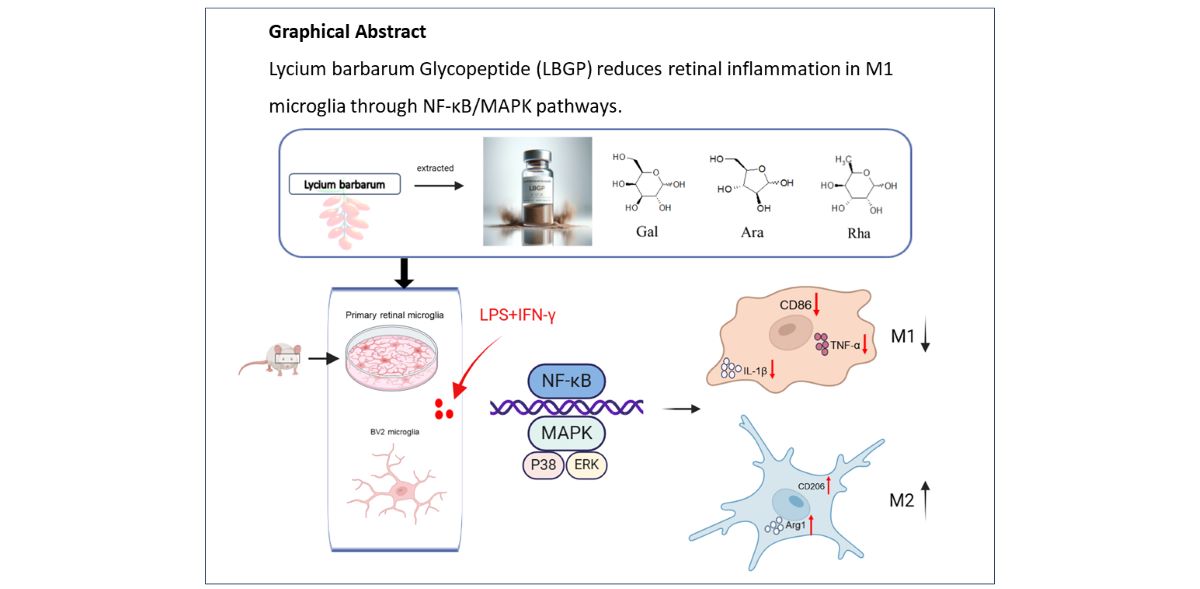
1. Introduction
Microglia, the principal innate immune cells in the retina, are a significant subset of glial cells [
1,
2]. Under normal conditions, these cells are pivotal for the protection of retinal tissue, removal of cellular debris, and the preservation of retinal homeostasis [
3,
4]. Upon retinal damage, microglia transition from a resting state to an activated form with an amoeboid shape, migrating to the site of damage and secreting pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6 [
5,
6]. Microglial reactivity is a common hallmark of retinal diseases.
These two microglial polarization are known as the classically activated M1 (pro-inflammatory) phenotype and the alternatively activated M2 (anti-inflammatory) phenotype [
7,
8]. The M1 activation phenotype in microglia elevated production of pro-inflammatory factors, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), inducible nitric oxide synthase (iNOS), CD16, and CD86, which can exacerbate retinal damage and impede cellular repair in retinal disorders. Conversely, the M2 phenotype is associated with an increase in anti-inflammatory mediators, such as arginase-1 (Arg-1), CD206, and transforming growth factor-β (TGF-β), which confer retinal protection and facilitate retinal recovery and remodeling [
9]. Dysregulation of these mediators in microglial cells has been implicated in the pathogenesis of retinal diseases in human patients and in retinal damage observed in experimental animal models [
10,
11].
The underlining pathways involved in microglial transformation are the nuclear factor-κB (NF-κB) p65 and mitogen-activated protein kinase (MAPK) p38, a set of signaling molecules that are pivotal in regulating the expression of pro-inflammatory cytokines [
12,
13]. Activation of the MAPK signaling pathway aids in mediating the production of pro-inflammatory factors by microglia following retinal injury [
14]. Previous studies have shown that hyper-phosphorylation of MAPK molecules ultimately activates the transcription factor NF-κB, leading to subsequent inflammatory responses [
15]. Moreover, retinal inflammation following retinal diseases can be induced by activated microglia through NF-κB signaling pathway [
16]. Thus, microglia can be regarded as a potential therapeutic target to be modulated in the treatment of retinal diseases.
Lycium barbarum polysaccharide (LBP), the primary bioactive compound extracted from Lycium barbarum berries, has been demonstrated to have anti-inflammatory, anti-oxidation, anti-aging properties, as well as benefits for reproductive protection, liver protection, eye protection, and immune regulation [
17]. Lycium barbarum glycopeptide (LBGP) is a glycoconjugate which is further purified from LBP and considered to be the most active component of Lycium barbarum [
18,
19]. However, research on the biology of LBGP remains limited. Whether LBGP has a beneficial effect on LPS/IFN-γ induced retinal microglial activation needs to be investigated. In this study, we investigated whether LBGP has anti-inflammatory effect in active microglia cells and then analyzed the potential signaling pathways of retinal diseases. These findings might provide new alternative avenues for the treatment of retinal-related disorders.
