1. Introduction
Phosphorus (P) is an essential mineral nutrient required for plant growth and development and plays important roles in cellular processes such as macromolecule synthesis, energy storage, and signal transduction [
1]. P deficiency in soils is a global problem with significant implications for long-term crop sustainability [
2], exacerbated by the misuse of rock phosphate fertilizers [
3]. To find solutions, researchers are learning from plants that are well adapted to nutrient deficiencies, such as white lupin (
Lupinus albus), which has become an illuminating model for the study of plant adaptations to P and iron (Fe) deficiency [
4,
5]. Under P and Fe deficiency, white lupin forms cluster roots, specialized roots that resemble bottle brushes, and that allow white lupin to acquire nutrients unavailable to most other plants [
6]. White lupin’s unique adaptations to P deficiency, such as the development of cluster roots to augment root surface area, have been elucidated through physiological studies [
7,
8,
9,
10,
11]) and transcriptomics [
5,
12,
13,
14,
15].
However, the signaling pathways involved in white lupin (or other plants) to sense P deficiency and to elicit responses are not thoroughly understood. Split root experiments in white lupin have revealed that P deficiency is sensed in the shoot, and communicated to the root [
16]. This realization started a search to identify the long-distance signal that is transported from shoot to root in response to P deficiency. Several studies in recent decades have revealed that sucrose acts not only as a metabolite, but also as the major long-distance signal sent from the shoot to the root to signal P deficiency [
17,
18]. Interestingly, sucrose has also been identified as a long-distance signal of Fe deficiency [
19]. While glucose is widely accepted as a signaling molecule, the concept of sucrose signaling is still not well understood, though it was first suggested many decades ago [
20].
In white lupin, external application of sucrose has been shown to induce the formation of cluster roots [
21,
22], which are usually formed only in response to P (and Fe) deficiency. The hypothesis that sucrose acts as a signal of nutrient deficiency is further supported by studies that revealed a role of sucrose in the signaling of other nutrient deficiencies, including Fe [
19] and nitrogen [
23]. The somewhat surprising finding that sucrose is involved in signaling of different nutrient deficiencies may explain the well-known observation of crosstalk, an overlap of signaling pathways and plant responses to various nutrient deficiencies [
24].
In addition, several studies have revealed a role of sucrose-signaling in plant defense [
25,
26]. The exogenous application of sucrose has been shown to induce the expression of defense-related transcription factors in rice [
27]. In soybean, genes involved in biotic and abiotic biotic stresses were enriched among up-regulated genes after short-term (20 min, 40 min) addition of sucrose to the roots [
28]. Taken together, many genes in response to biotic and abiotic stress are induced by sucrose, supporting a role of sucrose as a signaling molecule [
29].
RNA-seq is a well-established approach to assess differential gene expression, e.g. in response to nutrient deficiencies [
