Submitted:
24 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Method
2.1. Preparation of Microbial Inoculation
2.1.1. Cyanobacteria (Cyano)
2.1.2. Yeast (Y)
2.1.3. Arbuscular Mycorrhizal Fungi (AMF)
2.2. K-Humate Preparation (K-H)
2.3. Lab and Field Trials
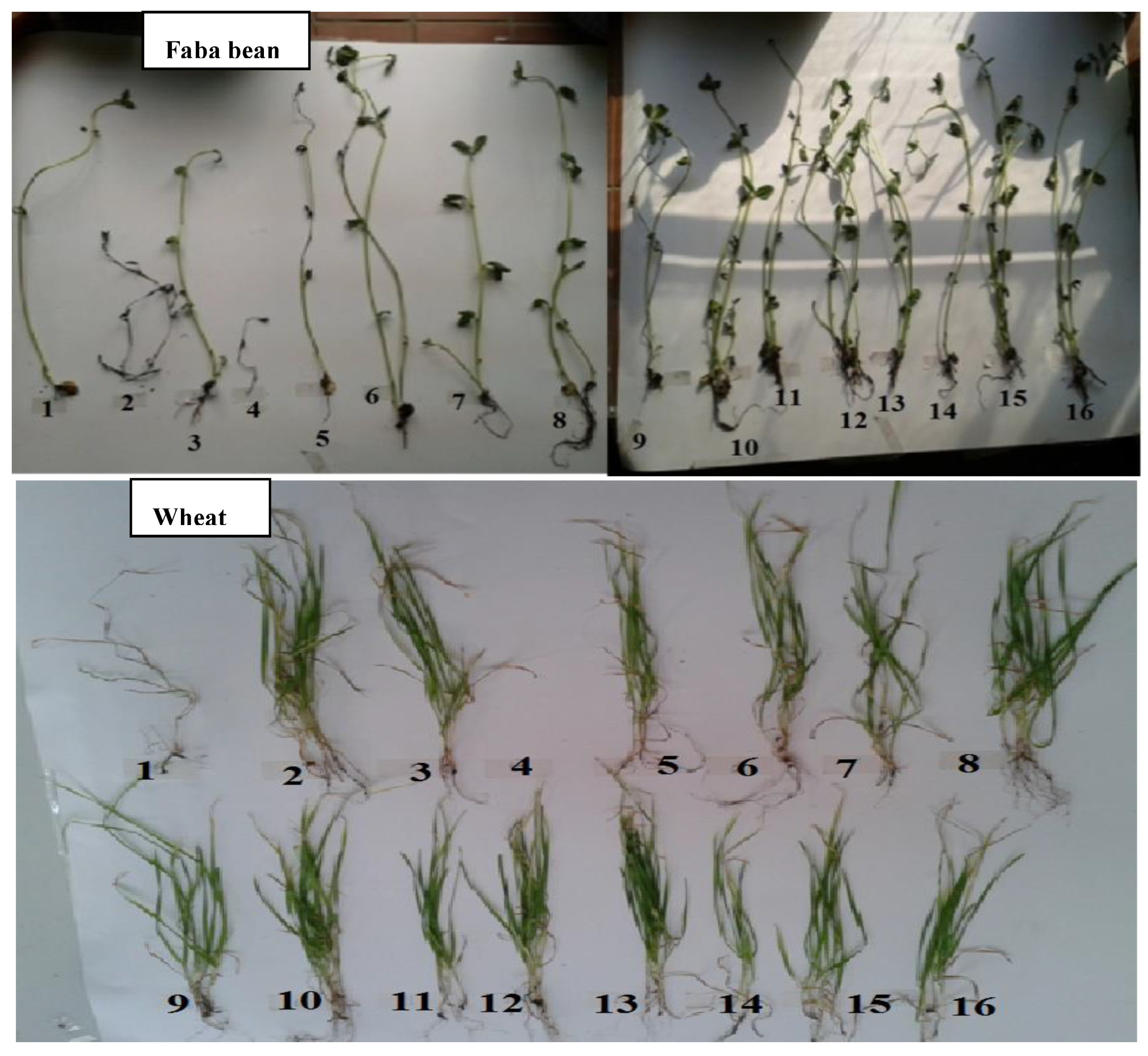
2.3.1. Preliminary Experiment
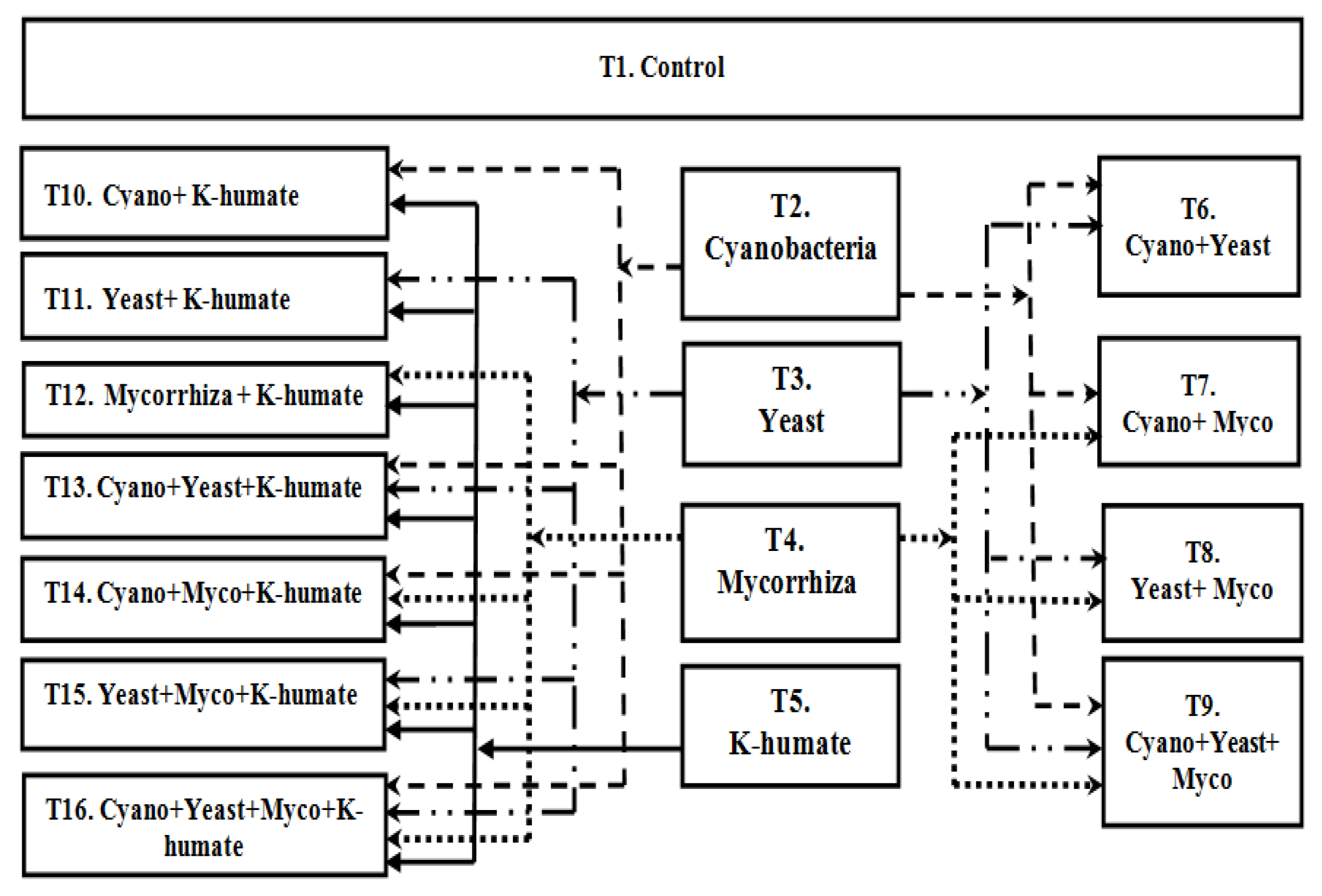
2.4. Field Experiment
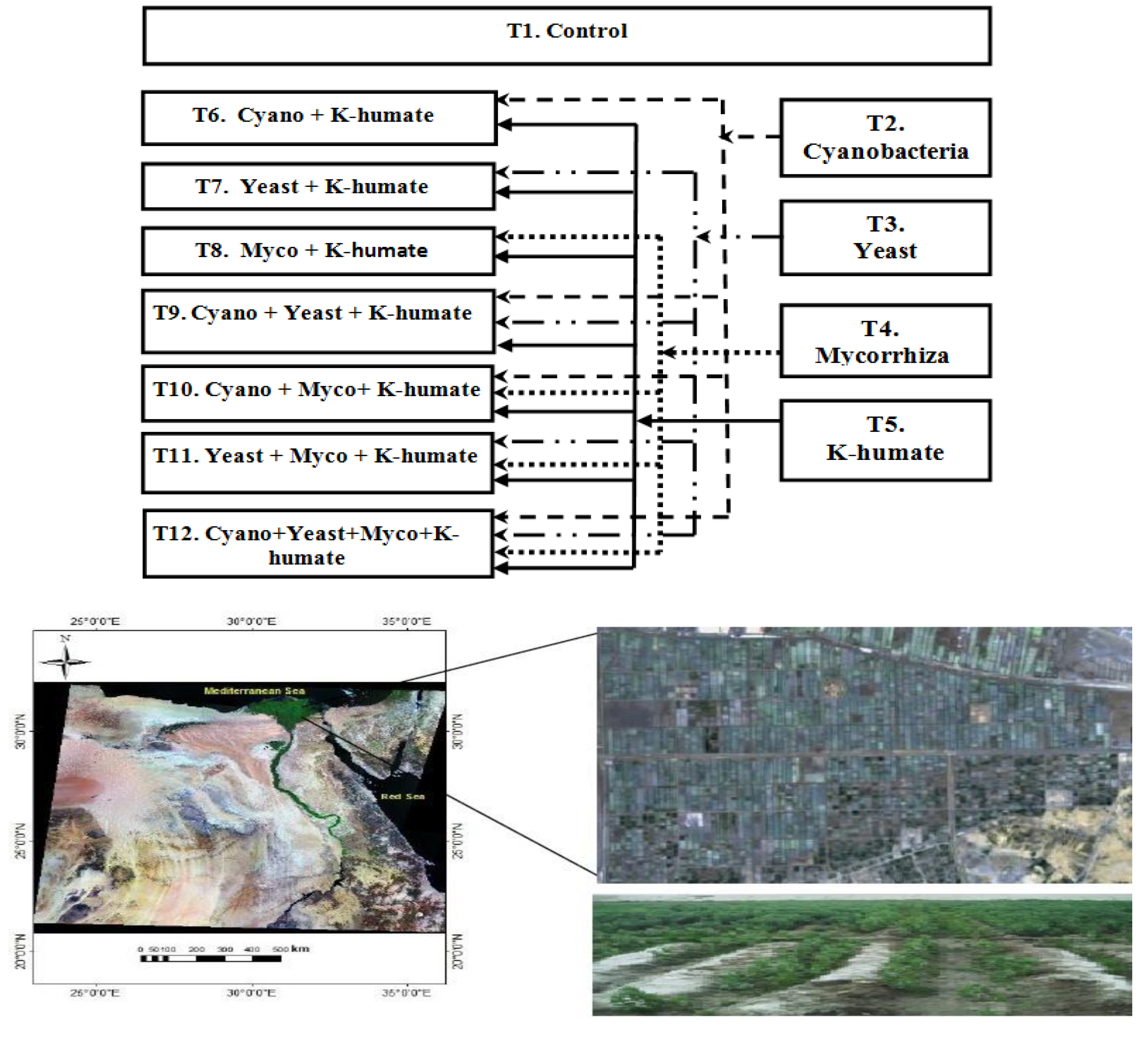
2.4.1. Experimental location and Soil Analysis
2.4.2. Soil Microbial Enzymes Analysis
2.4.3. Oxidative Enzymes Bioassay
2.4.4. Some Soil Biological Activity
2.4.5. Crop components and Some Chemical Analyses
2.4.6. Economic Evaluation of the Study
