Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
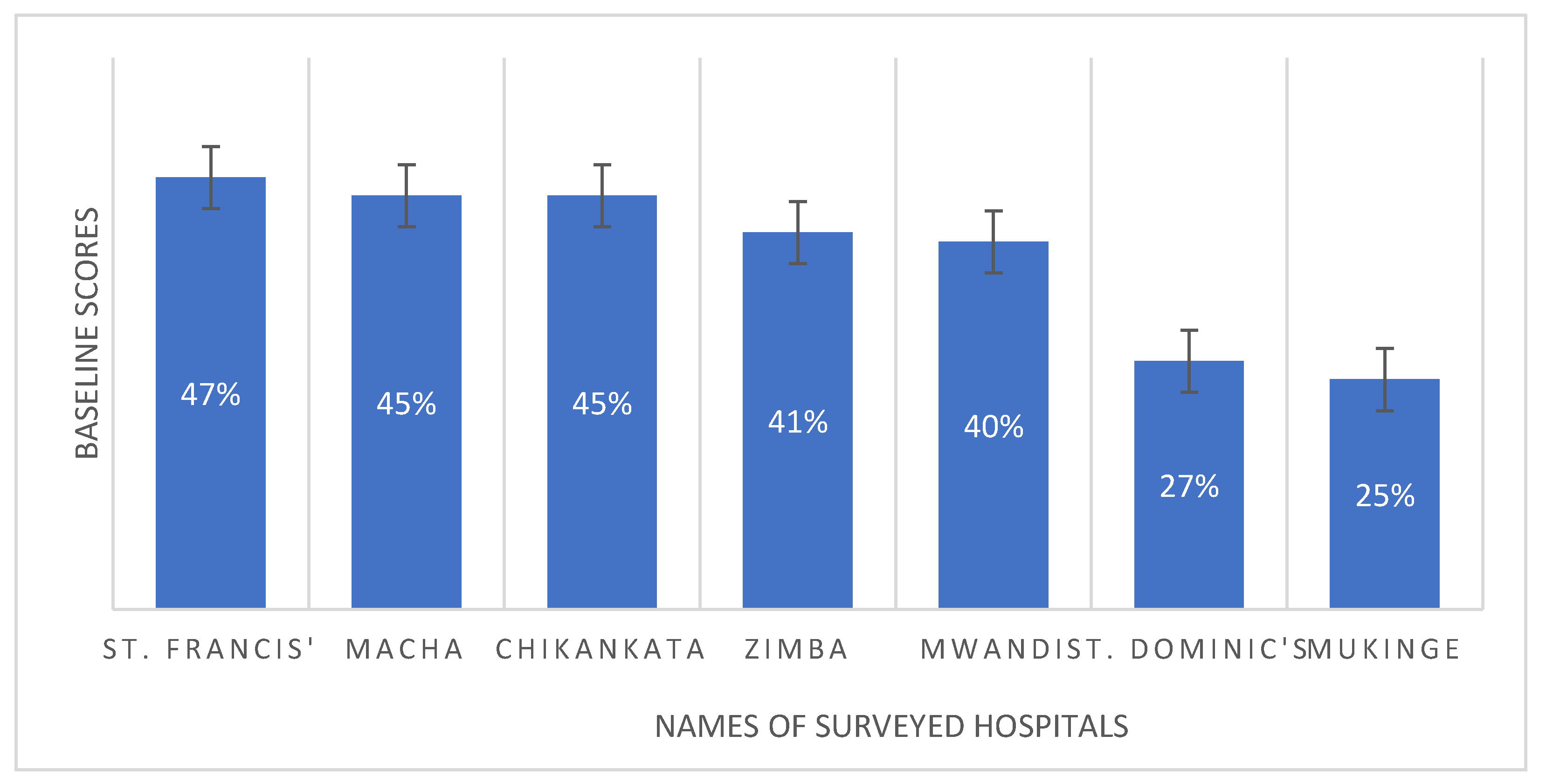
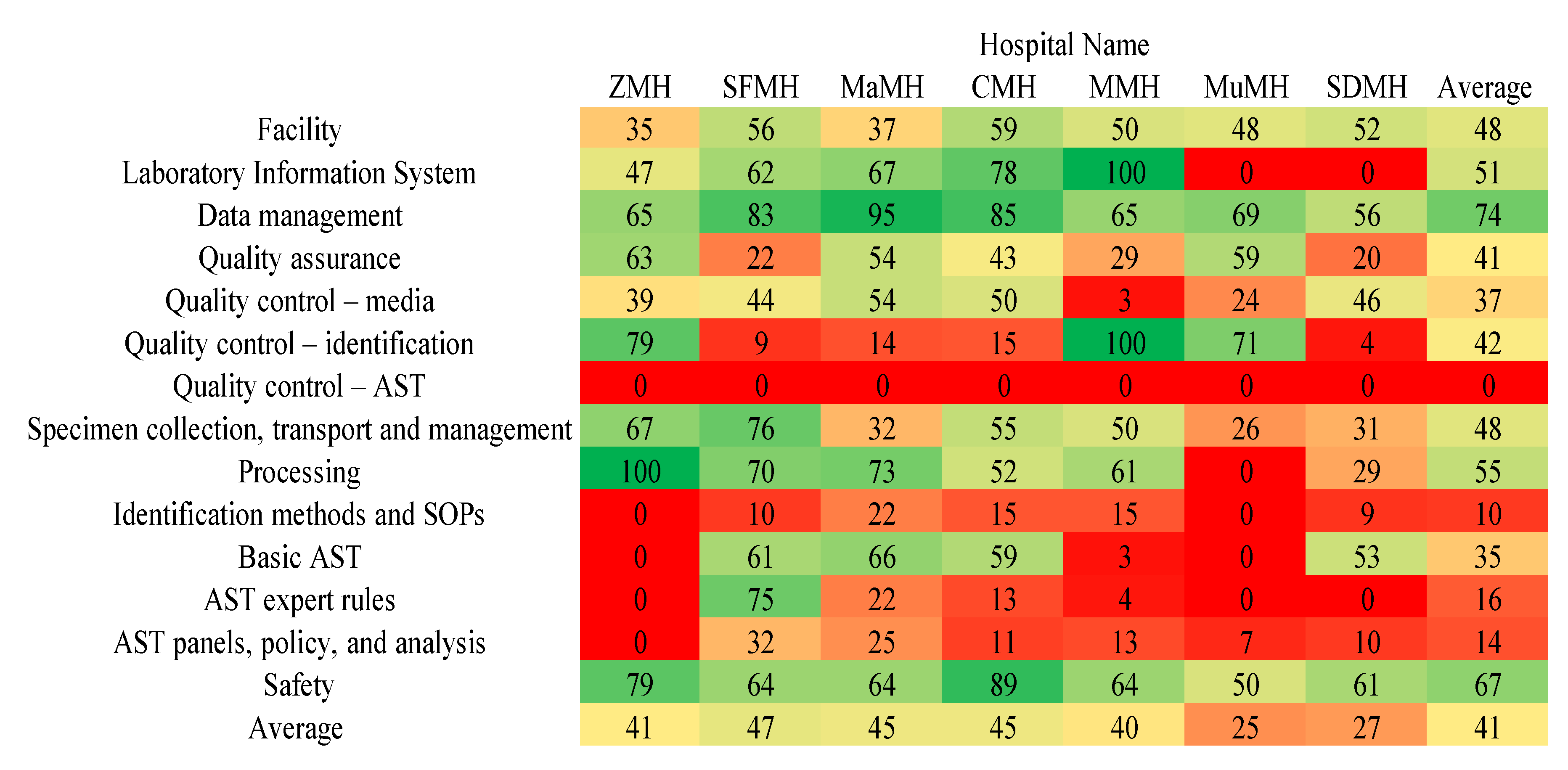
2. Results
3. Discussion
4. Materials and Methods
