1. Introduction
Physio and Manual therapists use various interventions that aims to improve muscle and joint functions. It is worth noting that physiotherapists, osteopaths, chiropractors, and massage therapists fall under the category of manual therapists. Manual therapists in general emphasize the evaluation and treatment of muscle flexibility and joint mobility that causes muscle-joint disfunction, and pain [
1,
2]. Fascia tissue manipulations as treatment intervention utilized gaining increased attention [
3,
4,
5,
6]. Fascia tissue manipulations encompass methods such as soft tissue mobilization, application of elastic tape, myofascial release techniques, and joint high-velocity thrust manipulations and mobilizations [
7]. Applying force during fascia tissue manipulation is expected to stress the fascial tissues underlying the skin, thereby expecting to modulate specific parts of tissues surrounding a muscle and/or joint [
8].
A comprehensive terminology was suggested at the International Fascia Research Congress regarding the classification of fasciae [
9], which has been updated [
10]. The integration of fasciae primarily focuses on the superficial fascia, deep fascia, and myofascia, largely due to its origins in massage therapy, which predominantly targets muscle treatments. In physiotherapy, a classic muscle-bone concept is used to translate basic and clinical anatomy [
11,
12]. This concept is based on the principles that muscle and bone are genetically, molecularly, and mechanically interconnected [
12].
Considering that muscles are epimuscularly attached to bones, joint capsules, and ligaments [
13], and that joint capsules and ligaments are also classified as fascia [
9], the muscle-bone concept is no longer adequate. This is further emphasized by the myofascial unit concept [
14]. The concept of the myofascial unit elucidates that in numerous motor tasks, active muscle engagement interact with periods of passive muscle behavior [
15]. In addition, it offers a potential explanation of how muscles interact with bones not only through the muscle-tendon unit but also through the extramuscular connective tissues linkages when the muscle actively contracts [
16]. Thereby it is suggested that extramuscular anatomical structures like joint capsules and ligaments (referred to as ‘arthrofascia’), as well as deep fascia and even superficial fascia, may have the capacity to influence skeletal muscle function, thereby impacting joint mobility [
13]. Therefore, it is hypothesized that these anatomical structures collectively contribute to optimal motor control
collaborating synergistically to optimize functional movements in the most effective manner [
13,
15]. Understanding the complex interplay between fascia, muscle, bone, and a joint during functional movements starts with integrating basic knowledge of fascia anatomy in the muscle-bone concept.
Since the muscle-bone concept is integrated in physiotherapy education courses, clinicians consequently continue to face challenges in defining fascia as an anatomical structure, essential for clinical applications and peer communication. Therefore, this narrative review aims to comprehensively describe the linkages from the skin to bone at the level of a joint, including anatomical structures such as skeletal muscle, fasciae, and neurovascular tracts. By defining the anatomical structures from superficial to deeper layers which frame the ArthroMyoFascial complex, our aim is to offer clinicians a comprehensive classification of fasciae within the muscle-bone concept. The significance of this work lies in enhancing the clinical concept of fascia, which is crucial for therapeutic testing, treatment, reporting, and multidisciplinary communication, important for comprehending musculoskeletal and orthopedic rehabilitation.
2. Anatomical Description of Fascia
First, this review will provide background information to describe the basics of fascia. Secondly, we briefly explain and classify each anatomical fascial structure and its linkages within this ArthroMyoFascial complex. Thirdly, we analyze the proof of concept through quantitative analyses of ultrasound images using skeletonization. While detailed explanations are omitted, this information is deemed essential for evaluating and discussing the proposed model.
2.1. Fascia Basics
Fascia is a specialized connective tissue sheath and is most often misplaced as a synonym for connective tissue, simply because it is not known that it is a general name for various phenotypic structures like the stratum membranosum, deep fascia, epi-peri-endomysium, capsules, and ligaments, which are interconnected (
Table 1) [
9,
10]. In other words, it is a fascial network that consists of different fasciae [
17]. A fascia is a sheath of connective tissue that forms beneath the skin to attach, enclose, and separate not only muscles but also bones, nerves, blood vessels, and organs [
10]. Each fascia within this network consists of a unique extracellular matrix encapsulating fibroblasts, myofibroblasts, and fasciacytes [
18,
19] that determines the fascial biomechanical content. This biomechanical content is expressed in viscoelasticity, which determines the stiffness and thereby the resistance to stress and strain. Consider these elements, this anatomical structure could be classified into 1) a fascia, 2) fasciae (multiple fascia), and 3) the fascial system [
9,
10,
17], connected from the skin to the bone within an ArthroMyoFascial complex.