5]. The use of Oxford Nanopore Technologies is making RNA-seq more affordable, and enables the sequencing of longer reads, which is helpful for mapping and for distinguishing splice variants.
Our current study focuses on using Oxford Nanopore cDNA sequencing of white lupin roots grown hydroponically with sufficient nutrients. After three weeks, plant roots were treated with external sucrose added directly into the hydroponics solution for 0 minutes (control), 10 minutes, 15 minutes, and 20 minutes. Our goal was to identify early key contributors within the sucrose signaling pathway, giving deeper insight into how white lupin plants are responding to sucrose signaling. In the long-term, a better understanding of sucrose signaling could help in the development of plants with increased tolerance of biotic and abiotic stresses.
2. Results
2.1. Nanopore cDNA Sequencing to Assess Short-Term Responses to Sucrose Resulted in 35.5 Million Reads
To mimic the sucrose signal that is transported from shoot to root in response to P and Fe deficiency, we added sucrose directly to the roots of hydroponically grown white lupin. Previous results in white lupin have shown that cluster root formation, a morphological response to P and Fe deficiency, can be mimicked by external sucrose application in a concentration range of 2.5 mM to 12.5 mM sucrose; further increase to 25 mM sucrose resulted in unusual root thickening [
22]. Internal sucrose concentrations at the initiation zone of cluster root formation were measured at 3.4 mM sucrose [
22]. Based on this data, we decided to add external sucrose at a concentration of 10 mM, which should be high enough to expect a strong response to the sucrose signal but not too high to expect non-physiological responses.
After hydroponic growth in full nutrient solution for three weeks, roots were subjected to external sucrose at a final concentration of 10 mM for 0 min (control), 10 min, 15 min, and 20 min in three biological replications, for a total of twelve samples. Twelve corresponding cDNA libraries were combined into three pools, one for each biological replication, and each biological replication was sequenced on a different Oxford Nanopore Minion flow cell, resulting in an initial 35,545,919 total reads.
2.2. A Set of 17 Genes Was Up-Regulated at All Three Time Points of Sucrose Exposure
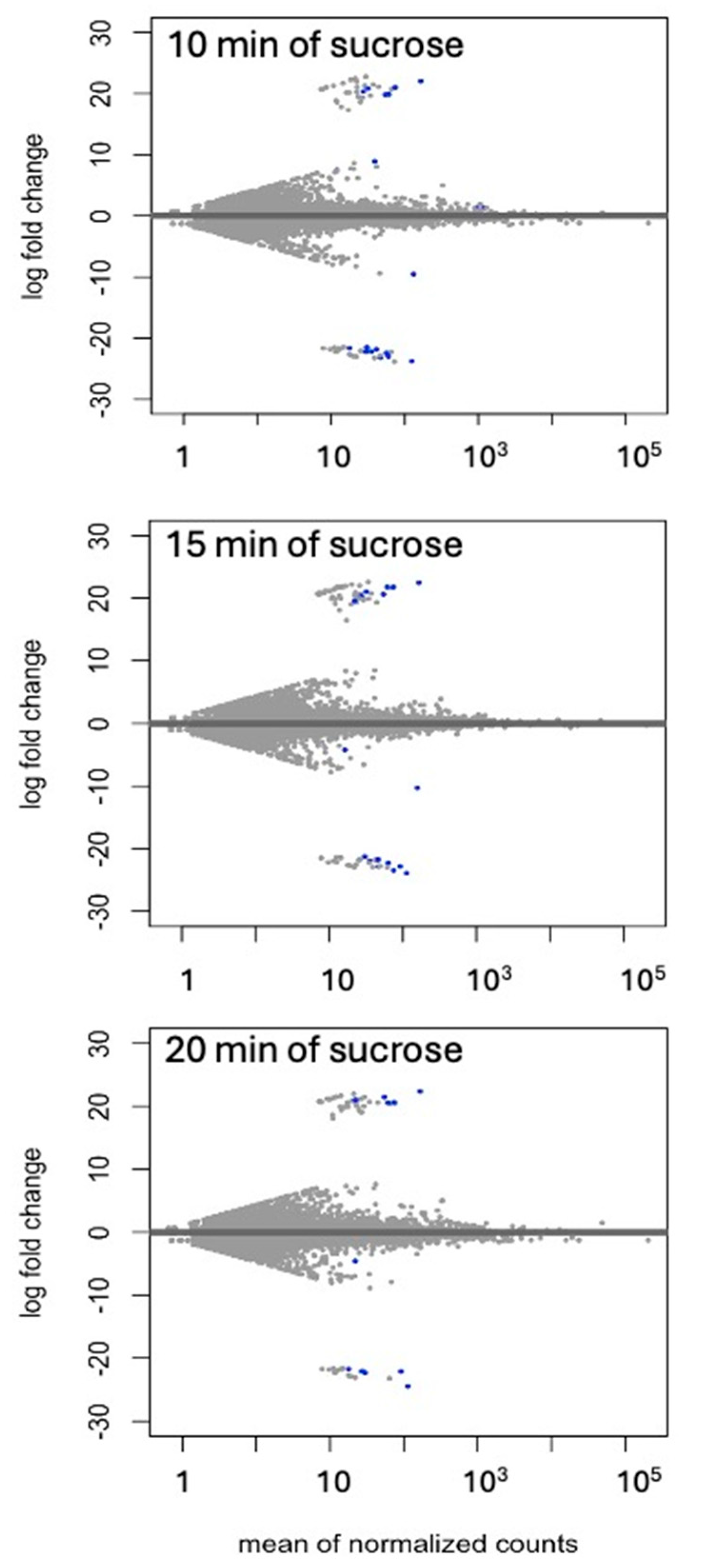
Differential expression of genes and transcripts were further analyzed with DESeq2 [
31]. MA (mean average) plots (
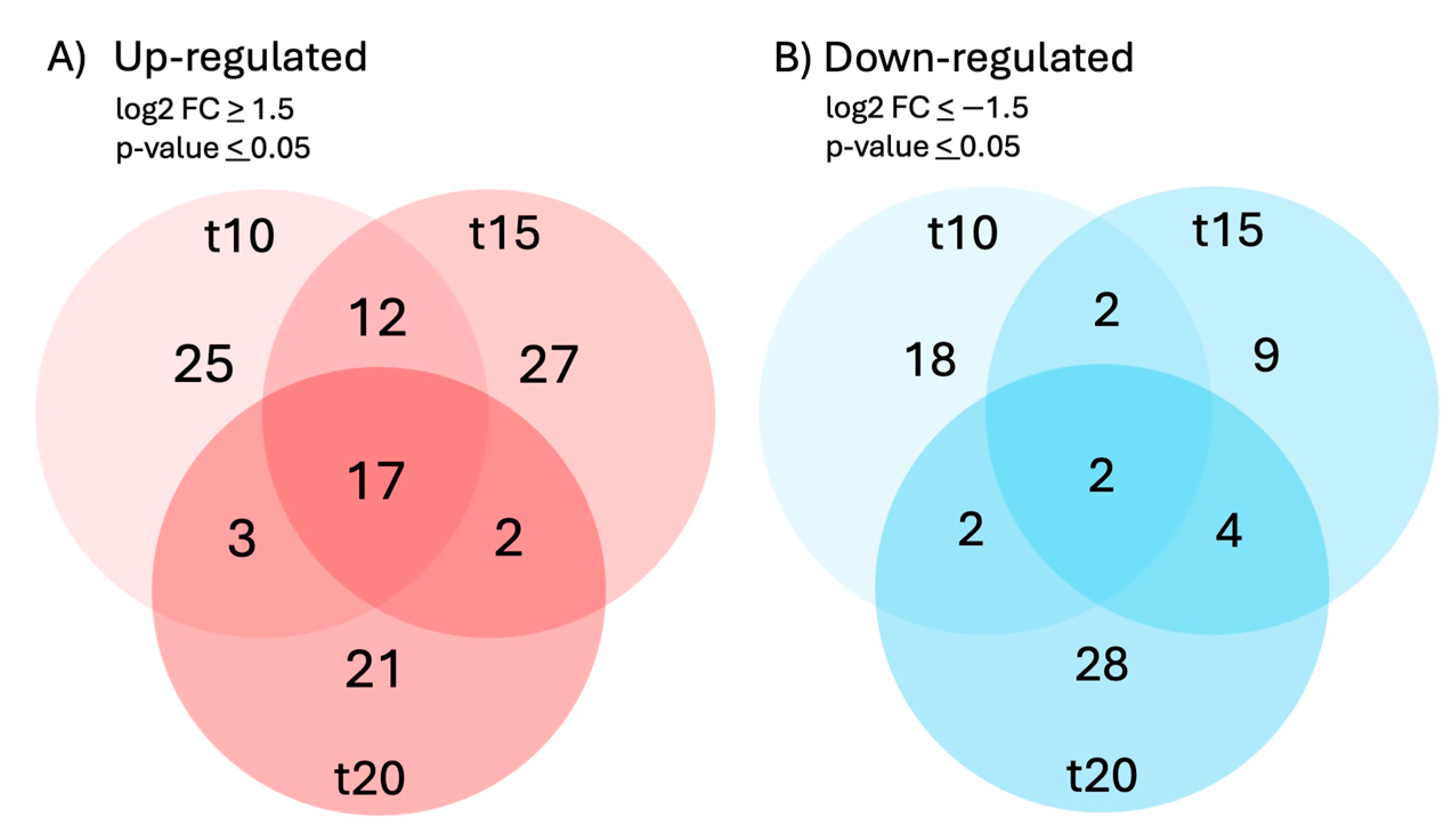
Figure 1) were used to visualize the resulting log2 FC (fold change) against normalized sequence counts at 10 min, 15 min, and 20 min of sucrose exposure, each compared to 0 min (control), revealing some differential gene expression already at 10 min after sucrose addition. A PCA plot (data not shown) did not reveal clear differences between the four time points used in this study, likely due to the relatively few changes at such short exposure. While longer exposure and bigger differences between time points would likely reveal larger numbers of differentially expressed genes and better separation between time points, we were interested in the earliest responses to sucrose, to identify potential key players of responses to sucrose. A Venn diagram (
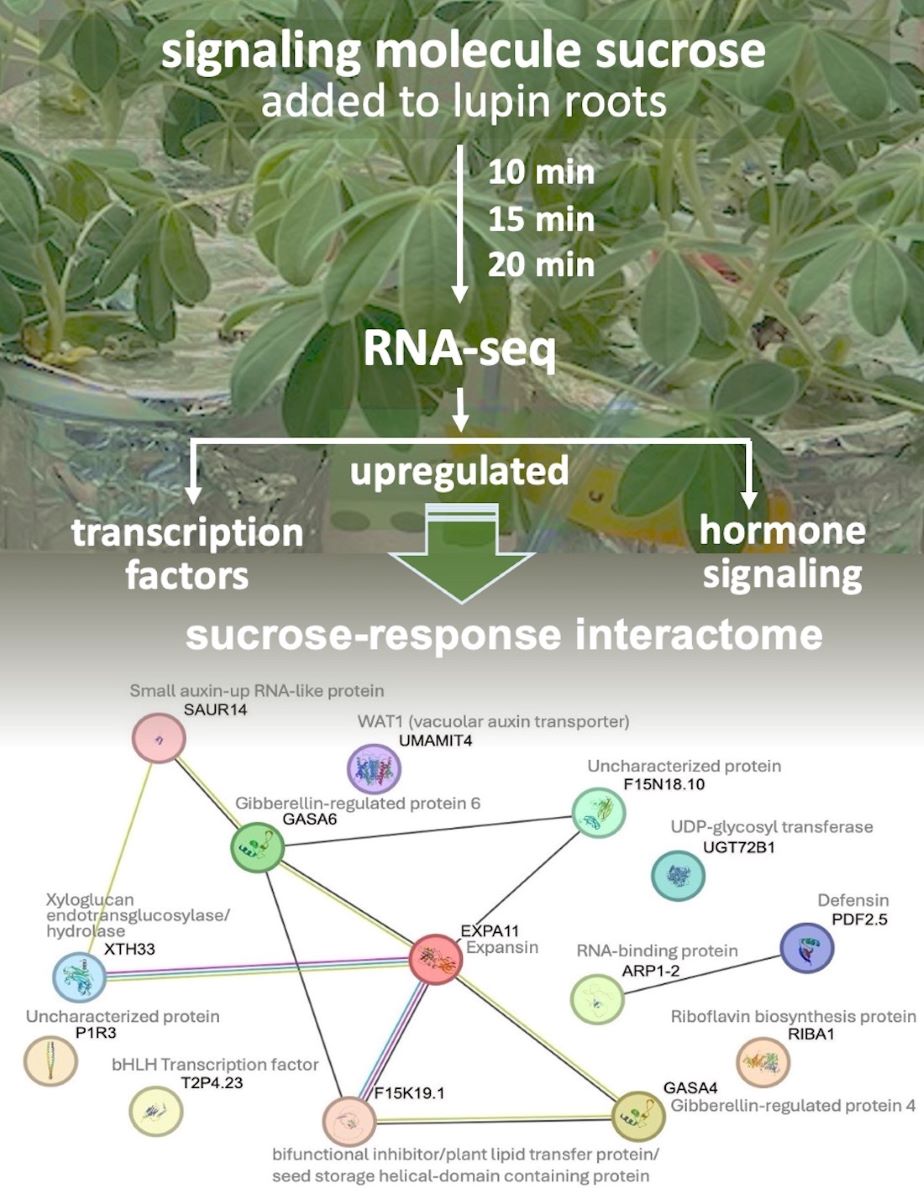
Figure 2) reveals a set of 17 genes that were up-regulated at all three time points of sucrose exposure.
