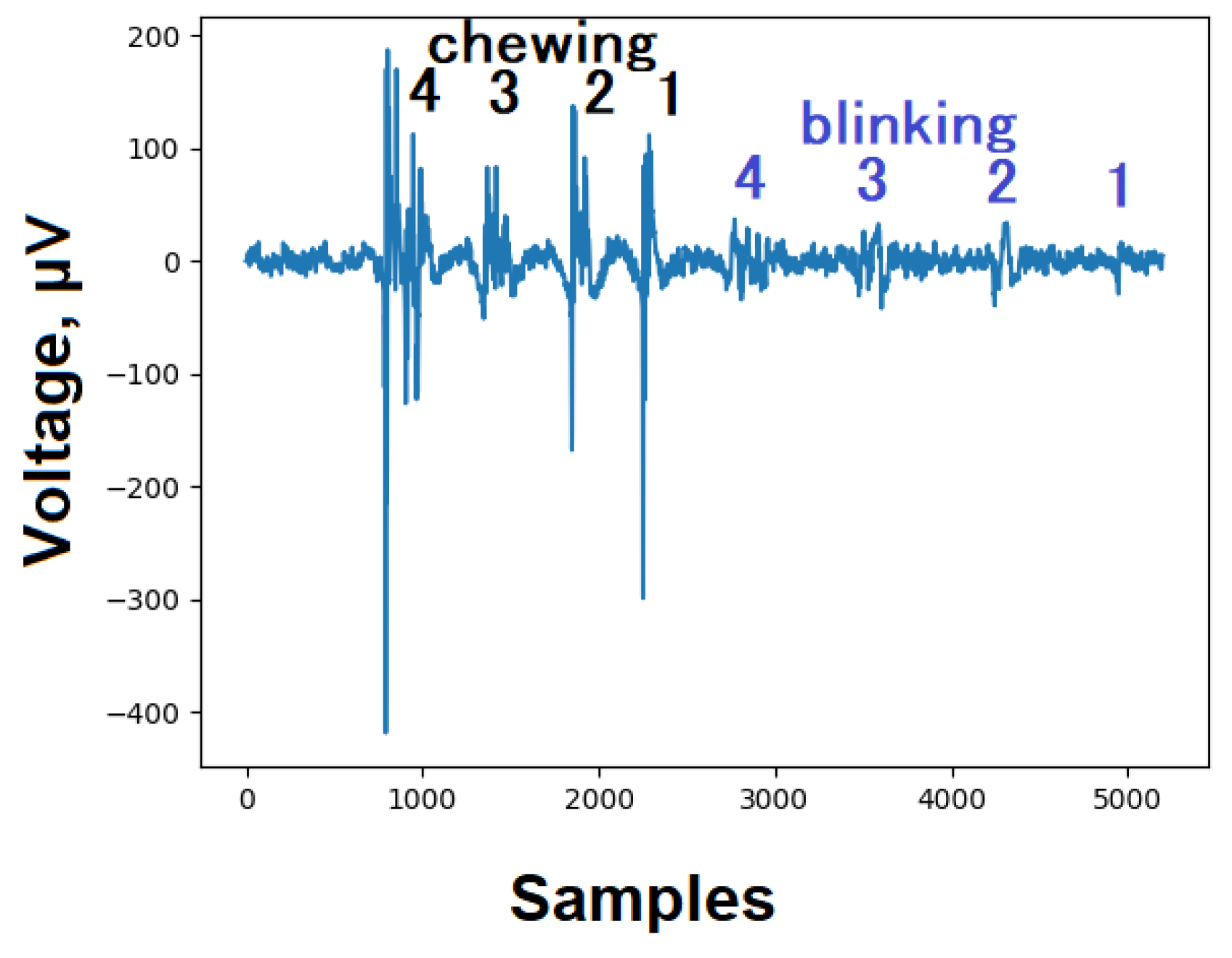
5.1. Artefacts
Internal noise for ardEEG lies within 1 µV, (dataset
https://github.com/Ildaron/ardEEG/tree/main/dataset/internal_noise ). We realized several tests for reading EEG signals for the presence of the simplest artifacts - chewing and blinking
. These artifacts are easy to use for testing a developed EEG device [
18]. Sheoran [
19] detailed how to detect it and how to deal with this artifact. In our experiments, the blinking and chewing artifact was strongly distinguished against the background of the EEG signal (
Figure 3).