2.2. Superficial Fascia
The skin consists of epithelium and is composed of three layers: the epidermis, dermis, and hypodermis [
20]. The layers known as the epidermis, dermis, and hypodermis are not categorized as fascia. Nevertheless, the stratum membranosum present within the hypodermis is commonly recognized as a type of fascia [
9]. The stratum membranosum is also known as the superficial fascia [
21]. The superficial fascia is a connective tissue network and varies in thickness [
22]. The superficial fascia is a connective tissue network with a membrane that separates the superficial adipose tissue from the deep adipose tissues. The superficial fascia membrane is superiorly attached to the dermis via retinacula cutis superior and inferiorly to the deep fascia via the retinacula cutis inferior [
17,
23]. For functional purposes, at some locations, this linkage is more firm via skin ligaments (cooper’s ligaments), and at other locations, it is filmier and loose [
24]. In addition, the superficial fascia is a dense innervated structure. Research revealed extensive innervation within the superficial fascia, emphasizing its significance in thermoregulation, sensation, and pain perception, which could enhance the understanding of factors impacting fascial sensitivity [
25].
2.3. Deep Fascia and Myofascia Including Muscle Fibers
Deep fascia also known as the fascia generalis is a strong and dense sheath of connective tissue [
26]. It forms a continuous sheath that covers muscles, bones, nerves, and blood vessels, helping to compartmentalize and protect these structures [
27].
For example, well-known deep fascia layers are the thoracolumbar fascia [
28] and fascia lata [
29]. The fascia lata is a dense connective tissue that surrounds the thigh muscles. It is the outermost layer of fascia in the thigh and extends from the hip to the knee [
29]. Within the fascia lata, there is a thick band of fibrous tissue known as the iliotibial tract. It runs along the outside of the thigh, extending from the pelvis (specifically, the iliac crest) down to the knee, where it attaches to the tibia and the fibula [
29]. The thoracolumbar fascia is a specific deep fascia located in the lower back region, covering the muscles of the lumbar and thoracic spine. It consists of several layers of fibrous tissue and serves as a critical attachment site for various muscles, including the latissimus dorsi, gluteus, and transverse abdominis [
30] facilitating force transmission among them [
31] via the so called lateral raphe [
32,
33]. Without further specific expansion, it is evident that deep fascia is often named based on its anatomical location. Examples include the brachialis intermuscular septa found in the upper arm (brachium), the popliteal fascia located at the back of the knee (poples), and the fascia plantaris at the sole of the foot (plantar). These names help to identify the precise areas where these fascial structures are prominent.
Skeletal muscles consist of a complex three-dimensional connective tissue scaffold resembling a honeycomb structure [
9]. This myofascial scaffold includes several interconnected layers: the epimysium, perimysium, endomysium, and tendon [
34]. The epimysium is the outermost layer that envelops the entire muscle, providing a protective covering and connecting it to surrounding structures. In certain areas, the epimysium thickens to form aponeurotic connective tissues, such as the erector spinae aponeurosis, which are known for their unique functions and mechanical properties, distinct from the typical epimysium [
34]. The perimysium is located within the muscle and divides it into bundles of muscle fibers, called fascicles. It acts as a supportive framework, enclosing and supporting these fascicles. Inside each fascicle, the endomysium is present, surrounding individual muscle fibers and facilitating their proper functioning. At the ends of skeletal muscles, the aponeuroses, together with the epimysium, merge to form the tendon [
35]. Tendons are strong, fibrous structures that attach the muscle to the bone, transmitting the force generated by muscle contractions, allowing tissue strains and joint movement [
35]. Histological studies have revealed that the endomysium, perimysium, epimysium, and tendons of skeletal muscles are tightly interconnected, for efficient force transmission from intermuscular to epimuscular tissues via the tendon, epimysium, and extramuscular connective tissue linkages [
35,
36,
37].
The deep fascia and muscles are densely innervated. A study analyzed the thoracolumbar fascia and epimisial gluteal fascia in adult mice using transmission electron microscopy and immunohistochemistry, revealing a dense network of nerves within these fascial tissues, with significant differences in innervation between the two types of fascia [
34]. In addition, skeletal muscles are provided by motor units and proprioceptors including muscle spindles and Golgi tendon organs, predominantly located near the myofascial elements within the muscle and adjacent to the deep fascia [
34,
38]. This suggests that myofascial force transmission potentially affects proprioception by transmitting muscle-generated forces through the fascial network, while sensory receptor cells in the fascia contribute to the body's awareness of its position and movement. However, this remains a topic of debate, and further research is needed to confirm this.