2.3. Auxin- and Gibberellin-Responsive Genes and Two bHLH Transcription Factors Are among the Earliest Up-Regulated Genes
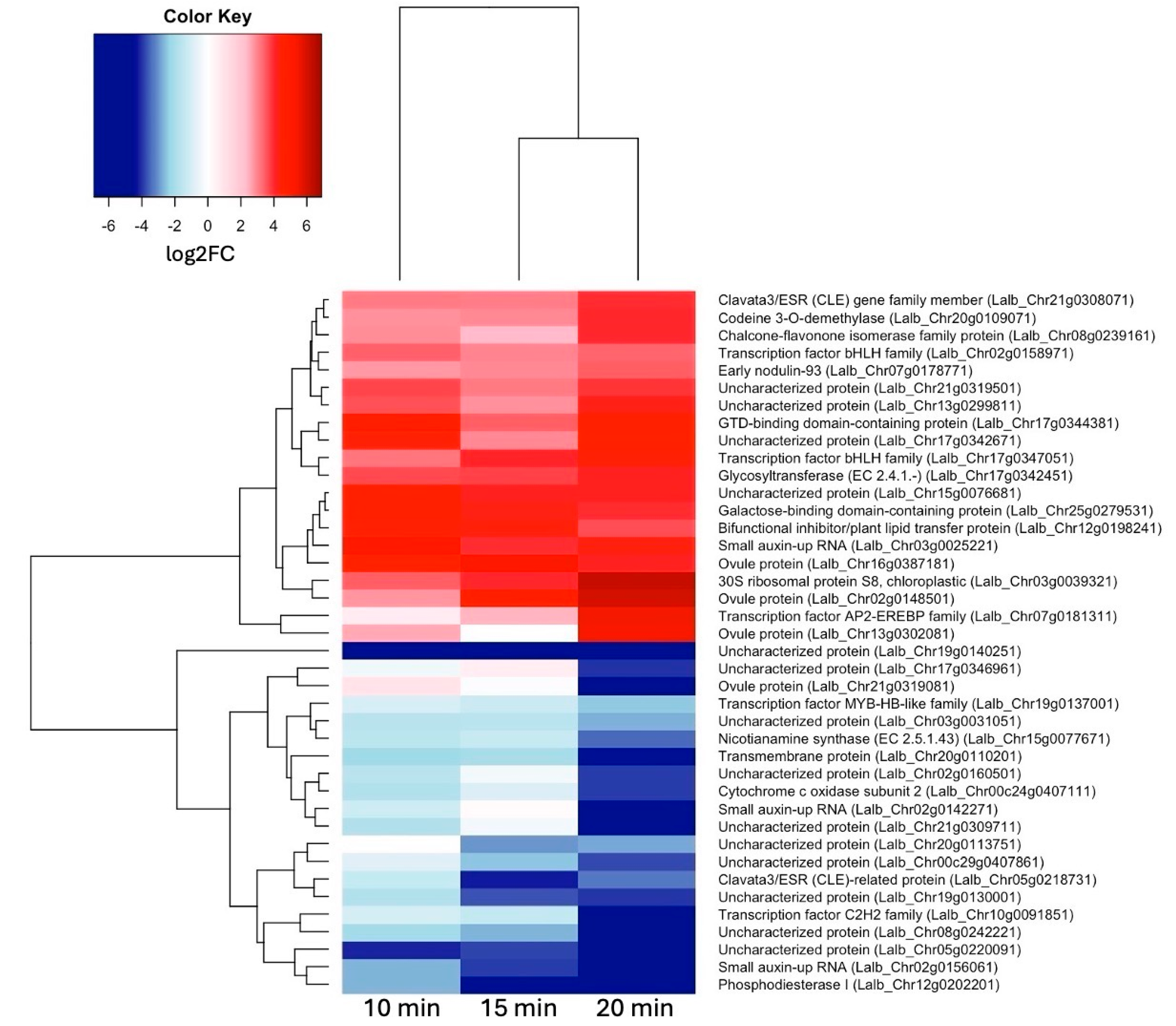
Table 2 shows a set of 17 genes that were up-regulated at all three time points of sucrose treatment. Two of these genes are involved with the plant hormone auxin: “small auxin-up RNA” is an auxin-induced protein of unknown function, while WAT1 is a vacuolar auxin transporter. Among the 17 up-regulated genes are also two gibberellin-responsive proteins. Because we are interested in key regulators of early responses to sucrose, we are especially interested in the two basic helix-loop-helix transcription factors. Expansin and xyloglucan endotransglucosylase/hydrolase, both involved in cell wall organization, were also up-regulated, as was defensin, part of biotic stress responses.
Because we are particularly interested in early responses to sucrose, we also looked at all genes that were up- or down-regulated at both 10 and 15 min of sucrose exposure, encompassing 29 up-regulated and 4 down-regulated genes (
Figure 3). In addition to the two bHLH transcription factors, a WRKY transcription factor shows significant up-regulation at these early time points.
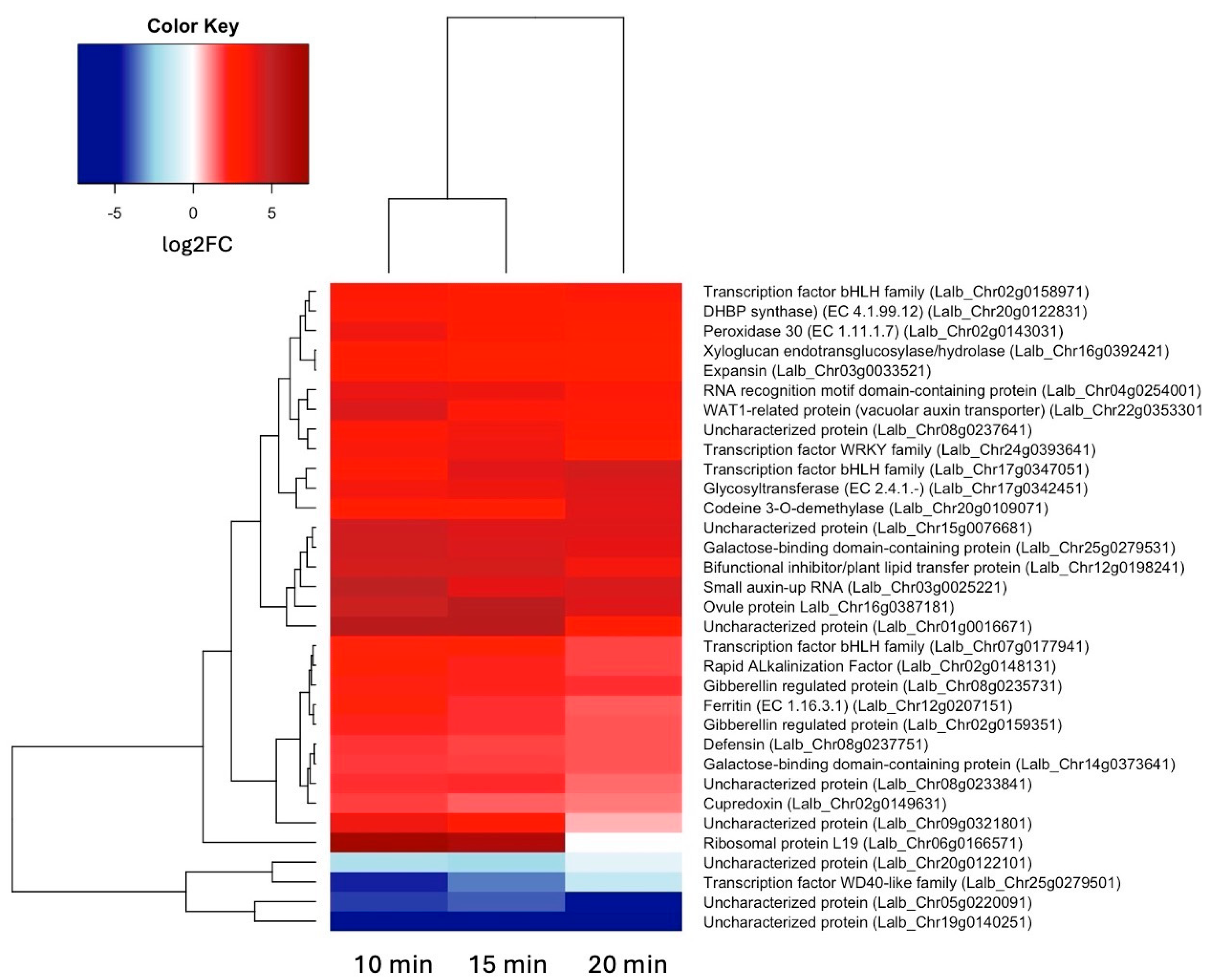
To see how these early genes may differ from slightly later activated genes, we also looked at the most up- and down-regulated genes at 20 min of sucrose treatment (
Figure 4). Interestingly, Clavata3/ESR (CLE) homologs are among the most up- and most down-regulated genes at 20 min of sucrose exposure. A Blast search revealed that the up-regulated CLE gene is most similar to CLE44, and the down-regulated CLE gene to CLE4. Also worth pointing out is the AP2-EREB (APETALA2-Ethylene-Responsive Element Binding Protein) family-type transcription factor, which becomes more up-regulated with increased time of sucrose exposure.
