Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Lücking, R. , “Fungal Diversity Revisited: 2.2 to 3.8 Million Species,” Microbiol Spectr, vol. 5, no. 4, 2017. [CrossRef]
- Burki, T. , “WHO publish fungal priority pathogens list,” Lancet Microbe, vol. 4, no. 2, 2023. [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. , “Global and multi-national prevalence of fungal diseases—estimate precision,” Journal of Fungi, vol. 3, no. 4. 2017. [CrossRef]
- Firacative, C. , “Invasive fungal disease in humans: Are we aware of the real impact?,” Mem Inst Oswaldo Cruz, vol. 115, no. 9, 2020. [CrossRef]
- Turner, J.H.; Soudry, E.; Nayak, J.V.; Hwang, P.H. , “Survival outcomes in acute invasive fungal sinusitis: A systematic review and quantitative synthesis of published evidence,” Laryngoscope, vol. 123, no. 5, 2013. [CrossRef]
- Roland, L.T. et al., “Diagnosis, Prognosticators, and Management of Acute Invasive Fungal Rhinosinusitis: Multidisciplinary Consensus Statement and Evidence-Based Review with Recommendations,” Int Forum Allergy Rhinol, 2023. [CrossRef]
- “Mycosis of the eye and its adnexa.,” Developments in ophthalmology, vol. 32. 1999.
- Klotz, S.A.; Penn, C.C.; Negvesky, G.J.; Butrus, S.I. , “Fungal and Parasitic Infections of the Eye,” Clin Microbiol Rev, vol. 13, no. 4, 2000. [CrossRef]