2.4. Arthrofascia
The deepest layer in the ArthroMyoFascial complex, is referred to as arthrofascia, pertains to the fibrous connective tissue connections between bones that constitute a joint. The arthrofascia consists of capsules, ligaments, periosteum, and cartilage fibers [
39]. The arthrofascial connections can be described as either segmental, involving two bones, or regional, involving three or more bones. The arthrofascia strongly determines passive joint motion [
40]. In general, there are three types of arthrofascial connections for describing joint mechanics (synovial capsules, ligamentous, and via cartilage fibers). The synovial capsules are dense fibrous connective tissues that merge with the periosteum and are attached at the ends of each of the involved bones forming a segmental joint. In certain areas, the capsule thickens to create capsular ligaments, which might also integrate tendons and other types of connective tissues [
41]. In addition, ligaments can also form a joint [
42]. Compared to capsules, ligamentous connections regionally connect bones that work together to achieve specific functions. At last, bone linkages are formed by cartilage fibers, such as the annulus fibers of the discus and ligamentum calcaneonaviculare plantare [
9,
43]. All these tissues, capsules, ligaments, and tendons attach to the periosteum via Sharpey’s fibers, which merge the periosteum with the underlying bone tissue [
44]. Consequently, we have classified it as arthrofascia, similar to how fascia from the muscles can be classified as myofascia.
3. ArthroMyoFascial Complex: Proposed Concept for Clinicians
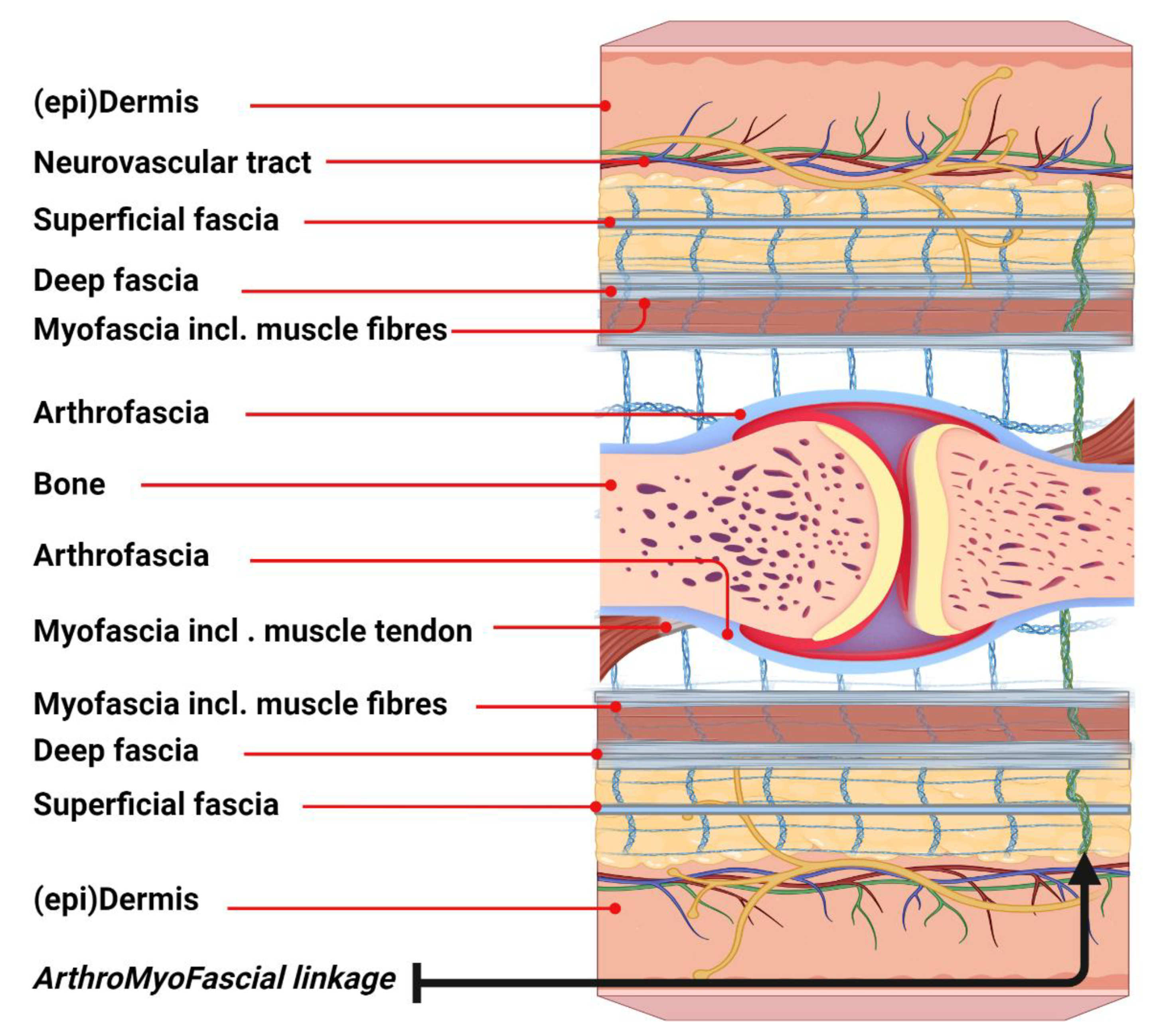
The ArthroMyoFascial complex (
Figure 1) represents a comprehensive and integrated concept in basic fascia anatomy, highlighting the interconnection of various anatomical structures surrounding a joint in both humans and animals. This model clarifies the continuity of fascia at the joint level. From superficial to deeper layers, the model delineates the skin, including the (epi)dermis and superficial fascia, followed by the deep fascia, which overlies the myofascia and skeletal muscles. In addition, the model illustrates lateral connections with the arthrofascia, as well as the muscle-tendon linkage with the arthrofascia. Subsequently, the model demonstrates the inverse connections of deeper tissues underneath the joint. Moreover, the connective tissue linkages depict the entire network in which these tissues are embedded. It is important to note that the neurovascular tract also links these tissues, although it is not fully represented in this model for clarity. This model could help expand our understanding of the myofascial unit, which plays a key role within the larger ArthroMyoFascial complex.