2.4. qRT-PCR Confirms Differential Expression of Selected Genes
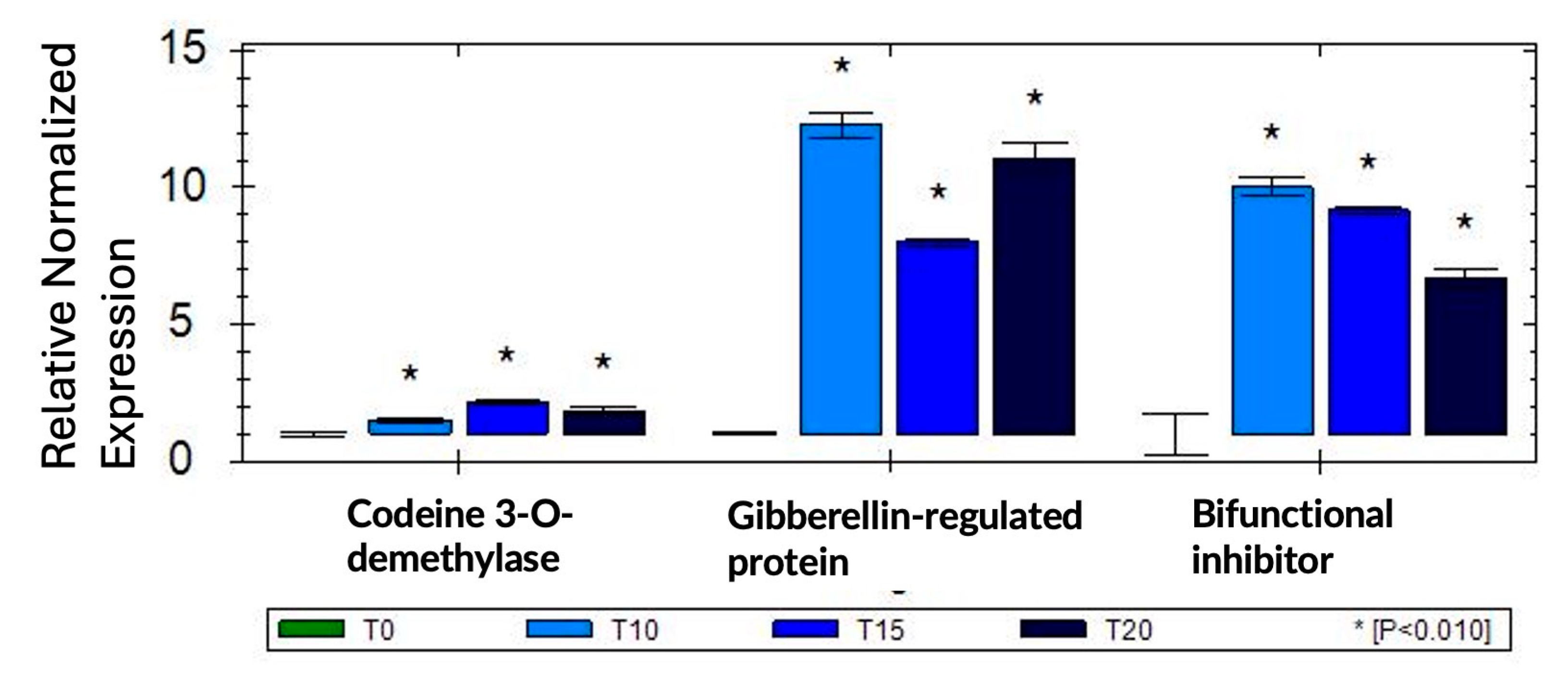
To validate our RNA-seq results, we selected three genes (codein 3-O demethylase, gibberellin-regulated protein, and bifunctional inhibitor/plant lipid transfer protein/seed storage helical-domain containing protein) that were up-regulated in response to sucrose exposure and tested these genes by qRT-PCR in one biological and three technical replications. To normalize gene expression, we used two reference genes (Histone H2A variant3 and proteasome endopeptidase complex) that we identified from a set of four potential reference genes as most stable in response to sucrose using geNorm [
32]. The qRT-PCR results confirmed our RNA-seq data, all three genes showed up-regulation in response to sucrose (
Figure 5).
2.5. GO Analysis Reveals Enrichment of Sugar- and Hormone-Responsive Genes
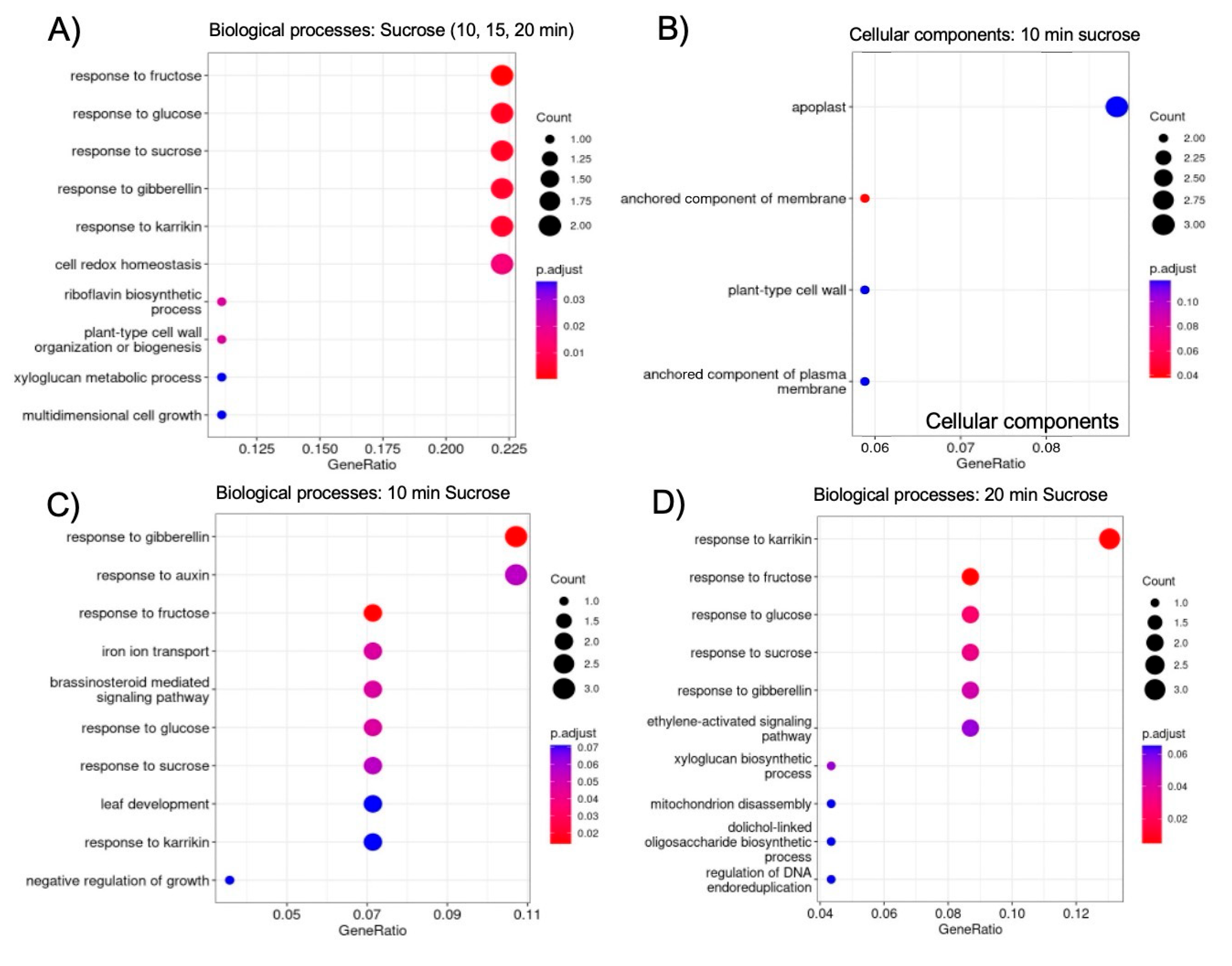
GO (Gene Ontology)-enrichment reveals responses to hormones and to sucrose as enriched biological processes (
Figure 6A). The apoplast, cell wall and membrane are revealed as enriched locations (
Figure 6B). To further delineate the timeline of biological processes that are activated in response to sucrose, we looked separately at enrichment at 10 minutes (
Figure 6C) and 20 minutes (
Figure 6D) of sucrose addition. At 10 minutes of sucrose exposure, responses to the plant hormone gibberellin are most significantly enriched; other responses include responses to auxin, brassinosteroids, and sucrose. In regard to nutrient deficiency signaling, it is of interest that iron ion transport is also an enriched term. At 20 min, the ethylene-activated signaling pathway becomes enriched, revealing a possible involvement of this signaling pathway later in the sucrose response.