2.4.7. Statistical Analysis
3. Results
3.1. Preliminary Experiment: Interactions of Plants, Microbiomes and K-Humate to Cope with Salinity Stress
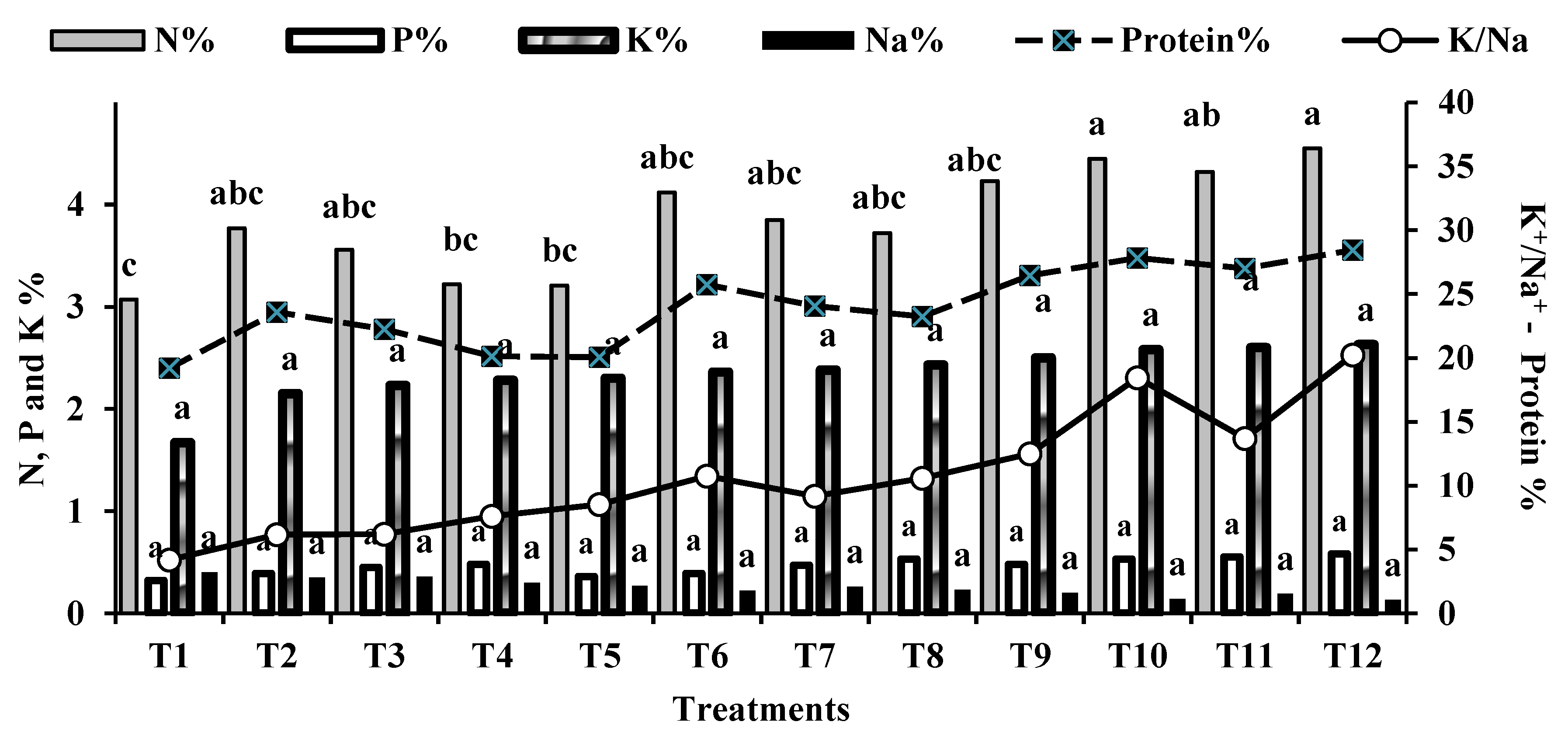
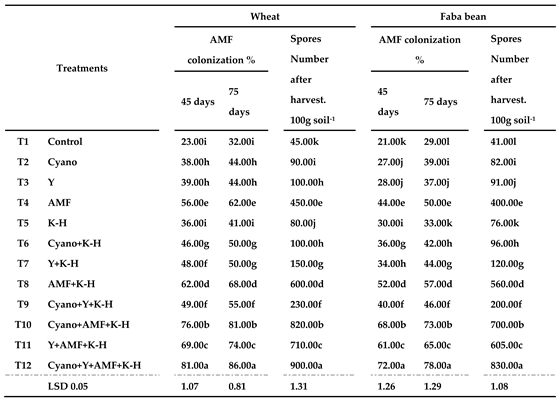
3.1.1. Faba Bean
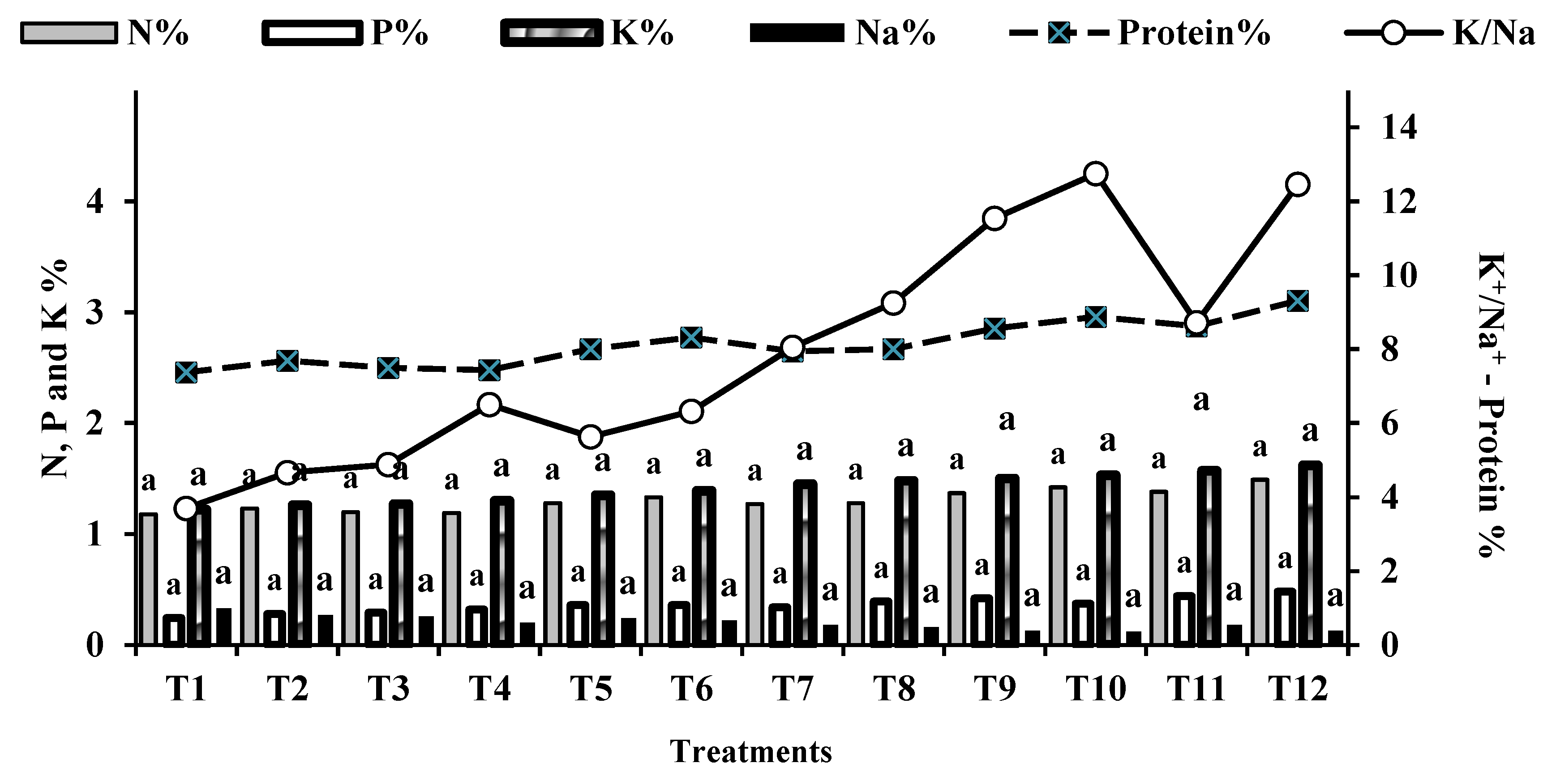
3.1.2. Wheat
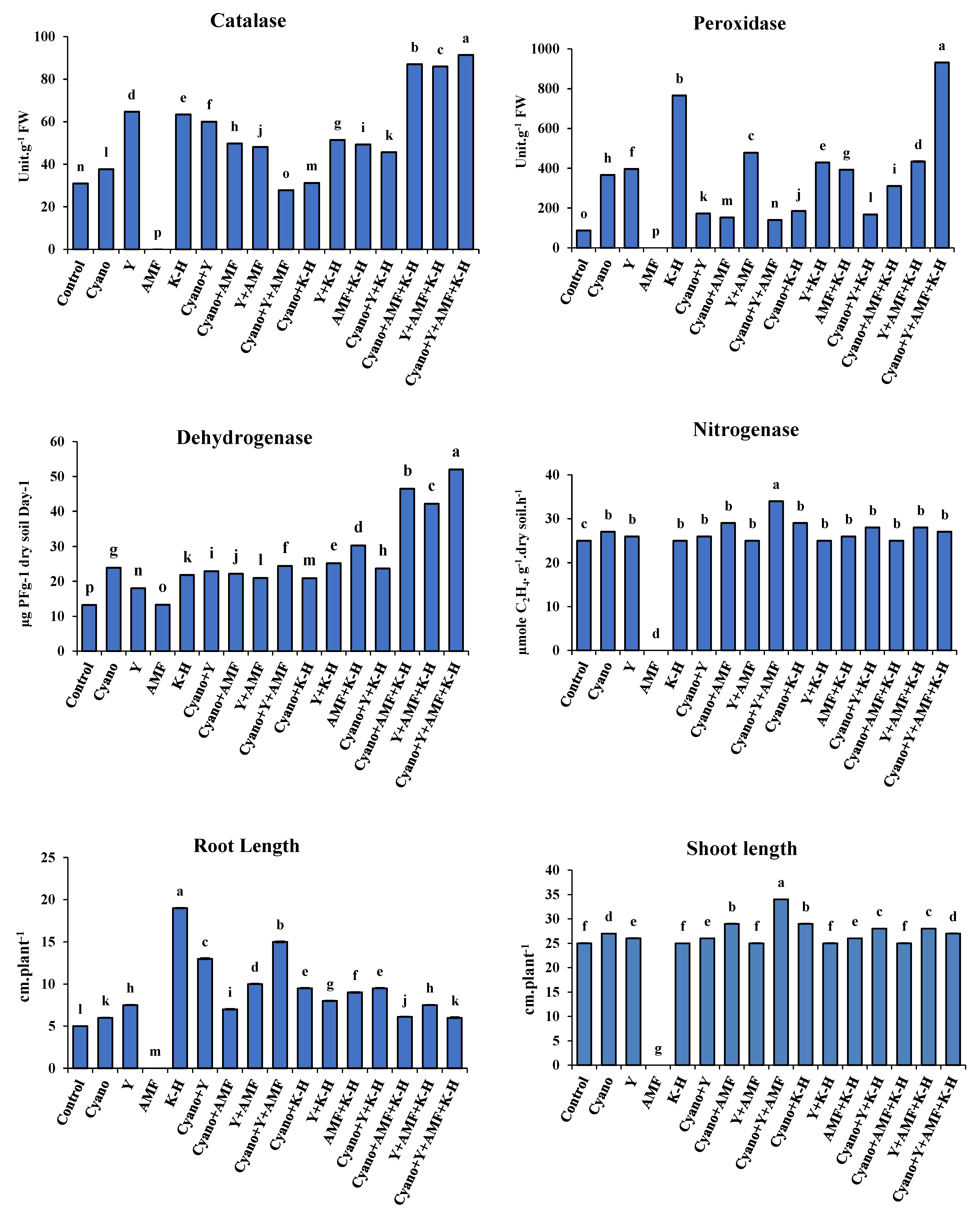
3.2. Field Experiment: Alleviating Effect of Salinity Stress via Microbiomes Inoculation and Organic Soil Amendment on Plants in Field Application
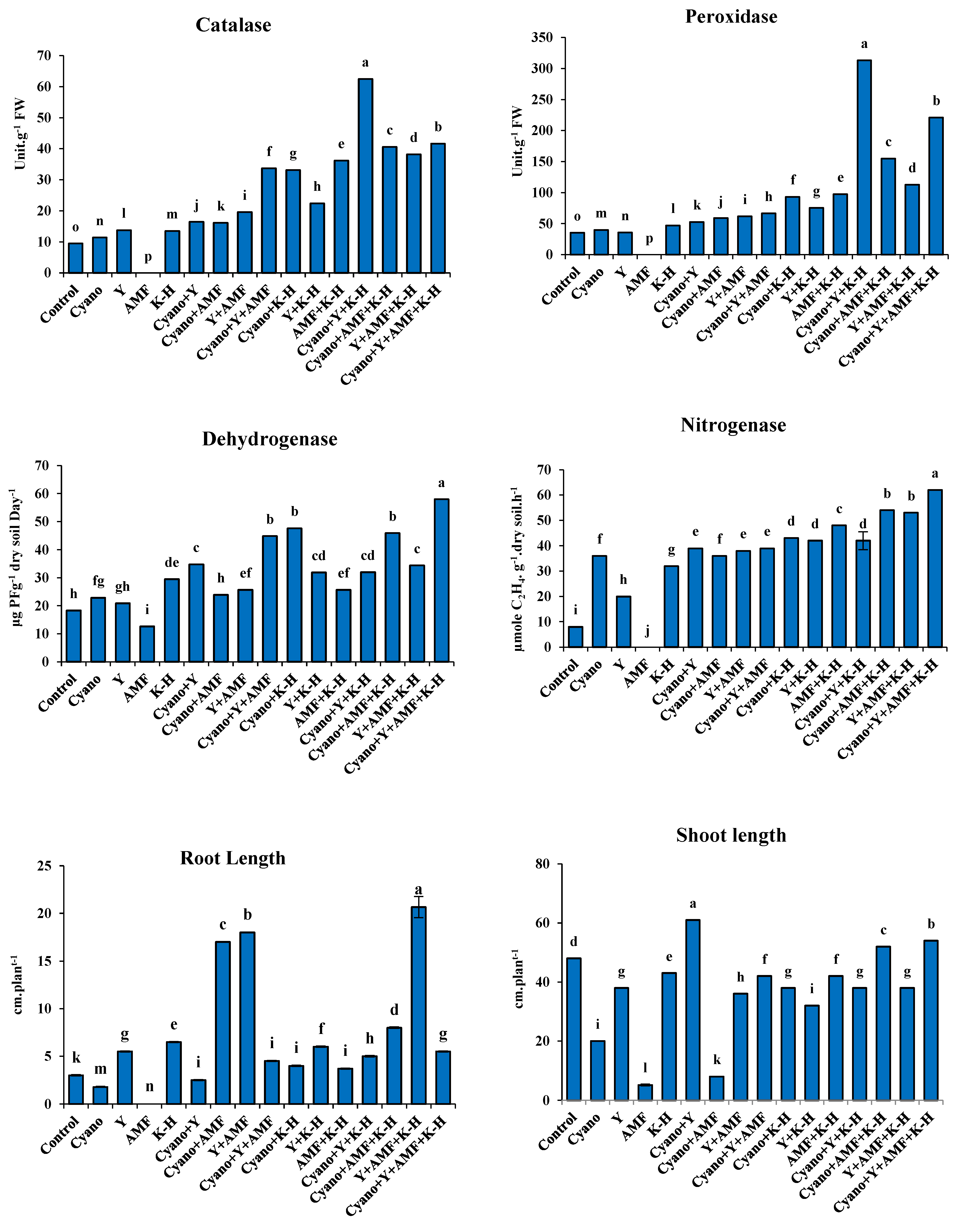
3.2.1. Soil Microbial Activity
3.2.2. Effects of Microbiomes and K-Humate on Wheat and Faba Bean Yield and Yield Components under Saline Soil Conditions