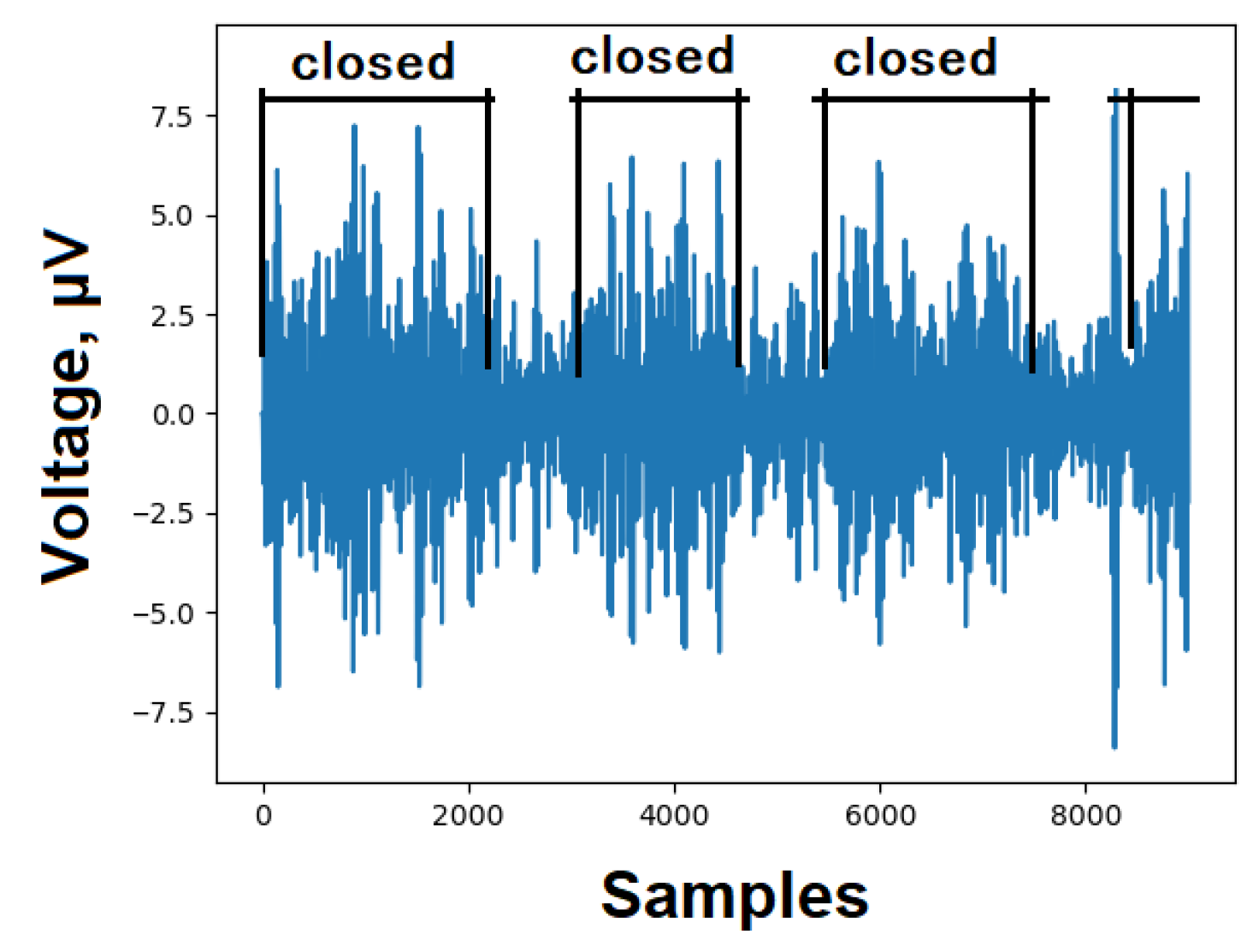
5.2. Alpha
An alpha wave (α-wave) brain signal, ranging from 8 to 12 Hz with an amplitude typically between 35 and 65 µV, serves as a suitable test for assessing the EEG recording system. Typically, individuals in an awake state with closed eyes, during relaxation, can detect alpha waves. Each EEG signal was measured for a duration of 8 seconds with both eyes closed and open. As anticipated, there was an observed increase in EEG signal amplitude within the frequency band of 8 to 12 Hz. Likewise, a decrease in alpha activity was noted when the eyes were open. These findings align with the anticipated alpha wave pattern in the occipital lobe of the brain, validating the proper design and functioning of the device,
Figure 4.
6. Conclusion and Discussion
This article highlights the distinctiveness of the presented device by emphasizing its combination of low cost and high accuracy. Additionally, it underscores the uniqueness of the work by confirming measurement accuracy through established methods involving a well-recognized artifact and alpha waves.