- Thomas, P.A. , “Current Perspectives on Ophthalmic Mycoses,” Clinical Microbiology Reviews, vol. 16, no. 4. 2003. [CrossRef]
- Coskuncan, N.M. et al., “The Eye in Bone Marrow Transplantation: VI. Retinal Complications,” Archives of Ophthalmology, vol. 112, no. 3, 1994. [CrossRef]
- Diamond, R.D. , “The growing problem of mycoses in patients infected with the human immunodeficiency virus,” Reviews of Infectious Diseases, vol. 13, no. 3. 1991. [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. , “Zygomycetes in human disease,” Clinical Microbiology Reviews, vol. 13, no. 2. 2000. [CrossRef]
- STRAATSMA, B.R.; ZIMMERMAN, L.E.; GASS, J.D. , “Phycomycosis. A clinicopathologic study of fifty-one cases.,” Lab Invest, vol. 11, 1962.
- Gass, J.D.M. , “Ocular Manifestations of Acute Mucormycosis,” Archives of Ophthalmology, vol. 65, no. 2, 1961. [CrossRef]
- Baum, J.L. , “Rhino-orbital mucormycosis. Occurring in an otherwise apparently healthy individual,” Am J Ophthalmol, vol. 63, no. 2, 1967. [CrossRef]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. , “Survival factors in rhino-orbital-cerebral mucormycosis,” Survey of Ophthalmology, vol. 39, no. 1. 1994. [CrossRef]