3.2.3. Influence of the Microbiomes and K-Humate on the Quality of Faba Bean Seeds and Wheat Grains
3.3. Economic Evaluation of the Study
4. Discussion
4.1. Impact of Microbiomes and K-Humate on the Resistance of Faba Bean and Wheat Seedlings to Salt Stress
4.2. The Integrated Effect of Microbiomes and K-Humate on Soil Microbial Activity under Salinity Stress
4.3. Effects of Microbiomes and K-Humate on Wheat and Faba Bean Yield in Saline Soil
4.4. Economic and Sustainability Evaluation of the Eco-Friendly Microbiomes Fertilizers
5. Conclusion
References
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. 2020. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agricultural Water Management 227: 105832.
- Negm, M. E., El- Kallawy, W. and Hefeina, A. (2019). Comparative study on rice germination and seedling growth under salinity and drought stresses. Env. Biodiv. Soil Security,, 3, 109-117. [CrossRef]
- Ouda, S. and Zohry A.E.H. (2020) Water Scarcity Leads to Food Insecurity. In: Deficit Irrigation. Springer, Cham,pp 1-13. [CrossRef]
- Abdellatif, K. F. ; E.A. El Absawy and Asmaa. M. Zakaria (2012). Drought stress tolerance of faba bean as studied by morphological traits and seed storage protein pattern. J. of Plant Studies, Vol. 1 (2): 47-54.
- Mohamed, A. I. (2013): Irrigation water quality evaluation In El-Salam canal project. International Journal of Engineering and Applied Sciences 3 (1): 21–28.