Furthermore, the article mentions that the noise characteristics of the proposed device align well with those of the ADS1299 from Texas Instruments, validating the quality of the affordable board. It also suggests a promising avenue for future research: practical application as a brain-computer interface. The article asserts that the proposed device ensures high-quality data transfer between the ADC and the processor without introducing any delays, making it suitable for real-time use.
Given the widespread popularity of Arduino, we anticipate that this article will capture the interest of numerous researchers. We express hope that the project will facilitate the accumulation of a substantial dataset, and to that end, we have made the software project in open-source format. We extend an invitation to others to collaborate on data processing efforts.
Conflicts of Interest
None.
References
- Li, G., et al. (2018). Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sensors and Actuators B: Chemical, 277, 250- 260.
- Rakhmatulin, I., Gan, Y. Review Dry and Non-Contact EEG Electrodes for 2010-2021 Years. Preprints 2021, 2021030555. [CrossRef]
- Meng, J. et al. Noninvasive Electroencephalogram Based Control of a Robotic Arm for Reach and Grasp Tasks. Sci. Rep. 6, 38565. (2016). [CrossRef]
- Shokur, S., et al. (2021). A modular strategy for next-generationupper limb sensory-motor neuroprostheses. Med, 2, Issue 8, 912-937. [CrossRef]
- Lacan, L. (2020). Fetal sheep cerebral electrical activity: A new technique to record EEG. Journal of Neuroscience Methods, 345, 108888.
- Kovács, S., et al. (2021). Cost-effectiveness analysis of invasive EEG monitoring in drug-resistant epilepsy. Epilepsy & Behavior, 114, 107488.
- Nithin, G. et al. (2021). Graph energy-based centrality measures to detect epileptogenic focal invasive EEG electrodes. Seizure, 85, 127- 137.
- Gunawan, A., Surya, K. Brainwave Classification of Visual Stimuli Based on Low Cost EEG Spectrogram Using DenseNet. Procedia Computer Science Volume 135, 2018, Pages 128-139.
- Ashby, C., Bhatia, A., Tenoreb, F., Vogelstein,J. Low-cost electroencephalogram (EEG) based authentication. Proceedings of the 5th InternationalIEEE EMBS Conference on Neural EngineeringCancun, Mexico, April 27 - May 1, 2011, 442-445.
- Senevirathna, B., Berman, L., Bertoni, N., & Pareschi, F. (2016). Low Cost Mobile EEG for Characterization of Cortical Auditory Responses, 2016 IEEE International Symposium on Circuits and Systems(ISCAS), 1102-1105.
- Nithin, G. et al. (2021). Graph energy-based centrality measures to detect epileptogenic focal invasive EEG electrodes. Seizure, 85, 127- 137.
- Chapman, R., & Bragdon, H. (1964). Evoked responses to numerical and non-numerical visual stimuli while problemsolving. Nature. 203 (4950): 1155-1157. [CrossRef]
- Dhillon, N., et al. (2021). A Raspberry Pi-Based Traumatic Brain Injury Detection System for Single-Channel Electroencephalogram. ensors 2021, 21(8), 2779. [CrossRef]
- Uktveris, T., & Jusas, V. (2018). Development of a Modular Board for EEG Signal Acquisition. Sensors (Basel, Switzerland), 18(7), 2140. [CrossRef]
- Usman, R., Imran, K., Nada, S., & Denise, T. An EEG Experimental Study Evaluating the Performance of Texas Instruments ADS1299, Sensors 2018, 18(11), 3721. [CrossRef]
- Rakhmatulin, I., Parfenov, A., Traylor, Z. et al. (2021). Low-cost brain computer interface for everyday use. Exp Brain Res. [CrossRef]
- Rakhmatulin, I. (2020). The electronic board to replace the reference voltage on the earlobe for EEG measurement. Measurement 173. [CrossRef]
- Allen, A., Jacob, T., Smith, A. (2014) Effects and after-effectsof chewing gum on vigilance, heart rate, EEG and mood. Physiology & Behavior, 133: 244-255.
- Sheoran, P., Saini, J. (2020). A New Method for Automatic Electrooculogram and Eye Blink Artifacts Correction of EEG Signals using CCA and NAPCT. Procedia Computer Science, 167, 1761-1770.
- Rakhmatuiln, I., et al. (2021). Raspberry PI Shield - for measure EEG (PIEEG). 2021 5th International Conference on Electrical, Electronics, Communication, Computer Technologies and Optimization Techniques (ICEECCOT), Mysuru, India, 2021, pp. 410-413. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).