2.6. Several Sucrose-Induced Genes Are Also Expressed in Cluster Roots
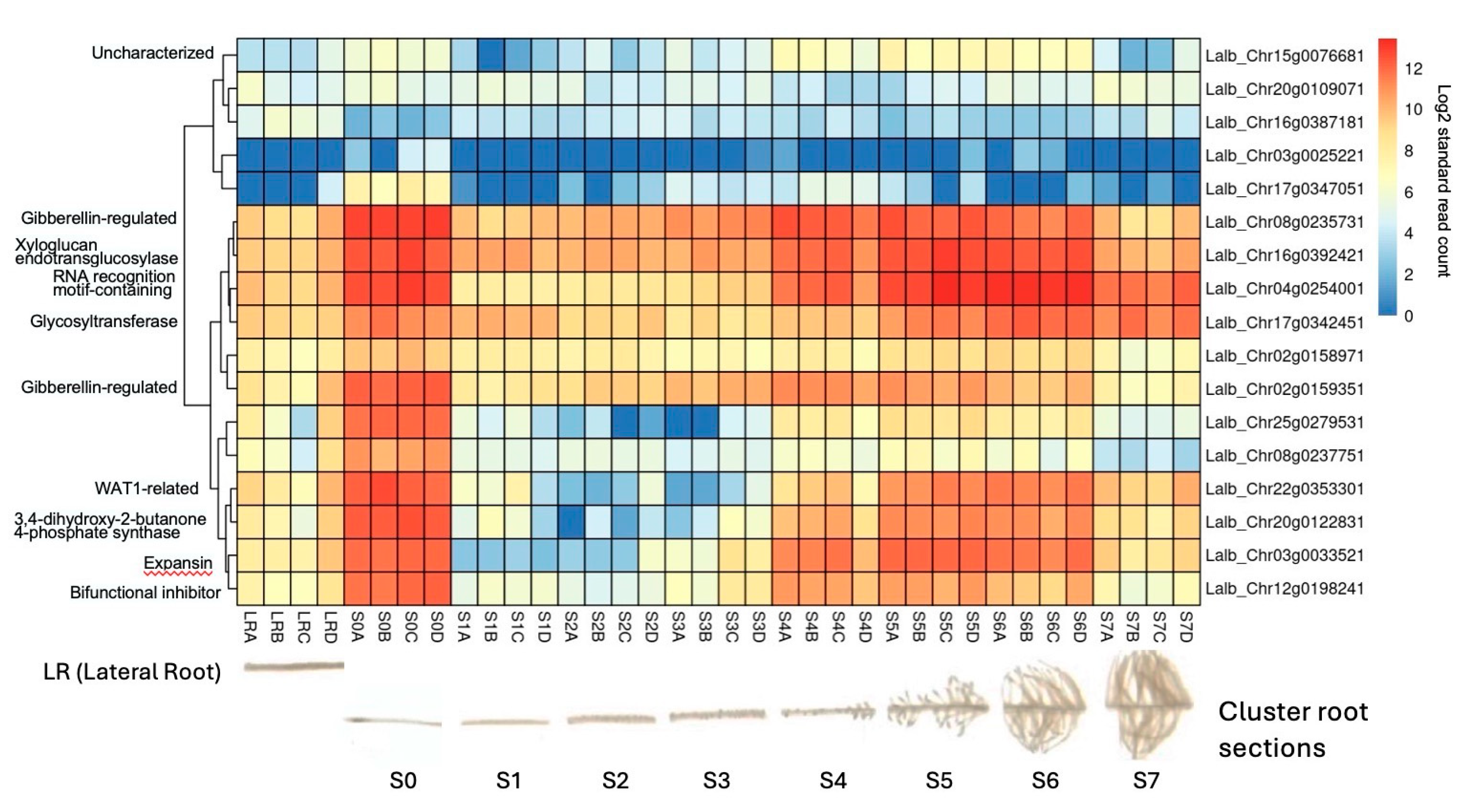
As the molecular mechanisms that control cluster root formation remains unknown, the search for key regulators in cluster root development is of great interest. Because external application of sucrose can induce cluster roots in white lupin [
21,
22], we were interested in identifying genes in our data set that were up-regulated in response to sucrose, and are also differentially expressed in cluster roots. Using the gene expression profile tool of the
Lupinus albus Genome Browser [
30], we performed hierarchical cluster analysis of the 17 genes up-regulated in response to sucrose treatment at all three time points (log2FC
> 1.5, p-value
< 0.05). This analysis identified 10/17 genes that are indeed also up-regulated in cluster root sections, compared to regular lateral roots (
Figure 7). These include the two gibberellin-regulated proteins and WAT1-related (vacuolar auxin transporter). Cluster root sections that show the most up-regulation include S4 (just emerging), S5 (premature) and S6 (mature) cluster roots.
3. Discussion
3.1. A Network of Sucrose-Responsive Genes Is Involved in Cell Growth and Differentiation
White lupin has become a model plant for adaptations to P and Fe deficiency because of its ability to form cluster roots, bottlebrush-like root structurers that enhance P and Fe solubilization and uptake [
4,
5,
33]. Sucrose, transported from the shoot to the roots, has been identified as a long-distance signal for P [
17] and Fe [
19] deficiency in plants, and acts as a signal of cluster root development [
21,
34,
35]. We were interested in unraveling the regulatory network that becomes activated in the root in response to sucrose signaling. To mimic the sucrose signal, we added sucrose directly to the roots of hydroponically grown lupin. Oxford Nanopore sequencing of cDNA proved useful as a method to look at the global gene expression changes in response to such short-term sucrose addition to the roots. Our results revealed significant up- and down-regulation already at 10 min of sucrose addition, and a set of 17 genes that were up-regulated at all three time points (10 min, 15 min, 20 min) of sucrose exposure.