4. Proof of Concept: Ultrasound-Based Evaluation
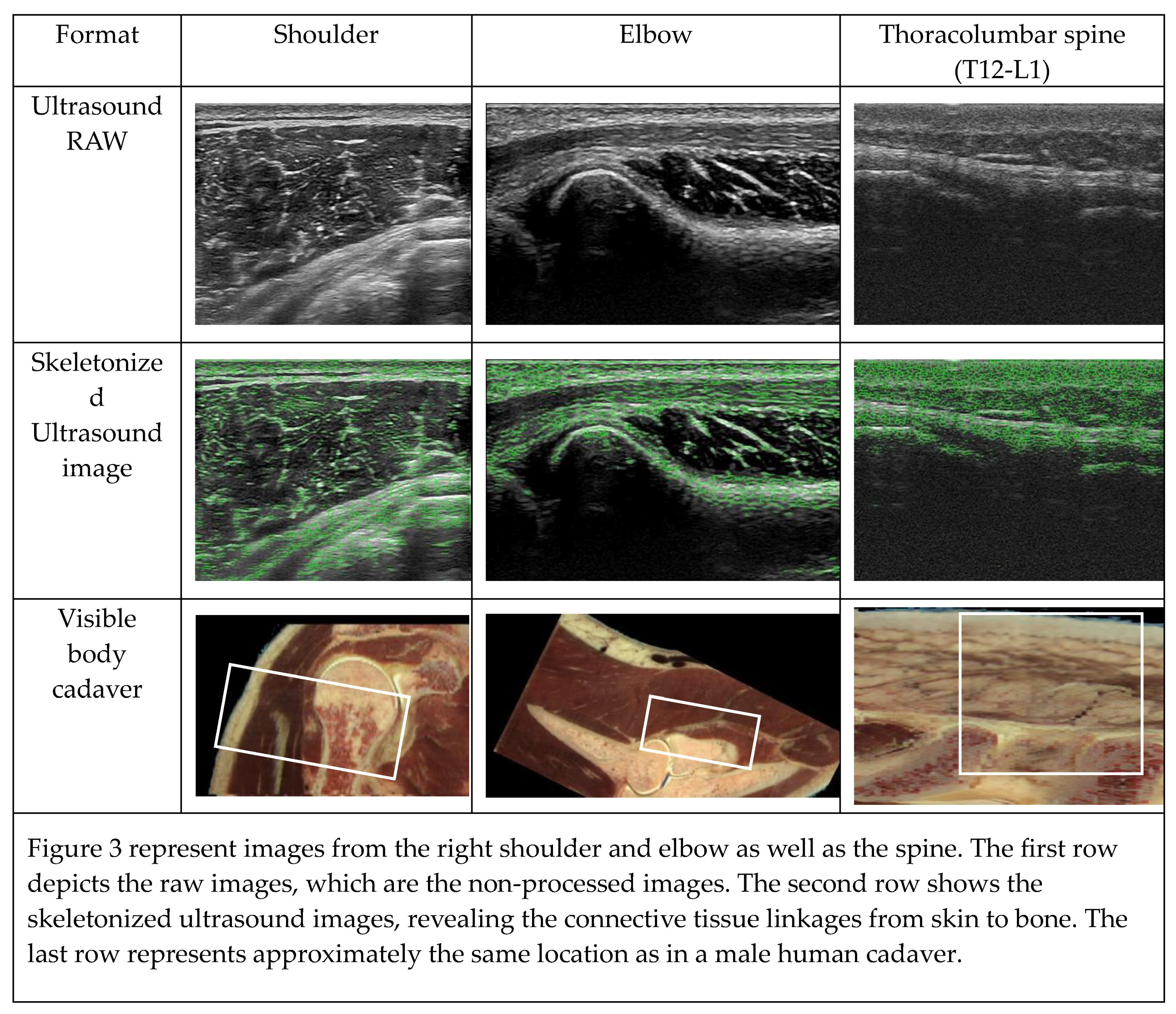
To analyze the linkages between the skin, superficial fascia, deep fascia, and arthrofascia, we assessed the visualization of anatomical structures and linkages of the shoulder (glenohumeral), elbow (humeroradial), and thoracolumbar spine (spinal processes T12-L1) using 2D ultrasound imaging analysis. The 2D ultrasound images were obtained from the right and left side of two male subjects (subject 1: age 41, 178 cm, 78 kg, BMI 24.5; subject 2: age 24, 180 cm, 73 kg, BMI 22.2).