- Daly, A.L.; Velazquez, L.A.; Bradley, S.F.; Kauffman, C.A. , “Mucormycosis: association with deferoxamine therapy,” Am J Med, vol. 87, no. 4, 1989. [CrossRef]
- Morriss, F.H.; Spock, A. , “Intracranial Aneurysm Secondary to Mycotic Orbital and Sinus Infection: Report of a Case Implicating Penicillium as an Opportunistic Fungus,” American Journal of Diseases of Children, vol. 119, no. 4, 1970. [CrossRef]
- Hale, L.M. , “Orbital-Cerebral Phycomycosis: Report of a Case and a Review of the Disease in Infants,” Archives of Ophthalmology, vol. 86, no. 1, 1971. [CrossRef]
- Hedges, T.R.; Leung, L.S.E. , “Parasellar and orbital apex syndrome caused by aspergillosis,” Neurology, vol. 26, no. 2, 1976. [CrossRef]
- Sponsler, T.A.; Sassani, J.W.; Johnson, L.N.; Towfighi, J. , “Ocular invasion in mucormycosis,” Survey of Ophthalmology, vol. 36, no. 5. 1992. [CrossRef]
- Schwartz, J.N.; Donnelly, E.H.; Klintworth, G.K. , “Ocular and orbital phycomycosis,” Survey of Ophthalmology, vol. 22, no. 1. 1977. [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. , “Epidemiology and diagnosis of mucormycosis: An update,” Journal of Fungi, vol. 6, no. 4. 2020. [CrossRef]