2. Result
2.1. Effects of LBGP on the Viability of Microglia Cells and Cell Morphology
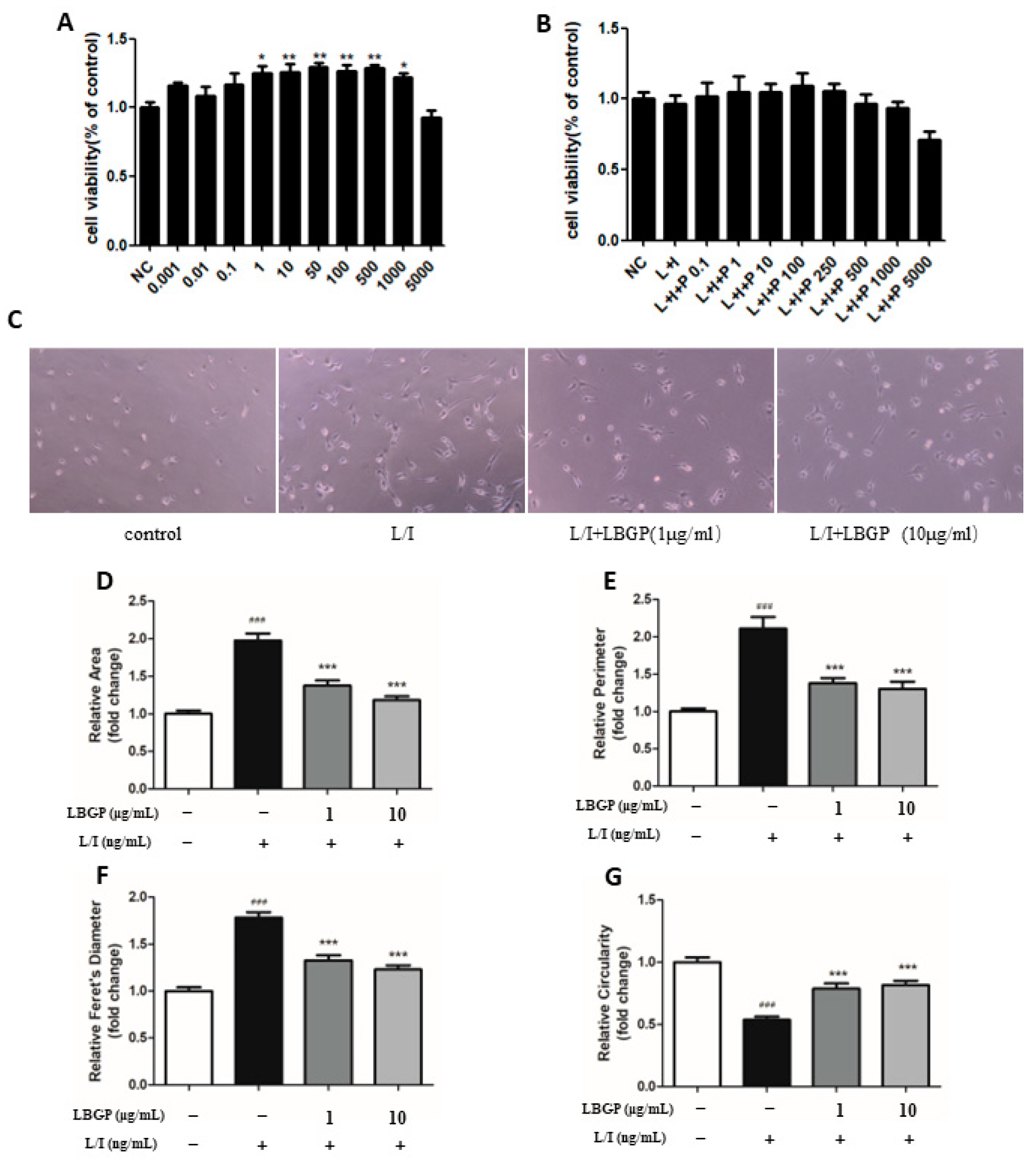
To assess the impact of LBGP on BV2 cells, a cell counting kit-8 (CCK-8) assay was conducted. The viability of BV2 cells was determined following treatments with various concentrations of LBGP for 24 hours. The results demonstrated a significant improvement in BV2 cell viability after treatment with various concentrations of LBGP ranging from 0.001μg/ml to 1000μg/ml (
Figure 1A). BV2 cells were pre-treated with various concentrations of LBGP for 2 hours, followed by incubation with L/I for an additional 24 hours. The viability of BV2 cells was decreased by LPS, whereas it was increased by LBGP (
Figure 1B). Therefore, we selected concentrations of 1 and 10 µg/mL of LBGP for the subsequent experiments.