Ultrasound observations were conducted by the 2
nd author using the Hitachi Arietta 850 SE, manufactured by Hitachi Ltd., Tokyo, Japan. Measurements were taken with a linear array probe (L64, 5-18 MHz) and ultrasonic gel (Aquasonic 100 Ultrasonic Gel, Parker Laboratories, Inc., Fairfield, NJ, USA). The feedback radio frequency of this signal was displayed in a two-dimensional ultrasound image (3*4 cm). The utilized gain (display value percentage) ranged between 55% and 80%, depending on the individual. The ultrasound images were analyzed in ImageJ to visualize the ArthroMyoFascial linkages [
44]. First, the original image was opened and filtered using the Laplacian filter, subsequently it was duplicated. The duplicate image was then converted to a binary image and subsequently skeletonized with the skeletonize function. The skeletonized lines, representing the connective tissue linkages, were colored, copied, and overlaid on the filtered ultrasound image. The skeletonized ultrasound images revealed a connective tissue matrix for all three locations (
Figure 3). The skeletonization was performed 3 times by the 2
nd author (ICC=.994). The validity of analyzing tissue networks has been ensured by skeletonized cells and original photomicrographs [
45]. Skeletonization is widely used in morphological analyses of biological structures, such as blood vessels, bony matrix, cell membranes, and more. It is useful for simplifying complex shapes and conducting quantitative & qualitative analyses [
46,47]. However, the quality of the skeletonization method used to analyze connective tissue linkages in ultrasound images should be further tested.
The connective tissue linkages that were identified in our initial ultrasound analysis are important proof of concept. These linkages between the joints, muscles, and fascial systems via the used evaluation techniques, might be an important frontier of musculoskeletal research and therapy. To fully validate these findings, more research is needed, and research is warranted.
5. Discussion and Clinical Consideration
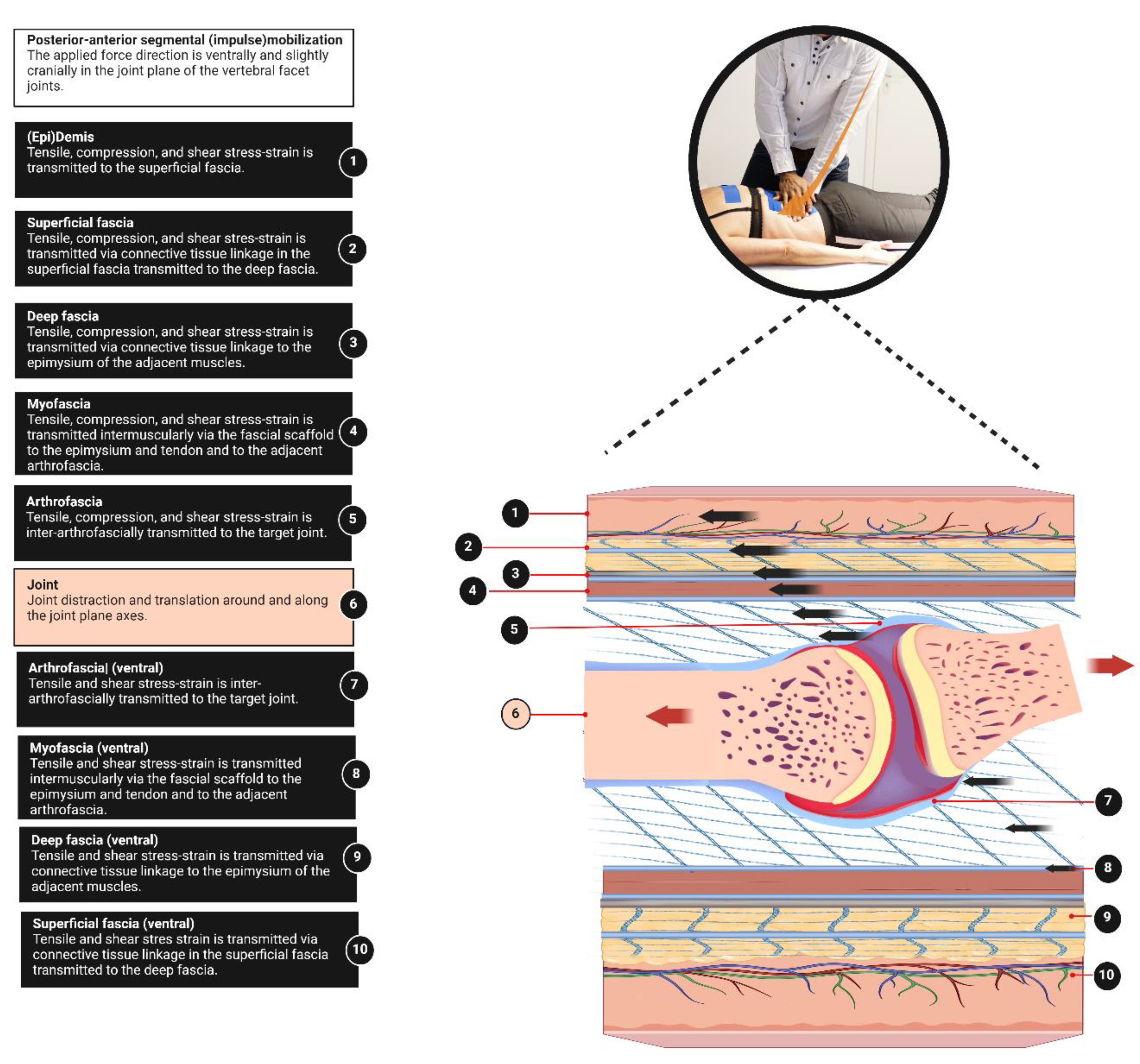
This perspective provides a basic anatomical model for clinicians, to better understand fascia anatomy to complete the muscle-bone concept. An integral aspect of the ArthroMyoFascial complex is the recognition of a force-transmitting pathway between anatomical structures within this complex. In the ArthroMyoFascial complex, the force-transmitting pathway refers to the continuous network of connective tissue linkages from superficial to deeper layers and vice versa. However, it should be acknowledged that our model does not fully explain the longitudinal intermuscular force-transmitting pathways. These pathways require other anatomical explanatory models, such as the myofascial trains [
31,