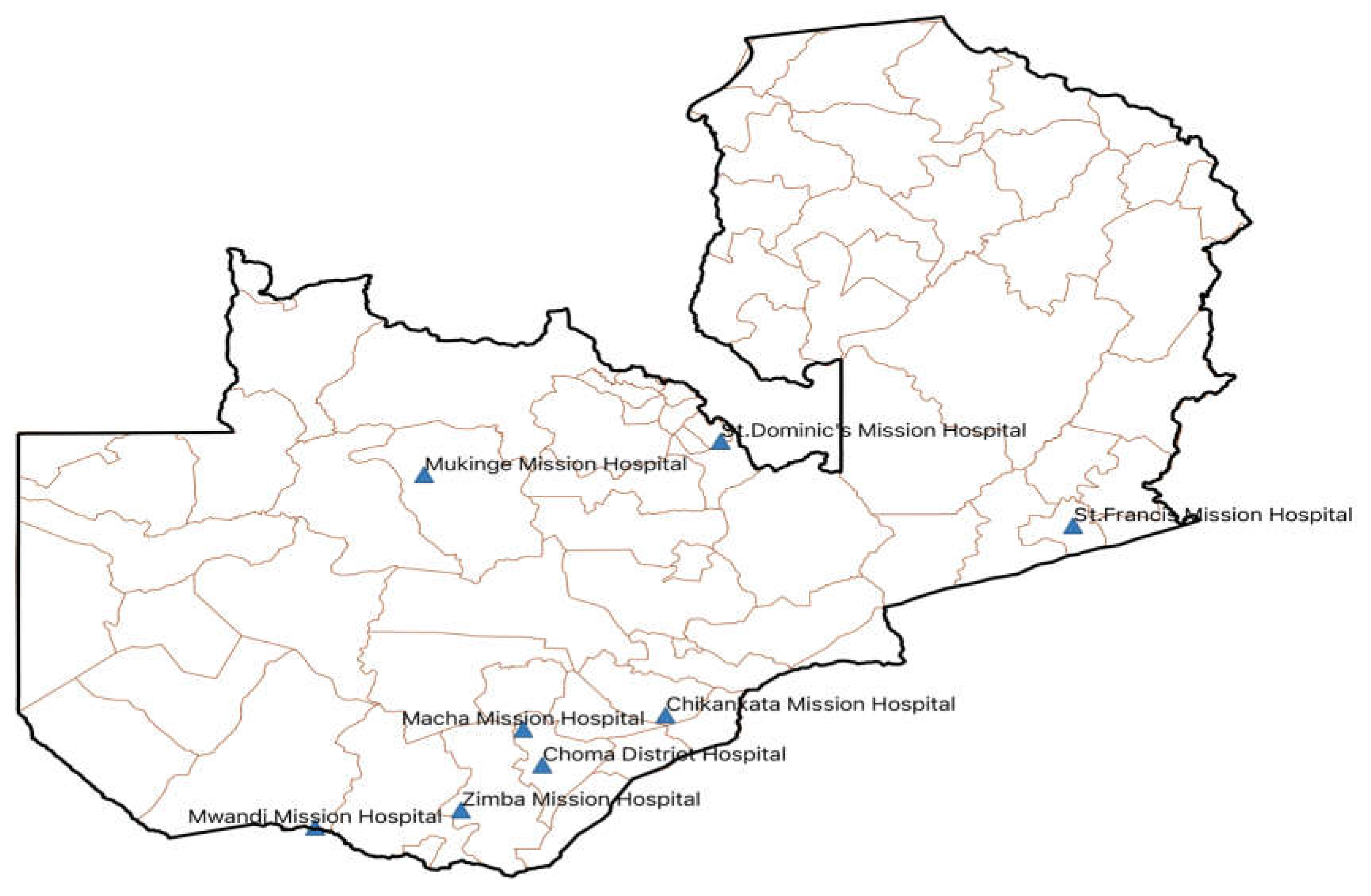
4.1. Study Design, Period, and Setting
4.2. Sample Size and Sampling Technique
4.3. Data Collection
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387.