To further verify the impact of LBGP on primary retinal microglial cells, primary microglia were cultured from mouse retinas. Microglial purity was assessed using the markers IBA1, CD11b, and F4/80 (Supplementary Figure). Upon microscopic observation, microglia exhibited 'amoeba-like' characteristics and cell body enlargement after being stimulated by L/I (
Figure 1C). Moreover, LBGP pre-treatment significantly reversed alterations of L/I-induced microglial in area (
Figure 1D), perimeter (
Figure 1E), Feret's diameter (
Figure 1F), and roundness (
Figure 1G) compared to the control group. These data showed that the optimal concentration of LBGP increases cell viability and plays a crucial role in L/I-induced microglial cell activation.
2.2. Effects of LBGP on the L/I-Induced Microglial Cell Migration
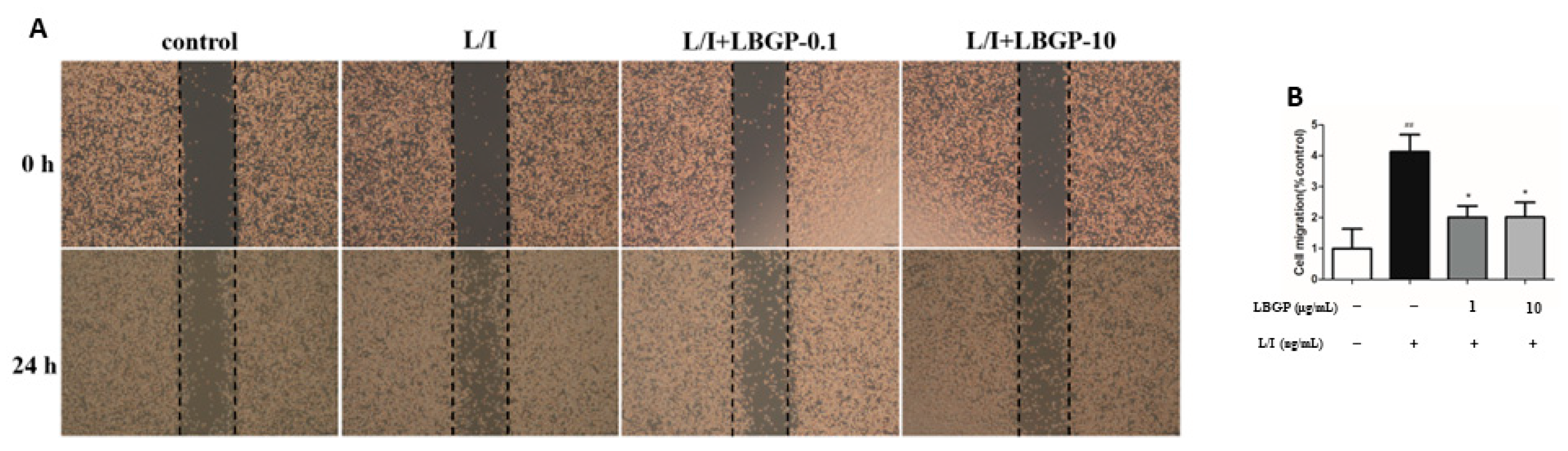
Inflammatory responses are associated with microglial cell movement [
23]. To elucidate the impact of LBGP on L/I-induced BV2 cell migration, we conducted wound healing assays. As shown in
Figure 2A, L/I markedly increased BV2 cell migration during 24 h of treatment compared with the control group. Interestingly, pre-treatment of LBGP repressed L/I-induced cell migration.
2.3. Effects of LBGP on the Production of NO and Inflammatory Cytokines in L/I-Induced Microglial Cells
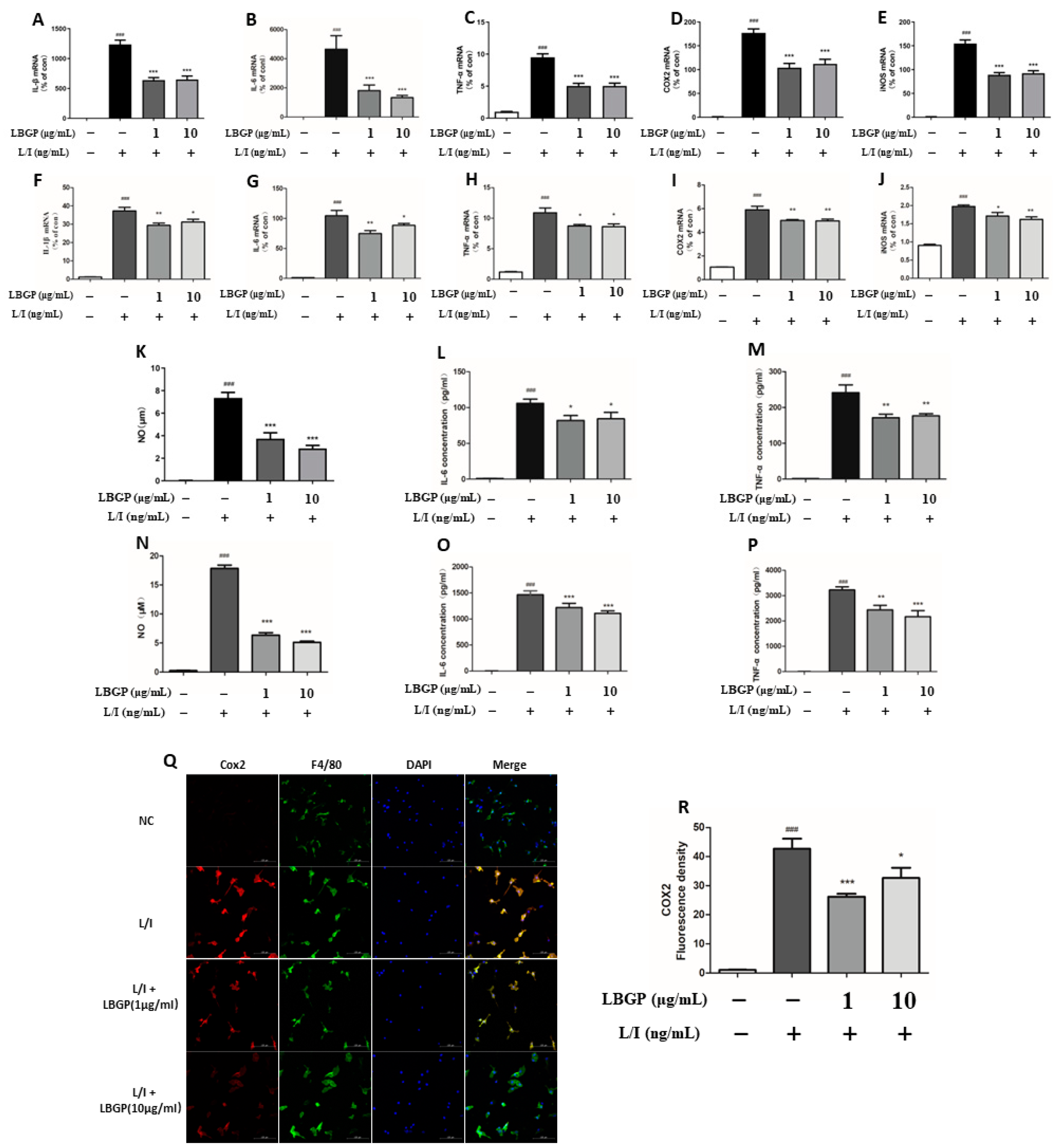
To investigate the anti-inflammatory effects of LBGP on L/I-induced BV2 microglia and retinal primary microglia cells, we initially assessed the production of NO and inflammatory cytokines. Results showed that LBGP significantly reduced the production of NO in BV2 and retinal primary microglia microglia cells (