45]. It's worth noting that these myofascial models typically do not encompass the skin, superficial fascia, and arthrofascia, which are covered in our ArthroMyoFascial model. This implies that passive stiffness surrounding a myofascial unit may arise not only by its myofascia but as well from more superficially located tissues such as the superficial fascia and deep fascia, as well as from deeper structures like the arthrofascia of joints. Additionally, understanding this concept may assist in elucidating the mechanism of force transmission from the skin to the bone and vice versa. For instance, it can explain how displacement of the skin results in the transmission of stress to anatomical structures beneath the skin (
Figure 4). This might be a key effect of various manual therapeutic practices, such as posterior-anterior joint mobilizations/manipulations, myofascial release techniques, and elastic tape application, where force is transmitted from the skin to the superficial fascia, deep fascia, myofascia, and the arthrofascia (
Figure 4). Furthermore, it may contribute to the clinical understanding of the interaction between joints, myofascia, and deep fascia. For instance, during secondary hip traction, the applied force stresses the arthrofascia but as well in less amount the deep fascia and myofascia [
13,
46]. It is significant that changes in relative stiffness will alter the stress transmitting pathways between fascia, muscles, and joints [
13]. The ArthroMyoFascial complex can enhance clinical understanding by highlighting how increased epi and extramuscular connective tissues may disrupt the force generated by the myofascial unit. In addition, it suggests that stiffness in the superficial fascia could hinder access to deeper layers of myofascia and/or arthrofascia during joint mobilizations or manipulations. For example, softening the superficially stiffened tissues through myofascial release may be crucial for achieving the desired therapeutic effects of other fascial tissue manipulations, such as elastic tape application and joint mobilizations/manipulations. However, we want to emphasize, that this is an anatomical model specifically designed for clinical therapists, and while it doesn't represent the complete anatomical truth, it provides a useful framework for understanding and approaching complex mechanical processes.
7. Conclusions
The ArthroMyofascial complex consists of multiple layers from superficial to deeper anatomical structures, namely the skin, superficial fascia, deep fascia, myofascia including muscle fibers, and arthrofascia, all linked within a connective tissue matrix. This model indicates that it is a force-transmitting system from the skin to the bone. This information is essential for manual therapists, including physiotherapists, osteopaths, chiropractors, and massage therapists, as they all work with fasciae within the musculoskeletal domain. Adopting this basic approach enables clinicians to effectively address various body tissues, enhancing the clinical understanding of fascia within the muscle-bone concept. This is essential for therapeutic testing, treatment, reporting, and multidisciplinary communication, which is important for comprehending musculoskeletal and orthopedic rehabilitation.