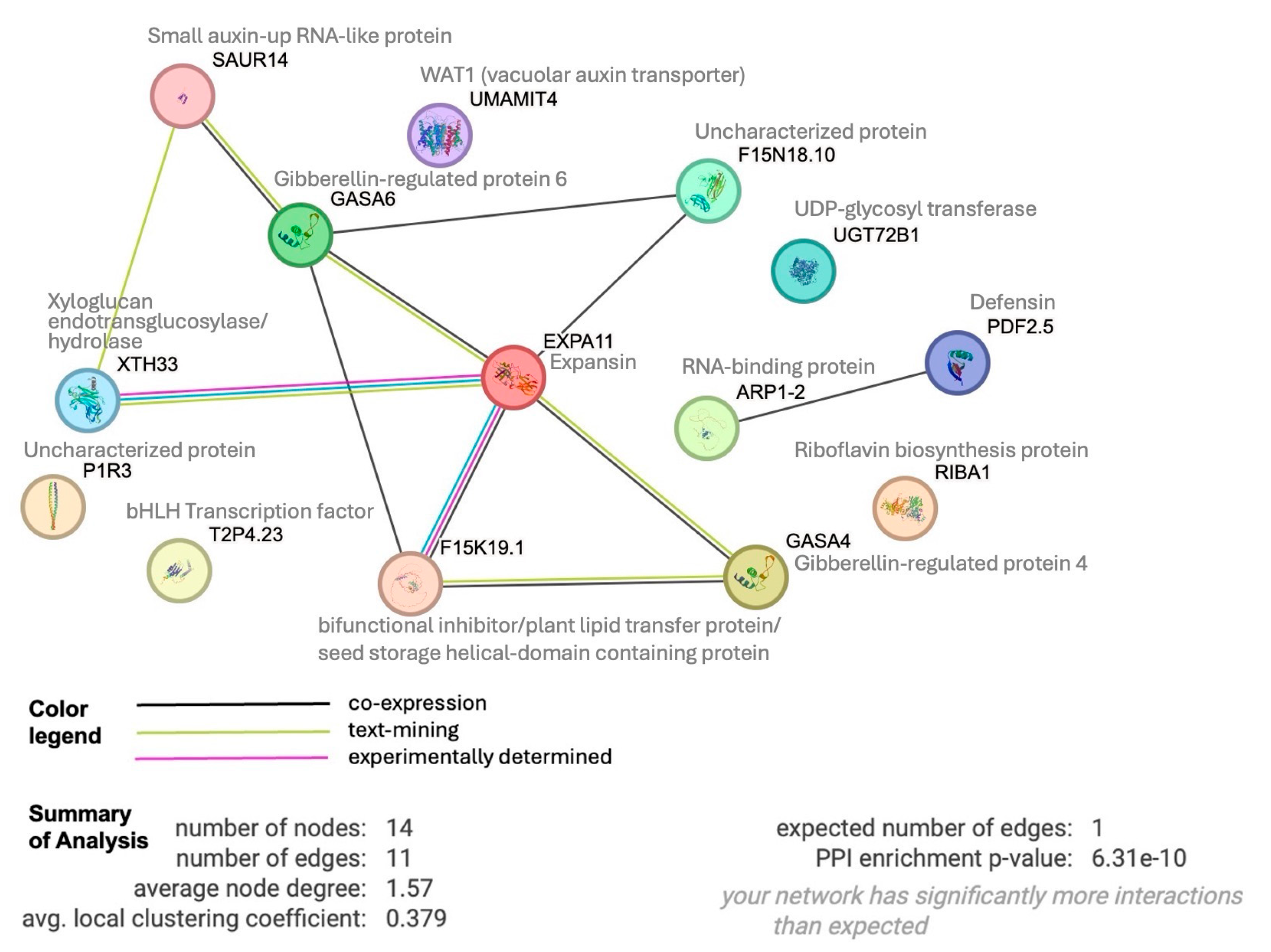
To see if these 17 up-regulated genes share any connections, we performed protein interactome analysis [
36] of the 14 Arabidopsis paralogues we could identify. This analysis revealed an enrichment (p-value 6
-10) of protein-protein associations between these 14 homologs (
Figure 8), indicating relevant biological connections. This interactome depicts expansin as a central hub, around which many interactions center around. Expansins, xyloglucan endotransglucosylase/hydrolase and SAUR (SMALL AUXIN UP-REGULATED RNA) proteins are all involved in auxin-induced cell growth [
37]. WAT1 (WALLS ARE THIN1), while not connected in the interactome, is a vacuolar auxin transport facilitator and has been shown to be important for maintaining cell wall thickness [
38]. These findings indicate a role of auxin in short-term sucrose responses, which is in line with recent findings in Arabidopsis that demonstrate sucrose acting as long-distance signal and regulating the local biosynthesis of auxin at the primary root tip [
39].
In the interactome, expansin also shows connection to a bifunctional inhibitor/plant lipid transfer protein/seed storage (BI/LTP/SS) helical domain-containing protein. Proteins with this domain typically are lipid transfer proteins located in the cell wall that can be involved in key cellular processes, such as the stabilization of membranes, cell wall organization, and signal transduction [
40,
41]. It is worth noting that two other BI/LTP/SS helical domain-containing proteins were also significantly up-regulated at 10 min of sucrose exposure, indicating an important role of members of this protein family in sucrose responses. Defensins, part of our set of 17 up-regulated genes, are also located in the cell wall and extracellular space. They can be induced by pathogen attack, wounding, and some abiotic stresses [
38]. To confirm apoplastic localization of BI/LTP/SS and defensin, we used DeepLoc 2 [
42], which indeed identified signal peptides for these proteins and predicted their likely location as extracellular.
Among the 17 shared up-regulated genes were two bHLH transcription factors. One of these showed highest homology to the bHLH transcription factor UPBEAT1 (UPB1), known to regulate the expression of a set of peroxidases, which in turn modulate the balance of reactive oxygen species (ROS) in the root [
43]. ROS signaling can activate other responses and determine the transition between the meristematic and elongation zones of roots. We looked for differentially expressed peroxidases and found indeed one peroxidase (Lalb_Chr02g0143031) up-regulated already at 10 min of sucrose exposure.
Another type of meristem regulators, Clavata3/ESR (CLE) paralogues were among the most up- and most down-regulated genes at 20 min of sucrose exposure. It is worth noting that the up-regulated and down-regulated genes encode different CLE peptides (CLE 44- and CLE 4-like). CLEs are peptide signals, often transported in the xylem or phloem, shown to bind to leucine-rich repeat receptor-like kinases, though the mechanisms of signal transduction is still largely unknown [
44]. Interestingly, mutant analyses have shown that CLE genes positively affected root sucrose level [
45]. CLEs are known to influence root architecture in other plants [
46], and a possible role of CLE in fine-tuning white lupin cluster root development has recently been suggested [
47].
3.2. Timing and Coordination of Sucrose Responses
GO (Gene Ontology) enrichment comparing 10 min and 20 min of sucrose exposure display much overlap, but also a few interesting differences between time points, which may help to delineate the timeline of sucrose responses. At 10 min of sucrose treatment, responses to auxin and gibberellin are the most enriched biological processes, while responses to ethylene are enriched only at 20 min, indicating that ethylene-mediated responses may act later in the network of sucrose signaling. This finding is in support with our recent work on sucrose-responses in soybean (20 min and 40 min of sucrose exposure), were responses to ethylene were highly enriched 40 min after sucrose addition [
28]. The study in soybean found an increase of ROS signaling and Ca
2+ signaling at 40 min of sucrose, but we were unable to determine the order (does ROS signaling lead to Ca
2+ signaling, or vice versa?). Our current study in white lupin shows enrichment of REDOX events and up-regulation of a peroxidase at 10 min of sucrose treatment, indicating that ROS signaling may be occurring early in the response to sucrose. We did not find any evidence for Ca
2+ signaling in our short-term study, indicating that Ca
2+ signaling – if involved in the sucrose response in white lupin – may occur later.
While our previous study in soybean identified many transcriptional factors, our current study on even earlier time points was successful in narrowing down the number of transcriptional regulators to a total of four up-regulated and one down-regulated transcription factors. Two bHLH transcription factors mentioned above were up-regulated at all three time points, while an AP2-EREB (APETALA2-Ethylene-Responsive Element Binding Proteins) family-type transcription factor was up-regulated at 20 min of sucrose. Interestingly, one WRKY transcription factor, was only up-regulated at 10 min (highest) and 15 min of sucrose exposure, indicating possible involved very early in the sucrose response. Another gene of interest that was upregulated early (10 and 15 min of sucrose) is a Rapid Alkalinization Factor (RALF). RALFs are peptide hormones that control cell wall integrity, cell-to-cell communication, and can act as sensors for regulating responses to environmental stimuli [
48], and thus may play a central part in regulating sucrose responses.
In future, it would be interesting to further analyze the potential function of these transcription factors and the Rapid Alkalinization Factor in response to sucrose. We are also interested in looking at even earlier time points of sucrose exposure, to identify the very earliest responses, which may identify one or few key regulators. We are also interested in analyzing later time points of white lupin responses to sucrose, to reveal if responses to biotic stresses become enriched, as is the case in soybean [
28]. Unraveling the complex network of sucrose responses in plants will be helpful in better understanding how plants integrate various nutrient deficiencies as well as other abiotic and even biotic stress responses, using sucrose not only as a metabolite, but also as a signal.
4. Materials and Methods
4.1. Plant Growth and Treatments
White lupin (
Lupinus albus cv. Amiga) seeds were sterilized by shaking for 3 minutes in 10% bleach, followed by several rinses with sterile water. Sterile seeds were then spread out in sterile petri dishes and covered about halfway with sterile water to germinate at room temperature in the dark for 3-4 days. Once the radicles reached a length of 2-3 cm, the seedlings were transferred to hydroponics containers filled with 850 ml of Hoagland solution [
49], which was changed about every 4 days. The temperature of the growth chamber was maintained at ~21°C with a light cycle of 16 hours and a dark cycle of 8 hours (Leggett et al., 1971).