Figure 3A and B). Furthermore, mRNA levels of pro-inflammatory cytokines, including COX-2, IL-6, IL-1β, iNOS, and TNF-α measured using qPCR were significantly induced by L/I. However, the level of pro-inflammatory cytokine was greatly decreased by pre-treatment with LBGP (
Figure 3G-P). It was further confirmed with ELISA assay for IL-6 and TNF-α. Consistent with the findings above, LBGP significantly reduced L/I-induced IL-1β, IL-6, and TNF-α levels compared with L/I treatment (
Figure 3C-F).
2.4. Effects of LBGP on L/I-Induced Microglial Polarization and Inflammatory Cytokines Production
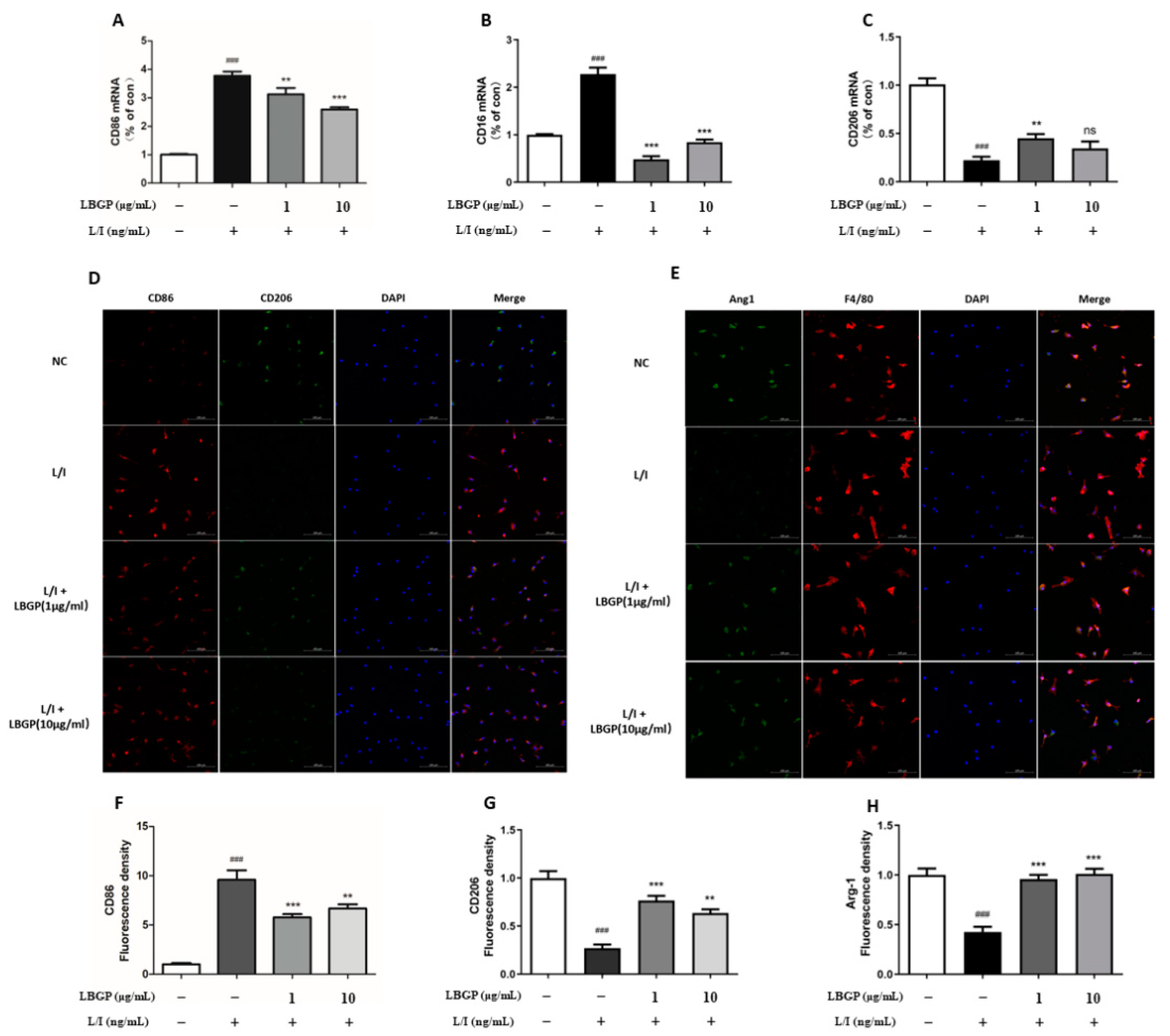
LBGP significantly reduced the mRNA levels of CD16 and CD86 and upregulated the CD206, suggesting that the LBGP could suppress the L/I-induced M1 polarization and promote M2 polarization (
Figure 4A-C). Moreover, CD86, CD16, and COX2 were used as the markers of M1 polarization, while CD206 and Arg1 were employed as the markers of M2 polarization, in primary retinal microglia. Upregulation of CD86 and COX2 immunoreactivity and diminished CD206 and Arg1 immunoreactivity were detected in L/I-induces cells, while partially reversed by LBGP intervention (
Figure 3Q and
Figure 4D-H).
2.5. Effects of LBGP on L/I-Induced NF-κB/MAPK Signaling Pathways
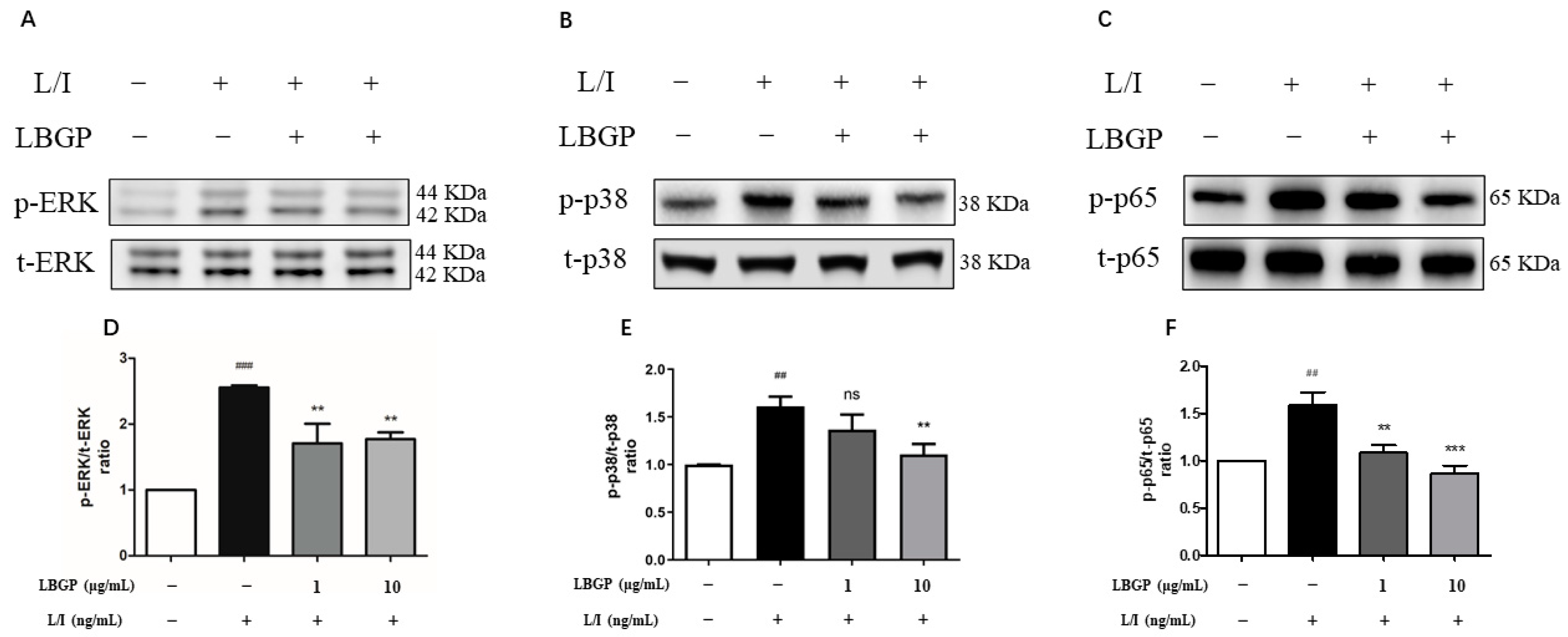
To further investigate the underlying mechanism of LBGP on L/I-induced microglial activation, the activation of NF-κB and MAPK pathways was analyzed, two key nuclear transcription factors that are essential in regulating inflammation. Levels of the phosphorylation and total proteins, p38, ERK, and p65, involved in these pathways were evaluated using western blotting. L/I treatment led to increased phosphorylation of p38, ERK, and p65, which were then suppressed by LBGP intervention in BV2 cells (
Figure 5A-C). These data suggest that the anti-inflammatory effects of LBGP in microglial cells may involve the MAPK and NF-κB signaling pathways.