Author Contributions
Both authors: Karl Noten and Robbert van Amstel contributed equally in all aspects of conceptualization; methodology; software; validation; formal analysis; investigation; resources; data curation; writing—original draft preparation; writing—review and editing; visualization; project administration. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent has been obtained from the two individuals.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like the thank the colleagues from the Vrije Universiteit Amsterdam, FGB FysioLab and Fysio Physics, department FysioScience for the assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swart, N.M.; Apeldoorn, A.T.; Conijn, D.; Meerhoff, G.A.; Ostelo, R. KNGF Clinical Practice Guideline for Low back pain and lumbosacral radicular syndrome. KNGF-richtlijn, 2021. [Google Scholar]
- Apeldoorn, A.T.; Swart, N.M.; Conijn, D.; Meerhoff, G.A.; Ostelo, R.W. Management of low back pain and lumbosacral radicular syndrome: the Guideline of the Royal Dutch Society for Physical Therapy (KNGF). European Journal of Physical and Rehabilitation Medicine 2024. [Google Scholar] [CrossRef]
- Stecco, C.; Day, J.A. The fascial manipulation technique and its biomechanical model: a guide to the human fascial system. International journal of therapeutic massage & bodywork 2010, 3, 38. [Google Scholar]
- Starich, M. Rolfing® Structural Integration. Integrative Geriatric Medicine 2017, 101. [Google Scholar]
- Kase, K. Clinical therapeutic applications of the Kinesio (! R) taping method, 3rd ed.; Ken Ikai Co., Ltd: Tokyo, Japan, 2008; Volume 3rd Edition. [Google Scholar]
- Simmonds, N.; Miller, P.; Gemmell, H. A theoretical framework for the role of fascia in manual therapy. Journal of bodywork and movement therapies 2012, 16, 83–93. [Google Scholar] [CrossRef]
- van Amstel, R.; Noten, K.; Malone, S.; Vaes, P. Fascia Tissue Manipulations in Chronic Low Back Pain: A Pragmatic Comparative Randomized Clinical Trial of the 4xT Method® and Exercise Therapy. Life 2023, 14, 7. [Google Scholar] [CrossRef]
- van Amstel, R.N.; Jaspers, R.T.; Pool-Goudzwaard, A.L. Skin Displacement as fascia tissue manipulation at the lower back affects instantaneously the flexion-and extension spine, pelvis, and hip range of motion. Frontiers in Physiology 2022, 13, 01–15. [Google Scholar] [CrossRef]
- Schleip, R.; Jäger, H.; Klingler, W. What is ‘fascia’? A review of different nomenclatures. Journal of bodywork and movement therapies 2012, 16, 496–502. [Google Scholar] [CrossRef]
- Schleip, R.; Hedley, G.; Yucesoy, C.A. Fascial nomenclature: Update on related consensus process. Clinical Anatomy 2019, 32, 929–933. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between muscle and bone—where physics meets biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef]
- Avin, K.G.; Bloomfield, S.A.; Gross, T.S.; Warden, S.J. Biomechanical aspects of the muscle-bone interaction. Current osteoporosis reports 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Maas, H. Significance of epimuscular myofascial force transmission under passive muscle conditions. Journal of Applied Physiology 2019, 126, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Stecco, L. Manipolazione della fascia: per il trattamento delle affezioni muscoloscheletriche; Piccin: 2002.
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. International Journal of Molecular Sciences 2023, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Huijing, P.A. Epimuscular myofascial force transmission: a historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei, 2007. Journal of biomechanics 2009, 42, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Adstrum, S.; Hedley, G.; Schleip, R.; Stecco, C.; Yucesoy, C.A. Defining the fascial system. Journal of bodywork and movement therapies 2017, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia is able to actively contract and may thereby influence musculoskeletal dynamics: a histochemical and mechanographic investigation. Frontiers in physiology 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Fede, C.; Macchi, V.; Porzionato, A.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial gliding regulation. Clinical Anatomy 2018, 31, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Carneiro, J. Basic histology: text & atlas; McGraw-Hill Professional: 2005.
- Ferreira, L.M.; Hochman, B.; Locali, R.F.; Rosa-Oliveira, L.M. A stratigraphic approach to the superficial musculoaponeurotic system and its anatomic correlation with the superficial fascia. Aesthetic plastic surgery 2006, 30, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Clarys, J.; Provyn, S.; Marfell-Jones, M. Cadaver studies and their impact on the understanding of human adiposity. Ergonomics 2005, 48, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Herlin, C.; Chica-Rosa, A.; Subsol, G.; Gilles, B.; Macri, F.; Beregi, J.P.; Captier, G. Three-dimensional study of the skin/subcutaneous complex using in vivo whole body 3T MRI: review of the literature and confirmation of a generic pattern of organization. Surgical and radiologic anatomy 2015, 37, 731–741. [Google Scholar] [CrossRef]
- Hedley, G. Interstitium? Perifascia, aka the fuzz! (video for 2018 Fascia Congress, Berlin). Available online: https://www.gilhedley.com/clips (accessed on.