After 21 days of cultivation in hydroponics, the plant roots were exposed to sucrose by adding 8.5 ml of 1 M sucrose (prepared in Hoagland solution) directly to the hydroponic solution, for a final concentration of 10 mM sucrose. Harvesting was done after exposing the plants to sucrose for different periods: 0 minutes (control), 10 minutes, 15 minutes and 20 minutes. All time points were done in 3 biological replications; each biological replication consists of one plant. For harvesting, about 100 mg of the root tip sections with a length of 5-6 cm were harvested in liquid nitrogen from each plant and stored immediately at -80 deg C.
4.2. RNA Isolation and Quality Check
RNA from white lupin samples was isolated following the protocol for “Purification of Total RNA from Plant Cells and Tissues, and Filamentous fungi” from RNasy Plant Mini kit (Qiagen). The Qubit 4 Fluorometer (Thermo Fisher) in conjunction with an RNA-High Sensitivity assay (Thermo Fisher) was used to assess RNA quantity. In addition, the RNA IQ assay was used to determine the RNA integrity number (RIN). This RIN is based on the ratio of large and/or structured RNA to small RNA in the sample. Only samples with RIN of 8 or higher were used for RNA sequencing.
4.3. cDNA Library Preparation and RNA-Sequencing
Using the PCR-cDNA sequencing-barcoding (SQK-PCB111) kit of Oxford Nanopore Technologies, the extracted RNA was converted into cDNA and uniquely barcoded, following the manufacturer’s instructions. Equal concentration of the four cDNA libraries for each biological replication were pooled and sequenced on three separate MinION FLO-MIN106 flow cells (Oxford Nanopore Technology), using a MinION MK1C running Minknow v20.06.5 and guppy v4.09. Basecalling was performed during the run using the fast-basecalling algorithm with a Q score cutoff >7.
4.4. RNA-seq Data Analysis
Demultiplexed sequencing reads in fastq format were transferred from the Mk1C device to a PC with Epi2me installed. The data was analyzed using the Epi2me wf-transcriptomes workflow version 1.1. available at
https://github.com/epi2me-labs/wf-transcriptomes. This pipeline consists of the following steps: First fastcat was used to concatenate files and generate read statistics, followed by Pychopper to orient, trim and rescue full length cDNA reads. Then Minimap2 was used to map reads to the
Lupinus albus reference genome CNRS_Lalb_1.0 (GCA_009771035.1 assembly; submitted. Dec 20, 2019, (Hufnagel, Marques et al. 2020) which we accessed in Jan 2024. Samtools converted and sorted BAM files, with Seqkit creating alignment statistics. Chunk BAM was used to split aligned BAMs into chunks using the bundle_min_reads parameter (we used the default of 50,000). StringTie was then used to assemble the transcripts based on the aligned segments in the chunked BAM files. The resulting transcript GFF files were merged via Merge Chunks, and GFFCompare was used to compare query and reference annotations, merging and annotating records. Transcriptome FASTA file from the final GFFs were generated using Gffread. The reads from all samples were aligned with the final non-redundant transcriptome using Minimap2 in a splice aware manner. Salmon (
https://github.com/COMBINE-lab/salmon) was used for transcript quantification, giving gene and transcript counts as output. Because the Epi2me workflow did not have a time-course option, we then used this output to analyze differential expression as a time course experiment in DeSeq2, which we also used to create MA plots. We used the UniProt browser-based mapping (
https://www.uniprot.org/id-mapping) to map UniProt gene names to Uniprot accession numbers, protein names, and GO terms. Raw and processed data were submitted to NCBI GEO (accession # GSE268152 ).
4.5. Bioinformatic Analysis
For analysis of interactions among genes, we used sequences of our selected proteins as input for STRING (
https://string-db.org) and selected the best hits based on E-values among
Arabidopsis thaliana homologues. We then used the analysis tab, with a confidence setting of P > 0.1.
4.6. Validation by qRT-PCR
1,500 ng of total RNA for each sample (t0, t10, t15, t20) was treated with RNase-free DNase to eliminate genomic DNA contamination and reverse-transcribed using iScript™ gDNA Clear cDNA Synthesis Kit (BioRad).
Using the Reference Gene Selection Tool of the CFX Maestro software (BioRad), which utilizes the geNorm algorithm [
32], we selected two reference genes (Histone H2A variant3 and proteasome endopeptidase complex) from a group of four candidates as most stable in response to sucrose addition.
Primers were designed using primer 3 [
50] for the two reference genes and three target genes: codein 3-O demethylase, gibberellin-regulated protein, and bifunctional inhibitor/plant lipid transfer protein/seed storage helical-domain containing protein, with amplicon sizes between 60-200 bp, and no or low 3’ complementarity to avoid primer dimers. Primer sequences were as follows:
Histone H2A variant3 (Lalb_Chr11g0072021) forward: GAAGTTGCTATTGTTGATCTTGG, reverse: GCTGCATTGTTAATCACCTTTT;
Proteasome endopeptidase complex (Lalb_Chr08g0243601) forward: TGCCTTTATGCCCTGCTGTA, reverse: CATCAAGCAACGCAAAACATG
Codeine 3-O-demethylase (Lalb_Chr20g0109071) forward: GGTGAGTTAGGTCCAGCATCT, reverse: ACTCCTGTTGTTTTGTACTGTGC;
Bifunctional inhibitor (Lalb_Chr05g0225671) forward: TCAACTACTGTGGAAAGGGTGT, reverse: GCCAACGAGCTTCAGAAACC;
Gibberellin regulated protein (Lalb_Chr02g0159351) forward: ACCTGGCAGTCTCAAAAGCT, reverse: TTTGTGGTACTGGGTCTGGC
qPCR was performed using SYBR Green Supermix (BioRad) on a BioRad CFX96 instrument set to 30s at 95°C followed by 40 cycles of 95°C (15s) and 60°C (30s). After amplification, a melt curve analysis was performed from 65°C to 95°C with a 0.5°C increment every 5s. Cq values were called using the CFX Maestro Software (BioRad). Standard curves for all five genes were prepared and amplification efficiency determined. Relative gene expression was calculated using the ∆∆Cq method.
Author Contributions
Conceptualization, T.S. and C.U.-S.; methodology, T.S., Y.S.L., J.T., A.M., J.M., M.W., M.M., I.C. and C.U.-S.; software, T.S., Y.S.L., J.T., A.M., J.M., M.W., M.M., I.C. and C.U.-S.; validation, Y.S.L., J.T., A.M., J.M., M.W., M.M., I.C. and C.U.-S.; data curation, C.U.-S. ; writing—original draft preparation, T.S. and C.U.-S.; writing—review and editing, T.S., Y.S.L., J.T., A.M., J.M., M.W., M.M., I.C. and C.U.-S.; software, T.S., Y.S.L., J.T., A.M., J.M., M.W., M.M., I.C. and C.U.-S.; supervision, C.U.-S.; project administration C.U.-S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Our data is freely accessible at NCBI GEO: GSE268152.
References
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Howarth, R.W. Phosphorus in all its formsThe Devil's Element: Phosphorus and a World Out of Balance Dan Egan Norton, 2023. 256 pp. Science 2023, 379, 1096. [Google Scholar] [CrossRef]
- Ahmad, N.; Usman, M.; Ahmad, H.R.; Sabir, M.; Farooqi, Z.U.R.; Shehzad, M.T. Environmental implications of phosphate-based fertilizer industrial waste and its management practices. Environ Monit Assess 2023, 195, 1326. [Google Scholar] [CrossRef]
- Uhde-Stone, C. White lupin: a model system for understanding plant adaptation to low phosphorus availability. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability; Springer: 2017; pp. 243-280.