3. Discussion
Retinal microglia inflammatory activation is one of the vital processes involved in retinal diseases, including diabetic retinopathy (DR), age-related macular degeneration (AMD), and retinitis pigmentosa (RP), et al. [
24,
25]. Consequently, this process is also considered as a promising target in the management of retinal diseases. In this study, we identified the anti-inflammatory effects of LBGP on L/I-induced retinal inflammation and elucidated the potential mechanisms involved in the inhibition of microglial activation.
During the progression of retinal diseases, activated microglia manifest in increasing activity of amoeba transformation, migration, proliferation, and polarization, a series of complex pathophysiological responses [
26,
27,
28,
29]. Meanwhile, polarization activation of retinal microglia results in the maintenance of an inflammatory balance between the release of proinflammatory (M1 microglia phenotype) and anti-inflammatory (M2 phenotype) mediators [
30,
31]. Several research studies have confirmed that the M1 microglia phenotype induces toxic effects in the retina to produce various inflammatory cytokines such as TNF-α, IL-1β, and IL-6, thereby inducing damage [
32,
33,
34]. Our findings have shown that LBGP exerts inhibitory effects on retinal microglial inflammation. Furthermore, LBGP demonstrated the capacity to modulate microglial M1/M2 phenotypic polarization in vitro.
M2 cells have been shown with the overexpression of Arg1 and CD206, which not only inhibite the expression of proinflammatory mediators but also provide protection to the retina [
35,
