Submitted:
25 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Basic Ad Structure
3. Basic Concepts of Antiviral Immune Responses
4. Natural Infection
4.1. Innate Immunity
4.2. Adaptive Immunity
5. Vector/OV-Induced Immunity
5.1. Widespread Ad Vector Development, Clinical Evaluation and Use
5.2. Innate Immunity
5.3. Adaptive Immunity
6. Engineering Ads for Immune Evasion
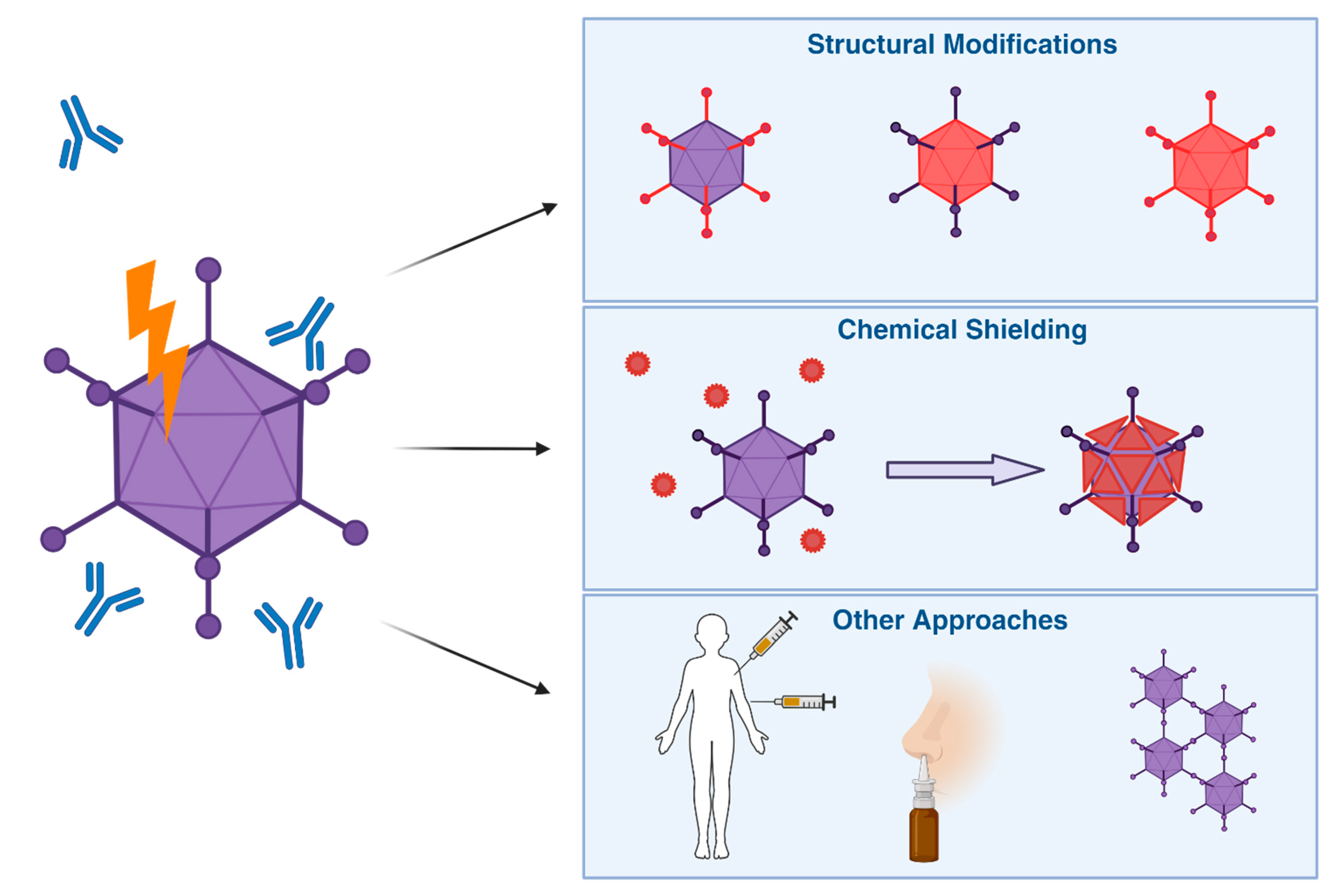
6.1. Structural Modifications
6.2. Chemical Shielding
6.3. Other Approaches
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A Review of 65 Years of Human Adenovirus Seroprevalence. Expert Rev Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Lemckert, A.A.C.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. J Virol 2007, 81, 4654–4663. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, A.C.; Stehle, T. Human Adenovirus Binding to Host Cell Receptors: A Structural View. Med Microbiol Immunol 2020, 209, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, Y.; Nakajima, E.; Ishikawa, M.; Kano, A.; Komane, A.; Fujimoto, T.; Hanaoka, N.; Okabe, N.; Shimizu, H. Construction of New Primer Sets for Corresponding to Genetic Evolution of Human Adenoviruses in Major Capsid Genes through Frequent Recombination. Japanese Journal of Infectious Diseases 2014, 67, 495–502. [Google Scholar] [CrossRef]
- Davies, J.A.; Marlow, G.; Uusi-Kerttula, H.K.; Seaton, G.; Piggott, L.; Badder, L.M.; Clarkson, R.W.E.; Chester, J.D.; Parker, A.L. Efficient Intravenous Tumor Targeting Using the Avβ6 Integrin-Selective Precision Virotherapy Ad5NULL-A20. Viruses 2021, 13, 864. [Google Scholar] [CrossRef]
- Coughlan, L.; Uusi-Kerttula, H.; Ma, J.; Degg, B.P.; Parker, A.L.; Baker, A.H. Retargeting Adenovirus Serotype 48 Fiber Knob Domain by Peptide Incorporation. Hum Gene Ther 2014, 25, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hiwasa, K.; Nagaya, H.; Terao, S.; Acharya, B.; Hamada, K.; Mizuguchi, H.; Gotoh, A. Improved Gene Transfer into Bladder Cancer Cells Using Adenovirus Vector Containing RGD Motif. Anticancer Research 2012. [Google Scholar]
- Uusi-Kerttula, H.; Davies, J.A.; Thompson, J.M.; Wongthida, P.; Evgin, L.; Shim, K.G.; Bradshaw, A.; Baker, A.T.; Rizkallah, P.J.; Jones, R.; et al. Ad5NULL-A20: A Tropism-Modified, Avβ6 Integrin-Selective Oncolytic Adenovirus for Epithelial Ovarian Cancer Therapies. Clinical Cancer Research 2018, 24, 4215–4224. [Google Scholar] [CrossRef]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. TheQ1 Influence of Innate and Pre-Existing Immunity on Adenovirus Therapy. J Cell Biochem 2009, 108, 778. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.C. Adenoviruses: Update on Structure and Function. Journal of General Virology 2009, 90, 1–20. [Google Scholar] [CrossRef]
- Gallardo, J.; Pérez-Illana, M.; Martín-González, N.; San Martín, C. Adenovirus Structure: What Is New? Int J Mol Sci 2021, 22, 5240. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Pache, L.; Von Seggern, D.J.; Mullen, T.-M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the Adenovirus Fiber Is Required for Efficient Receptor Interaction. J Virol 2003, 77, 7225–7235. [Google Scholar] [CrossRef] [PubMed]
- Marttila, M.; Persson, D.; Gustafsson, D.; Liszewski, M.K.; Atkinson, J.P.; Wadell, G.; Arnberg, N. CD46 Is a Cellular Receptor for All Species B Adenoviruses except Types 3 and 7. J Virol 2005, 79, 14429–14436. [Google Scholar] [CrossRef] [PubMed]
- Nestić, D.; Božinović, K.; Pehar, I.; Wallace, R.; Parker, A.L.; Majhen, D. The Revolving Door of Adenovirus Cell Entry: Not All Pathways Are Equal. Pharmaceutics 2021, 13, 1585. [Google Scholar] [CrossRef] [PubMed]
- Arnberg, N. Adenovirus Receptors: Implications for Targeting of Viral Vectors. Trends in Pharmacological Sciences 2012, 33, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Palm, E.; Trulsson, F.; Mundigl, S.; Becker, M.; Persson, B.D.; Frängsmyr, L.; Lenman, A. Heparan Sulfate Is a Cellular Receptor for Enteric Human Adenoviruses. Viruses 2021, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, R.L.; Volkwein, W.; Gao, J.; Schiwon, M.; Bahlmann, N.; Dittmar, T.; Hagedorn, C.; Ehrke-Schulz, E.; Zhang, W.; Baiker, A.; et al. A Human In Vitro Model to Study Adenoviral Receptors and Virus Cell Interactions. Cells 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Howitt, J.; Anderson, C.W.; Freimuth, P. Adenovirus Interaction with Its Cellular Receptor CAR. In Adenoviruses: Model and Vectors in Virus-Host Interactions: Virion-Structure, Viral Replication and Host-Cell Interactions; Doerfler, W., Böhm, P., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin, Heidelberg, 2003; pp. 331–364. ISBN 978-3-662-05597-7. [Google Scholar]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a Common Receptor for Coxsackie B Viruses and Adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Lieber, A. Dependence of Adenovirus Infectivity on Length of the Fiber Shaft Domain. J Virol 2000, 74, 10274–10286. [Google Scholar] [CrossRef] [PubMed]
- Law, L.K.; Davidson, B.L. Adenovirus Serotype 30 Fiber Does Not Mediate Transduction via the Coxsackie-Adenovirus Receptor. Journal of Virology 2002, 76, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Greenshields-Watson, A.; Coughlan, L.; Davies, J.A.; Uusi-Kerttula, H.; Cole, D.K.; Rizkallah, P.J.; Parker, A.L. Diversity within the Adenovirus Fiber Knob Hypervariable Loops Influences Primary Receptor Interactions. Nat Commun 2019, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-D.; Liu, X.-L.; Chen, D.-L.; Zou, X.-H.; Wang, M.; Qu, J.-G.; Lu, Z.-Z.; Hung, T. Human Adenovirus Type 41 Possesses Different Amount of Short and Long Fibers in the Virion. Virology 2012, 432, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Davies, J.A.; Bates, E.A.; Moses, E.; Mundy, R.M.; Marlow, G.; Cole, D.K.; Bliss, C.M.; Rizkallah, P.J.; Parker, A.L. The Fiber Knob Protein of Human Adenovirus Type 49 Mediates Highly Efficient and Promiscuous Infection of Cancer Cell Lines Using a Novel Cell Entry Mechanism. J Virol 2021, 95, e01849–20. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, W.P.; Guilligay, D.; Cusack, S.; Wadell, G.; Arnberg, N. Crystal Structure of Species D Adenovirus Fiber Knobs and Their Sialic Acid Binding Sites. J Virol 2004, 78, 7727–7736. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Mundy, R.M.; Davies, J.A.; Rizkallah, P.J.; Parker, A.L. Human Adenovirus Type 26 Uses Sialic Acid–Bearing Glycans as a Primary Cell Entry Receptor. Science Advances 2019, 5, eaax3567. [Google Scholar] [CrossRef] [PubMed]
- Mundy, R.M.; Baker, A.T.; Bates, E.A.; Cunliffe, T.G.; Teijeira-Crespo, A.; Moses, E.; Rizkallah, P.J.; Parker, A.L. Broad Sialic Acid Usage amongst Species D Human Adenovirus. npj Viruses 2023, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.D.; John, L.; Rafie, K.; Strebl, M.; Frängsmyr, L.; Ballmann, M.Z.; Mindler, K.; Havenga, M.; Lemckert, A.; Stehle, T.; et al. Human Species D Adenovirus Hexon Capsid Protein Mediates Cell Entry through a Direct Interaction with CD46. Proceedings of the National Academy of Sciences 2021, 118. [Google Scholar] [CrossRef]
- Hemsath, J.R.; Liaci, A.M.; Rubin, J.D.; Parrett, B.J.; Lu, S.-C.; Nguyen, T.V.; Turner, M.A.; Chen, C.Y.; Cupelli, K.; Reddy, V.S.; et al. Ex Vivo and In Vivo CD46 Receptor Utilization by Species D Human Adenovirus Serotype 26 (HAdV26). J Virol 2022, 96, e0082621. [Google Scholar] [CrossRef] [PubMed]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 Is a Cellular Receptor for Group B Adenoviruses. Nat Med 2003, 9, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Fleischli, C.; Sirena, D.; Lesage, G.; Havenga, M.J.E.; Cattaneo, R.; Greber, U.F.; Hemmi, S. Species B Adenovirus Serotypes 3, 7, 11 and 35 Share Similar Binding Sites on the Membrane Cofactor Protein CD46 Receptor. J Gen Virol 2007, 88, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Kawabata, K.; Koizumi, N.; Inoue, N.; Okabe, M.; Yamaguchi, T.; Hayakawa, T.; Mizuguchi, H. Adenovirus Serotype 35 Vector-Mediated Transduction into Human CD46-Transgenic Mice. Gene Ther 2006, 13, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liaw, Y.-C.; Stone, D.; Kalyuzhniy, O.; Amiraslanov, I.; Tuve, S.; Verlinde, C.L.M.J.; Shayakhmetov, D.; Stehle, T.; Roffler, S.; et al. Identification of CD46 Binding Sites within the Adenovirus Serotype 35 Fiber Knob. J Virol 2007, 81, 12785–12792. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, D.J.; Segerman, A.; Lindman, K.; Mei, Y.-F.; Wadell, G. The Arg279Gln [Corrected] Substitution in the Adenovirus Type 11p (Ad11p) Fiber Knob Abolishes EDTA-Resistant Binding to A549 and CHO-CD46 Cells, Converting the Phenotype to That of Ad7p. J Virol 2006, 80, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.D.; Reiter, D.M.; Marttila, M.; Mei, Y.-F.; Casasnovas, J.M.; Arnberg, N.; Stehle, T. Adenovirus Type 11 Binding Alters the Conformation of Its Receptor CD46. Nat Struct Mol Biol 2007, 14, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.-B.; et al. Desmoglein 2 Is a Receptor for Adenovirus Serotypes 3, 7, 11, and 14. Nat Med 2011, 17, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vassal-Stermann, E.; Effantin, G.; Zubieta, C.; Burmeister, W.; Iseni, F.; Wang, H.; Lieber, A.; Schoehn, G.; Fender, P. CryoEM Structure of Adenovirus Type 3 Fibre with Desmoglein 2 Shows an Unusual Mode of Receptor Engagement. Nat Commun 2019, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Singh, G.; Lee, J.Y.; Dehghan, S.; Rajaiya, J.; Liu, E.B.; Yousuf, M.A.; Betensky, R.A.; Jones, M.S.; Dyer, D.W.; et al. Molecular Evolution of Human Adenoviruses. Sci Rep 2013, 3, 1812. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; et al. Identification of Coagulation Factor (F)X Binding Sites on the Adenovirus Serotype 5 Hexon: Effect of Mutagenesis on FX Interactions and Gene Transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.D.; Lenman, A.; Frängsmyr, L.; Schmid, M.; Ahlm, C.; Plückthun, A.; Jenssen, H.; Arnberg, N. Lactoferrin-Hexon Interactions Mediate CAR-Independent Adenovirus Infection of Human Respiratory Cells. J Virol 2020, 94, e00542–20. [Google Scholar] [CrossRef]
- Morris, S.J.; Sebastian, S.; Spencer, A.J.; Gilbert, S.C. Simian Adenoviruses as Vaccine Vectors. Future Virol 2016, 11, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.J.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.S.; Cottingham, M.G. A Novel Chimpanzee Adenovirus Vector with Low Human Seroprevalence: Improved Systems for Vector Derivation and Comparative Immunogenicity. PLoS One 2012, 7, e40385. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Boyd, R.J.; Sarkar, D.; Teijeira-Crespo, A.; Chan, C.K.; Bates, E.; Waraich, K.; Vant, J.; Wilson, E.; Truong, C.D.; et al. ChAdOx1 Interacts with CAR and PF4 with Implications for Thrombosis with Thrombocytopenia Syndrome. Science Advances 2021, 7, eabl8213. [Google Scholar] [CrossRef] [PubMed]
- Reeh, M.; Bockhorn, M.; Görgens, D.; Vieth, M.; Hoffmann, T.; Simon, R.; Izbicki, J.R.; Sauter, G.; Schumacher, U.; Anders, M. Presence of the Coxsackievirus and Adenovirus Receptor (CAR) in Human Neoplasms: A Multitumour Array Analysis. Br J Cancer 2013, 109, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Asher, D.R.; Cerny, A.M.; Finberg, R.W. The Erythrocyte Viral Trap: Transgenic Expression of Viral Receptor on Erythrocytes Attenuates Coxsackievirus B Infection. Proceedings of the National Academy of Sciences 2005, 102, 12897–12902. [Google Scholar] [CrossRef] [PubMed]
- Hensen, L.C.M.; Hoeben, R.C.; Bots, S.T.F. Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy. Int J Mol Sci 2020, 21, 6828. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Waddington, S.N.; Buckley, S.M.K.; Custers, J.; Havenga, M.J.E.; van Rooijen, N.; Goudsmit, J.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Effect of Neutralizing Sera on Factor X-Mediated Adenovirus Serotype 5 Gene Transfer. J Virol 2009, 83, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.B.; Sun, L.; Chen, Z.J. Antiviral Innate Immunity Pathways. Cell Res 2006, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.E.; Goodbourn, S. Interferons and Viruses: An Interplay between Induction, Signalling, Antiviral Responses and Virus Countermeasures. J Gen Virol 2008, 89, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Weber, F. Antiviral Innate Immunity: Introduction. Encyclopedia of Virology 2021, 577–583. [Google Scholar] [CrossRef]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The Complement System: History, Pathways, Cascade and Inhibitors. Eur J Microbiol Immunol (Bp) 2012, 2, 103–111. [Google Scholar] [CrossRef]
- Charles A Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Complement System and Innate Immunity. Immunobiology: The Immune System in Health and Disease. 5th edition 2001.