- Khalil, Y.M.M. , Abd El-Ghani, S.S. & Mansour, T.G.I. A standard analysis of Egyptian foreign trade structure for wheat. Bull Natl Res Cent 44, 20 (2020). [CrossRef]
- Royo, A., Abió, D. (2003). Salt tolerance in durum wheat cultivars. Spanish Journal of Agriculture Research, 1, 27–35. Phyton, 2021. [CrossRef]
- Vogelsang-O’dwyer, M. , Petersen, I. L., Joehnke, M. S., Sørensen, J. C., Bez, J., Detzel, A., et al. (2020). Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: techno-functional, nutritional and environmental performance. Foods 9:322. [CrossRef]
- Mass, E.V. , Hoffman, G.J., 1977. Crop salt tolerance: current assessment. American Society of Civil Engineers 103, 115–134.
- Al-Ashkar, I., Alderfasi, A., Romdhane, W., Seleiman, M. F., El-Said, R. A. et al. (2020). Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants, 9, 287. [CrossRef]
- Inbaraj MP (2021) Plant-Microbe Interactions in Alleviating Abiotic Stress—A Mini Review. Front. Agron. 3:667903. [CrossRef]
- Alharbi, K.; Rashwan, E.; Hafez, E.; Omara, A.E.-D.; Mohamed, H.H.; Alshaal, T. Potassium Humate and Plant Growth-Promoting Microbes Jointly Mitigate Water Deficit Stress in Soybean Cultivated in Salt-Affected Soil. Plants 2022, 11, 3016. [CrossRef] [PubMed]
- Rippka, R., J. Deruelles, J. B. Waterburg, M. Herdman and R.Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteira. J. General Microbiol. 111:1-16.
- Zarrouk, C. 1966. Contribution á l’étuded’unecyanophycée. Influence de divers facteurs physiques et chimiquessur la croissance et la photosynthése de Spirulina maxima (Setch. et Gardner) Geitler. Ph. D. Thesis, University of Paris, France.
- Vonshak, A. 1986. Laboratory techniques for the cultivation of microalgae. In Handbook of microalgal mass culture. (Ed. A. Richmond).Boca Raton: CRC Press.
- Leduy, A. and N. Therien. 1977. An improved method for optical density measurement of semimcro blue-green alga Spirulina maxima. Biotechnol. Bioeng. 19:1219-1224.
- Difco Manual, 1985. Dehydrated culture media and reagents for microbiology. laboratories incorporated Detroit. Michigan, 48232 USS, p: 621.
- Sanchez-Monedero, M. A., Roid, A., Cegarra, J., Bernal,M.P. and Paredes, C. Effects of HCL-HF purification treatment on chemical composition and structure of humic acids. Eur. J. Soil Sci. 2002, 53, 375–381. [Google Scholar] [CrossRef]
- Page, A. L., R. H. Miller and D. R. Keeny. 1982. Methods of Soil Analysis. Part 2. Chemical and Microbiological properties. Second Edition. Madison, Wisconsin, USA.
- Glathe', H. and A. Thalmann (1970). Uber die microbielloactivitat and iherBeziehungenzuFruchtbrkeitsmerkmaleneinigerAcherbodenunterbesondererBerucksichtigung der dehydrogenase akativitat (TCC. Redukation).Zbl. Bakt. Abt. II, 124: 1-23.
- Dilworth MJ (1966) Acetylene reduction by nitrogen-fxing preparations from Clostridium pasteurianum. Biochimicaet Biophysica Acta 127:285– 294. [CrossRef]
- Allam, A.I. and Hollis, J.P. (1972) Sulphide Inhibition of Oxidases in Rice Roots. Phytopathology, 62, 634-639. [CrossRef]
- Góth, L. 1991. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta, 196, 143–151. [CrossRef]
- Casida, L.E., D. A. Klien and T. Suntoro. 1964. Soil dehydrogenase activity. Soil Sci. 98:371-376.
- Dilworth MJ (1966) Acetylene reduction by nitrogen-fxing preparations from Clostridium pasteurianum. Biochimicaet Biophysica Acta 127:285– 294. [CrossRef]
- APHA (1992) Standard Methods for the Examination of Water and Wastewater. 18th Edition, American Public Health Association (APHA), American Water Works Association (AWWA) and Water Pollution Control Federation (WPCF), Washington DC.
- Stanier, R. Y. , Kunisawa, R., Mandel, M., & Cohen-Bazire, G. (1971). Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriological reviews, 35(2), 171–205. [CrossRef]
- Watanabe, I. W. L. , and Barraque. 1979. Low levels of fixed N required for free-living organisms from rice roots. Nature 277:565-566.
- Phillips, J.M. and Hayman, D.A. (1970) Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Transactions of the British Mycological Society, 55, 158-161. [CrossRef]
- Gerdemann, J.W and Nicolson, T.H. (1963) Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Transactions of the British Mycological Society,46,235-244. [CrossRef]
- Van Schouwenburg,l. C. & Walinga I. (1967) The rapid determination of phosphorus in the presence of arsenic, silicon and germanium. Analytica. chim. Acta 37,269-271.
- Kay, J. A. (1986). Farm management, 2nd edition, Mc Graw-Hill, New York.
- Gomez MA, Gomez AA (1993) Statistical procedure for agricultural research (2nded). John Wile & Sons, New York, p 680.
- Kthiri, Z.; Ben Jabeur, M.; Machraoui, M.; Gargouri, S.; Hiba, K.; Hamada, W. Coating seeds with Trichoderma strains promotes plant growth and enhance the systemic resistance against Fusarium crown rot in durum wheat. Egypt. J. Biol. Pest Control 2020, 30, 139.
- Lau, SE. , Teo, W.F.A., Teoh, E.Y. et al. Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discov Food 2, 9 (2022). [CrossRef]
- Phour M, Sindhu SS, Salama KHA, Morsy AA. 2022. Mitigating abiotic stress: microbiome engineering for improving agricultural production and environmental sustainability. Planta. 256(5):85. [CrossRef]
- Türkmen Ö, Şensoy S, Erdal İ. Effect of Potassium on Emergence and Seedling Growth of Cucumber Grown in Salty Conditions. Yuzuncu Yil University, J. Agric. Sci. 2000, 10, 113–117. [Google Scholar]
- Pinali N, Kaplan M. Investigation of effect on nutrient uptake of humic acid applications of different forms to strawberry plant. J. Plant Nutri. 2003, 26, 835–843. [Google Scholar] [CrossRef]
- Aydın, A., Kant, C., & Turan, M. Humic acid application alleviate salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. African Journal of Agricultural Research 2012, 7, 1073–1086. [Google Scholar]
- Parial, R., Mohajan, S., Mt, H., Hashem, M., Das, M., & Islam, M. Isolation of arbuscular mycorrhizal Fungi and evaluation its effect on plant Growthover chemical fertilizers for better human health. SMU Medical Journal 2014, 1, 192–206. [Google Scholar]
- Sharma, J. , Zettler, L.W., Van Sambeek, J.W., Ellersieck, M.R., & Starbuck, C.J. (2003). Symbiotic Seed Germination and Mycorrhizae of Federally Threatened Platanthera praeclara (Orchidaceae).
- Kumar, A. , Sharma, S. & Mishra, S. Influence of Arbuscular Mycorrhizal (AM) Fungi and Salinity on Seedling Growth, Solute Accumulation, and Mycorrhizal Dependency of Jatropha curcas L.. J Plant Growth Regul 29, 297–306 (2010). [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167 [CrossRef]. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S., & Lee, K.D. (2005). Plant Growth Promoting Rhizobacteria Effect on Antioxidant Status , Photosynthesis , Mineral Uptake and Growth of Lettuce under Soil Salinity.