- Zanin, L.; Venuti, S.; Marroni, F.; Franco, A.; Morgante, M.; Pinton, R.; Tomasi, N. Physiological and RNA sequencing data of white lupin plants grown under Fe and P deficiency. Data Brief 2019, 25, 104069. [Google Scholar] [CrossRef]
- Neumann, G.; Massonneau, A.; Langlade, N.; Dinkelaker, B.; Hengeler, C.; Römheld, V.; Martinoia, E. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of botany 2000, 85, 909–919. [Google Scholar] [CrossRef]
- Massonneau, A.; Langlade, N.; Léon, S.; Smutny, J.; Vogt, E.; Neumann, G.; Martinoia, E. Metabolic changes associated with cluster root development in white lupin (Lupinus albus L.): relationship between organic acid excretion, sucrose metabolism and energy status. Planta 2001, 213, 534–542. [Google Scholar] [CrossRef]
- Sas, L.; Rengel, Z.; Tang, C. Excess cation uptake, and extrusion of protons and organic acid anions by Lupinus albus under phosphorus deficiency. Plant Sci 2001, 160, 1191–1198. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Cheng, L.; Zhang, A.; Zhang, W.; Zhang, F.; Liu, J.; Cao, Y.; Allan, D.; Vance, C. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytologist 2010, 187, 1112–1123. [Google Scholar] [CrossRef]
- Johnson, J.; Allan, D.; Vance, C. Phosphorus Stress-Induced Proteoid Roots Show Altered Metabolism in Lupinus albus. Plant physiology 1994, 104, 657–665. [Google Scholar] [CrossRef]
- Zhou, Y.; Olt, P.; Neuhauser, B.; Moradtalab, N.; Bautista, W.; Uhde-Stone, C.; Neumann, G.; Ludewig, U. Loss of LaMATE impairs isoflavonoid release from cluster roots of phosphorus-deficient white lupin. Physiol Plant 2021, 173, 1207–1220. [Google Scholar] [CrossRef]
- Uhde-Stone, C.; Zinn, K.E.; Ramirez-Yanez, M.; Li, A.; Vance, C.P.; Allan, D.L. Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant physiology 2003, 131, 1064–1079. [Google Scholar] [CrossRef]
- O'Rourke, J.A.; Yang, S.S.; Miller, S.S.; Bucciarelli, B.; Liu, J.; Rydeen, A.; Bozsoki, Z.; Uhde-Stone, C.; Tu, Z.J.; Allan, D.; et al. An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant physiology 2013, 161, 705–724. [Google Scholar] [CrossRef]
- Secco, D.; Shou, H.; Whelan, J.; Berkowitz, O. RNA-seq analysis identifies an intricate regulatory network controlling cluster root development in white lupin. BMC Genomics 2014, 15, 230. [Google Scholar] [CrossRef]
- Wang, Z.; Straub, D.; Yang, H.; Kania, A.; Shen, J.; Ludewig, U.; Neumann, G. The regulatory network of cluster-root function and development in phosphate-deficient white lupin (Lupinus albus) identified by transcriptome sequencing. Physiologia plantarum 2014, 151, 323–338. [Google Scholar] [CrossRef]
- Shu, L.; Shen, J.; Rengel, Z.; Tang, C.; Zhang, F. Cluster root formation by Lupinus albus is modified by stratified application of phosphorus in a split-root system. Journal of plant nutrition 2007, 30, 271–288. [Google Scholar] [CrossRef]
- Hammond, J.; White, P. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. J Exp Bot 2008, 59, 93–109. [Google Scholar] [CrossRef]
- Hammond, J.P.; White, P.J. Sugar signaling in root responses to low phosphorus availability. Plant physiology 2011, 156, 1033–1040. [Google Scholar] [CrossRef]
- Lin, X.Y.; Ye, Y.Q.; Fan, S.K.; Jin, C.W.; Zheng, S.J. Increased Sucrose Accumulation Regulates Iron-Deficiency Responses by Promoting Auxin Signaling in Arabidopsis Plants. Plant physiology 2016, 170, 907–920. [Google Scholar] [CrossRef]
- Pontis, H.G. On the scent of the riddle of sucrose. Trends Biochem Sci 1978, 3, 137–139. [Google Scholar] [CrossRef]
- Zhou, K.; Yamagishi, M.; Osaki, M.; Masuda, K. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. J Exp Bot 2008, 59, 2749–2756. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J.; Ludewig, U.; Neumann, G. A re-assessment of sucrose signaling involved in cluster-root formation and function in phosphate-deficient white lupin (Lupinus albus). Physiologia plantarum 2015, 154, 407–419. [Google Scholar] [CrossRef]
- Lei, M.; Liu, D. Sucrose regulates plant responses to deficiencies in multiple nutrients. Plant Signal Behav 2011, 6, 1247–1249. [Google Scholar] [CrossRef]
- Ruffel, S. Nutrient-related Long-Distance Signals: common players and possible crosstalk. Plant Cell Physiol 2018. [CrossRef]
- Thibaud, M.C.; Gineste, S.; Nussaume, L.; Robaglia, C. Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. Plant Physiol Biochem 2004, 42, 81–88. [Google Scholar] [CrossRef]
- Morkunas, I.; Marczak, L.; Stachowiak, J.; Stobiecki, M. Sucrose-induced lupine defense against Fusarium oxysporum. Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem 2005, 43, 363–373. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Vo, K.T.X.; Cho, L.H.; Hoang, T.V.; Peng, X.; Kim, E.J.; Win, K.; Lee, S.W.; Jung, K.H.; et al. Sucrose preferentially promotes expression of OsWRKY7 and OsPR10a to enhance defense response to blast fungus in rice. Front Plant Sci 2023, 14, 1117023. [Google Scholar] [CrossRef]
- Nidumolu, L.C.M.; Lorilla, K.M.; Chakravarty, I.; Uhde-Stone, C. Soybean Root Transcriptomics: Insights into Sucrose Signaling at the Crossroads of Nutrient Deficiency and Biotic Stress Responses. Plants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci 2021, 302, 110703. [Google Scholar] [CrossRef]
- Hufnagel, B.; Marques, A.; Soriano, A.; Marques, L.; Divol, F.; Doumas, P.; Sallet, E.; Mancinotti, D.; Carrere, S.; Marande, W.; et al. High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nature communications 2020, 11, 492. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014, 15, 550. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Johnson, J.; Vance, C.; Allan, D. Phosphorus deficiency in Lupinus albus. Altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant physiology 1996, 112, 31–41. [Google Scholar]
- Wang, Z.; Rahman, A.B.; Wang, G.; Ludewig, U.; Shen, J.; Neumann, G. Hormonal interactions during cluster-root development in phosphate-deficient white lupin (Lupinus albus L.). J Plant Physiol 2015, 177, 74–82. [Google Scholar] [CrossRef]
- Liu, J.; Samac, D.; Bucciarelli, B.; Allan, D.; Vance, C. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. the Plant journal : for cell and molecular biology 2005, 41, 257–268. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic acids research 2022. [CrossRef] [PubMed]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: the plant's toolbox for adaptation of growth and development. J Exp Bot 2019, 70, 17–27. [Google Scholar] [CrossRef]
- Bacete, L.; Melida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. The Plant journal : for cell and molecular biology 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose drives the lateral root clock in Arabidopsis seedlings. Current biology : CB 2023, 33, 2201-2212 e2203. [CrossRef]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: presenting new advances and an integrated functional analysis. J Exp Bot 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [PubMed]
- Kader, J.C. Lipid-Transfer Proteins in Plants. Annu Rev Plant Physiol Plant Mol Biol 1996, 47, 627–654. [Google Scholar] [CrossRef] [PubMed]
- Thumuluri, V.; Almagro Armenteros, J.J.; Johansen, A.R.; Nielsen, H.; Winther, O. DeepLoc 2. Nucleic acids research 2022, 50, W228–W234. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Li, C.; Yu, G. Modulatory Role of Reactive Oxygen Species in Root Development in Model Plant of Arabidopsis thaliana. Front Plant Sci 2020, 11, 485932. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, M.; Nilsson, O. CLE peptide signaling in plants–the power of moving around. Physiologia plantarum 2015, 155, 74–87. [Google Scholar] [CrossRef]
- Okamoto, S.; Kawasaki, A.; Makino, Y.; Ishida, T.; Sawa, S. Long-distance translocation of CLAVATA3/ESR-related 2 peptide and its positive effect on roots sucrose status. Plant physiology 2022, 189, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, S.; Aoyama, T.; Sato, Y.; Kajiwara, T.; Ishida, T.; Sawa, S. CLE3 and its homologs share overlapping functions in the modulation of lateral root formation through CLV1 and BAM1 in Arabidopsis thaliana. The Plant journal : for cell and molecular biology 2023, 113, 1176-1191. [CrossRef]
- Olt, P.; Ding, W.; Schulze, W.X.; Ludewig, U. The LaCLE35 peptide modifies rootlet density and length in cluster roots of white lupin. Plant Cell Environ 2024, 47, 1416–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, P.T.; Zhou, M.; Liu, H.Z.; Xu, X.J.; Liu, W.T.; Chen, K.M. Rapid alkalinization factor: function, regulation, and potential applications in agriculture. Stress Biol 2023, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. California agricultural experiment station 1950, 347. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic acids research 2012, 40, e115. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).