- Smith, J.G.; Silvestry, M.; Lindert, S.; Lu, W.; Nemerow, G.R.; Stewart, P.L. Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization. PLoS Pathog 2010, 6, e1000959. [Google Scholar] [CrossRef] [PubMed]
- Land, W.G. Prologue: About DAMPs, PAMPs, and MAMPs. In Damage-Associated Molecular Patterns in Human Diseases: Volume 1: Injury-Induced Innate Immune Responses; Land, W.G., Ed.; Springer International Publishing: Cham, 2018; pp. 191–217. ISBN 978-3-319-78655-1. [Google Scholar]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu Rev Immunol 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory Cytokines as a Third Signal for T Cell Activation. Current Opinion in Immunology 2010, 22, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.L.; Bradley, L.M. Lessons in Antiviral Immunity. Science 2021. [Google Scholar] [CrossRef] [PubMed]
- Charles A Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunological Memory. In Immunobiology: The Immune System in Health and Disease. 5th edition; Garland Science, 2001.
- Charles A Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. B-Cell Activation by Armed Helper T Cells. In Immunobiology: The Immune System in Health and Disease. 5th edition; Garland Science, 2001.
- Charles A Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Structure of a Typical Antibody Molecule. In Immunobiology: The Immune System in Health and Disease. 5th edition; Garland Science, 2001.
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-Cell Subsets: Heterogeneity, Functions, and Therapeutic Potential. Exp Mol Med 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Cavacini, L. Structure and Function of Immunoglobulins. J Allergy Clin Immunol 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Stavnezer, J.; Guikema, J.E.J.; Schrader, C.E. Mechanism and Regulation of Class Switch Recombination. Annu Rev Immunol 2008, 26, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Forthal, D.N. Functions of Antibodies. Microbiol Spectr 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J. Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives. Adv Biol 2014, 2014, 157895. [Google Scholar] [CrossRef]
- Top, F.H. Control of Adenovirus Acute Respiratory Disease in U.S. Army Trainees. Yale J Biol Med 1975, 48, 185–195. [Google Scholar] [PubMed]
- Fox, J.P.; Brandt, C.D.; Wassermann, F.E.; Hall, C.E.; Spigland, I.; Kogon, A.; Elveback, L.R. The Virus Watch Program: A Continuing Surveillance of Viral Infections in Metropolitan New York Families: Observations of Adenovirus Infections: Virus Excretion Patterns, Antibody Response, Efficiency of Surveillance, Patterns of Infection, and Relation to Illness. American Journal of Epidemiology 1969, 89, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, M. Adenoviruses in Immunocompromised Hosts. Clin Microbiol Rev 2008, 21, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.P.; Chintakuntlawar, A.; Robinson, C.M.; Madisch, I.; Harrach, B.; Hudson, N.R.; Schnurr, D.; Heim, A.; Chodosh, J.; Seto, D.; et al. Evidence of Molecular Evolution Driven by Recombination Events Influencing Tropism in a Novel Human Adenovirus That Causes Epidemic Keratoconjunctivitis. PLOS ONE 2009, 4, e5635. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Muzerie, C.; Wermenbol, A.; Wigand, R.; Keller, D.; Wadell, G.; Schaap, G. Adenovirus 37: Identification and Characterization of a Medically Important New Adenovirus Type of Subgroup D. Journal of Medical Virology 1981, 7, 105–118. [Google Scholar] [CrossRef]
- Lei, Z.; Zhu, Z.; wang, B. ma ci; mei, H.; Li, H.; ga, D. zeng gong; jie, G.; chi, M. ma bu; Zhang, S.; Ma, C.; et al. Outbreaks of Epidemic Keratoconjunctivitis Caused by Human Adenovirus Type 8 in the Tibet Autonomous Region of China in 2016. PLoS One 2017, 12, e0185048. [Google Scholar] [CrossRef] [PubMed]
- Toovey, O.T.R.; Kulkarni, P.; David, J.; Patel, A.; Lai, F.Y.; Burns, J.; Thompson, C.; Ellis, J.; Tang, J.W. An Outbreak of Adenovirus D8 Keratoconjunctivitis in Leicester, United Kingdom, from March to August 2019. J Med Virol 2021, 93, 3969–3973. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.K.; Caul, E.O.; Porter, H.J.; Oakhill, A. Fatal Adenovirus 32 Infection in a Bone Marrow Transplant Recipient. Journal of Clinical Pathology 1995, 48, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veen, J.; Van Der Ploeg, G. Adenovirus Type-15 Infection. The Lancet 1960, 275, 1024. [Google Scholar] [CrossRef]
- James, L.; Vernon, M.O.; Jones, R.C.; Stewart, A.; Lu, X.; Zollar, L.M.; Chudoba, M.; Westercamp, M.; Alcasid, G.; Duffee-Kerr, L.; et al. Outbreak of Human Adenovirus Type 3 Infection in a Pediatric Long-Term Care Facility—Illinois, 2005. Clinical Infectious Diseases 2007, 45, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.I.; Erdman, D.D.; Pur, S.L.; Diaz, P.S.; Segreti, J.; Kajon, A.E.; Belkengren, R.P.; Jones, R.C. Outbreak of Adenovirus Genome Type 7d2 Infection in a Pediatric Chronic-Care Facility and Tertiary-Care Hospital. Clinical Infectious Diseases 2001, 32, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Rozwadowski, F. Notes from the Field: Fatalities Associated with Human Adenovirus Type 7 at a Substance Abuse Rehabilitation Facility — New Jersey, 2017. MMWR Morb Mortal Wkly Rep 2018, 67. [Google Scholar] [CrossRef]
- Atasheva, S.; Shayakhmetov, D.M. Adenovirus Sensing by the Immune System. Curr Opin Virol 2016, 21, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Doronin, K.; Flatt, J.W.; Di Paolo, N.C.; Khare, R.; Kalyuzhniy, O.; Acchione, M.; Sumida, J.P.; Ohto, U.; Shimizu, T.; Akashi-Takamura, S.; et al. Coagulation Factor X Activates Innate Immunity to Human Species C Adenovirus. Science 2012, 338, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Stein, S.; Falck-Pedersen, E. Adenovirus Detection by the cGAS/STING/TBK1 DNA Sensing Cascade. J Virol 2014, 88, 974–981. [Google Scholar] [CrossRef]
- Iacobelli-Martinez, M.; Nemerow, G.R. Preferential Activation of Toll-Like Receptor Nine by CD46-Utilizing Adenoviruses. J Virol 2007, 81, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Teigler, J.E.; Kagan, J.C.; Barouch, D.H. Late Endosomal Trafficking of Alternative Serotype Adenovirus Vaccine Vectors Augments Antiviral Innate Immunity. J Virol 2014, 88, 10354–10363. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Björkström, N.K.; Petrovas, C.; Liang, F.; Gall, J.G.D.; Loré, K.; Koup, R.A. Type I Interferon-Dependent Activation of NK Cells by rAd28 or rAd35, but Not rAd5, Leads to Loss of Vector-Insert Expression. Vaccine 2014, 32, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.; Schäfer, H. Human α-Defensin 1 (HNP-1) Inhibits Adenoviral Infection in Vitro. Regulatory Peptides 2001, 101, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Cassany, A.; Gerace, L.; Ralston, R.; Nemerow, G.R. Neutralizing Antibody Blocks Adenovirus Infection by Arresting Microtubule-Dependent Cytoplasmic Transport. J Virol 2008, 82, 6492–6500. [Google Scholar] [CrossRef] [PubMed]
- Mistchenko, A.S.; Diez, R.A.; Mariani, A.L.; Robaldo, J.; Maffey, A.F.; Bayley-Bustamante, G.; Grinstein, S. Cytokines in Adenoviral Disease in Children: Association of Interleukin-6, Interleukin-8, and Tumor Necrosis Factor Alpha Levels with Clinical Outcome. J Pediatr 1994, 124, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb Perspect Biol 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular Immune Privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.C.; Lakhai, W.; Koudstaal, W.; Verhoeven, M.; Koel, B.F.; Vogels, R.; Goudsmit, J.; Havenga, M.J.E.; Kostense, S. Quantifying Adenovirus-Neutralizing Antibodies by Luciferase Transgene Detection: Addressing Preexisting Immunity to Vaccine and Gene Therapy Vectors. J Clin Microbiol 2003, 41, 5046–5052. [Google Scholar] [CrossRef] [PubMed]
- Vogels, R.; Zuijdgeest, D.; van Rijnsoever, R.; Hartkoorn, E.; Damen, I.; de Béthune, M.-P.; Kostense, S.; Penders, G.; Helmus, N.; Koudstaal, W.; et al. Replication-Deficient Human Adenovirus Type 35 Vectors for Gene Transfer and Vaccination: Efficient Human Cell Infection and Bypass of Preexisting Adenovirus Immunity. J Virol 2003, 77, 8263–8271. [Google Scholar] [CrossRef] [PubMed]
- Dakin, R.S.; Parker, A.L.; Delles, C.; Nicklin, S.A.; Baker, A.H. Efficient Transduction of Primary Vascular Cells by the Rare Adenovirus Serotype 49 Vector. Hum Gene Ther 2015, 26, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Klann, P.J.; Wang, X.; Elfert, A.; Zhang, W.; Köhler, C.; Güttsches, A.-K.; Jacobsen, F.; Weyen, U.; Roos, A.; Ehrke-Schulz, E.; et al. Seroprevalence of Binding and Neutralizing Antibodies against 39 Human Adenovirus Types in Patients with Neuromuscular Disorders. Viruses 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Lynch, D.M.; Iampietro, M.J.; Borducchi, E.N.; Barouch, D.H. Adenovirus Serotype 5 Neutralizing Antibodies Target Both Hexon and Fiber Following Vaccination and Natural Infection. J Virol 2012, 86, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Dong, J.; Wang, C.; Zhan, Y.; Zhang, H.; Wu, J.; Kong, W.; Yu, X. Characteristics of Neutralizing Antibodies to Adenovirus Capsid Proteins in Human and Animal Sera. Virology 2013, 437, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Gahéry-Ségard, H.; Farace, F.; Godfrin, D.; Gaston, J.; Lengagne, R.; Tursz, T.; Boulanger, P.; Guillet, J.-G. Immune Response to Recombinant Capsid Proteins of Adenovirus in Humans: Antifiber and Anti-Penton Base Antibodies Have a Synergistic Effect on Neutralizing Activity. J Virol 1998, 72, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Tsai, V.; Goudreau, A.; Shinoda, J.Y.; Wen, S.F.; Ramachandra, M.; Ralston, R.; Maneval, D.; LaFace, D.; Shabram, P. Specific Depletion of Human Anti-Adenovirus Antibodies Facilitates Transduction in an in Vivo Model for Systemic Gene Therapy. Mol Ther 2001, 3, 768–778. [Google Scholar] [CrossRef]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.C.; Vogels, R.; Custers, J.H.H.V.; Addo, M.M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; et al. Neutralizing Antibodies to Adenovirus Serotype 5 Vaccine Vectors Are Directed Primarily against the Adenovirus Hexon Protein1. The Journal of Immunology 2005, 174, 7179–7185. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Maxfield, L.F.; Lynch, D.M.; Iampietro, M.J.; Borducchi, E.N.; Barouch, D.H. Adenovirus Serotype 5-Specific Neutralizing Antibodies Target Multiple Hexon Hypervariable Regions. J Virol 2012, 86, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Mikyas, Y.; Stewart, P.L.; Ralston, R. Postentry Neutralization of Adenovirus Type 5 by an Antihexon Antibody. J Virol 2004, 78, 12320–12332. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, C. Neutralization of Adenoviruses: Kinetics, Stoichiometry, and Mechanisms. J Virol 1988, 62, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Everitt, E.; Lutter, L.; Philipson, L. Structural Proteins of Adenoviruses: XII. Location and Neighbor Relationship among Proteins of Adenovirion Type 2 as Revealed by Enzymatic Lodination, Immunoprecipitation and Chemical Cross-Linking. Virology 1975, 67, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tian, J.; Smith, J.S.; Byrnes, A.P. Clearance of Adenovirus by Kupffer Cells Is Mediated by Scavenger Receptors, Natural Antibodies, and Complement. J Virol 2008, 82, 11705–11713. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Vilaysane, A.; Cotter, M.J.; Clark, S.A.; Meijndert, H.C.; Colarusso, P.; Yates, R.M.; Petrilli, V.; Tschopp, J.; Muruve, D.A. Antiviral Antibodies Target Adenovirus to Phagolysosomes and Amplify the Innate Immune Response1. The Journal of Immunology 2009, 182, 7058–7068. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies (Basel) 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.; Eisenlohr, L.; Flomenberg, N.; Hsu, S.; Flomenberg, P. The Adenovirus Capsid Protein Hexon Contains a Highly Conserved Human CD4+ T-Cell Epitope. Hum Gene Ther 2002, 13, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Olive, M.; Champagne, K.; Flomenberg, N.; Eisenlohr, L.; Hsu, S.; Flomenberg, P. Adenovirus Hexon T-Cell Epitope Is Recognized by Most Adults and Is Restricted by HLA DP4, the Most Common Class II Allele. Gene Ther 2004, 11, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Onion, D.; Crompton, L.J.; Milligan, D.W.; Moss, P.A.H.; Lee, S.P.; Mautner, V. The CD4+ T-Cell Response to Adenovirus Is Focused against Conserved Residues within the Hexon Protein. Journal of General Virology 2007, 88, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Veltrop-Duits, L.A.; Heemskerk, B.; Sombroek, C.C.; van Vreeswijk, T.; Gubbels, S.; Toes, R.E.M.; Melief, C.J.M.; Franken, K.L.M.C.; Havenga, M.; van Tol, M.J.D.; et al. Human CD4+ T Cells Stimulated by Conserved Adenovirus 5 Hexon Peptides Recognize Cells Infected with Different Species of Human Adenovirus. European Journal of Immunology 2006, 36, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Olive, M.; Pulmanausahakul, R.; Schnell, M.; Flomenberg, N.; Eisenlohr, L.; Flomenberg, P. Human CD8+ Cytotoxic T Cell Responses to Adenovirus Capsid Proteins. Virology 2006, 350, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.M.; Sili, U.; Vanin, E.F.; Jewell, A.M.; Xie, W.; Vignali, D.; Piedra, P.A.; Brenner, M.K.; Rooney, C.M. Conserved CTL Epitopes on the Adenovirus Hexon Protein Expand Subgroup Cross-Reactive and Subgroup-Specific CD8+ T Cells. Blood 2004, 104, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Tischer, S.; Geyeregger, R.; Kwoczek, J.; Heim, A.; Figueiredo, C.; Blasczyk, R.; Maecker-Kolhoff, B.; Eiz-Vesper, B. Discovery of Immunodominant T-Cell Epitopes Reveals Penton Protein as a Second Immunodominant Target in Human Adenovirus Infection. J Transl Med 2016, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Kremer, E.J. Frequency, Proliferation, and Activation of Human Memory T Cells Induced by a Nonhuman Adenovirus. J Virol 2005, 79, 14595–14605. [Google Scholar] [CrossRef] [PubMed]
- Hutnick, N.A.; Carnathan, D.; Ertl, H.; Betts, M.R. Adenovirus-Specific Human T Cells Are Pervasive, Polyfunctional, and Cross Reactive. Vaccine 2010, 28, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin Microbiol Rev 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Ison, M.G.; Hayden, R.T. Adenovirus. Microbiology Spectrum 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Li, J.; Yang, G.; Huang, Q.; Li, J.; Wang, H.; He, S.; Li, E. Histone Deacetylase Inhibitors Promote Latent Adenovirus Reactivation from Tonsillectomy Specimens. J Virol 2020, 94, e00100–20. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.R.; Cook, J.L. Human Adenovirus Infections: Update and Consideration of Mechanisms of Viral Persistence. Curr Opin Infect Dis 2018, 31, 251–256. [Google Scholar] [CrossRef] [PubMed]
- King, C.R.; Zhang, A.; Mymryk, J.S. The Persistent Mystery of Adenovirus Persistence. Trends in Microbiology 2016, 24, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.P.; Eichholz, K.; Amelio, P.; Moyer, C.; Nemerow, G.R.; Perreau, M.; Mennechet, F.J.D.; Kremer, E.J. Humoral Immune Response to Adenovirus Induce Tolerogenic Bystander Dendritic Cells That Promote Generation of Regulatory T Cells. PLoS Pathog 2018, 14, e1007127. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Stamminger, T.; Hearing, P. E2F/Rb Family Proteins Mediate Interferon Induced Repression of Adenovirus Immediate Early Transcription to Promote Persistent Viral Infection. PLoS Pathog 2016, 12, e1005415. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.C.; Gujer, C.; McInerney, G.; Gall, J.G.D.; Petrovas, C.; Hedestam, G.B.K.; Koup, R.A.; Loré, K. Adenovirus Type-35 Vectors Block Human CD4+ T-Cell Activation via CD46 Ligation. Proc Natl Acad Sci U S A 2011, 108, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Iacobelli-Martinez, M.; Nepomuceno, R.R.; Connolly, J.; Nemerow, G.R. CD46-Utilizing Adenoviruses Inhibit C/EBPβ-Dependent Expression of Proinflammatory Cytokines. J Virol 2005, 79, 11259–11268. [Google Scholar] [CrossRef] [PubMed]
- Membrane Cofactor Protein (CD46) Protects Cells from Complement- Mediated Attack by an Intrinsic Mechanism. J Exp Med 1992, 175, 1547–1551. [CrossRef] [PubMed]
- Baker, A.T.; Aguirre-Hernández, C.; Halldén, G.; Parker, A.L. Designer Oncolytic Adenovirus: Coming of Age. Cancers (Basel) 2018, 10, 201. [Google Scholar] [CrossRef]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic Virotherapy: Basic Principles, Recent Advances and Future Directions. Sig Transduct Target Ther 2023, 8, 1–29. [Google Scholar] [CrossRef]
- He, T.-C.; Zhou, S.; da Costa, L.T.; Yu, J.; Kinzler, K.W.; Vogelstein, B. A Simplified System for Generating Recombinant Adenoviruses. Proc Natl Acad Sci U S A 1998, 95, 2509–2514. [Google Scholar] [CrossRef]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; McCormick, F.; Von Hoff, D.D.; Kirn, D.H. ONYX-015, an E1B Gene-Attenuated Adenovirus, Causes Tumor-Specific Cytolysis and Antitumoral Efficacy That Can Be Augmented by Standard Chemotherapeutic Agents. Nat Med 1997, 3, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int J Mol Sci 2021, 22, 10522. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Li, G.; Li, P.; Wang, H.; Fang, X.; He, T.; Li, J. Twenty Years of Gendicine® rAd-P53 Cancer Gene Therapy: The First-in-Class Human Cancer Gene Therapy in the Era of Personalized Oncology. Genes & Diseases 2024, 11, 101155. [Google Scholar] [CrossRef] [PubMed]
- Research, C. for B.E. and ADSTILADRIN. FDA 2023.
- Akamis Bio A Multicentre, Open-Label, Non-Randomised First in Human Study of NG-641, a Tumour Selective Transgene Expressing Adenoviral Vector, in Patients With Metastatic or Advanced Epithelial Tumours (STAR); clinicaltrials.gov, 2023.
- Champion, B.R.; Besneux, M.; Patsalidou, M.; Silva, A.; Zonca, M.; Marino, N.; Genova, G. di; Illingworth, S.; Fedele, S.; Slater, L.; et al. Abstract 5013: NG-641: An Oncolytic T-SIGn Virus Targeting Cancer-Associated Fibroblasts in the Stromal Microenvironment of Human Carcinomas. Cancer Research 2019, 79, 5013. [Google Scholar] [CrossRef]
- Orca Therapeutics B.V. A Phase I/IIa Study Evaluating the Safety and Tolerability of Intratumoral Administration of ORCA-010 in Treatment-Naïve Patients With Localized Prostate Cancer.; clinicaltrials.gov, 2023.
- Dong, W.; van Ginkel, J.-W.H.; Au, K.Y.; Alemany, R.; Meulenberg, J.J.M.; van Beusechem, V.W. ORCA-010, a Novel Potency-Enhanced Oncolytic Adenovirus, Exerts Strong Antitumor Activity in Preclinical Models. Hum Gene Ther 2014, 25, 897–904. [Google Scholar] [CrossRef] [PubMed]
- GeoVax, Inc. Phase 1/2, Open-Label Study Evaluating Safety of Repeat Administration of Ad/PNP-F-araAMP (Ad/PNP Administered Intratumorally Followed by Intravenous Fludarabine Phosphate) in Subjects With Recurrent, Local Head and Neck Cancer; clinicaltrials.gov, 2023.
- Rab, R.; Ehrhardt, A.; Achyut, B.R.; Joshi, D.; Gilbert-Ross, M.; Huang, C.; Floyd, K.; Borovjagin, A.V.; Parker, W.B.; Sorscher, E.J.; et al. Evaluating Antitumor Activity of Escherichia Coli Purine Nucleoside Phosphorylase against Head and Neck Patient-Derived Xenografts. Cancer Reports 2023, 6, e1708. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Phase II Window of Opportunity Trial of Stereotactic Body Radiation Therapy and In Situ Oncolytic Virus Therapy in Metastatic Triple Negative Breast Cancer and Metastatic Non-Small Cell Lung Cancer Followed by Pembrolizumab; clinicaltrials.gov, 2022.
- Nasu, Y.; Saika, T.; Ebara, S.; Kusaka, N.; Kaku, H.; Abarzua, F.; Manabe, D.; Thompson, T.C.; Kumon, H. Suicide Gene Therapy With Adenoviral Delivery of HSV-tK Gene for Patients With Local Recurrence of Prostate Cancer After Hormonal Therapy. Molecular Therapy 2007, 15, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Henry Ford Health System Phase I Study of Replication-Competent Adenovirus-Mediated Double Suicide Gene Therapy With Stereotactic Radiosurgery in Patients With Recurrent or Progressive High Grade Astrocytomas; clinicaltrials.gov, 2023.
- Lokon Pharma AB Phase I/II Trial Investigating an Immunostimulatory Oncolytic Adenovirus for Cancer; clinicaltrials.gov, 2023.
- Araújo, N.M.; Rubio, I.G.S.; Toneto, N.P.A.; Morale, M.G.; Tamura, R.E. The Use of Adenoviral Vectors in Gene Therapy and Vaccine Approaches. Genet. Mol. Biol. 2022, 45, e20220079. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Ng, P. Improved System for Helper-Dependent Adenoviral Vector Production. Mol Ther 2003, 8, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.; Beauchamp, C.; Evelegh, C.; Parks, R.; Graham, F.L. Development of a FLP/Frt System for Generating Helper-Dependent Adenoviral Vectors. Mol Ther 2001, 3, 809–815. [Google Scholar] [CrossRef]
- Liu, J.; Seol, D.-W. Helper Virus-Free Gutless Adenovirus (HF-GLAd): A New Platform for Gene Therapy. BMB Rep 2020, 53, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Liu, J.; Junn, H.J.; Lee, E.-J.; Jeong, K.-S.; Seol, D.-W. No More Helper Adenovirus: Production of Gutless Adenovirus (GLAd) Free of Adenovirus and Replication-Competent Adenovirus (RCA) Contaminants. Exp Mol Med 2019, 51, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Peng, P.D.; Ehrhardt, A.; Storm, T.A.; Kay, M.A. Comparison of Adenoviral and Adeno-Associated Viral Vectors for Pancreatic Gene Delivery in Vivo. Hum Gene Ther 2004, 15, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int J Mol Sci 2023, 24, 7736. [Google Scholar] [CrossRef] [PubMed]
- Herantis Pharma Plc. Phase 2, Double-Blind, Placebo-Controlled, Randomized Efficacy, Safety and Tolerability Study of Lymfactin in Combination With Surgical Lymph Node Transfer To Patients With Secondary Lymphedema Associated With the Treatment of Breast Cancer; clinicaltrials.gov, 2023.
- Leppäpuska, I.-M.; Hartiala, P.; Suominen, S.; Suominen, E.; Kaartinen, I.; Mäki, M.; Seppänen, M.; Kiiski, J.; Viitanen, T.; Lahdenperä, O.; et al. Phase 1 Lymfactin® Study: 24-Month Efficacy and Safety Results of Combined Adenoviral VEGF-C and Lymph Node Transfer Treatment for Upper Extremity Lymphedema. Journal of Plastic, Reconstructive & Aesthetic Surgery 2022, 75, 3938–3945. [Google Scholar] [CrossRef] [PubMed]
- XyloCor Therapeutics, Inc. A Phase 1/2 Trial of Direct Administration of AdVEGF-All6A+, a Replication Deficient Adenovirus Vector Expressing a cDNA/Genomic Hybrid of Human VEGF, to the Ischemic Myocardium of Subjects With Angina Pectoris; clinicaltrials.gov, 2024.