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and Economic Impact of Antibiotic Resistance in Developing Countries: A Systematic Review and Meta-Analysis. PLoS One 2017, 12, e0189621. [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309. [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [CrossRef]
- Beyene, A.M.; Andualem, T.; Dagnaw, G.G.; Getahun, M.; LeJeune, J.; Ferreira, J.P. Situational Analysis of Antimicrobial Resistance, Laboratory Capacities, Surveillance Systems and Containment Activities in Ethiopia: A New and One Health Approach. One Heal. 2023, 16, 100527. [CrossRef]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the Economic Cost of Antimicrobial Resistance per Antibiotic Consumed to Inform the Evaluation of Interventions Affecting Their Use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [CrossRef]
- Aa, J.; Ab, U.; Cd, U. Upaganlawar AB, et Al. Impact of Antimicrobial Resistance in Health and Economic Outcomes: A Review. Adv. Pharmacol. Clin. Trials 2024, 9, 000234. [CrossRef]
- Matee, M.; Mshana, S.E.; Mtebe, M.; Komba, E.V.; Moremi, N.; Lutamwa, J.; Kapona, O.; Sekamatte, M.; Mboera, L.E.G. Mapping and Gap Analysis on Antimicrobial Resistance Surveillance Systems in Kenya, Tanzania, Uganda and Zambia. Bull. Natl. Res. Cent. 2023, 47, 12. [CrossRef]
- Trollip, A.; Gadde, R.; Datema, T.; Gatwechi, K.; Oskam, L.; Katz, Z.; Whitelaw, A.; Kinyanjui, P.; Njukeng, P.; Wendifraw, D.A.; et al. Implementation of a Customised Antimicrobial Resistance Laboratory Scorecard in Cameroon Ethiopia and Kenya. Afr. J. Lab. Med. 2022, 11, 1476. [CrossRef]
- Sartorius, B.; Gray, A.P.; Davis Weaver, N.; Robles Aguilar, G.; Swetschinski, L.R.; Ikuta, K.S.; Mestrovic, T.; Chung, E.; Wool, E.E.; Han, C.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO African Region in 2019: A Cross-Country Systematic Analysis. Lancet Glob. Heal. 2024, 12, e201–e216. [CrossRef]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [CrossRef]
- Malania, L.; Wagenaar, I.; Karatuna, O.; Tambic Andrasevic, A.; Tsereteli, D.; Baidauri, M.; Imnadze, P.; Nahrgang, S.; Ruesen, C. Setting up Laboratory-Based Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Lessons Learned from Georgia. Clin. Microbiol. Infect. 2021, 27, 1409–1413.
- Ombelet, S.; Ronat, J.B.; Walsh, T.; Yansouni, C.P.; Cox, J.; Vlieghe, E.; Martiny, D.; Semret, M.; Vandenberg, O.; Jacobs, J.; et al. Clinical Bacteriology in Low-Resource Settings: Today’s Solutions. Lancet Infect. Dis. 2018, 18, e248–e258. [CrossRef]
- Mathew, P.; Ranjalkar, J.; Chandy, S.J. Challenges in Implementing Antimicrobial Stewardship Programmes at Secondary Level Hospitals in India: An Exploratory Study. Front. Public Heal. 2020, 8, 493904. [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10. [CrossRef]
- Barbé, B.; Yansouni, C.P.; Affolabi, D.; Jacobs, J. Implementation of Quality Management for Clinical Bacteriology in Low-Resource Settings. Clin. Microbiol. Infect. 2017, 23, 426–433. [CrossRef]
- Dakorah, M.P.; Agyare, E.; Acolatse, J.E.E.; Akafity, G.; Stelling, J.; Chalker, V.J.; Spiller, O.B.; Aidoo, N.B.; Kumi-Ansah, F.; Azumah, D.; et al. Utilising Cumulative Antibiogram Data to Enhance Antibiotic Stewardship Capacity in the Cape Coast Teaching Hospital, Ghana. Antimicrob. Resist. Infect. Control 2022, 11, 122. [CrossRef]
- Tornimbene, B.; Eremin, S.; Abednego, R.; Abualas, E.O.; Boutiba, I.; Egwuenu, A.; Fuller, W.; Gahimbare, L.; Githii, S.; Kasambara, W.; et al. Global Antimicrobial Resistance and Use Surveillance System on the African Continent: Early Implementation 2017–2019. Afr. J. Lab. Med. 2022, 11, 1–11. [CrossRef]
- Mudenda, S.; Chabalenge, B.; Daka, V.; Mfune, R.L.; Salachi, K.I.; Mohamed, S.; Mufwambi, W.; Kasanga, M.; Matafwali, S.K. Global Strategies to Combat Antimicrobial Resistance: A One Health Perspective. Pharmacol. Pharm. 2023, 14, 271–328. [CrossRef]
- Jacobs, J.; Hardy, L.; Semret, M.; Lunguya, O.; Phe, T.; Affolabi, D.; Yansouni, C.; Vandenberg, O. Diagnostic Bacteriology in District Hospitals in Sub-Saharan Africa: At the Forefront of the Containment of Antimicrobial Resistance. Front. Med. 2019, 6, 20. [CrossRef]
- Yadav, S.K.; Shrestha, L.; Acharya, J.; Gompo, T.R.; Chapagain, S.; Jha, R. Integrative Digital Tools to Strengthen Data Management for Antimicrobial Resistance Surveillance in the “One Health” Domain in Nepal. Trop. Med. Infect. Dis. 2023, 8. [CrossRef]
- Cantón, R. Role of Microbiology Laboratory in Infectious Disease Surveillance, Alert and Response. Clin. Microbiol. Infect. Suppl. 2005, 11, 3–8. [CrossRef]