- Parody, R.; Martino, R.; Sánchez, F.; Subirá, M.; Hidalgo, A.; Sierra, J. , “Predicting survival in adults with invasive aspergillosis during therapy for hematological malignancies or after hematopoietic stem cell transplantation: Single-center analysis and validation of the Seattle, French, and Strasbourg prognostic indexes,” Am J Hematol, vol. 84, no. 9, 2009. [CrossRef]
- Benedict, K.; Thompson, G.R.; Jackson, B.R. , “Cannabis use and fungal infections in a commercially insured population, United States, 2016,” Emerg Infect Dis, vol. 26, no. 6, 2020. [CrossRef]
- Hartnett, K.P. et al., “Bacterial and Fungal Infections in Persons Who Inject Drugs — Western New York, 2017,” MMWR Morb Mortal Wkly Rep, vol. 68, no. 26, 2019. [CrossRef]
- Hamilton, J.D.; Lai, S.Y.; Ginsberg, L.E. , “Superimposed infection in mandibular osteoradionecrosis: Diagnosis and outcomes,” J Comput Assist Tomogr, vol. 36, no. 6, 2012. [CrossRef]
- Trang, P.T.L.; Thi, D.D.; Son, N.T. , “Clinical symptoms, endoscopic imaging and stroboscopic imaging in patients with laryngeal fungal infection : An evaluation in 48 patients,” Biomedical Research and Therapy, vol. 7, no. 9, 2020. [CrossRef]
- Telles, D.R.; Karki, N.; Marshall, M.W. , “Oral Fungal Infections: Diagnosis and Management,” Dental Clinics of North America, vol. 61, no. 2. 2017. [CrossRef]