- Anand Mohan, Baidyanath Kumar and Dina Nath. Cyanobacterial Consortium in the Improvement of Maize Crop. Int.J.Curr.Microbiol.App.Sci 2015, 4, 26. [Google Scholar]
- Ali, Laila K.M. and Mostafa, Soha S.M. (2009). Evaluation of potassium humate and Spirulina platensis as a bio-organic fertilizer for sesame plants grown under salinity stress. The 7th International Conference of Organic Agriculture, 13-15 December, Egypt. J. Res., 87(1):369-388.
- Mostafa, Soha S.M.; AbouElkhair, Amal W. and Shhata, Heba Sh. Effect of plant growth promoting substances from rhizo- and cyano-bacteria on sugar beet growth, yield and yield quality in saline soil. Intr. J. of Acad. Res. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Badawi, H. Mona; Mostafa, S. M. Soha; Abo El-Rakha, S. Eman; Mostafa, A. A. and Fayez, M. Cereal-PGPR interweave in salt-affected environments: towards plant persistence and growth promotion. International Journal of Scientific & Engineering Research, Volume 7, Issue 11, November-2016.
- Morais, P.B., M.B. Martins, A.N., Hagler, L.C. Mendonça-Hagler and L.B. Klaczko. 1995. Yeast succession in the Amazon fruit Parahancornia amapa as resource partitioning among Drosophila spp. Applied and Environmental Microbiology 1995, 61, 4251–4257. [Google Scholar]
- Ganter, P.F. 2006. Yeast and invertebrate associations. In: Biodiversity and Ecophysiology of Yeasts. The Yeast Handbook, (eds). C.A. Rosa and G. Peter (pp. 303-370). Heidelberg: Springer.
- Sampedro, I., E. Aranda, J. M. scervino, S. Fracchia, I. García-Romera, J. A. Ocampo and S. Godeas. Improvement by soil yeasts of arbuscular mycorrhizal symbiosis of soybean (Glycine max) colonized by Glomus mosseae. Mycorrhiza 2004, 14, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Boby, V.U., A.N. Balakrishna and D.J. Bagyaroj. Effect of combined inoculation of an AM fungus with soil yeasts on growth and nutrition of cowpea in sterilized soil. World Journal of Agriculture Sciences 2007, 3, 423–429. [Google Scholar]
- Hesham, A. and H.M. Mohamed. Molecular genetic identification of yeast strains isolation from Egyption soils for solubilization of inorganic phosphates and growth promotion of corn plants. J. Microbiolo. Biotechnol. 2011, 21, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M. Effect of Arbuscular Mycorrhizal Fungus (Glomus Mosseae) and Soil Yeasts Interaction on Root Nodulation, N-Fixation and Growth of Faba Bean (Vichia faba). Malaysian Journal of Soil Science, 2015; 19, 157–168. [Google Scholar]
- Dastogeer KMG, Zahan MI, Tahjib-Ul-Arif M, Akter MA and Okazaki S (2020) Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 11:588550. [CrossRef]
- Singh, C.S., A. Kapoor and S.S. Wange. The enhancement of root colonization of legumes by vesicular-arbuscular mycorrhizal (VAM) fungi through the inoculation of the legume seed with commercial yeast (Saccharomyces cerevisiae). Plant Soil 1991, 131, 129–133. [Google Scholar] [CrossRef]
- Larsen, J. and I. Jacobsen. Interactions between a mycophagus Collembola, dry yeast and the external mycelium of an arbuscular mycorrhizal fungus. Mycorrhiza 1996, 6, 259–264. [Google Scholar] [CrossRef]
- Hamed, S.M. , El-Gaml, N.M. & Eissa, S.T. Integrated biofertilization using yeast with cyanobacteria on growth and productivity of wheat. Beni-Suef Univ J Basic Appl Sci 11, 112 (2022). [CrossRef]
- Ghazal FM, Moussa LAA, Fetyan NAH (2010) Cyanobacteria and Rhizobium radiobacter as possible biofertilizers in wheat production. J Agric Chemi Biotechnol 1(7):383–399. [CrossRef]
- Hussain A, Hasnain S (2011) Phytostimulation and biofertilization in wheat by cyanobacteria. J Ind Microbiol Biotechnol 38:85–92. [CrossRef]
- Chittapun S, Limbipichai S, Amnuaysin N, Boonkerd R, Charoensook M (2017) Efects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: a pot experiment. J Appl Phycol 30(1):79–85. [CrossRef]
- Singh, R. R. and K. Prasad, (2011). Effect of bio-fertilizers on growth and productivity of wheat (Triticum aestivum). J. of Farm Sci.,1(1): 1-8.
- Arif, Y. , Singh, P., Siddiqui, H., Bajguz, A., and Hayat, S. (2020). Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol. Biochem. 156, 64–77. [CrossRef]
- Acosta-Motos, J. R. , Ortuño, M. F., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M. J., and Hernandez, J. A. (2017). Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. [CrossRef]
- Yang, Y. , Guo Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018;60:796–804. [CrossRef]
- Van Zelm, E. , Zhang Y., Testerink C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020;71:403–433. [CrossRef]
- Mutale-joan, C. , Rachidi, F., Mohamed, H. A., Mernissi, N. E., Aasfar, A., Barakate, M., et al. (2021). Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 33, 3779–3795. [CrossRef]
- Aguégué, M. R. , Ahoyo Adjovi, N. R., Houédjofonon, E. M., Djinadou, K. A., Koda, D. A., Adoko, M. Y., et al. (2021a). Financial profitability of maize production with bio-fertilizer based on arbuscular mycorrhizal fungi native to Benin. J. Dev. Agric. Economics. 13 (1), 56–64. [CrossRef]
- Shiferaw B, Hellin J, Muricho G (2011). "Improving market access and agricultural productivity growth in Africa: what role for producer organizations and collective action institutions? Food Security 3(4):475-489. [CrossRef]
- Di-Marcantonio F, Morales-Opazo C, Barreiro-Hurlé J, Demeke M (2014). Determinants of food production in Sub Saharan Africa: The impact of policy, market access and governance. No. 727:2016- 50321. [CrossRef]