- Povsic, T.J.; Nakamura, K.; Henry, T.D.; Traverse, J.H.; Anderson, R.D.; Bakaeen, F.; Latter, D.A.; Ohman, E.M.; Peterson, M.W.; Byrnes, D.; et al. Angiogenic Gene Therapy for Refractory Angina: Results of the Epicardial Delivery of XC001 Gene Therapy for Refractory Angina Coronary Treatment (EXACT) Phase 2 Trial. European Heart Journal 2023, 44, ehad655–1307. [Google Scholar] [CrossRef]
- Stewart, D.J.; Gianchetti, A.; Byrnes, D.; Dittrich, H.C.; Thorne, B.; Manza, L.L.; Reinhardt, R.R. Safety and Biodistribution of XC001 (Encoberminogene Rezmadenovec) Gene Therapy in Rats: A Potential Therapy for Cardiovascular Diseases. Gene Ther 2024, 31, 45–55. [Google Scholar] [CrossRef] [PubMed]
- University of Pennsylvania Phase I Trial to Evaluate the Safety of Platelet Derived Growth Factor B (PDGF-B) and a Limb Compression Bandage in Venous Leg Ulcers; clinicaltrials.gov, 2013.
- Margolis, D.J.; Crombleholme, T.; Herlyn, M. Clinical Protocol: Phase I Trial to Evaluate the Safety of H5.020CMV.PDGF-B for the Treatment of a Diabetic Insensate Foot Ulcer. Wound Repair and Regeneration 2000, 8, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Study Details | Phase I Pilot Study of Ad5-CB-CFTR, an Adenovirus Vector Containing the Cystic Fibrosis Transmembrane Conductance Regulator Gene, in Patients With Cystic Fibrosis | ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT00004779 (accessed on 25 March 2024).
- Crosby, C.M.; Barry, M.A. IIIa Deleted Adenovirus as a Single-Cycle Genome Replicating Vector. Virology 2014, 0, 158–165. [Google Scholar] [CrossRef]
- Crosby, C.M.; Matchett, W.E.; Anguiano-Zarate, S.S.; Parks, C.A.; Weaver, E.A.; Pease, L.R.; Webby, R.J.; Barry, M.A. Replicating Single-Cycle Adenovirus Vectors Generate Amplified Influenza Vaccine Responses. J Virol 2017, 91, e00720–16. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World in Data 2020. [Google Scholar]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A Comprehensive Review of SARS-CoV-2 Vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother 18, 2002083. [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. New England Journal of Medicine 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T. Intranasal COVID-19 Vaccine Fails to Induce Mucosal Immunity. Nature Medicine 2022, 28, 2439–2440. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal Comparative Clinical Trial of Intranasal (iNCOVACC) and Intramuscular COVID 19 Vaccine (Covaxin®). NPJ Vaccines 2023, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Migueles, S.A.; Huang, J.; Bolkhovitinov, L.; Stuccio, S.; Griesman, T.; Pullano, A.A.; Kang, B.H.; Ishida, E.; Zimmerman, M.; et al. A Replication-Competent Adenovirus-Vectored Influenza Vaccine Induces Durable Systemic and Mucosal Immunity. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Houser, K.V.; Gaudinski, M.R.; Happe, M.; Narpala, S.; Verardi, R.; Sarfo, E.K.; Corrigan, A.R.; Wu, R.; Rothwell, R.S.; Novik, L.; et al. Safety and Immunogenicity of an HIV-1 Prefusion-Stabilized Envelope Trimer (Trimer 4571) Vaccine in Healthy Adults: A First-in-Human Open-Label, Randomized, Dose-Escalation, Phase 1 Clinical Trial. eClinicalMedicine 2022, 48, 101477. [Google Scholar] [CrossRef] [PubMed]
- Jcovden (Previously COVID-19 Vaccine Janssen) | European Medicines Agency Available online:. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jcovden-previously-covid-19-vaccine-janssen (accessed on 25 March 2024).
- MHRA Products | Home Available online:. Available online: https://products.mhra.gov.uk/ (accessed on 25 March 2024).
- Research, C. for B.E. and Janssen COVID-19 Vaccine. FDA 2023.
- Vaxzevria (Previously COVID-19 Vaccine AstraZeneca) | European Medicines Agency Available online:. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca (accessed on 25 March 2024).
- Bharat Biotech International Limited A Phase III Randomized Open Label Multi-Center Study to Compare Immunogenicity and Safety of BBV154 With COVAXIN®, and to Assess Lot to Lot Consistency of BBV154 in Healthy Volunteers; clinicaltrials.gov, 2022.
- Sunagar, R.; Prasad, S.D.; Ella, R.; Vadrevu, K.M. Preclinical Evaluation of Safety and Immunogenicity of a Primary Series Intranasal COVID-19 Vaccine Candidate (BBV154) and Humoral Immunogenicity Evaluation of a Heterologous Prime-Boost Strategy with COVAXIN (BBV152). Front Immunol 2022, 13, 1063679. [Google Scholar] [CrossRef]
- Albert B. Sabin Vaccine Institute A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate Safety, Tolerability, and Immune Responses of an Investigational Monovalent Chimpanzee Adenoviral-Vectored Marburg Virus Vaccine in Healthy Adults; clinicaltrials.gov, 2023.
- Ankara City Hospital Bilkent Phase I Randomized Double-Blind Clinical Trial With New SARS-CoV-2 CoVacHGMix Type 5 Adenoviral Vector Vaccine in Healthy Volunteers Aged 18-45 Years; clinicaltrials.gov, 2022.
- McMaster University Phase 1, Open Label Study to Evaluate the Safety and Immunogenicity of ChAd68 and AdHu5 Vector-Based Trivalent COVID-19 Vaccines Delivered Via Inhaled Aerosol; clinicaltrials.gov, 2023.
- Huang, J. A Randomized, Open-Label, Parallel-Controlled Clinical Study on Booster Immunization of Two COVID-19 Vaccines Constructed From Different Technical Routes Among People Aged 18 Years and Older; clinicaltrials.gov, 2023.
- National Institute of Allergy and Infectious Diseases (NIAID) Phase I Open-Label Study of Safety and Immunogenicity of AD4-HIV Envelope Vaccine Vectors in Healthy Volunteers; clinicaltrials.gov, 2024.
- LICHTENSTEIN, D.L.; TOTH, K.; DORONIN, K.; TOLLEFSON, A.E.; WOLD, W.S.M. Functions and Mechanisms of Action of the Adenovirus E3 Proteins. International Reviews of Immunology 2004, 23, 75–111. [Google Scholar] [CrossRef]
- Kumar, V.; McNerney, M.E. A New Self: MHC-Class-I-Independent Natural-Killer-Cell Self-Tolerance. Nat Rev Immunol 2005, 5, 363–374. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Lundholm-Beauchamp, U.; Horswood, R.L.; Pernis, B.; Wold, W.S.; Chanock, R.M.; Prince, G.A. Role of Early Region 3 (E3) in Pathogenesis of Adenovirus Disease. Proc Natl Acad Sci U S A 1989, 86, 3823–3827. [Google Scholar] [CrossRef]
- Kirn, D. Clinical Research Results with Dl1520 (Onyx-015), a Replication-Selective Adenovirus for the Treatment of Cancer: What Have We Learned? Gene Ther 2001, 8, 89–98. [Google Scholar] [CrossRef]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and Its Update in China. Curr Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-Designing Interleukin-12 to Enhance Its Safety and Potential as an Anti-Tumor Immunotherapeutic Agent. Nat Commun 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Fejer, G.; Drechsel, L.; Liese, J.; Schleicher, U.; Ruzsics, Z.; Imelli, N.; Greber, U.F.; Keck, S.; Hildenbrand, B.; Krug, A.; et al. Key Role of Splenic Myeloid DCs in the IFN-Aβ Response to Adenoviruses In Vivo. PLoS Pathog 2008, 4, e1000208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Yang, Y. A Critical Role for Type I IFN–Dependent NK Cell Activation in Innate Immune Elimination of Adenoviral Vectors In Vivo. Molecular Therapy 2008, 16, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Miao, E.A.; Iwakura, Y.; Kaja, M.-K.; Aderem, A.; Flavell, R.A.; Papayannopoulou, T.; Shayakhmetov, D.M. Virus Sensing at the Plasma Membrane Triggers Interleukin-1α–Mediated Pro-Inflammatory Macrophage Response in Vivo. Immunity 2009, 31, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Baldwin, L.K.; Irons, E.E.; Papayannopoulou, T.; Tomlinson, S.; Shayakhmetov, D.M. IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells. PLoS Pathog 2014, 10, e1004035. [Google Scholar] [CrossRef] [PubMed]
- Shashkova, E.V.; Doronin, K.; Senac, J.S.; Barry, M.A. Macrophage Depletion Combined with Anticoagulant Therapy Increases Therapeutic Window of Systemic Treatment with Oncolytic Adenovirus. Cancer Res 2008, 68, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Khare, R.; May, S.M.; Vetrini, F.; Weaver, E.A.; Palmer, D.; Rosewell, A.; Grove, N.; Ng, P.; Barry, M.A. Generation of a Kupffer Cell-Evading Adenovirus for Systemic and Liver-Directed Gene Transfer. Mol Ther 2011, 19, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.A.; Vishnevskiy, D.A.; Stepanenko, A.A.; Sosnovtseva, A.O.; Chernysheva, A.A.; Abakumova, T.O.; Valikhov, M.P.; Lipatova, A.V.; Abakumov, M.A.; Chekhonin, V.P. In Vivo Tracking for Oncolytic Adenovirus Interactions with Liver Cells. Biomedicines 2022, 10, 1697. [Google Scholar] [CrossRef]
- Kiang, A.; Hartman, Z.C.; Everett, R.S.; Serra, D.; Jiang, H.; Frank, M.M.; Amalfitano, A. Multiple Innate Inflammatory Responses Induced after Systemic Adenovirus Vector Delivery Depend on a Functional Complement System. Molecular Therapy 2006, 14, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xu, Z.; Smith, J.S.; Hofherr, S.E.; Barry, M.A.; Byrnes, A.P. Adenovirus Activates Complement by Distinctly Different Mechanisms in Vitro and in Vivo: Indirect Complement Activation by Virions in Vivo. J Virol 2009, 83, 5648–5658. [Google Scholar] [CrossRef] [PubMed]