- Perovic, O.; Schultsz, C. Stepwise Approach for Implementation of Antimicrobial Resistance Surveillance in Africa. Afr. J. Lab. Med. 2016, 5, 482. [CrossRef]
- Fabre, V.; Davis, A.; Diekema, D.J.; Granwehr, B.; Hayden, M.K.; Lowe, C.F.; Pfeiffer, C.D.; Sick-Samuels, A.C.; Sullivan, K. V.; Van Schooneveld, T.C.; et al. Principles of Diagnostic Stewardship: A Practical Guide from the Society for Healthcare Epidemiology of America Diagnostic Stewardship Task Force. Infect. Control Hosp. Epidemiol. 2023, 44, 178–185. [CrossRef]
- Morgan, D.J.; Malani, P.; Diekema, D.J. Diagnostic Stewardship - Leveraging the Laboratory to Improve Antimicrobial Use. JAMA - J. Am. Med. Assoc. 2017, 318, 607–608. [CrossRef]
- Dik, J.H.; Poelman, R.; Friedrich, A.W.; Niesters, H.G.M.; Rossen, J.W.A.; Sinha, B. Integrated Stewardship Model Comprising Antimicrobial, Infection Prevention, and Diagnostic Stewardship (AID Stewardship). J. Clin. Microbiol. 2017, 55, 3306–3307. [CrossRef]
- Patel, R.; Fang, F.C. Diagnostic Stewardship: Opportunity for a Laboratory-Infectious Diseases Partnership. Clin. Infect. Dis. 2018, 67, 799–801. [CrossRef]
- Dik, J.W.H.; Poelman, R.; Friedrich, A.W.; Panday, P.N.; Lo-Ten-Foe, J.R.; Assen, S. Van; Van Gemert-Pijnen, J.E.W.C.; Niesters, H.G.M.; Hendrix, R.; Sinha, B. An Integrated Stewardship Model: Antimicrobial, Infection Prevention and Diagnostic (AID). Future Microbiol. 2016, 11, 93–102. [CrossRef]
- Iregbu, K.C.; Osuagwu, C.S.; Umeokonkwo, C.D.; Fowotade, A.A.; Ola-Bello, O.I.; Nwajiobi-Princewill, P.I.; Taiwo, S.S.; Olayinka, A.T.; Oduyebo, O.O. Underutilization of the Clinical Microbiology Laboratory by Physicians in Nigeria. African J. Clin. Exp. Microbiol. 2019, 21, 53–59. [CrossRef]
- Yansouni, C.P.; Seifu, D.; Libman, M.; Alemayehu, T.; Gizaw, S.; Johansen, Ø.H.; Abebe, W.; Amogne, W.; Semret, M. A Feasible Laboratory-Strengthening Intervention Yielding a Sustainable Clinical Bacteriology Sector to Support Antimicrobial Stewardship in a Large Referral Hospital in Ethiopia. Front. Public Heal. 2020, 8, 258. [CrossRef]
- Turner, P.; Rupali, P.; Opintan, J.A.; Jaoko, W.; Feasey, N.A.; Peacock, S.J.; Ashley, E.A. Laboratory Informatics Capacity for Effective Antimicrobial Resistance Surveillance in Resource-Limited Settings. Lancet Infect. Dis. 2021, 21, e170–e174.
- Khadse, S.N.; Ugemuge, S.; Singh, C. Impact of Antimicrobial Stewardship on Reducing Antimicrobial Resistance. Cureus 2023, 15, e49935. [CrossRef]
- Agyare, E.; Acolatse, J.E.E.; Dakorah, M.P.; Akafity, G.; Chalker, V.J.; Spiller, O.B.; Schneider, K.A.; Yevutsey, S.; Aidoo, N.B.; Blankson, S.; et al. Antimicrobial Stewardship Capacity and Antibiotic Utilisation Practices in the Cape Coast Teaching Hospital, Ghana: A Point Prevalence Survey Study. PLoS One 2024, 19, e0297626. [CrossRef]
- Mudenda, S.; Chabalenge, B.; Daka, V.; Jere, E.; Sefah, I.; Wesangula, E.; Yamba, K.; Nyamupachitu, J.; Mugenyi, N.; Mustafa, Z.U.; et al. Knowledge, Awareness and Practices of Healthcare Workers Regarding Antimicrobial Use, Resistance and Stewardship in Zambia: A Multi-Facility Cross-Sectional Study. JAC-Antimicrobial Resist. 2024, 6, dlae076. [CrossRef]
- Gebretekle, G.B.; Mariam, D.H.; Abebe, W.; Amogne, W.; Tenna, A.; Fenta, T.G.; Libman, M.; Yansouni, C.P.; Semret, M. Opportunities and Barriers to Implementing Antibiotic Stewardship in Low and Middle-Income Countries: Lessons from a Mixed-Methods Study in a Tertiary Care Hospital in Ethiopia. PLoS One 2018, 13, e0208447. [CrossRef]
- Watson, K.J.; Trautner, B.; Russo, H.; Phe, K.; Lasco, T.; Pipkins, T.; Lembcke, B.; Al Mohajer, M. Using Clinical Decision Support to Improve Urine Culture Diagnostic Stewardship, Antimicrobial Stewardship, and Financial Cost: A Multicenter Experience. Infect. Control Hosp. Epidemiol. 2020, 41, 564–570. [CrossRef]
- Langford, B.J.; Seah, J.; Chan, A.; Downing, M.; Johnstone, J.; Matukas, L.M. Antimicrobial Stewardship in the Microbiology Laboratory: Impact of Selective Susceptibility Reporting on Ciprofloxacin Utilization and Susceptibility of Gram-Negative Isolates to Ciprofloxacin in a Hospital Setting. J. Clin. Microbiol. 2016, 54, 2343–2347. [CrossRef]
- Morency-Potvin, P.; Schwartz, D.N.; Weinstein, R.A. Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship. Clin. Microbiol. Rev. 2017, 30, 381–407. [CrossRef]
- O. Popoola, O. Implementing Antimicrobial Stewardship in Various Healthcare Settings. In Antimicrobial Stewardship [Working Title]; IntechOpen, 2023; pp. 1–22 ISBN 978-1-83769-091-6.