36]. U Under the stimulation via LPS and IFN-γ, primary microglia and BV2 cells become polarized towards the M1 phenotype accompanied by overexpression of CD16 and CD86, while pretreatment with LBGP induced the overexpression Arg1 and CD206, suggesting the polarization of microglia shift from the M1 to the M2 phenotype. Collectively, these data emphasize that LBGP-elicited microglial M1/M2 polarization may have an impact on retinal inflammatory diseases. LBGP might be a potential agent to treat microglia-mediated retinal inflammatory disorders.
Current research suggests that various signaling pathways are involved in microglial activation [
37,
38,
39]. As evidenced by our previous study, which demonstrated that NF-κB and MAPK pathways are significantly involved in activated microglia [
40]. NF-κB-mediated inflammatory cascade leads to a substantial release of inflammatory cytokines, chemokines, and adhesion molecules, contributing to pathological changes in retinal tissues and resulting in intractable chronic inflammation [
41,
42]. While MAPK signaling pathway is known to play an important role in inducing proinflammatory cytokines in retinal diseases through controlling NF-κB activity, with the functional expression and secretion of various inflammatory cytokines including TNF-α, IL-6, IL-1β, COX-2, and iNOS [
43,
44,
45]. These signaling activation has also been associated with inflammatory diseases such as DR, AMD and RP [
46]. Targeting these pathways could have potential in preventing progression in these retinal diseases [
47]. Our study further indicated that LBGP suppressed the phosphorylation of p38 and ERK1/2 induced by L/I. Herein, the anti-inflammatory properties of the LBGP are mediated by the inhibition of the phosphorylation of NF-κB, and the activation of MAPK proteins (p38 and ERK), suggesting the potential mechanism of anti-inflammatory effect of LBGP in retinal inflammation.