- Bojanović, M. et al., “Etiology, Predisposing Factors, Clinical Features and Diagnostic Procedure of Otomycosis: A Literature Review,” Journal of Fungi, vol. 9, no. 6. 2023. [CrossRef]
- Cornely, O.A. et al., “Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium,” The Lancet Infectious Diseases, vol. 19, no. 12. 2019. [CrossRef]
- Skiada, A. et al., “Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007,” Clinical Microbiology and Infection, vol. 17, no. 12, 2011. [CrossRef]
- Patterson, T.F. et al., “Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America,” Clinical Infectious Diseases, vol. 63, no. 4. 2016. [CrossRef]
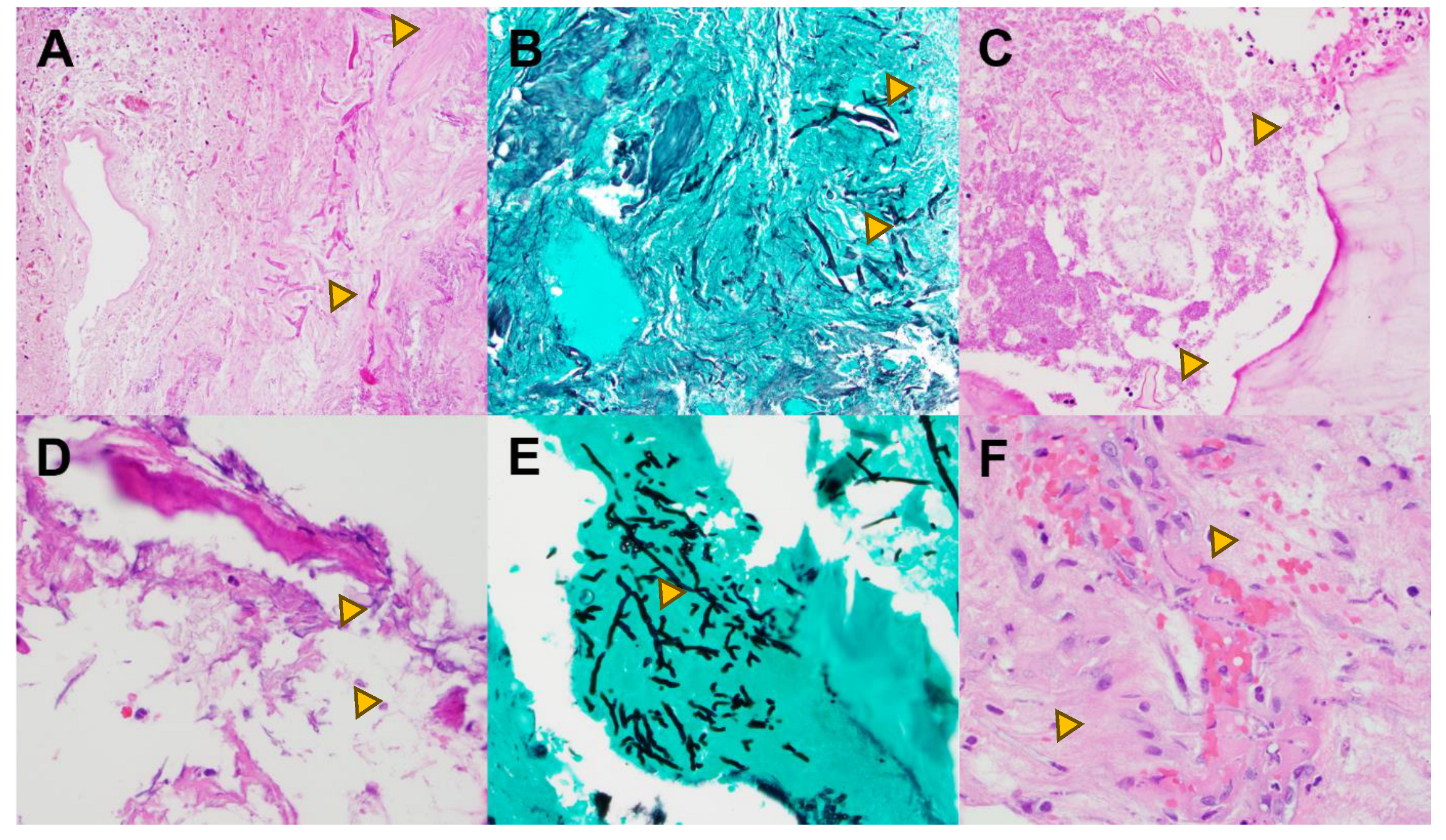
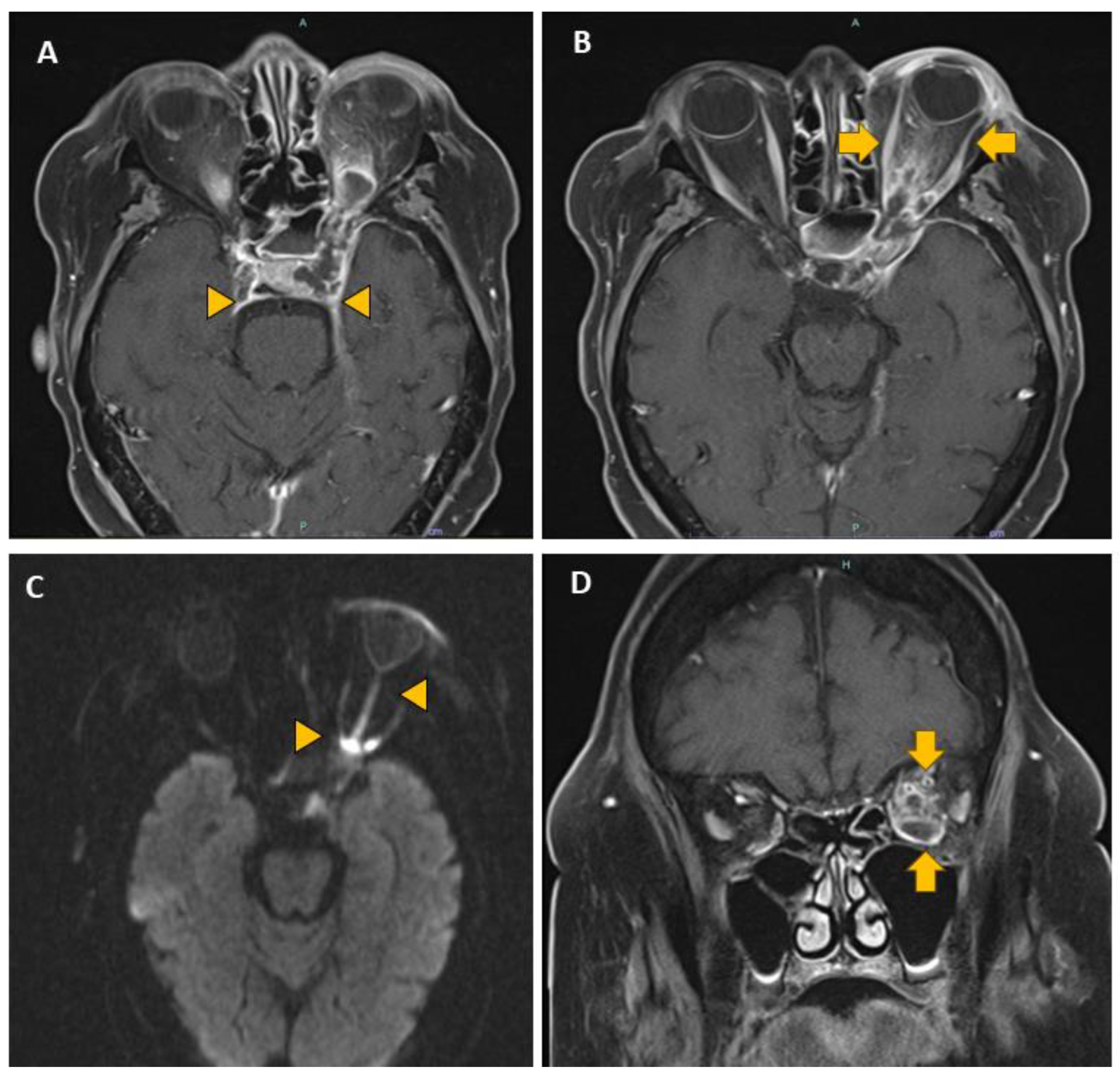
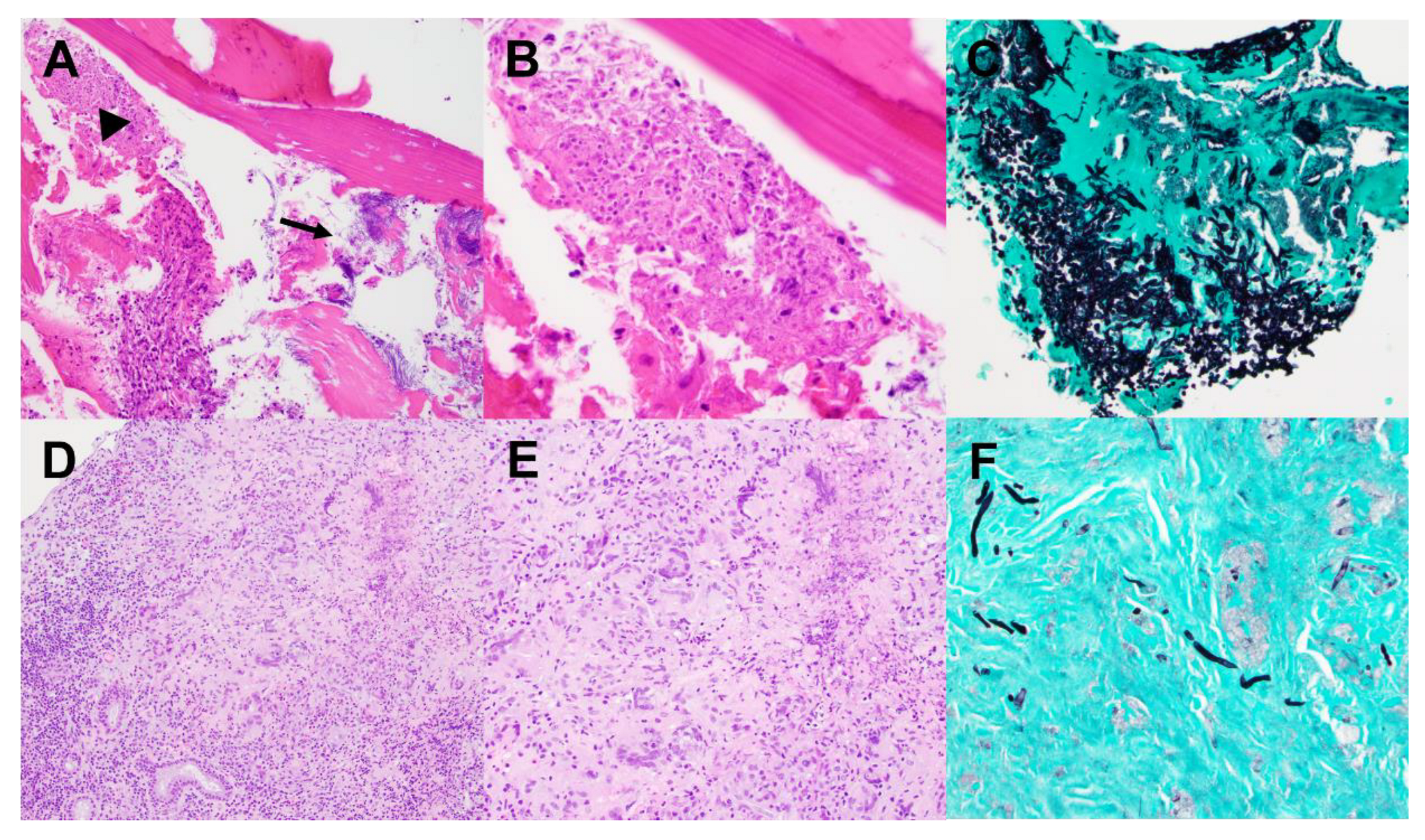
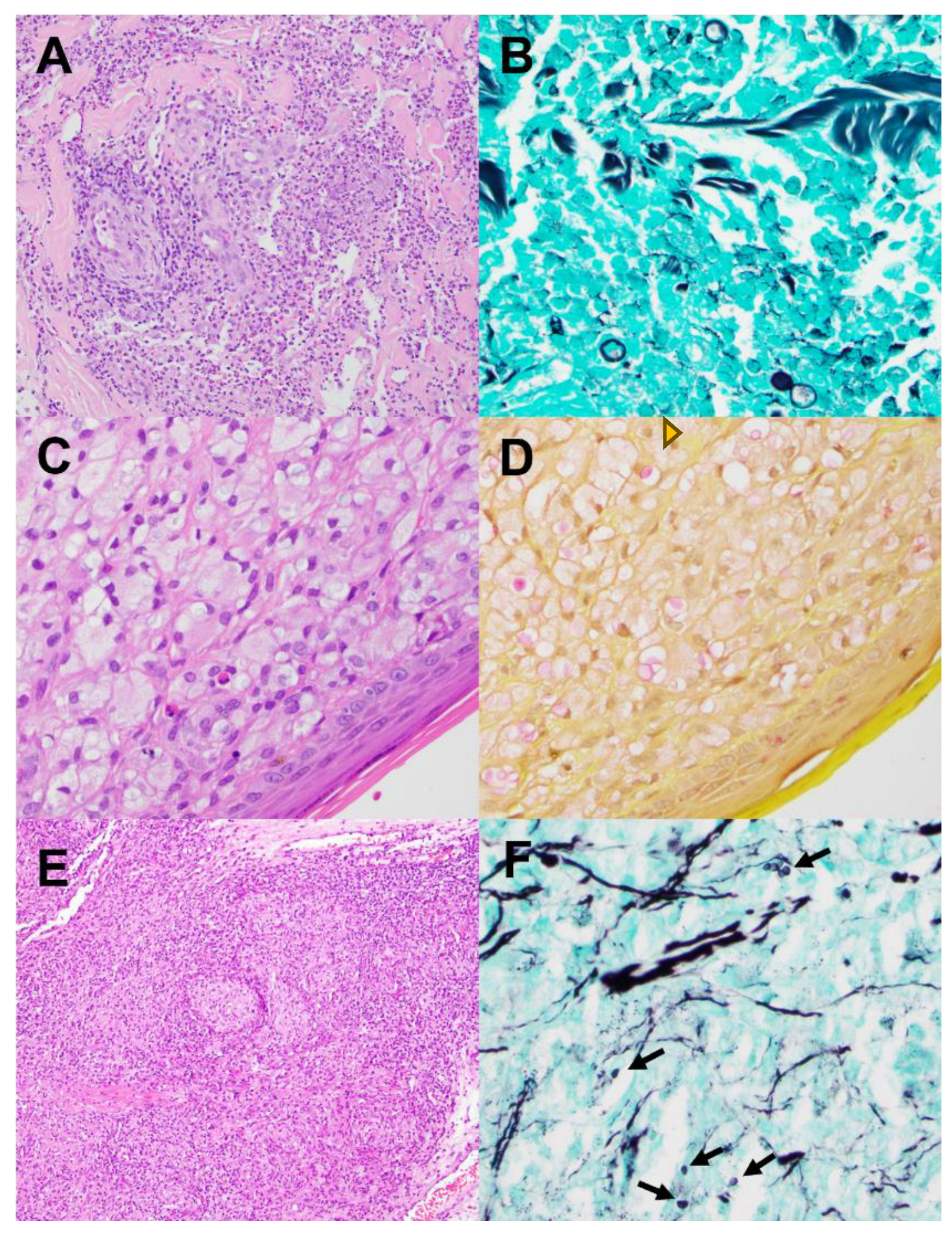
| Invasive (Inv) | Non-invasive (Non-inv) | Ratio Non-Inv : Inv | |||
|---|---|---|---|---|---|
| Site | n | % | n | % | |
| Sinonasal | 45 | 58.4 | 203 | 60.4 | 4.5 |
| Ear/mastoid | 3 | 3.9 | 7 | 2.1 | 2.3 |
| Larynx | 2 | 2.6 | 10 | 3.0 | 5 |
| Oral cavity | 3 | 3.9 | 86 | 25.6 | 28.3 |
| Oropharynx/esophagus | 0 | 0 | 30 | 8.9 | >30 |
| Base of skull | 2 | 2.6 | - | - | - |
| Orbit | 14 | 18.2 | - | - | - |
| Mandible | 8 | 10.4 | - | - | - |
| Morphology | Genus/species | n (%) | N (%) |