- Bhowmick, T.; Kirn, T.J.; Hetherington, F.; Takavarasha, S.; Sandhu, S.S.; Gandhi, S.; Narayanan, N.; Weinstein, M.P. Collaboration between an Antimicrobial Stewardship Team and the Microbiology Laboratory Can Shorten Time to Directed Antibiotic Therapy for Methicillin-Susceptible Staphylococcal Bacteremia and to Discontinuation of Antibiotics for Coagulase-Negative Stap. Diagn. Microbiol. Infect. Dis. 2018, 92, 214–219. [CrossRef]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; Mcneil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to Improve Antibiotic Prescribing Practices for Hospital Inpatients. Cochrane Database Syst. Rev. 2017, 2017, CD003543. [CrossRef]
- Steinberg, D.I. Review: Interventions Improve Hospital Antibiotic Prescribing and Reduce Hospital Stay but Do Not Affect Mortality. Ann. Intern. Med. 2017, 166, JC59. [CrossRef]
- Valderrama-Rios, M.C.; Álvarez-Moreno, C.A.; Cortes, J.A. Interventions to Improve Antibiotic Use in Hospitals with Different Levels of Complexity in Colombia: Findings from a Before-and-After Study and Suggestions for the Future. Antibiotics 2023, 12, 867. [CrossRef]
- Crayton, E.; Richardson, M.; Fuller, C.; Smith, C.; Liu, S.; Forbes, G.; Anderson, N.; Shallcross, L.; Michie, S.; Hayward, A.; et al. Interventions to Improve Appropriate Antibiotic Prescribing in Long-Term Care Facilities: A Systematic Review. BMC Geriatr. 2020, 20, 237. [CrossRef]
- Gruber, M.M.; Weber, A.; Jung, J.; Strehlau, A.; Tsilimparis, N.; Draenert, R. The Impact of Antibiotic Stewardship Interventions and Patient Related Factors on Antibiotic Prescribing in a Vascular Surgical Department. Infection 2024, 52, 83–91. [CrossRef]
- Loevinsohn, G.; Hardick, J.; Sinywimaanzi, P.; Fenstermacher, K.Z.J.; Shaw-Saliba, K.; Monze, M.; Gaydos, C.A.; Rothman, R.E.; Pekosz, A.; Thuma, P.E.; et al. Respiratory Pathogen Diversity and Co-Infections in Rural Zambia. Int. J. Infect. Dis. 2021, 102, 291–298. [CrossRef]
- Fwoloshi, S.; Hines, J.Z.; Barradas, D.T.; Yingst, S.; Siwingwa, M.; Chirwa, L.; Zulu, J.E.; Banda, D.; Wolkon, A.; Nikoi, K.I.; et al. Prevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Among Healthcare Workers-Zambia, July 2020. Clin. Infect. Dis. 2021, 73, e1321–e1328. [CrossRef]
- Lungu, P.; Kasapo, C.; Mihova, R.; Chimzizi, R.; Sikazwe, L.; Banda, I.; Mucheleng’anga, L.A.; Chanda-Kapata, P.; Kapata, N.; Zumla, A.; et al. A 10-Year Review of TB Notifications and Mortality Trends Using a Joint Point Analysis in Zambia - a High TB Burden Country. Int. J. Infect. Dis. 2022, 124, S30–S40. [CrossRef]
- Yamba, K.; Lukwesa-Musyani, C.; Samutela, M.T.; Kapesa, C.; Hang’ombe, M.B.; Mpabalwani, E.; Hachaambwa, L.; Fwoloshi, S.; Chanda, R.; Mpundu, M.; et al. Phenotypic and Genotypic Antibiotic Susceptibility Profiles of Gram-Negative Bacteria Isolated from Bloodstream Infections at a Referral Hospital, Lusaka, Zambia. PLOS Glob. Public Heal. 2023, 3, e0001414. [CrossRef]
- Mwansa, T.N.; Kamvuma, K.; Mulemena, J.A.; Phiri, C.N.; Chanda, W. Antibiotic Susceptibility Patterns of Pathogens Isolated from Laboratory Specimens at Livingstone Central Hospital in Zambia. PLOS Glob. Public Heal. 2022, 2, e0000623. [CrossRef]
- Bumbangi, F.N.; Llarena, A.-K.; Skjerve, E.; Hang’ombe, B.M.; Mpundu, P.; Mudenda, S.; Mutombo, P.B.; Muma, J.B. Evidence of Community-Wide Spread of Multi-Drug Resistant Escherichia Coli in Young Children in Lusaka and Ndola Districts, Zambia. Microorganisms 2022, 10, 1684. [CrossRef]
- Kasanga, M.; Mudenda, S.; Siyanga, M.; Chileshe, M.; Mwiikisa, M.J.; Kasanga, M.; Solochi, B.B.; Gondwe, T.; Kantenga, T.; L Shibemba, A.; et al. Antimicrobial Susceptibility Patterns of Bacteria That Commonly Cause Bacteremia at a Tertiary Hospital in Zambia. Future Microbiol. 2020, 15, 1735–1745. [CrossRef]
- Kasanga, M.; Kwenda, G.; Wu, J.; Kasanga, M.; Mwikisa, M.J.; Chanda, R.; Mupila, Z.; Yankonde, B.; Sikazwe, M.; Mwila, E.; et al. Antimicrobial Resistance Patterns and Risk Factors Associated with ESBL-Producing and MDR Escherichia Coli in Hospital and Environmental Settings in Lusaka, Zambia: Implications for One Health, Antimicrobial Stewardship and Surveillance Systems. Microorganisms 2023, 11, 1951. [CrossRef]