- Fede, C.; Petrelli, L.; Pirri, C.; Neuhuber, W.; Tiengo, C.; Biz, C.; De Caro, R.; Schleip, R.; Stecco, C. Innervation of human superficial fascia. Frontiers in Neuroanatomy 2022, 73. [Google Scholar] [CrossRef]
- Stecco, C.; Porzionato, A.; Lancerotto, L.; Stecco, A.; Macchi, V.; Day, J.A.; De Caro, R. Histological study of the deep fasciae of the limbs. Journal of bodywork and movement therapies 2008, 12, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Gagey, O.; Belloni, A.; Pozzuoli, A.; Porzionato, A.; Macchi, V.; Aldegheri, R.; De Caro, R.; Delmas, V. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie 2007, 91, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Vleeming, A.; Schuenke, M.; Danneels, L.; Willard, F. The functional coupling of the deep abdominal and paraspinal muscles: the effects of simulated paraspinal muscle contraction on force transfer to the middle and posterior layer of the thoracolumbar fascia. Journal of anatomy 2014, 225, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M. The fascia of the limbs and back–a review. Journal of anatomy 2009, 214, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.; Vleeming, A.; Schuenke, M.; Danneels, L.; Schleip, R. The thoracolumbar fascia: anatomy, function and clinical considerations. Journal of anatomy 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Krause, F.; Wilke, J.; Vogt, L.; Banzer, W. Intermuscular force transmission along myofascial chains: a systematic review. Journal of anatomy 2016, 228, 910–918. [Google Scholar] [CrossRef]
- Schuenke, M.; Vleeming, A.; Van Hoof, T.; Willard, F. A description of the lumbar interfascial triangle and its relation with the lateral raphe: anatomical constituents of load transfer through the lateral margin of the thoracolumbar fascia. Journal of anatomy 2012, 221, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N.; Macintosh, J.E. The applied anatomy of the thoracolumbar fascia. Spine 1984, 9, 164–170. [Google Scholar] [CrossRef]
- Fede, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Pirri, C.; Fan, C.; De Caro, R.; Stecco, C. Evidence of a new hidden neural network into deep fasciae. Scientific Reports 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Purslow, P.P. The structure and role of intramuscular connective tissue in muscle function. Frontiers in physiology 2020, 11, 495. [Google Scholar] [CrossRef]
- Sensini, A.; Massafra, G.; Gotti, C.; Zucchelli, A.; Cristofolini, L. Tissue engineering for the insertions of tendons and ligaments: an overview of electrospun biomaterials and structures. Frontiers in bioengineering and biotechnology 2021, 9, 645544. [Google Scholar] [CrossRef] [PubMed]
- Huijing, P. Intramuscular myofascial force transmission. In Encyclopedia of Neuroscience; Springer Verlag: 2008.
- James, G.; Stecco, C.; Blomster, L.; Hall, L.; Schmid, A.B.; Shu, C.C.; Little, C.B.; Melrose, J.; Hodges, P.W. Muscle spindles of the multifidus muscle undergo structural change after intervertebral disc degeneration. European Spine Journal 2022, 31, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Noten, K.; Amstel, R.N.v. Introducing the Arthro-myofascial complex. (4xT®Method the ArthroMyofascial Therapy: Chapter 3)1. Fascia more than myofascia! Available online: https://osf.io/d85k3/wiki/ArthroMyofascial%20Complex/ (accessed on.
- Widmer, J.; Cornaz, F.; Scheibler, G.; Spirig, J.M.; Snedeker, J.G.; Farshad, M. Biomechanical contribution of spinal structures to stability of the lumbar spine—novel biomechanical insights. The Spine Journal 2020, 20, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.D.; Savage, A.; Braly, B.A.; Palmer, I.J.; Beall, D.P.; Kelly, B. The function of the hip capsular ligaments: a quantitative report. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2008, 24, 188–195. [Google Scholar]
- Bejarano-Pineda, L.; Guss, D.; Waryasz, G.; DiGiovanni, C.W.; Kwon, J.Y. The Syndesmosis, Part I: Anatomy, Injury Mechanism, Classification, and Diagnosis. Orthopedic Clinics 2021, 52, 403–415. [Google Scholar] [PubMed]
- Bartoníček, J.; Rammelt, S.; Naňka, O. Anatomy of the subtalar joint. Foot and ankle clinics 2018, 23, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Petermann, H.; Sander, M. Histological evidence for muscle insertion in extant amniote femora: implications for muscle reconstruction in fossils. Journal of Anatomy 2013, 222, 419–436. [Google Scholar] [CrossRef]
- Myers, T.W. Anatomy trains e-book: myofascial meridians for manual and movement therapists; Elsevier Health Sciences: 2013.
- Estébanez-de-Miguel, E.; López-de-Celis, C.; Caudevilla-Polo, S.; González-Rueda, V.; Bueno-Gracia, E.; Pérez-Bellmunt, A. The effect of high, medium and low mobilization forces applied during a hip long-axis distraction mobilization on the strain on the inferior ilio-femoral ligament and psoas muscle: A cadaveric study. Musculoskeletal Science and Practice 2020, 47, 102148. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).