LBGP, a natural compound, is extracted from Lycium barbarum berries. Previous studies have found that it possesses numerous pharmacological properties, particularly including anti-inflammation effects [
48,
49,
50]. LBGP attenuates aversive stimuli (AS)-induced inflammation and activation of microglia in vitro [
51], which is consistent with our data. Moreover, Huang et al. demonstrated that LBGP reduced inflammation in dextran sulfate sodium (DSS)-induced mouse models [
52]. hese findings and our data together suggest that LBGP is effective in decreasing pro-inflammation responses. In ophthalmology, LBGP has been sparingly used to treat inflammation-relative eye diseases. However, the potential of LBGP in treating ophthalmic conditions like DR, AMD, and RP remains largely unexplored, although this study confirms that regulating microglial activation is a viable therapeutic target for preserving retinal function. Therefore, how to employ LBGP to function specifically and in a targeted manner needs to be addressed in further studies.
Taken together, these results indicate that LBGP suppresses the production of proinflammatory cytokines after L/I treatment by downregulating the phosphorylation of MAPKs (p38MAPK, ERK) and NF-кB. These are key signaling molecules and transcription factors involved in the production of pro-inflammatory cytokines in microglial cells. The limitations of our study include the absence of in vivo experiments on acute inflammation due to time constraints. Therefore, we used retinal primary microglia in vitro to supplement the methodology. However, it is regrettable in this study that the validation of signaling pathways could not be conducted. Future work may be necessary to elucidate the specific signaling pathway for further in-depth investigation into the intrinsic mechanisms of this subject.
4. Methods
4.1. Cell Culture and Treatment
The BV2 microglial cell line was obtained from the National Infrastructure of Cell Line Resource (China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) (Sigma, USA) and 1% penicillin-streptomycin, under a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
Primary retinal microglial cultures were derived from postnatal (8–10 days) C57BL/J mice. Retinas were dissected under a microscope and subjected to papain digestion for 15 minutes at 37°C. After filtration through a 40 μm filter, cells were resuspended in F12/DMEM medium containing 20% FBS, 1% penicillin-streptomycin, and 10 μg/mL recombinant macrophage colony-stimulating factor (bio-techne, Shanghai, China) before being seeded into T25 culture flasks [
20,
21]. After 14 days of culture, microglial cells were exposed to mild trypsinization (0.0625%) for 30 minutes at 37°C, followed by 0.25% trypsin for 10 minutes at 37°C to eliminate macroglia [
22]. Microglial purity was determined by staining with the microglia-specific markers IBA1, F4/80, and CD11b [
20].
We divided into three groups including Control, LPS+IFN-γ(L/I), and L/I+LBGP groups. BV2 cells and primary microglia were pre-treated with different concentrations of LBGP for 2 hours (1 μg/ml and 10 μg/ml). At the end of pre-treatment, 100 ng/ml LPS (Sigma, USA) and 20 ng/ml IFN-γ (Sigma, USA) was added for further 24 h.
4.2. Cell Viability Assay and Morphological Analysis
Cell viability was assessed using the CCK8 assay (MCE, USA) in 96-well plates, with a cell density of 5000 cells per well. Different concentrations of LBGP with or without LPS and IFN-γ (L/I) for 24 h. Subsequently, 10 μL of CCK8 reagent was added to each well and incubated for 2 hours at 37°C. Absorbance was measured at 450 nm using a microplate reader (Biotek, Winooski, VT, USA). For morphological analysis, cells were observed under a light microscope (Nikon, Tokyo, Japan) at 20× magnification.
4.3. NO Assay
BV2 cells were seeded in 96-well plates at a density of 2.0 × 104 cells per well and cultured for 24 hours. Cells were pretreated with the indicated concentrations of LBGP (1μg/ml and 10μg/ml) for 2h, and then L/I was used to stimulate primary retinal microglia and BV2 microglia cells for 24 h. Concentrations of NO in the culture media were determined using the Nitric Oxide Assay Kit (Beyotime, China), based on Griess reaction.
4.4. Wound-Healing Assay
BV2 cells were seeded onto 6-well plates at a density of 5 × 105 cells per well and incubated at 37°C until reaching 80–90% confluence. The cells were scratched with a 10μl pipette tips to create a wound. The unattached cells were removed and treated with LBGP (1μg/ml and 10μg/ml) with or without L/I in serum free culture medium for 24h. Images at 0 and 24 h were captured under a light microscope (Nikon, Tokyo, Japan). Cell migration was determined using ImageJ software and was presented as a percentage of the control cells. (Percentage of migration (%) = (A1-A0)/A1 × 100, where A1 represents the scratch area and A0 represents the remaining area of the wound at the metering point).
4.5. Quantitative PCR (qPCR)
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen, USA) following the manufacturer’s instructions. cDNA was synthesized from the extracted RNA using HiScript II Q Select RT SuperMix for qPCR (Vazyme, China).The qPCR was conducted with ChamQ Universal SYBR qPCR Master Mix (Vazyme, China) on a Roche 96 system (Roche, USA). The results were analyzed by the 2
-ΔΔCt method and given as a ratio compared with the control. All primer sequences used are listed in
Table 1.