|---|---|---|---|
| Mucorales | Mucor sp. Rhizopus sp. Rhizomucor sp. Sincephalastrum racemosum |
18 (64) 5 (18) 4 (14) 1 (4) |
28 (51) |
| Hyaline molds | Aspergillus (various species) Fusarium sp. Scedosporium sp. |
14 (88) 1 (6) 1 (6) |
16 (29) |
| Candida | C. albicans C. parasilopsis C. glabrata C. dubliniensis C. sp. |
2 (33) 1 (17) 1 (17) 1 (17) 1 (17) |
6 (11) |
| Dematiaceous | Curvularia Dematiaceous sp. |
1 (50) 1 (50) |
2 (4) |
| Others | Blastomyces dermatitides Kluyveromyces marxianus Mycelia sterilia |
1 (33) 1 (33) 1 (33) |
3 (6) |
| Extension | n | % |
|---|---|---|
| Sinonasal only | 12 | 27 |
| Beyond sinonasal | 33 | 73 |
| Extrasinonasal extension | ||
| Orbit | 19 | 42 |
| Intracranial | 5 | 11 |
| Base of skull | 5 | 11 |
| Facial soft tissue | 3 | 7 |
| Palate | 3 | 7 |
| Nasopharynx | 2 | 4 |
| Oropharynx | 1 | 2 |
| Risk Factor | Subgroup n (%) | n (%) | Dead n (%) |
All Risk Factors | n (%) | Dead n (%) |
|---|---|---|---|---|---|---|
| Malignancy | Hematolymphoid 19 (83) Somatic 4 (17) |
23 (51) | 19 (83) | Malignancy only Malign. + transplant Malignancy + DM Malignancy + Drugs Malign. + transpl.+ DM Transplant only Transplant + DM Transplant + Drugs DM only DM + Drugs Drugs only |