- Yamba, K.; Mudenda, S.; Mpabalwani, E.; Mainda, G.; Mukuma, M.; Samutela, M.T.; Lukwesa, C.; Chizimu, J.; Kaluba, C.K.; Mutalange, M.; et al. Antibiotic Prescribing Patterns and Carriage of Antibiotic-Resistant Escherichia Coli and Enterococcus Species in Healthy Individuals from Selected Communities in Lusaka and Ndola Districts, Zambia. JAC-Antimicrobial Resist. 2024, 6, dlae027. [CrossRef]
- Shawa, M.; Paudel, A.; Chambaro, H.; Kamboyi, H.; Nakazwe, R.; Alutuli, L.; Zorigt, T.; Sinyawa, T.; Samutela, M.; Chizimu, J.; et al. Trends, Patterns and Relationship of Antimicrobial Use and Resistance in Bacterial Isolates Tested between 2015–2020 in a National Referral Hospital of Zambia. PLoS One 2024, 19, e0302053. [CrossRef]
- Chileshe, C.; Shawa, M.; Phiri, N.; Ndebe, J.; Khumalo, C.S.; Nakajima, C.; Kajihara, M.; Higashi, H.; Sawa, H.; Suzuki, Y.; et al. Detection of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Enterobacteriaceae from Diseased Broiler Chickens in Lusaka District, Zambia. Antibiotics 2024, 13, 259. [CrossRef]
- Kasanga, M.; Shempela, D.M.; Daka, V.; Mwikisa, M.J.; Sikalima, J.; Chanda, D.; Mudenda, S. Antimicrobial Resistance Profiles of Escherichia Coli Isolated from Clinical and Environmental Samples: Findings and Implications. JAC-Antimicrobial Resist. 2024, 6, dlae061. [CrossRef]
- 2020; 59. Republic of Zambia AMRCC Zambia’s Integrated Antimicrobial Resistance Surveillance Framework; 2020;
- 2017; 60. Government of the Republic of Zambia Multi-Sectoral National Action Plan on Antimicrobial Resistance; 2017;
- Moirongo, R.M.; Aglanu, L.M.; Lamshöft, M.; Adero, B.O.; Yator, S.; Anyona, S.; May, J.; Lorenz, E.; Eibach, D. Laboratory-Based Surveillance of Antimicrobial Resistance in Regions of Kenya: An Assessment of Capacities, Practices, and Barriers by Means of Multi-Facility Survey. Front. Public Heal. 2022, 10, 1003178. [CrossRef]
- Mao, S.; Soputhy, C.; Lay, S.; Jacobs, J.; Ku, G.M.; Chau, D.; Chhea, C.; Ir, P. The Barriers and Facilitators of Implementing a National Laboratory-Based AMR Surveillance System in Cambodia: Key Informants’ Perspectives and Assessments of Microbiology Laboratories. Front. Public Heal. 2023, 11, 1332423. [CrossRef]
- Yamba, K.; Chizimu, J.Y.; Mudenda, S.; Lukwesa, C.; Chanda, R.; Nakazwe, R.; Simunyola, B.; Shawa, M.; Kalungia, A.C.; Chanda, D.; et al. Assessment of Antimicrobial Resistance Laboratory-Based Surveillance Capacity of Hospitals in Zambia: Findings and Implications for System Strengthening. J. Hosp. Infect. 2024, 148, 129–137. [CrossRef]
- Zongo, E.; Dama, E.; Yenyetou, D.; Muhigwa, M.; Nikiema, A.; Dahourou, G.A.; Ouedraogo, A.S. On-Site Evaluation as External Quality Assessment of Microbiology Laboratories Involved in Sentinel Laboratory-Based Antimicrobial Resistance Surveillance Network in Burkina Faso. Antimicrob. Resist. Infect. Control 2024, 13, 3. [CrossRef]
- Musa, K.; Okoliegbe, I.; Abdalaziz, T.; Aboushady, A.T.; Stelling, J.; Gould, I.M. Laboratory Surveillance, Quality Management, and Its Role in Addressing Antimicrobial Resistance in Africa: A Narrative Review. Antibiotics 2023, 12, 1313.
- Okolie, O.J.; Igwe, U.; Ismail, S.U.; Ighodalo, U.L.; Adukwu, E.C. Systematic Review of Surveillance Systems for AMR in Africa. J. Antimicrob. Chemother. 2023, 78, 31–51. [CrossRef]
- Umutesi, G.; Velin, L.; Muwanguzi, M.; Faktor, K.; Mugabo, C.; Rukundo, G.; Rucogoza, A.; Yankurije, M.; Mazimpaka, C.; Gatete, J.D.D.; et al. Strengthening Antimicrobial Resistance Diagnostic Capacity in Rural Rwanda: A Feasibility Assessment. Ann. Glob. Heal. 2021, 87, 1–13. [CrossRef]
- Gulumbe, B.H.; Haruna, U.A.; Almazan, J.; Ibrahim, I.H.; Faggo, A.A.; Bazata, A.Y. Combating the Menace of Antimicrobial Resistance in Africa: A Review on Stewardship, Surveillance and Diagnostic Strategies. Biol. Proced. Online 2022, 24, 19.