- Ahmed. E., J. Sulaiman and S. Mohd. Wheat Production and Economics. Am. J. Agric. Biol. Sci. 2011, 6, 332–338. [Google Scholar] [CrossRef]
- Soni, S. N. Economics of Technical Change of Wheat Production in Sagar District. M. P. Crop-Research-Hisar. 2000, 19, 452–456. [Google Scholar]
- Habib, W. Z. and I, Iskandar and A. Aziz. Economic efficiency of orange production in Syria. Damascus University Journal for Agricultural Sciences 2013, 29, 375–391. [Google Scholar]
- Maoba, S. (2016) Farmers’ Perception of Agricultural Extension Service Delivery in Germiston Region, Gauteng Province, South Africa. South African Journal of Agricultural Extension, 44, 167-173. [CrossRef]
| Ser. | Microalgal strains | NCBI* Accession no. | Family | pH | Optical density at 560 nm | Total chlorophyll (mg.l-1) |
Dry weight (mg.l-1) |
|---|---|---|---|---|---|---|---|
| 1 | Nostoc muscorum isolate HSSASE1 | KT277784.1 | Nostocaceae | 8.11 | 1.19 | 5.26 | 760.96 |
| 2 | Spirulina platensis isolate HSSASE5 | KT277788.1 | Spirulinaceae | 10.16 | 2.77 | 11.63 | 1772.80 |
| 3 | Anabaena oryzae isolate HSSASE6 | KT277789.1 | Nostocaceae | 7.14 | 0.87 | 4.03 | 557.76 |
| 4 | Wollea saccata isolate HSSASE7 | KT277790.1 | Nostocaceae | 6.82 | 2.40 | 9.82 | 1532.80 |
| 5 | Phormidium fragilis isolate HSSASE9 | KT277792.1 | Phormidiaceae | 8.67 | 2.09 | 3.00 | 1334.40 |
| 6 | Anabaena sp. HSSASE11 | KT277794.1 | Nostocaceae | 8.05 | 1.67 | 7.56 | 1065.60 |
| Coarse sand (%) | Fin sand (%) | Silt(%) | Clay (%) | Texture | O.M(%) | CaCO3 (%) | |||||||
| 3.14 | 8.29 | 28.76 | 59.81 | Clay | 0.48 | 5.19 | |||||||
| pH (1:2.5) | EC(dS.m-1) | Cations (meq/l) | Anions (meq/l) | ||||||||||
| Ca++ | Mg++ | Na+ | K+ | HCO-3 | Cl- | SO--4 | |||||||
| 8.25 | 18.57 | 12.46 | 21.73 | 150 | 0.76 | 8.25 | 132 | 44.70 | |||||
| Macronutrients (ppm) | Micronutrients (ppm) | ||||||||||||
| N | P | K | Fe | Mn | Zn | Cu | |||||||
| 37 | 5.67 | 189 | 1.37 | 3.25 | 0.73 | 0.048 | |||||||
| pH | EC (dS.m-1) | Cations (meq/l) | Anions (meq/l) | R.S.C. | SAR | ||||||||
| Ca++ | Mg++ | Na+ | K+ | HCO-3 | CO--3 | Cl- | SO--4 | 14.64 | 26.25 | ||||
| 9.00 | 4.94 | 2.12 | 2.29 | 39.00 | 2.40 | 12.45 | 6.60 | 16.27 | 10.49 | ||||
 |
| Treatments | Wheat | Faba bean | ||||||||||
|
Cyano count c.f.u.* 10-3.g-1 dry soil |
Bacteria count c.f.u.* 10-6.g-1 dry soil |
Yeast count c.f.u.* 10-3.g-1 dry soil |
N-ase µmole C2H4. g-1 dry soil.h-1 |
DHA-ase μgTPF. g-1 dry soil.day-1 |
Cyano count c.f.u.* 10-3.g-1 dry soil |
Bacteria count c.f.u.* 10-6.g-1 dry soil |
Yeast count c.f.u.* 10-3.g-1 dry soil |
N-ase µmole C2H4. g-1 dry soil.h-1 |
DHA-ase μgTPF. g-1 dry soil.day-1 |
|||
| T1 | 10.00j | 21.50k | 1.00k | 0.85f | 5.29g | 15.02jk | 108.00j | 2.00l | 3.46gh | 4.67h | ||
| T2 | 36.00f | 61.50g | 3.00j | 1.60f | 14.08d | 30.00g | 250.25d | 4.00k | 1.13i | 9.33f | ||
| T3 | 17.00h | 60.00h | 7.00h | 2.70de | 3.71ghi | 24.00h | 245.25e | 5.00j | 2.84h | 7.46g | ||
| T4 | 12.00i | 26.00j | 6.00i | 0.70f | 2.87i | 14.00k | 147.33i | 7.00i | 4.8fg | 4.01h | ||
| T5 | 12.00i | 35.00i | 3.00j | 2.34de | 3.36hi | 32.00f | 108.50j | 10.00h | 8.41e | 8.95f | ||
| T6 | 38.00e | 95.50d | 9.00g | 5.89c | 11.45e | 36.00e | 207.00g | 11.00g | 12.76c | 10.85e | ||
| T7 | 23.00g | 79.00e | 12.00f | 5.52c | 7.19f | 16.00j | 250.00d | 17.00e | 17.46b | 5.01h | ||
| T8 | 18.00h | 66.00f | 13.00e | 3.12d | 4.84gh | 20.00i | 221.25f | 16.00f | 5.13f | 5.38h | ||
| T9 | 60.00c | 95.50d | 18.00c | 5.48c | 21.82b | 50.00b | 194.00h | 32.00c | 11.14d | 18.19b | ||
| T10 | 64.00b | 126.00c | 14.00d | 8.42b | 19.69c | 42.00c | 307.00b | 23.00d | 25.30a | 12.92d | ||
| T11 | 48.00d | 194.00b | 22.00b | 16.98a | 18.22c | 38.00d | 287.00c | 35.00b | 25.11a | 14.42c | ||
| T12 | 78.00a | 200.00a | 25.00a | 17.27a | 30.88a | 61.00a | 378.50a | 38.00a | 26.51a | 24.15a | ||
| LSD | 1.06 | 0.74 | 0.92 | 1.11 | 1.64 | 1.09 | 0.85 | 0.86 | 1.39 | 1.39 | ||
| Treatments | Number of pods. plant-1 | Number of Seeds. plant-1 | Weight of seeds. plant-1 (g) | Weight of 100 seeds (g) | Seeds yield (ton.ha-1) | Straw yield (ton.ha-1) | Biological yield (ton.ha-1) |
Harvest index (%) | |
|---|---|---|---|---|---|---|---|---|---|
| T1 | Control | 6.83g | 16.10j | 5.83g | 31.56l | 1.21j | 1.45k | 2.66 | 0.45 |
| T2 | Cyano | 14.00d | 33.75g | 19.00c | 50.41h | 1.33g | 1.63h | 2.96 | 0.45 |
| T3 | Y | 11.00ef | 23.43i | 11.67f | 46.61j | 1.22i | 1.62i | 2.84 | 0.43 |
| T4 | AMF | 10.00f | 14.00k | 6.76g | 42.92k | 0.98k | 1.41l | 2.39 | 0.41 |
| T5 | K-H | 13.50de | 28.00h | 17.76e | 48.78i | 1.29h | 1.45j | 2.75 | 0.47 |
| T6 | Cyano+K-H | 19.73c | 55.24c | 33.50c | 61.90e | 1.42e | 1.66f | 3.08 | 0.46 |
| T7 | Y+K-H | 18.20c | 42.22f | 23.57d | 54.04g | 1.42e | 1.71e | 3.13 | 0.45 |
| T8 | AMF+K-H | 20.30c | 52.78e | 33.42c | 59.28f | 1.38f | 1.66g | 3.04 | 0.45 |
| T9 | Cyano+Y+K-H | 24.20b | 54.21d | 39.10b | 70.75c | 1.93d | 2.11d | 4.03 | 0.48 |
| T10 | Cyano+AMF+K-H | 25.74b | 57.14b | 43.87a | 75.45b | 2.36b | 2.53b | 4.89 | 0.48 |
| T11 | Y+AMF+K-H | 24.97b | 52.94e | 37.74b | 69.88d | 2.21c | 2.55a | 4.77 | 0.46 |
| T12 | Cyano+Y+AMF+K-H | 28.86a | 58.09a | 46.20a | 78.24a | 2.77a | 2.51k | 5.28 | 0.52 |
| LSD 0.05 | 2.96 | 0.65 | 3.82 | 0.59 | 0.57 | 0.72 |
| Treatments | Number of spike. plant-1 | Number of grains. spike-1 | Weight of 1000-grain (g) | Grain yield (ton.ha-1) | Straw yield (ton.ha-1) |
Biological yield (ton.ha-1) |