- Teigler, J.E.; Iampietro, M.J.; Barouch, D.H. Vaccination with Adenovirus Serotypes 35, 26, and 48 Elicits Higher Levels of Innate Cytokine Responses than Adenovirus Serotype 5 in Rhesus Monkeys. J Virol 2012, 86, 9590–9598. [Google Scholar] [CrossRef] [PubMed]
- Bottermann, M.; Foss, S.; van Tienen, L.M.; Vaysburd, M.; Cruickshank, J.; O’Connell, K.; Clark, J.; Mayes, K.; Higginson, K.; Hirst, J.C.; et al. TRIM21 Mediates Antibody Inhibition of Adenovirus-Based Gene Delivery and Vaccination. Proc Natl Acad Sci U S A 2018, 115, 10440–10445. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, M.A.; Jensen, B.A.H.; Holst, P.J.; Bassi, M.R.; Christensen, J.P.; Thomsen, A.R. Pre-Existing Vector Immunity Does Not Prevent Replication Deficient Adenovirus from Inducing Efficient CD8 T-Cell Memory and Recall Responses. PLoS One 2012, 7, e34884. [Google Scholar] [CrossRef] [PubMed]
- Vlachaki, M.T.; Hernandez-Garcia, A.; Ittmann, M.; Chhikara, M.; Aguilar, L.K.; Zhu, X.; The, B.S.; Butler, E.B.; Woo, S.; Thompson, T.C.; et al. Impact of Preimmunization on Adenoviral Vector Expression and Toxicity in a Subcutaneous Mouse Cancer Model. Molecular Therapy 2002, 6, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Nishimae, F.; Wakida, T.; Sakurai, F.; Mizuguchi, H. Effects of Pre-Existing Anti-Adenovirus Antibodies on Transgene Expression Levels and Therapeutic Efficacies of Arming Oncolytic Adenovirus. Sci Rep 2022, 12, 21560. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Gall, J.G.D.; Nason, M.; King, C.R.; Koup, R.A.; Roederer, M.; McElrath, M.J.; Morgan, C.A.; Churchyard, G.; Baden, L.R.; et al. Differential Specificity and Immunogenicity of Adenovirus Type 5 Neutralizing Antibodies Elicited by Natural Infection or Immunization. J Virol 2010, 84, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fan, Y.; Li, X.; Gu, S.; Zhou, Z.; Xu, D.; Qiu, S.; Li, C.; Zhou, R.; Tian, X. Identification of Adenovirus Neutralizing Antigens Using Capsid Chimeric Viruses. Virus Research 2018, 256, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, L.; Gall, J.G.D.; Nason, M.; Schwartz, R.M.; McElrath, M.J.; DeRosa, S.C.; Hural, J.; Corey, L.; Buchbinder, S.P.; et al. Decreased Pre-Existing Ad5 Capsid and Ad35 Neutralizing Antibodies Increase HIV-1 Infection Risk in the Step Trial Independent of Vaccination. PLoS One 2012, 7, e33969. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel Adenovirus-Based Vaccines Induce Broad and Sustained T Cell Responses to HCV in Man. Sci Transl Med 2012, 4, 115ra1. [Google Scholar] [CrossRef]
- O’Hara, G.A.; Duncan, C.J.A.; Ewer, K.J.; Collins, K.A.; Elias, S.C.; Halstead, F.D.; Goodman, A.L.; Edwards, N.J.; Reyes-Sandoval, A.; Bird, P.; et al. Clinical Assessment of a Recombinant Simian Adenovirus ChAd63: A Potent New Vaccine Vector. The Journal of Infectious Diseases 2012, 205, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; O’Hara, G.A.; Duncan, C.J.A.; Collins, K.A.; Sheehy, S.H.; Reyes-Sandoval, A.; Goodman, A.L.; Edwards, N.J.; Elias, S.C.; Halstead, F.D.; et al. Protective CD8+ T-Cell Immunity to Human Malaria Induced by Chimpanzee Adenovirus-MVA Immunisation. Nat Commun 2013, 4, 2836. [Google Scholar] [CrossRef] [PubMed]
- Kimani, D.; Jagne, Y.J.; Cox, M.; Kimani, E.; Bliss, C.M.; Gitau, E.; Ogwang, C.; Afolabi, M.O.; Bowyer, G.; Collins, K.A.; et al. Translating the Immunogenicity of Prime-Boost Immunization With ChAd63 and MVA ME-TRAP From Malaria Naive to Malaria-Endemic Populations. Mol Ther 2014, 22, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 nCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. The Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.; Lee, J.A.; Lee, S.J.; Lee, K.H.; Kim, J.H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Yeom, J.-S.; et al. Immunogenicity Differences of the ChAdOx1 nCoV-19 Vaccine According to Pre-Existing Adenovirus Immunity. Vaccines (Basel) 2023, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bello, J.; Morales-Núñez, J.J.; Machado-Sulbarán, A.C.; Díaz-Pérez, S.A.; Torres-Hernández, P.C.; Balcázar-Félix, P.; Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.A.; Muñoz-Valle, J.F. Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2. Vaccines (Basel) 2021, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sandoval, A.; Fitzgerald, J.C.; Grant, R.; Roy, S.; Xiang, Z.Q.; Li, Y.; Gao, G.P.; Wilson, J.M.; Ertl, H.C.J. Human Immunodeficiency Virus Type 1-Specific Immune Responses in Primates upon Sequential Immunization with Adenoviral Vaccine Carriers of Human and Simian Serotypes. J Virol 2004, 78, 7392–7399. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, Tolerability, and Immunogenicity of a Recombinant Adenovirus Type-5 Vectored COVID-19 Vaccine: A Dose-Escalation, Open-Label, Non-Randomised, First-in-Human Trial. The Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Bliss, C.M.; Bowyer, G.; Anagnostou, N.A.; Havelock, T.; Snudden, C.M.; Davies, H.; de Cassan, S.C.; Grobbelaar, A.; Lawrie, A.M.; Venkatraman, N.; et al. Assessment of Novel Vaccination Regimens Using Viral Vectored Liver Stage Malaria Vaccines Encoding ME-TRAP. Sci Rep 2018, 8, 3390. [Google Scholar] [CrossRef]
- Uddbäck, I.; Cartwright, E.K.; Schøller, A.S.; Wein, A.N.; Hayward, S.L.; Lobby, J.; Takamura, S.; Thomsen, A.R.; Kohlmeier, J.E.; Christensen, J.P. Long-Term Maintenance of Lung Resident Memory T Cells Is Mediated by Persistent Antigen. Mucosal Immunol 2021, 14, 92–99. [Google Scholar] [CrossRef]
- Gracht, E.T. van der; Schoonderwoerd, M.J.; Duikeren, S. van; Yilmaz, A.N.; Behr, F.M.; Colston, J.M.; Lee, L.N.; Yagita, H.; Gisbergen, K.P. van; Hawinkels, L.J.; et al. Original Research: Adenoviral Vaccines Promote Protective Tissue-Resident Memory T Cell Populations against Cancer. Journal for Immunotherapy of Cancer 2020, 8. [Google Scholar] [CrossRef]
- Zou, P.; Zhang, P.; Deng, Q.; Wang, C.; Luo, S.; Zhang, L.; Li, C.; Li, T. Two Novel Adenovirus Vectors Mediated Differential Antibody Responses via Interferon-α and Natural Killer Cells. Microbiol Spectr 2023, 11, e0088023. [Google Scholar] [CrossRef] [PubMed]
- Penaloza-MacMaster, P.; Provine, N.M.; Ra, J.; Borducchi, E.N.; McNally, A.; Simmons, N.L.; Iampietro, M.J.; Barouch, D.H. Alternative Serotype Adenovirus Vaccine Vectors Elicit Memory T Cells with Enhanced Anamnestic Capacity Compared to Ad5 Vectors. Journal of Virology 2013, 87, 1373–1384. [Google Scholar] [CrossRef]
- Holst, P.J.; Ørskov, C.; Thomsen, A.R.; Christensen, J.P. Quality of the Transgene-Specific CD8+ T Cell Response Induced by Adenoviral Vector Immunization Is Critically Influenced by Virus Dose and Route of Vaccination. J Immunol 2010, 184, 4431–4439. [Google Scholar] [CrossRef]
- Heemskerk, B.; Veltrop-Duits, L.A.; van Vreeswijk, T.; ten Dam, M.M.; Heidt, S.; Toes, R.E.M.; van Tol, M.J.D.; Schilham, M.W. Extensive Cross-Reactivity of CD4+ Adenovirus-Specific T Cells: Implications for Immunotherapy and Gene Therapy. Journal of Virology 2003, 77, 6562–6566. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, R.; Reimann, J.; Kochanek, S.; Kreppel, F. The Immunogenicity of Adenovirus Vectors Limits the Multispecificity of CD8 T-Cell Responses to Vector-Encoded Transgenic Antigens. Molecular Therapy 2008, 16, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.; Liu, F.; Niu, Q.; Hou, W.; Churchyard, G.; Morgan, C.; Frahm, N.; Nitayaphan, S.; Pitisuthithum, P.; Rerks-Ngarm, S.; et al. Distinct Susceptibility of HIV Vaccine Vector-Induced CD4 T Cells to HIV Infection. PLoS Pathog 2018, 14, e1006888. [Google Scholar] [CrossRef] [PubMed]
- Frahm, N.; DeCamp, A.C.; Friedrich, D.P.; Carter, D.K.; Defawe, O.D.; Kublin, J.G.; Casimiro, D.R.; Duerr, A.; Robertson, M.N.; Buchbinder, S.P.; et al. Human Adenovirus-Specific T Cells Modulate HIV-Specific T Cell Responses to an Ad5-Vectored HIV-1 Vaccine. J Clin Invest 2012, 122, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hutnick, N.A.; Carnathan, D.G.; Dubey, S.A.; Cox, K.S.; Kierstead, L.; Makadonas, G.; Ratcliffe, S.J.; Lasaro, M.O.; Robertson, M.N.; Casimiro, D.R.; et al. Vaccination with Ad5 Vectors Expands Ad5-Specific CD8+ T Cells without Altering Memory Phenotype or Functionality. PLoS One 2010, 5, e14385. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Q.; Ertl, H.C.; Wilson, J.M. Cellular and Humoral Immune Responses to Viral Antigens Create Barriers to Lung-Directed Gene Therapy with Recombinant Adenoviruses. J Virol 1995, 69, 2004–2015. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.M.; Truitt, D.M.; Kishko, M.G.; Arthur, J.C.; Jackson, S.S.; Gorgone, D.A.; Lifton, M.A.; Koudstaal, W.; Pau, M.G.; Kostense, S.; et al. Neutralizing Antibodies and CD8+ T Lymphocytes Both Contribute to Immunity to Adenovirus Serotype 5 Vaccine Vectors. Journal of Virology 2004, 78, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Appledorn, D.M.; McBride, A.; Seregin, S.; Scott, J.M.; Schuldt, N.; Kiang, A.; Godbehere, S.; Amalfitano, A. Complex Interactions with Several Arms of the Complement System Dictate Innate and Humoral Immunity to Adenoviral Vectors. Gene Ther 2008, 15, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Colloca, S.; Folgori, A.; Ammendola, V.; Capone, S.; Cirillo, A.; Siani, L.; Naddeo, M.; Grazioli, F.; Esposito, M.L.; Ambrosio, M.; et al. Generation and Screening of a Large Collection of Novel Simian Adenovirus Allows the Identification of Vaccine Vectors Inducing Potent Cellular Immunity in Humans: A Range of Novel Simian Adenoviral Vectors, Which Are Capable of Priming High Levels of T Cell Responses in Man, Has Been Defined. Science translational medicine 2012, 4, 115ra2. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med 2021, 384, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Leunig, A.; Pekayvaz, K.; Esefeld, M.; Anjum, A.; Rath, J.; Riedlinger, E.; Ehreiser, V.; Mader, M.; Eivers, L.; et al. Thrombocytopenia and Splenic Platelet-Directed Immune Responses after IV ChAdOx1 nCov-19 Administration. Blood 2022, 140, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Hunt, B.J.; Horner, D.; Bewley, S.; Karpusheff, J. Vaccine Induced Immune Thrombocytopenia and Thrombosis: Summary of NICE Guidance. BMJ 2021, 375, n2195. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Vaccine - Summary of Yellow Card Reporting Available online:. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 2 May 2024).