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [CrossRef]
- Mtonga, T.M.; Choonara, F.E.; Espino, J.U.; Kachaje, C.; Kapundi, K.; Mengezi, T.E.; Mumba, S.L.; Douglas, G.P. Design and Implementation of a Clinical Laboratory Information System in a Low-Resource Setting. Afr. J. Lab. Med. 2019, 8, 2225–2002. [CrossRef]
- Lukić, V. Laboratory Information System-Where Are We Today? J. Med. Biochem. 2017, 36, 220–224. [CrossRef]
- Kammergruber, R.; Durner, J. Laboratory Information System and Necessary Improvements in Function and Programming. J. Lab. Med. 2018, 42, 277–287. [CrossRef]
- Ibrahim, R.A.; Teshale, A.M.; Dinku, S.F.; Abera, N.A.; Negeri, A.A.; Desta, F.G.; Seyum, E.T.; Gemeda, A.W.; Keficho, W.M. Antimicrobial Resistance Surveillance in Ethiopia: Implementation Experiences and Lessons Learned. Afr. J. Lab. Med. 2018, 7, 2225–2002. [CrossRef]
- Do, P.C.; Assefa, Y.A.; Batikawai, S.M.; Reid, S.A. Strengthening Antimicrobial Resistance Surveillance Systems: A Scoping Review. BMC Infect. Dis. 2023, 23, 593. [CrossRef]
- Hazim, C.; Abubeker Ibrahim, R.; Westercamp, M.; Belete, G.A.; Amare Kibret, B.; Kanter, T.; Yimer, G.; Adem, T.S.; Stevenson, K.B.; Urrego, M.; et al. Establishment of a Sentinel Laboratory-Based Antimicrobial Resistance Surveillance Network in Ethiopia. Heal. Secur. 2018, 16, S30–S36. [CrossRef]
- Katawa, G.; Adziyno Agbemanyole, K.; Nguepou Tchopba, C.; Ataba, E.; Oukoé Amessoudji, M.; Adjoa Ameyapoh, H.; Edlom Tchadié, P.; Fagdéba Bara, D.; Karou, D.; Ameyapoh, Y. Antimicrobial Susceptibility Testing: Evaluation of the Conformity of 3 Medical Bacteriology Laboratories of Togo According to EUCAST/CA-SFM Guidelines. J. Appl. Biosci. 2021, 162, 16795–16803. [CrossRef]
- Allen, L.C. Role of a Quality Management System in Improving Patient Safety - Laboratory Aspects. Clin. Biochem. 2013, 46, 1187–1193. [CrossRef]
- Owusu, M.; Nkrumah, B.; Acheampong, G.; Mensah, E.K.; Komei, A.A.K.; Sroda, F.K.; David, S.; Emery, S.; Robinson, L.M.; Asante, K.; et al. Improved Detection of Microbiological Pathogens: Role of Partner and Non-Governmental Organizations. BMC Infect. Dis. 2021, 21, 303. [CrossRef]
- Barbé, B.; Verdonck, K.; Mukendi, D.; Lejon, V.; Lilo Kalo, J.R.; Alirol, E.; Gillet, P.; Horié, N.; Ravinetto, R.; Bottieau, E.; et al. The Art of Writing and Implementing Standard Operating Procedures (SOPs) for Laboratories in Low-Resource Settings: Review of Guidelines and Best Practices. PLoS Negl. Trop. Dis. 2016, 10, e0005053. [CrossRef]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of Antimicrobial Resistance in Low- and Middle-Income Countries: A Scattered Picture. Antimicrob. Resist. Infect. Control 2021, 10, 63.
- Chaplain, D.; Asutaku, B. Ben; Mona, M.; Bulafu, D.; Aruhomukama, D. The Need to Improve Antimicrobial Susceptibility Testing Capacity in Ugandan Health Facilities: Insights from a Surveillance Primer. Antimicrob. Resist. Infect. Control 2022, 11, 23. [CrossRef]
- Peng, H.; Bilal, M.; Iqbal, H.M.N. Improved Biosafety and Biosecurity Measures and/or Strategies to Tackle Laboratory-Acquired Infections and Related Risks. Int. J. Environ. Res. Public Health 2018, 15, 2697. [CrossRef]
- Sodjinou, V.D.; Ayelo, P.A.; Achade, A.G.A.; Affolabi, D.; Ouendo, D.E.M. Assessment of the Biosafety and Biosecurity in the Reference Veterinary Laboratory of Parakou in Benin. Trop. Med. Infect. Dis. 2021, 6, 146. [CrossRef]
- Mouillé, B.; Dauphin, G.; Wiersma, L.; Blacksell, S.D.; Claes, F.; Kalpravidh, W.; Kabore, Y.; Hietala, S. A Tool for Assessment of Animal Health Laboratory Safety and Biosecurity: The Safety Module of the Food and Agriculture Organization’s Laboratory Mapping Tool. Trop. Med. Infect. Dis. 2018, 3, 33. [CrossRef]
- Rutebemberwa, E.; Aku, F.Y.; Al Zein, E.I.K.; Bellali, H. Reasons for and Barriers to Biosafety and Biosecurity Training in Health-Related Organizations in Africa, Middle East and Central Asia: Findings from GIBACHT Training Needs Assessments 2018-2019. Pan Afr. Med. J. 2020, 37, 1–14. [CrossRef]
- Odetokun, I.A.; Jagun-Jubril, A.T.; Onoja, B.A.; Wungak, Y.S.; Raufu, I.A.; Chen, J.C. Status of Laboratory Biosafety and Biosecurity in Veterinary Research Facilities in Nigeria. Saf. Health Work 2017, 8, 49–58. [CrossRef]
- Orelle, A.; Nikiema, A.; Zakaryan, A.; Albetkova, A.A.; Keita, M.S.; Rayfield, M.A.; Peruski, L.F.; Pierson, A. A Multilingual Tool for Standardized Laboratory Biosafety and Biosecurity Assessment and Monitoring. Heal. Secur. 2022, 20, 488–496. [CrossRef]