Harvest index (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| T1 | Control | 18.00f | 35.57f | 55.80j | 3.07g | 4.44i | 7.51 | 0.41 | |
| T2 | Cyano | 26.00a | 60.17a | 62.30f | 3.53def | 5.28g | 8.81 | 0.40 | |
| T3 | Y | 19.00ef | 33.96g | 61.00g | 3.34efg | 4.92h | 8.26 | 0.40 | |
| T4 | AMF | 23.00b | 33.90g | 59.50h | 3.14fg | 5.02h | 8.16 | 0.39 | |
| T5 | K-H | 19.00ef | 44.89d | 60.98g | 3.43efg | 5.64ef | 9.07 | 0.38 | |
| T6 | Cyano+K-H | 22.00bc | 40.76e | 64.90e | 3.90bcd | 5.86de | 9.77 | 0.40 | |
| T7 | Y+K-H | 26.00a | 29.80h | 62.60f | 3.70cde | 5.59f | 9.31 | 0.40 | |
| T8 | AMF+K-H | 25.00a | 29.21h | 58.26i | 3.56def | 5.30g | 8.86 | 0.40 | |
| T9 | Cyano+Y+K-H | 14.00g | 34.81fg | 66.50c | 4.06bc | 6.07cd | 10.13 | 0.40 | |
| T10 | Cyano+AMF+K-H | 21.00cd | 44.20d | 68.10b | 4.10bc | 6.34ab | 10.44 | 0.39 | |
| T11 | Y+AMF+K-H | 20.00de | 48.23c | 65.80d | 4.10bc | 6.17bc | 10.27 | 0.40 | |
| T12 | Cyano+Y+AMF+K-H | 18.00f | 52.24b | 70.20a | 4.75a | 6.43a | 11.18 | 0.42 | |
| LSD 0.05 | 1.47 | 1.17 | 0.46 | 0.18 | 0.11 | ||||
| Items | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 |
| Seed yield (ton.ha-1) | 1.21 | 1.33 | 1.22 | 0.98 | 1.29 | 1.42 | 1.42 | 1.38 | 1.93 | 2.36 | 2.21 | 2.77 |
| Price ($.ton-1) | 640 | 640 | 640 | 640 | 640 | 640 | 640 | 640 | 640 | 640 | 640 | 640 |
| Straw yield (ton.ha-1) | 1.45 | 1.63 | 1.62 | 1.41 | 1.45 | 1.66 | 1.71 | 1.66 | 2.11 | 2.53 | 2.55 | 1.45 |
| Price ($.ton-1) | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
| Revenue ($.ha-1) | 890.40 | 981.60 | 910.40 | 740.00 | 941.60 | 1041.60 | 1045.60 | 1016.00 | 1404.00 | 1712.80 | 1618.40 | 1888.80 |
| Seeds ($.ha-1) | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 | 46.54 |
| Labor ($.ha-1) | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 | 116.3 |
| Machine ($.ha-1) | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 | 193.85 |
| Menial Fertilizers ($.ha-1) | 193.85 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 | 48.46 |
| Cyanobacteria ($.ha-1) | 0 | 13.97 | 0 | 0 | 0 | 13.97 | 0 | 0 | 13.97 | 13.97 | 0 | 13.97 |
| Yeast ($.ha-1) | 0 | 0 | 6.98 | 0 | 0 | 0 | 6.98 | 0 | 6.98 | 0 | 6.98 | 6.98 |
| AMF($.ha-1) | 0 | 0 | 0 | 9.29 | 0 | 0 | 0 | 9.29 | 0 | 9.29 | 9.29 | 9.29 |
| K-humate ($.ha-1) | 0 | 0 | 0 | 0 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 |
| Pesticides ($.ha-1) | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 | 16.06 |
| Variable Cost ($.ha-1) | 566.60 | 435.18 | 428.19 | 430.50 | 430.50 | 444.47 | 437.48 | 439.79 | 451.45 | 453.76 | 446.77 | 460.74 |
| Fixed Cost ($.ha-1) | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 | 193.87 |
| Total Cost ($.ha-1) | 760.47 | 629.05 | 622.06 | 624.37 | 624.37 | 638.34 | 631.35 | 633.66 | 645.32 | 647.63 | 640.64 | 654.61 |
| Profit ($.ha-1) | 129.93 | 352.55 | 288.34 | 115.63 | 317.23 | 403.26 | 414.25 | 382.34 | 758.68 | 1065.17 | 977.76 | 1234.19 |
| Profit /cost ratio | 0.17 | 0.56 | 0.46 | 0.19 | 0.51 | 0.63 | 0.66 | 0.60 | 1.18 | 1.64 | 1.53 | 1.89 |
| Break-even yield | 1.06 | 0.87 | 0.86 | 0.87 | 0.87 | 0.89 | 0.88 | 0.88 | 0.90 | 0.90 | 0.89 | 0.91 |
| Break-even price | 285.89 | 212.52 | 219.04 | 261.24 | 227.87 | 207.25 | 201.71 | 208.44 | 159.73 | 132.44 | 134.59 | 155.12 |
| Items | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 |
| Grain yield (ton.ha-1) | 3.07 | 3.53 | 3.34 | 3.14 | 3.43 | 3.91 | 3.72 | 3.55 | 4.06 | 4.10 | 4.10 | 4.75 |
| Price ($.ton-1) | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 |
| Straw yield (ton.ha-1) | 4.44 | 5.28 | 4.92 | 5.02 | 5.64 | 5.86 | 5.59 | 5.30 | 6.07 | 6.34 | 6.17 | 6.43 |
| Price ($.ton-1) | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 |
| Revenue ($.ha-1) | 5621.00 | 6655.00 | 6214.00 | 6276.00 | 7027.00 | 7384.00 | 7041.80 | 6682.00 | 7651.00 | 7958.00 | 7771.00 | 8213.00 |
| Seeds ($.ha-1) | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 | 38.78 |
| Labor ($.ha-1) | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 |
| Machine ($.ha-1) | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 | 155.09 |
| Menial Fertilizers ($.ha-1) | 77.54 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 | 19.39 |
| Cyanobacteria ($.ha-1) | 0 | 13.97 | 0 | 0 | 0 | 13.97 | 0 | 0 | 13.97 | 13.97 | 0 | 13.97 |
| Yeast ($.ha-1) | 0 | 0 | 6.98 | 0 | 0 | 0 | 6.98 | 0 | 6.98 | 0 | 6.98 | 6.98 |
| AMF($.ha-1) | 0 | 0 | 0 | 9.29 | 0 | 0 | 0 | 9.29 | 0 | 9.29 | 9.29 | 9.29 |
| K-humate ($.ha-1) | 0 | 0 | 0 | 0 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 | 9.29 |
| Pesticides ($.ha-1) | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 | 13.56 |
| Variable Cost ($.ha-1) | 440.10 | 395.90 | 388.90 | 391.20 | 391.20 | 405.20 | 398.20 | 400.50 | 412.20 | 414.50 | 407.50 | 421.44 |
| Fixed Cost ($.ha-1) | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 | 232.63 |
| Total Cost ($.ha-1) | 672.69 | 628.51 | 621.52 | 623.83 | 623.83 | 637.80 | 630.81 | 633.12 | 644.78 | 647.09 | 640.10 | 654.07 |
| Profit ($.ha-1) | 4948.11 | 6026.69 | 5592.08 | 5651.77 | 6403.37 | 6746.60 | 6410.99 | 6048.88 | 7006.62 | 7310.91 | 7130.90 | 7558.93 |
| Profit /cost ratio | 7.36 | 9.59 | 9.00 | 9.06 | 10.26 | 10.58 | 10.16 | 9.55 | 10.87 | 11.30 | 11.14 | 11.56 |
| Break-even yield | 0.50 | 0.47 | 0.46 | 0.47 | 0.47 | 0.48 | 0.47 | 0.47 | 0.48 | 0.48 | 0.48 | 0.49 |
| Break-even price | 89.57 | 71.34 | 75.24 | 76.45 | 68.78 | 65.28 | 67.76 | 71.54 | 63.65 | 61.98 | 62.33 | 58.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