- Pai, M. Epidemiology of VITT. Seminars in Hematology 2022, 59, 72–75. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Medvedev, N.; Delcea, M.; Greinacher, A. Anti-Platelet Factor 4/Polyanion Antibodies Mediate a New Mechanism of Autoimmunity. Nat Commun 2017, 8, 14945. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCoV-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia. Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Pembaur, D.; Schröer, K.; Schellhorn, S.; Koukou, G.; Schmidt, N.; Zhang, W.; Kreppel, F.; Ehrhardt, A. Adenovirus Type 34 and HVR1-Deleted Adenovirus Type 5 Do Not Bind to PF4: Clearing the Path towards Vectors without Thrombosis Risk 2022, 2022. 11.07.51 5483.
- Baker, A.T.; Boyd, R.J.; Sarkar, D.; Vant, J.; Crespo, A.T.; Waraich, K.; Truong, C.D.; Bates, E.; Wilson, E.; Chan, C.K.; et al. The Structure of ChAdOx1/AZD-1222 Reveals Interactions with CAR and PF4 with Implications for Vaccine-Induced Immune Thrombotic Thrombocytopenia 2021, 2021. 05.19.44 4882.
- Ehrhardt, A.; Kay, M.A. A New Adenoviral Helper–Dependent Vector Results in Long-Term Therapeutic Levels of Human Coagulation Factor IX at Low Doses in Vivo. Blood 2002, 99, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, D.B.; Himeda, C.L.; Winther, B.L.; Wilson, C.B.; Kay, M.A. Implication of Interfering Antibody Formation and Apoptosis as Two Different Mechanisms Leading to Variable Duration of Adenovirus-Mediated Transgene Expression in Immune-Competent Mice. Journal of Virology 1999, 73, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Balagué, C.; Zhou, J.; Dai, Y.; Alemany, R.; Josephs, S.F.; Andreason, G.; Hariharan, M.; Sethi, E.; Prokopenko, E.; Jan, H.; et al. Sustained High-Level Expression of Full-Length Human Factor VIII and Restoration of Clotting Activity in Hemophilic Mice Using a Minimal Adenovirus Vector. Blood 2000, 95, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Ramsey, B.W.; Meeker, D.P.; Aitken, M.L.; Balfour, R.P.; Gibson, R.L.; Launspach, J.; Moscicki, R.A.; Richards, S.M.; Standaert, T.A. Repeat Administration of an Adenovirus Vector Encoding Cystic Fibrosis Transmembrane Conductance Regulator to the Nasal Epithelium of Patients with Cystic Fibrosis. J Clin Invest 1996, 97, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Ng, T.; Iannitti, D.; Cioffi, W.; Stapleton, G.; Law, M.; Breinholt, J.; Palmer, D.; Grove, N.; Rice, K.; et al. Transgene Expression up to 7 Years in Nonhuman Primates Following Hepatic Transduction with Helper-Dependent Adenoviral Vectors. Human Gene Therapy 2013, 24, 761. [Google Scholar] [CrossRef]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front Immunol 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic Viruses as Engineering Platforms for Combination Immunotherapy. Nat Rev Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and Immunogenicity of Heterologous versus Homologous Prime-Boost Schedules with an Adenoviral Vectored and mRNA COVID-19 Vaccine (Com-COV): A Single-Blind, Randomised, Non-Inferiority Trial. The Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Sig Transduct Target Ther 2023, 8, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Torres, J.; Cabello-Gutiérrez, C.; Ayón-Núñez, D.-A.; Soldevila, G.; Olguin-Alor, R.; Diaz, G.; Acero, G.; Segura-Velázquez, R.; Huerta, L.; Gracia-Mora, I.; et al. Caveats of Chimpanzee ChAdOx1 Adenovirus-Vectored Vaccines to Boost Anti-SARS-CoV-2 Protective Immunity in Mice. Appl Microbiol Biotechnol 2024, 108, 179. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, J.E.; Costner, P.; Desai, N.; Holman, L.; Enama, M.E.; Yamshchikov, G.; Mulangu, S.; Hu, Z.; Andrews, C.A.; Sheets, R.A.; et al. A Replication Defective Recombinant Ad5 Vaccine Expressing Ebola Virus GP Is Safe and Immunogenic in Healthy Adults. Vaccine 2010, 29, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-X.; Hou, L.-H.; Meng, F.-Y.; Wu, S.-P.; Hu, Y.-M.; Liang, Q.; Chu, K.; Zhang, Z.; Xu, J.-J.; Tang, R.; et al. Immunity Duration of a Recombinant Adenovirus Type-5 Vector-Based Ebola Vaccine and a Homologous Prime-Boost Immunisation in Healthy Adults in China: Final Report of a Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Glob Health 2017, 5, e324–e334. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.; Buchbinder, S.; Duerr, A. Overview of STEP and Phambili Trial Results: Two Phase IIb Test-of-Concept Studies Investigating the Efficacy of MRK Adenovirus Type 5 Gag/Pol/Nef Subtype B HIV Vaccine. Current Opinion in HIV and AIDS 2010, 5, 357. [Google Scholar] [CrossRef] [PubMed]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. npj Vaccines 2022, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, S.; Hays, J.; HogenEsch, H.; Mittal, S.K. Circumvention of Vector-Specific Neutralizing Antibody Response by Alternating Use of Human and Non-Human Adenoviruses: Implications in Gene Therapy. Virology 2000, 272, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mastrangeli, A.; Harvey, B.-G.; Yao, J.; Wolff, G.; Kovesdi, I.; Crystal, R.G.; Falck-Pedersen, E. “Sero-Switch” Adenovirus-Mediated In Vivo Gene Transfer: Circumvention of Anti-Adenovirus Humoral Immune Defenses Against Repeat Adenovirus Vector Administration by Changing the Adenovirus Serotype. Human Gene Therapy 1996, 7, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.-L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A Single Intranasal Dose of Chimpanzee Adenovirus-Vectored Vaccine Protects against SARS-CoV-2 Infection in Rhesus Macaques. Cell Rep Med 2021, 2, 100230. [Google Scholar] [CrossRef] [PubMed]
- Uchino, J.; Curiel, D.T.; Ugai, H. Species D Human Adenovirus Type 9 Exhibits Better Virus-Spread Ability for Antitumor Efficacy among Alternative Serotypes. PLOS ONE 2014, 9, e87342. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Senac, J.S.; Weaver, E.A.; May, S.M.; Jelinek, D.F.; Greipp, P.; Witzig, T.; Barry, M.A. Species D Adenoviruses as Oncolytics Against B Cell Cancers. Clin Cancer Res 2011, 17, 6712–6722. [Google Scholar] [CrossRef] [PubMed]
- Holterman, L.; Vogels, R.; van der Vlugt, R.; Sieuwerts, M.; Grimbergen, J.; Kaspers, J.; Geelen, E.; van der Helm, E.; Lemckert, A.; Gillissen, G.; et al. Novel Replication-Incompetent Vector Derived from Adenovirus Type 11 (Ad11) for Vaccination and Gene Therapy: Low Seroprevalence and Non-Cross-Reactivity with Ad5. Journal of Virology 2004, 78, 13207–13215. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.A.; Davies, J.A.; Váňová, J.; Nestić, D.; Meniel, V.S.; Koushyar, S.; Cunliffe, T.G.; Mundy, R.M.; Moses, E.; Uusi-Kerttula, H.K.; et al. Development of a Low-Seroprevalence, Avβ6 Integrin-Selective Virotherapy Based on Human Adenovirus Type 10. Molecular Therapy - Oncolytics 2022, 25, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Sadoff Jerald; Gray Glenda; Vandebosch An; Cárdenas Vicky; Shukarev Georgi; Grinsztejn Beatriz; Goepfert Paul A; Truyers Carla; Fennema Hein; Spiessens Bart. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. New England Journal of Medicine 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Havenga, M.; Vogels, R.; Zuijdgeest, D.; Radosevic, K.; Mueller, S.; Sieuwerts, M.; Weichold, F.; Damen, I.; Kaspers, J.; Lemckert, A.; et al. Novel Replication-Incompetent Adenoviral B-Group Vectors: High Vector Stability and Yield in PER.C6 Cells. Journal of General Virology 2006, 87, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Seymour, L.; Fisher, K. Activity of a Group B Oncolytic Adenovirus (ColoAd1) in Whole Human Blood. Gene Ther 2014, 21, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Penaloza-MacMaster, P.; Provine, N.M.; Ra, J.; Borducchi, E.N.; McNally, A.; Simmons, N.L.; Iampietro, M.J.; Barouch, D.H. Alternative Serotype Adenovirus Vaccine Vectors Elicit Memory T Cells with Enhanced Anamnestic Capacity Compared to Ad5 Vectors. J Virol 2013, 87, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hetzel, M.; Zhang, W.; Ehrhardt, A.; Bayer, W. Comparative Analysis of the Impact of 40 Adenovirus Types on Dendritic Cell Activation and CD8+ T Cell Proliferation Capacity for the Identification of Favorable Immunization Vector Candidates. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Youil, R.; Toner, T.J.; Su, Q.; Chen, M.; Tang, A.; Bett, A.J.; Casimiro, D. Hexon Gene Switch Strategy for the Generation of Chimeric Recombinant Adenovirus. Hum Gene Ther 2002, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.E.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-Chimaeric Adenovirus Serotype 5 Vectors Circumvent Pre-Existing Anti-Vector Immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Bradshaw, A.C.; Parker, A.L.; Robinson, H.; White, K.; Custers, J.; Goudsmit, J.; Van Roijen, N.; Barouch, D.H.; Nicklin, S.A.; et al. Ad5:Ad48 Hexon Hypervariable Region Substitutions Lead to Toxicity and Increased Inflammatory Responses Following Intravenous Delivery. Mol Ther 2012, 20, 2268–2281. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Su, X.; Li, H.; Li, X.; Zhou, Z.; Liu, W.; Zhou, R. Construction and Characterization of Human Adenovirus Serotype 3 Packaged by Serotype 7 Hexon. Virus Research 2011, 160, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Crosby, C.M.; Heller, G.J.; Mendel, Z.I.; Barry, M.E.; Barry, M.A. Oncolytic Adenovirus Ad657 for Systemic Virotherapy against Prostate Cancer. Oncolytic Virother 2018, 7, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Nehete, P.N.; Shelton, K.; Nehete, B.P.; Yang, G.; Dorta-Estremera, S.; Barnette, P.; Xiao, P.; Byrareddy, S.N.; et al. Divergent HIV-1-Directed Immune Responses Generated by Systemic and Mucosal Immunization with Replicating Single-Cycle Adenoviruses in Rhesus Macaques. J Virol 2019, 93, e02016–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-C.; Hansen, M.J.; Hemsath, J.R.; Parrett, B.J.; Zell, B.N.; Barry, M.A. Modulating Oncolytic Adenovirus Immunotherapy by Driving Two Axes of the Immune System by Expressing 4-1BBL and CD40L. Hum Gene Ther 2022, 33, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Jiang, H.; Gillard, A.; Kim, D.; Fan, X.; Singh, S.; Nguyen, T.T.; Sohoni, S.; Lopez-Rivas, A.; Parthasarathy, A.; et al. Chimeric Oncolytic Adenovirus Evades Neutralizing Antibodies from Human Patients and Exhibits Enhanced Anti-Glioma Efficacy in Immunized Mice. Molecular Therapy 2024. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Alba, R.; Parker, A.L.; Wright, A.F.; Bradshaw, A.C.; Delles, C.; McDonald, R.A.; Baker, A.H. Assessment of a Novel, Capsid-Modified Adenovirus with an Improved Vascular Gene Transfer Profile. J Cardiothorac Surg 2013, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.; Kass-Eisler, A.; Leinwand, L.; Falck-Pedersen, E. Adenovirus Type 5 and 7 Capsid Chimera: Fiber Replacement Alters Receptor Tropism without Affecting Primary Immune Neutralization Epitopes. J Virol 1996, 70, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Nociari, M.; Philpott, N.; Falck-Pedersen, E. Influence of Fiber Detargeting on Adenovirus-Mediated Innate and Adaptive Immune Activation. J Virol 2005, 79, 11627–11637. [Google Scholar] [CrossRef] [PubMed]
- Ophorst, O.J.A.E.; Kostense, S.; Goudsmit, J.; de Swart, R.L.; Verhaagh, S.; Zakhartchouk, A.; van Meijer, M.; Sprangers, M.; van Amerongen, G.; Yüksel, S.; et al. An Adenoviral Type 5 Vector Carrying a Type 35 Fiber as a Vaccine Vehicle: DC Targeting, Cross Neutralization, and Immunogenicity. Vaccine 2004, 22, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, N.; Leopold, P.L.; Hackett, N.R.; Ferris, B.; Worgall, S.; Falck-Pedersen, E.; Crystal, R.G. Fiber Swap between Adenovirus Subgroups B and C Alters Intracellular Trafficking of Adenovirus Gene Transfer Vectors. J Virol 1999, 73, 6056–6065. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J. Adenovirus Protein IX: A New Look at an Old Protein. Molecular Therapy 2005, 11, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Seregin, S.S.; Aldhamen, Y.A.; Appledorn, D.M.; Hartman, Z.C.; Schuldt, N.J.; Scott, J.; Godbehere, S.; Jiang, H.; Frank, M.M.; Amalfitano, A. Adenovirus Capsid-Display of the Retro-Oriented Human Complement Inhibitor DAF Reduces Ad Vector–Triggered Immune Responses in Vitro and in Vivo. Blood 2010, 116, 1669–1677. [Google Scholar] [CrossRef]
- Kreppel, F.; Kochanek, S. Modification of Adenovirus Gene Transfer Vectors With Synthetic Polymers: A Scientific Review and Technical Guide. Molecular Therapy 2008, 16, 16–29. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of Adenovirus with Retention of Infectivity and Protection from Neutralizing Antibody in Vitro and in Vivo. Hum Gene Ther 1999, 10, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, A.; Vöhringer, S.; Engler, T.; Corjon, S.; Schirmbeck, R.; Reimann, J.; Kochanek, S.; Kreppel, F. Fully Detargeted Polyethylene Glycol-Coated Adenovirus Vectors Are Potent Genetic Vaccines and Escape from Pre-Existing Anti-Adenovirus Antibodies. Mol Ther 2008, 16, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Han, J.; Zhao, D.; Gong, T.; Zhang, Z.; Sun, X. Protection of Adenovirus from Neutralizing Antibody by Cationic PEG Derivative Ionically Linked to Adenovirus. Int J Nanomedicine 2012, 7, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ichihara, M.; Yoshioka, Y.; Ishida, T.; Nakagawa, S.; Kiwada, H. Intravenous Administration of Polyethylene Glycol-Coated (PEGylated) Proteins and PEGylated Adenovirus Elicits an Anti-PEG Immunoglobulin M Response. Biol Pharm Bull 2012, 35, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Yuan, C.; Xu, X.; Zhou, W.; Huang, Y.; Lu, H.; Zheng, Y.; Luo, G.; Shang, J.; et al. Polyethylene Glycol (PEG)-Associated Immune Responses Triggered by Clinically Relevant Lipid Nanoparticles in Rats. npj Vaccines 2023, 8, 1–13. [Google Scholar] [CrossRef]
- Fisher, K.D.; Stallwood, Y.; Green, N.K.; Ulbrich, K.; Mautner, V.; Seymour, L.W. Polymer-Coated Adenovirus Permits Efficient Retargeting and Evades Neutralising Antibodies. Gene Ther 2001, 8, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Subr, V.; Kostka, L.; Selby-Milic, T.; Fisher, K.; Ulbrich, K.; Seymour, L.W.; Carlisle, R.C. Coating of Adenovirus Type 5 with Polymers Containing Quaternary Amines Prevents Binding to Blood Components. Journal of Controlled Release 2009, 135, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Green, N.K.; Herbert, C.W.; Hale, S.J.; Hale, A.B.; Mautner, V.; Harkins, R.; Hermiston, T.; Ulbrich, K.; Fisher, K.D.; Seymour, L.W. Extended Plasma Circulation Time and Decreased Toxicity of Polymer-Coated Adenovirus. Gene Ther 2004, 11, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Ríhová, B.; Kovár, M. Immunogenicity and Immunomodulatory Properties of HPMA-Based Polymers. Adv Drug Deliv Rev 2010, 62, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, G.; HogenEsch, H.; North, A.; Hays, J.; Mittal, S.K. Encapsulation of Recombinant Adenovirus into Alginate Microspheres Circumvents Vector-Specific Immune Response. Gene Ther 2002, 9, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of Alginate Microspheres in Therapeutics Delivery and Cell Culture: Past, Present and Future. Int J Pharm 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L.; et al. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Zhang, H.; Wei, J.; Wu, J. Extracellular Vesicles-Mimetic Encapsulation Improves Oncolytic Viro-Immunotherapy in Tumors With Low Coxsackie and Adenovirus Receptor. Front Bioeng Biotechnol 2020, 8, 574007. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, C.; Li, P.; Wu, T.; Zhou, H.; Yang, D.; Liu, Y.; Ma, X.; Song, Z.; Nian, Q.; et al. Vaccine Engineering with Dual-Functional Mineral Shell: A Promising Strategy to Overcome Preexisting Immunity. Advanced Materials 2016, 28, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Ernst, P.; Honegger, A.; Suomalainen, M.; Zimmermann, M.; Braun, L.; Stauffer, S.; Thom, C.; Dreier, B.; Eibauer, M.; et al. Adenoviral Vector with Shield and Adapter Increases Tumor Specificity and Escapes Liver and Immune Control. Nat Commun 2018, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Prill, J.-M.; Šubr, V.; Pasquarelli, N.; Engler, T.; Hoffmeister, A.; Kochanek, S.; Ulbrich, K.; Kreppel, F. Traceless Bioresponsive Shielding of Adenovirus Hexon with HPMA Copolymers Maintains Transduction Capacity In Vitro and In Vivo. PLoS One 2014, 9, e82716. [Google Scholar] [CrossRef]
- Krutzke, L.; Prill, J.M.; Engler, T.; Schmidt, C.Q.; Xu, Z.; Byrnes, A.P.; Simmet, T.; Kreppel, F. Substitution of Blood Coagulation Factor X-Binding to Ad5 by Position-Specific PEGylation: Preventing Vector Clearance and Preserving Infectivity. Journal of Controlled Release 2016, 235, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers (Basel) 2022, 14, 4924. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Kovesdi, I.; Bruder, J.T. Effective Repeat Administration with Adenovirus Vectors to the Muscle. Gene Ther 2000, 7, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Patel, A.; Tran, K.N.; Gray, M.; Zhang, Y.; Strong, J.E.; Feldmann, H.; Kobinger, G.P. Nasal Delivery of an Adenovirus-Based Vaccine Bypasses Pre-Existing Immunity to the Vaccine Carrier and Improves the Immune Response in Mice. PLoS One 2008, 3, e3548. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Z.; Yu, R.; Zhang, J.; Liu, Y.; Song, X.; Yi, S.; Liu, J.; Chen, J.; Yin, Y.; et al. Intramuscular Delivery of Adenovirus Serotype 5 Vector Expressing Humanized Protective Antigen Induces Rapid Protection against Anthrax That May Bypass Intranasally Originated Preexisting Adenovirus Immunity. Clin Vaccine Immunol 2014, 21, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bramson, J.L.; Hitt, M.; Gauldie, J.; Graham, F.L. Pre-Existing Immunity to Adenovirus Does Not Prevent Tumor Regression Following Intratumoral Administration of a Vector Expressing IL-12 but Inhibits Virus Dissemination. Gene Ther 1997, 4, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Worgall, S.; Ely, S.; Leopold, P.L.; Crystal, R.G. Cellular Immune Responses of Healthy Individuals to Intradermal Administration of an E1-E3- Adenovirus Gene Transfer Vector. Hum Gene Ther 1999, 10, 2823–2837. [Google Scholar] [CrossRef] [PubMed]