12 (27) 8 (18) 1 (2) 0 2 (4) 1 (2) 2 (4) 0 11 (24) 2 (4) 3 (7) |
9 (75) 7 (87.5) 1 (100) 0 2 (100) 1 (100) 1 (50) 0 3 (27.3) 1 (50) 0 |
| Transplant | Bone marrow 9 (69) Pancreas & kidney 1 (8) Pancreas 1 (8) Kidney 1 (8) Liver 1 (8) |
13 (29) | 11 (85) | |||
| Diabetes mellitus (DM) | Type 1 5 (28) Type 2 13 (72) |
18 (40) | 7 (39) | |||
| Illicit Drug | Marijuana 2 (40) Methamphetamines 3 (60) Opioids* 3 (60) |
5 (11) | 1 (20) | |||
| None | - | 3 (7) | 0 |
| Risk Factor | Pearson Chi2 test (2-sided) |
Fisher’s exact test (2-sided) |
Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| Malignancy | < 0.001*** | < 0.001*** | 16.150 | 3.718 - 70.142* |
| Transplant | 0.007** | 0.009** | 8.038 | 1.523 - 42.430* |
| Diabetes mellitus | 0.113 | 0.138 | 0.374 | 0.110 - 1.278 |
| Illicit Drug | 0.113 | 0.169 | 0.185 | 0.019 - 1.805 |
| Therapy | n | % |
|---|---|---|
| Surgery | 40 | 89 |
| Multiple surgeries | 15 | 33 |
| Biopsy only | 5 | 11 |
| No antifungals | 3 | 7 |
| Amphotericin B | 27 | 60 |
| Triazoles | 35 | 78 |
| Voriconazole (40%) | 14 | 31 |
| Itraconazole (3%) | 1 | 2 |
| Posaconazole (26%) | 9 | 20 |
| Isavuconazonium (26%) | 9 | 20 |
| Fluconazole (6%) | 2 | 4 |
| Micafungin | 3 | 7 |
| Terbinafine | 2 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