- Dama, E.; Orelle, A.; Nikiema, A.; Mandingar, P.D.; Naby, A.; Bationo, G.B.; Kéita, M.S.; Pierson, A.; Sawadogo, C.; Koné, R.G. Strengthening Biorisk Management Capacities in Burkina Faso: Contribution of the Global Health Security Agenda. Heal. Secur. 2022, 20, 479–487. [CrossRef]
- Klinker, K.P.; Hidayat, L.K.; DeRyke, C.A.; DePestel, D.D.; Motyl, M.; Bauer, K.A. Antimicrobial Stewardship and Antibiograms: Importance of Moving beyond Traditional Antibiograms. Ther. Adv. Infect. Dis. 2021, 8, 20499361211011372. [CrossRef]
- Var, S.K.; Hadi, R.; Khardori, N.M. Evaluation of Regional Antibiograms to Monitor Antimicrobial Resistance in Hampton Roads, Virginia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 22. [CrossRef]
- Roth, B.M.; Laps, A.; Yamba, K.; Heil, E.L.; Johnson, J.K.; Stafford, K.; Hachaambwa, L.M.; Kalumbi, M.; Mulenga, L.; Patel, D.M.; et al. Antibiogram Development in the Setting of a High Frequency of Multi-Drug Resistant Organisms at University Teaching Hospital, Lusaka, Zambia. Antibiotics 2021, 10, 782. [CrossRef]
- Darboe, S.; Mirasol, R.; Adejuyigbe, B.; Muhammad, A.K.; Nadjm, B.; De St. Maurice, A.; Dogan, T.L.; Ceesay, B.; Umukoro, S.; Okomo, U.; et al. Using an Antibiogram Profile to Improve Infection Control and Rational Antimicrobial Therapy in an Urban Hospital in The Gambia, Strategies and Lessons for Low- and Middle-Income Countries. Antibiotics 2023, 12, 790. [CrossRef]
- Truong, W.R.; Hidayat, L.; Bolaris, M.A.; Nguyen, L.; Yamaki, J. The Antibiogram: Key Considerations for Its Development and Utilization. JAC-Antimicrobial Resist. 2021, 3, dlab060. [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What Is Antimicrobial Stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798.
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to Start an Antimicrobial Stewardship Programme in a Hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [CrossRef]
- Shamseddine, J.; Sadeq, A.; Yousuf, K.; Abukhater, R.; Yahya, L.O.; Espil, M.A.; Hassan, M.E.; Fadl, R.E.; Ahmed, R.T.E.; Elkonaissi, I.; et al. Impact of Antimicrobial Stewardship Interventions on Days of Therapy and Guideline Adherence: A Comparative Point-Prevalence Survey Assessment. Front. Trop. Dis. 2023, 3, 1050344. [CrossRef]
- Chukwu, E.E.; Abuh, D.; Idigbe, I.E.; Osuolale, K.A.; Chuka-Ebene, V.; Awoderu, O.; Audu, R.A.; Ogunsola, F.T. Implementation of Antimicrobial Stewardship Programs: A Study of Prescribers’ Perspective of Facilitators and Barriers. PLoS One 2024, 19, e0297472. [CrossRef]
- Tahoon, M.A.; Khalil, M.M.; Hammad, E.; Morad, W.S.; Awad, S.M.; Ezzat, S. The Effect of Educational Intervention on Healthcare Providers’ Knowledge, Attitude, & Practice towards Antimicrobial Stewardship Program at, National Liver Institute, Egypt. Egypt. Liver J. 2020, 10, 5. [CrossRef]
- Al-Omari, A.; Al Mutair, A.; Alhumaid, S.; Salih, S.; Alanazi, A.; Albarsan, H.; Abourayan, M.; Al Subaie, M. The Impact of Antimicrobial Stewardship Program Implementation at Four Tertiary Private Hospitals: Results of a Five-Years Pre-Post Analysis. Antimicrob. Resist. Infect. Control 2020, 9, 95. [CrossRef]
- Siachalinga, L.; Mufwambi, W.; Lee, I.-H. Impact of Antimicrobial Stewardship Interventions to Improve Antibiotic Prescribing for Hospital Inpatients in Africa: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2022, 129, 124–143.
- Cui, Y.; Liu, J.; Zhang, X. Effects of Laboratory Capabilities on Combating Antimicrobial Resistance, 2013–2016: A Static Model Panel Data Analysis. J. Glob. Antimicrob. Resist. 2019, 19, 116–121. [CrossRef]
- Eliopoulos, G.M.; Schwaber, M.J.; Carmeli, Y. An Ongoing National Intervention to Contain the Spread of Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2014, 58, 697–703. [CrossRef]
- Lesho, E.P.; Waterman, P.E.; Chukwuma, U.; McAuliffe, K.; Neumann, C.; Julius, M.D.; Crouch, H.; Chandrasekera, R.; English, J.F.; Clifford, R.J.; et al. The Antimicrobial Resistance Monitoring and Research (ARMoR) Program: The US Department of Defense Response to Escalating Antimicrobial Resistance. Clin. Infect. Dis. 2014, 59, 390–397. [CrossRef]
- Goddard, L.; Wozniak, T.M. Antimicrobial Resistance Surveillance to Support Decision-Making in a High-Prevalence Region: An Evaluation. Front. Trop. Dis. 2021, 2, 772491. [CrossRef]
- Centers for Disease Control and Prevention Laboratory Assessment of Antibiotic Resistance Testing Capacity. Centers Dis. Control Prev. 2020, 1–91.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