- Iscaro, A.; Jones, C.; Forbes, N.; Mughal, A.; Howard, F.N.; Janabi, H.A.; Demiral, S.; Perrie, Y.; Essand, M.; Weglarz, A.; et al. Targeting Circulating Monocytes with CCL2-Loaded Liposomes Armed with an Oncolytic Adenovirus. Nanomedicine: Nanotechnology, Biology and Medicine 2022, 40, 102506. [Google Scholar] [CrossRef]
- Muthana, M.; Giannoudis, A.; Scott, S.D.; Fang, H.-Y.; Coffelt, S.B.; Morrow, F.J.; Murdoch, C.; Burton, J.; Cross, N.; Burke, B.; et al. Use of Macrophages to Target Therapeutic Adenovirus to Human Prostate Tumors. Cancer Research 2011, 71, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, S.; Wang, Y.; Shao, F.; Lv, P.; Li, R.; Zhao, X.; Zhang, J.; Zhang, X.; Li, J.; et al. Lung-Targeted Transgene Expression of Nanocomplexed Ad5 Enhances Immune Response in the Presence of Preexisting Immunity. Engineering (Beijing) 2023. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Condezo, G.N.; Moreno, R.; Fajardo, C.A.; Arias-Badia, M.; San Martín, C.; Alemany, R. Albumin-Binding Adenoviruses Circumvent Pre-Existing Neutralizing Antibodies upon Systemic Delivery. Journal of Controlled Release 2016, 237, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Mato-Berciano, A.; Morgado, S.; Maliandi, M.V.; Farrera-Sal, M.; Gimenez-Alejandre, M.; Ginestà, M.M.; Moreno, R.; Torres-Manjon, S.; Moreno, P.; Arias-Badia, M.; et al. Oncolytic Adenovirus with Hyaluronidase Activity That Evades Neutralizing Antibodies: VCN-11. Journal of Controlled Release 2021, 332, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Peregrino, M.; Garcia-Carbonero, R.; Laquente, B.; Álvarez, R.; Mato-Berciano, A.; Gimenez-Alejandre, M.; Morgado, S.; Rodríguez-García, A.; Maliandi, M.V.; Riesco, M.C.; et al. VCN-01 Disrupts Pancreatic Cancer Stroma and Exerts Antitumor Effects. J Immunother Cancer 2021, 9, e003254. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martín, M.; Álvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillén-Ponce, C.; Farrera-Sal, M.; Moreno, R.; et al. Phase I, Multicenter, Open-Label Study of Intravenous VCN-01 Oncolytic Adenovirus with or without Nab-Paclitaxel plus Gemcitabine in Patients with Advanced Solid Tumors. J Immunother Cancer 2022, 10, e003255. [Google Scholar] [CrossRef] [PubMed]
- Muruve, D.A.; Cotter, M.J.; Zaiss, A.K.; White, L.R.; Liu, Q.; Chan, T.; Clark, S.A.; Ross, P.J.; Meulenbroek, R.A.; Maelandsmo, G.M.; et al. Helper-Dependent Adenovirus Vectors Elicit Intact Innate but Attenuated Adaptive Host Immune Responses In Vivo. Journal of Virology 2004, 78, 5966–5972. [Google Scholar] [CrossRef] [PubMed]
- Koup, R.A.; Lamoreaux, L.; Zarkowsky, D.; Bailer, R.T.; King, C.R.; Gall, J.G.D.; Brough, D.E.; Graham, B.S.; Roederer, M. Replication-Defective Adenovirus Vectors with Multiple Deletions Do Not Induce Measurable Vector-Specific T Cells in Human Trials. J Virol 2009, 83, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Gabitzsch, E.S.; Xu, Y.; Balint, J.P.; Hartman, Z.C.; Lyerly, H.K.; Jones, F.R. Anti-Tumor Immunotherapy despite Immunity to Adenovirus Using a Novel Adenoviral Vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother 2010, 59, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Seaman, M.S.; Xu, L.; Barouch, D.H.; Lord, C.I.; Lifton, M.A.; Gorgone, D.A.; Beaudry, K.R.; Svehla, K.; Welcher, B.; et al. Replication-Defective Adenovirus Serotype 5 Vectors Elicit Durable Cellular and Humoral Immune Responses in Nonhuman Primates. J Virol 2005, 79, 6516–6522. [Google Scholar] [CrossRef] [PubMed]
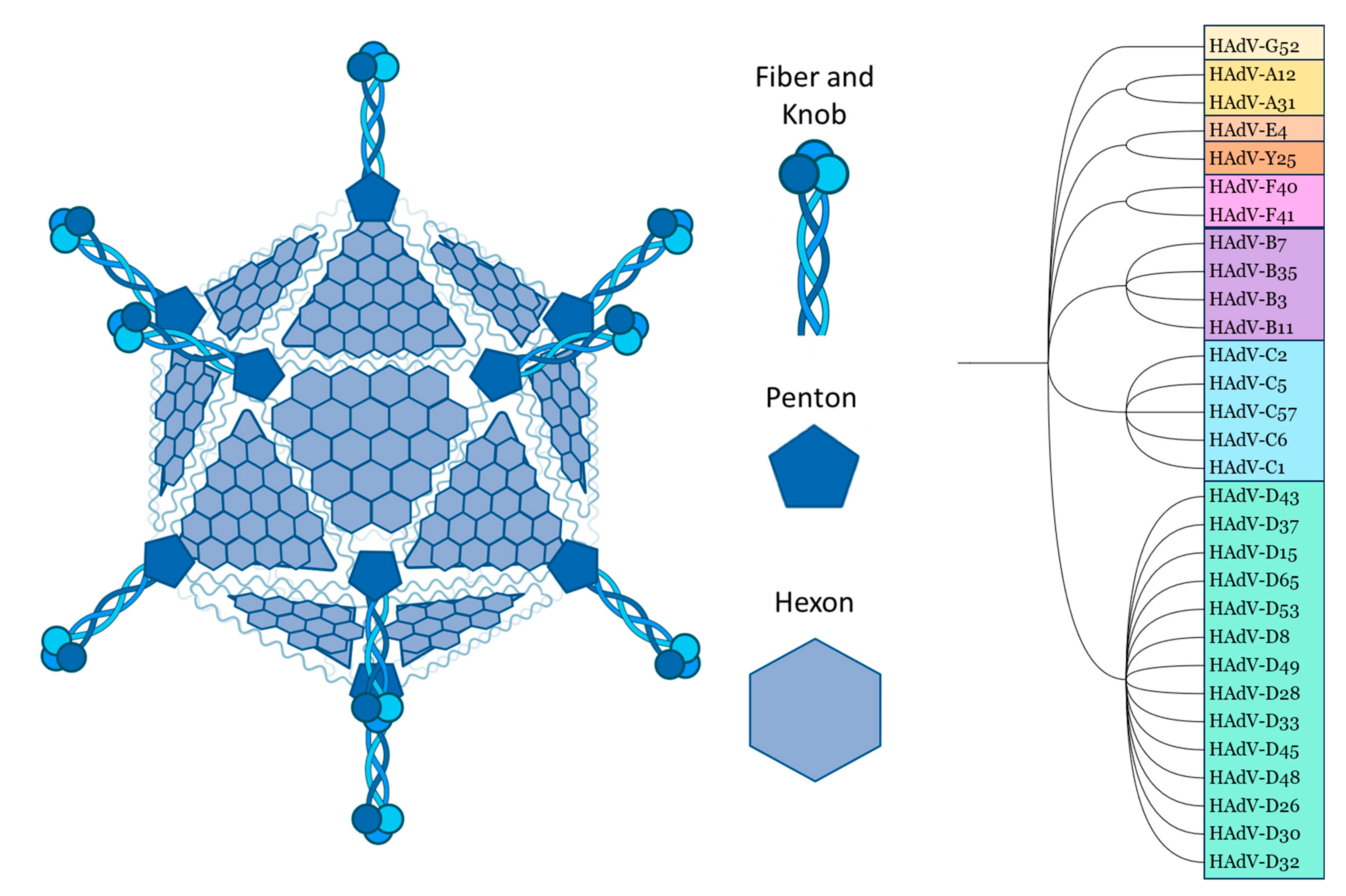
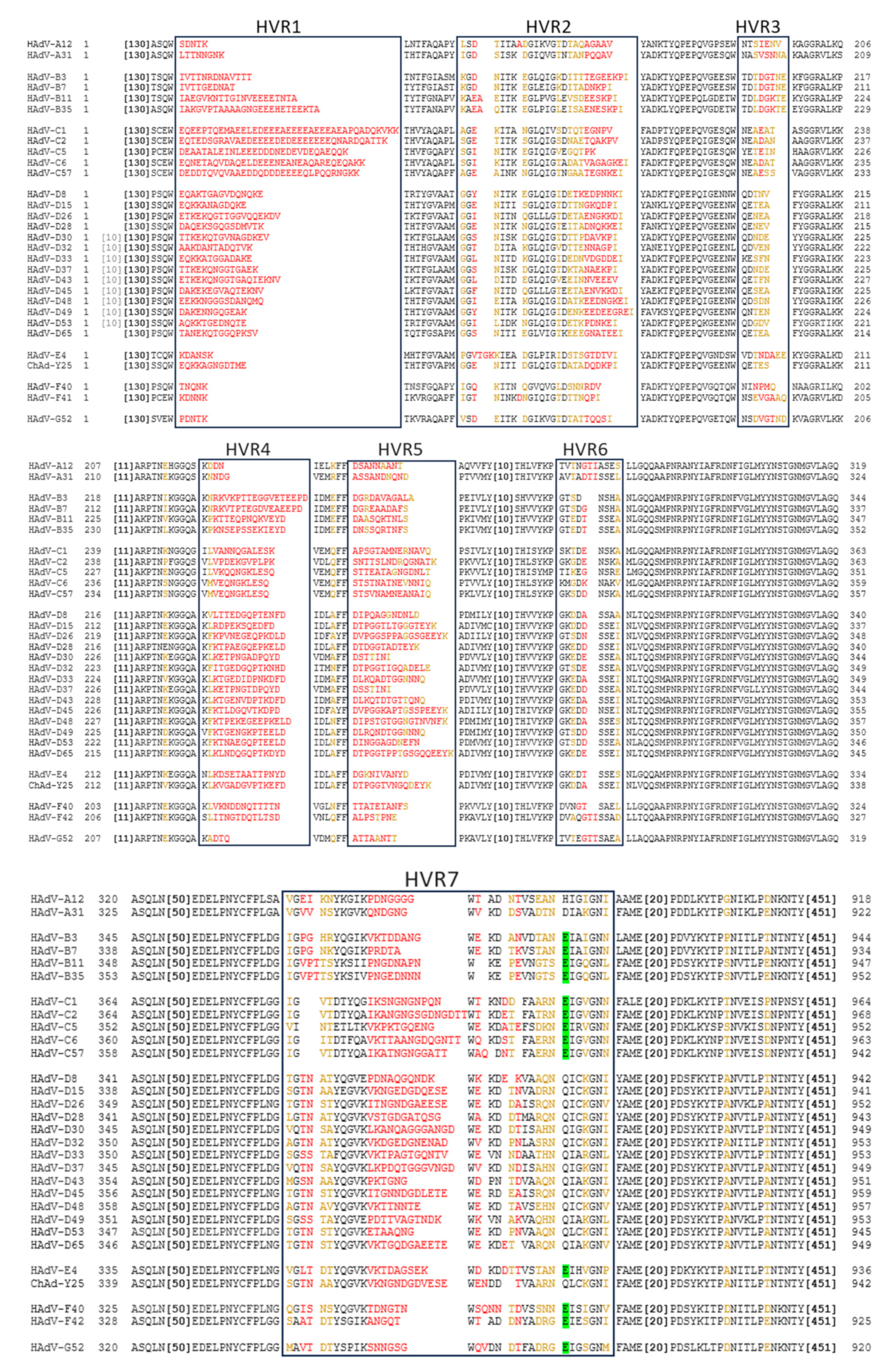
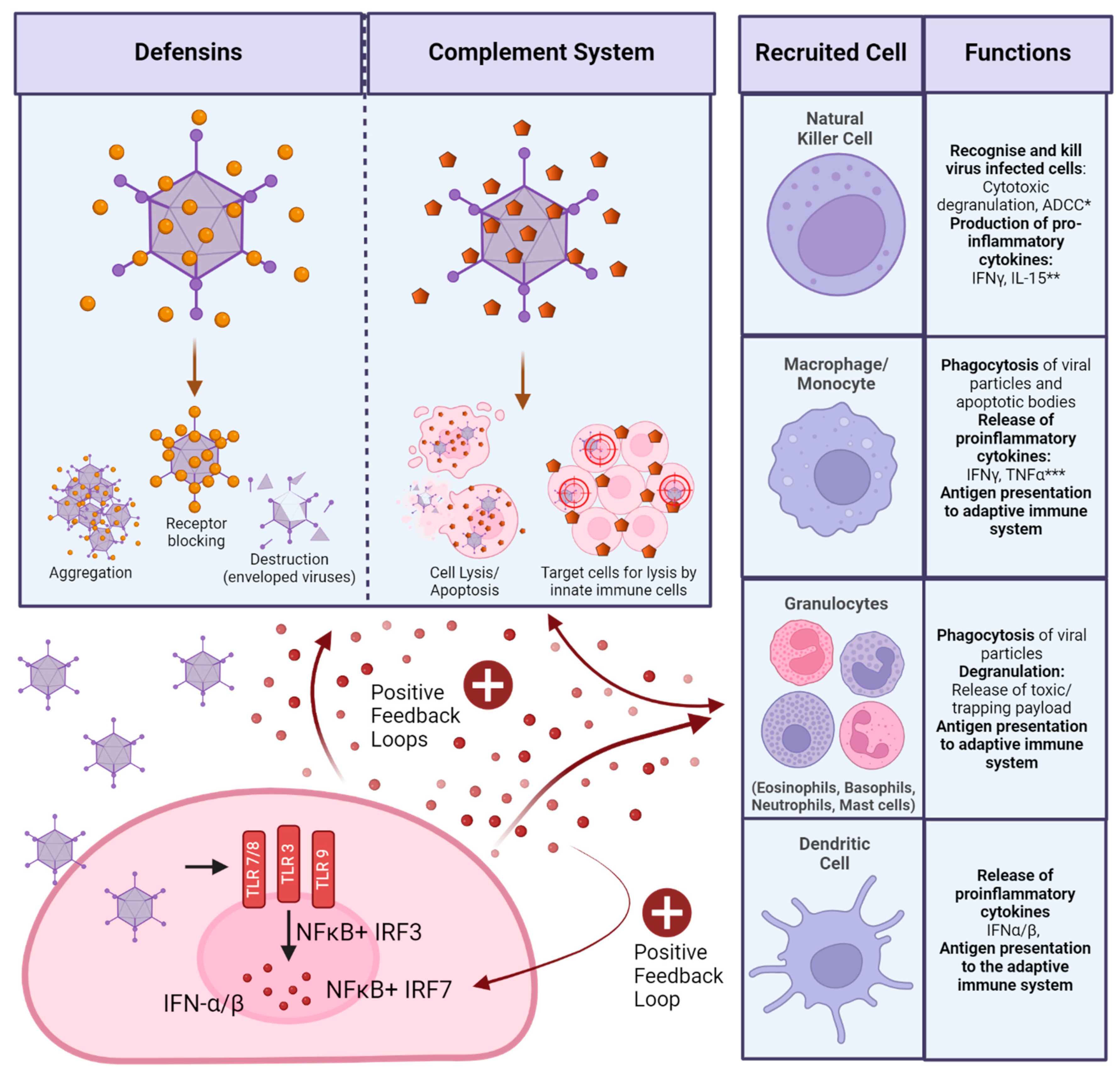
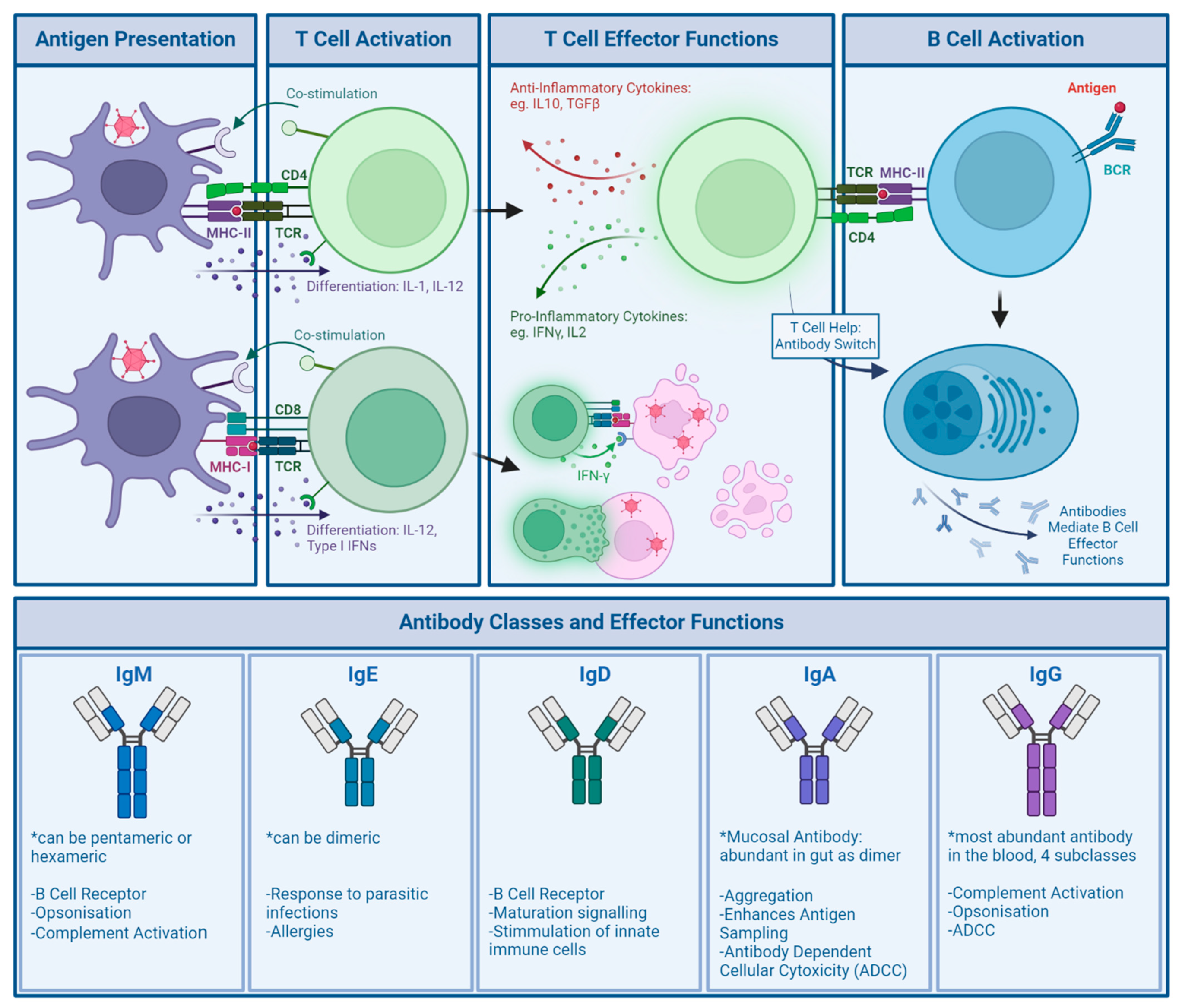
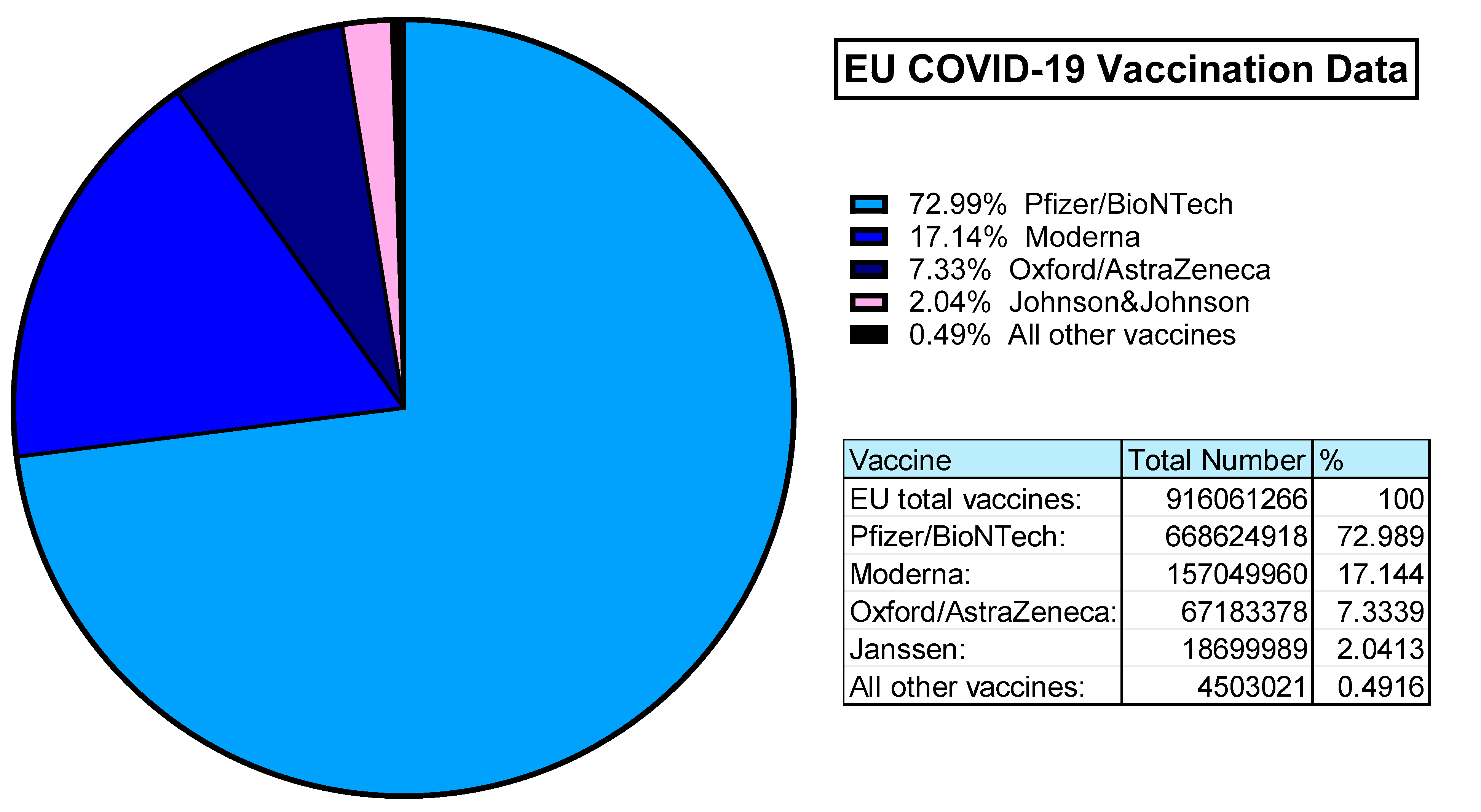
| Study Title/Drug Name | Status | Mechanism of Action | Vector based on | Targeted Cancer |
|---|---|---|---|---|
| Gendicine[129] | CFDA* approved (2003) |
Oncolytic, Suicide Gene Therapy (p53 restorative gene therapy) | HAdV-C5 | Head and Neck Squamous Cell Carcinoma |
| Adstiladrin[130] | FDA** approved (2022) | Immunomodulatory: IFN-α2b Transgene expression | HAdV-C5 (rep def) | Bladder Cancer Unresponsive to First Line Treatment |
| First in Human Study With NG-641, a Tumour Selective Transgene Expressing Adenoviral Vector (STAR)[131] | Phase I, recruiting |
Oncolytic, Immunostimulatory: FAP-directed bi-specific T-cell activator, Ifα2, CXC9 and CXC10 | HAdV-B11pHAdV-B3(EnAd)[132] | Metastatic or Advanced Epithelial Tumours |
| First in Man Clinical Study to Evaluate Safety and Tolerability of an Oncolytic Adenovirus in Prostate Cancer Patients.[133] | Phase I/IIa, active |
Oncolytic | HAdV-C5[134] | Prostate Cancer |
| Safety and Efficacy of Repeat Administration of Ad/PNP and Fludarabine Phosphate in Patients With Local Head/Neck Cancer[135] | Phase I/II, | Prodrug Conversion | HAdV-C5[136] (rep def) | Recurrent Local Head and Neck Cancer |
| SBRT and Oncolytic Virus Therapy Before Pembrolizumab for Metastatic TNBC and NSCLC (STOMP)[137] | Phase II, active |
Prodrug Conversion | HAdV-C5[138] | Metastatic Triple Negative Breast Cancer, Metastatic Non-Small Cell Lung Cancer |
| Adenovirus Mediated Suicide Gene Therapy With Radiotherapy in Progressive Astrocytoma [139] | Phase I, recruiting |
Prodrug Conversion | HAdV-C5 (rep def) | Different Brain Cancers |
| Trial Investigating an Immunostimulatory Oncolytic Adenovirus for Cancer [140] | Phase I/II, active |
Oncolytic, Immunostimulatory |
HAdV-C5/HAdV-B35 | Pancreatic Adenocarcinoma, Ovarian Cancer, Biliary Carcinoma, Colorectal Cancer |
| Study Title | Status | Mechanism of Action | Vector based on | Targeted Disease |
|---|---|---|---|---|
| Clinical Study With Lymfactin® in the Treatment of Patients With Secondary Lymphedema (AdeLE)[148] | Phase II, not yet recruiting |
Transgene: Vascular Endothelial growth Factor C (VEGF-C) |
HAdV-C5 (Lymfactin)[149] (rep def) | Secondary Lymphedema (after radiotherapy for breast cancer) |
| Epicardial Delivery of XC001 Gene Therapy for Refractory Angina Coronary Treatment (The EXACT Trial) (EXACT)[150] | Phase I/II, completed (2023) |
Transgene: VEGF |
HAdV-C5[151,152] (rep def) | Angina caused by Coronary Artery Disease |
| Preliminary Testing of New Treatment for Chronic Leg Wounds[153] | Phase I, completed (2011) |
Transgene: Platelet-Derived Growth Factor B (PDGF-B) |
HAdV-C5 (rep def (E1+E3 deleted[154])) | Chronic wounds of the leg/Venous Ulcers |
| Phase I Pilot Study of Ad5-CB-CFTR, an Adenovirus Vector Containing the Cystic Fibrosis Transmembrane Conductance Regulator Gene, in Patients With Cystic Fibrosis[155] | Phase I, completed (2001) |
Transgene: Cystic Fibrosis Transmembrane conductance Regulator (CFTR) |
HAdV-C5 (rep def) | Cystic Fibrosis |
| Study Title/Drug Name | Status | Route of Administration | Vector based on | Disease |
|---|---|---|---|---|
| Jcovden | Approved (EMA* March 2021 [165]) Approved (MHRA** December 2022 [166])Revoked (FDA*** March 2023 [167]) |
Intramuscular | HAdV-D26 | SARS-CoV-2 |
| Covishield (UK), Vaxzevria(EU) | Approved (EMA* January 2021[168])Approved (MHRA** July 2021 [166]) |
Intramuscular | ChAdOx1 (ChAd-Y25) | SARS-CoV-2 |
| Phase III Study of BBV154 Intranasal Vaccine in Healthy Volunteers (Nasal154PH3)[169] | Phase III, active |
Intranasal | ChAd36[170] | SARS-CoV-2 |
| Monovalent Chimpanzee Adenoviral-Vectored Marburg Virus Vaccine in Healthy Adults[171] | Phase II, not yet recruiting |
Intramuscular | ChAd-3 | Marburg Virus |
| Phase I Clinical Trial With New SARS-CoV-2 CoVacHGMix Type 5 Adenoviral Vector Vaccine[172] | Phase I, recruiting |
Intramuscular | HAdV-C5 | SARS-CoV-2 |
| Phase 1 Trial of ChAd68 and Ad5 Adenovirus COVID-19 Vaccines Delivered by Aerosol[173] | Phase I, recruiting |
Intranasal | ChAd-68HAdV-C5 | SARS-CoV-2 |
| A Clinical Trial on Booster Immunization of Two COVID-19 Vaccines Constructed From Different Technical Routes[174] | Phase I, recruiting |
Intranasal | HAdV-C5 | SARS-CoV-2 |
| Safety and Immunogenicity of Ad4-HIV Envelope Vaccine Vectors in Healthy Volunteers[175] | Phase I, recruiting |
Intranasal, Intramuscular (boost) | HAdV-E4 | HIV |
| Monovalent Chimpanzee Adenoviral-Vectored Marburg Virus Vaccine in Healthy Adults[171] | Phase II, not yet recruiting |
Intramuscular | ChAd-3 | Marburg Virus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