4.6. Enzyme-Linked Immunosorbent Assay
The supernatants from primary retinal microglia and BV2 microglia cells were collected by centrifugation at 850g at 4°C for 15 min, levels of IL-6 and TNF-α were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits (Absin, Shanghai, China & NEOBIOSCIENCE, China) according to manufacturer’s instructions.
4.7. Western Blotting
Cellular proteins were extracted from BV2 cells using RIPA lysis buffer (Beyotime, China) containing protease and phosphatase inhibitors. Protein concentration was determined using a bicinchoninic acid (BCA) Protein Kit (Vazyme, China). Equal amounts of protein from each sample were separated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking at room temperature with 5% blocking buffer (5% BSA in Tris-buffered saline with 0.1% Tween 20) for 1 hour, membranes were incubated with primary antibodies, followed by secondary antibodies (1:10000, LI-COR Bioscience, USA). The primary antibodies included anti-p-ERK (1:1000, Abcam, UK), anti-t-ERK (1:1000, Abcam, UK), anti-p-NF-κB p65 (1:1000, Abcam, UK), anti-t-NF-κB p65 (1:1000, Abcam, UK), anti-p-p38 (1:1000, CST, USA), and anti-t-p38 (1:1000, Abcam, USA). The membranes were washed with TBS-T and then incubated with the secondary antibody. The proteins on the membranes were detected by ChemiDocTM MP imaging system (Thermo Fisher Scientific, USA) and then quantified using ImageJ software.
4.8. Immunofluorescence
Primary retinal microglia cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 1% Triton X-100 for 15 min, and blocked with 3% BSA for 1h at room temperature. The cells were incubated with anti-rat F4/80 (1:200, Abcam, UK), anti-mouse COX2 (1:200, Abcam, UK), anti-rabbit CD206 (1:100, Abcam, UK), and anti-rabbit CD86 (1:100, CST, USA) at 4 °C overnight. The following day, the cells were washed with PBS and then incubated with Alexa Flour 488 or 594 conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, USA) (1:500) for 1 h. Then, the cells were counterstained with DAPI for nuclei visualization. Images were captured with an LSM800 confocal microscope (Carl Zeiss, Jena, Germany).
4.9. Statistical Analysis
All experiments were performed in triplicate and repeated at least three times. Data were expressed as mean ± standard error (SE) and analyzed using SPSS Statistics V22.0 (IBM, Armonk, NY, USA). Statistical methods included one-way ANOVA, followed by Student’s t-test for two-group comparisons or LSD post-hoc test for multiple group comparisons. A p-value less than 0.05 was considered statistically significant.
5. Conclusion
In summary, the present study demonstrates for the first time that LBGP can effectively inhibit the production of pro-inflammatory mediators in L/I-induced retinal microglia by blocking the activation of NF-κB and MAPK signaling pathways. This study provides a theoretical basis for the translation of LBGP in the clinical treatment of retinal inflammatory diseases.
Authors Contributions
Conceptualization, Z.Y.; Data curation, Y.O., Y.H., X.Y. and L.L.; Funding acquisition, S.T. and J.-H.M.; Methodology, Y.O., Y.H. and J.C.; Resources, K.-F. S., J.C., S.T. and J.-H.M.; Supervision, S.T.; Validation, Y.O. and R.M.; Writing – original draft, Y.O.; Writing – review & editing, K.-F. S.
Commercial relationships
None.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure: Immunofluorescent staining of primary retinal microglia. (A-C) The purity of primary retinal microglia was assessed by immunostaining of CD11b (A), F4/80 (B) and IBA1 (C).
Funding
This study was supported by the Medical Scientific Research Foundation of Guangdong Province of China (C2018041) and Science Research Grant of Aier Eye Institute, China (AEI202301ZD05).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data collected and analysed during the current study are available from the corresponding author on reasonable request.
References
- O’koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Church, K.A.; Pietramale, A.N.; Cardona, S.M.; Vanegas, D.; Rorex, C.; Leary, M.C.; Muzzio, I.A.; Nash, K.R.; Cardona, A.E. Fractalkine isoforms differentially regulate microglia-mediated inflammation and enhance visual function in the diabetic retina. J. Neuroinflammation 2024, 21, 1–18. [Google Scholar] [CrossRef]
- Silverman, S.M.; Wong, W.T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.W.; Walsh, J.T.; Santeford, A.; Lin, J.B.; Beatty, W.L.; Terao, R.; Liu, Y.A.; Hase, K.; Ruzycki, P.A.; Apte, R.S. Dysregulated CD200-CD200R signaling in early diabetes modulates microglia- mediated retinopathy. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, W.; Chen, J.; Li, N.; Mao, L.; Hou, S. Retinal microglia: Functions and diseases. Immunology 2022, 166, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.; Lam, E.; Alvarez, D.; Sun, Y. Ocular Vascular Diseases: From Retinal Immune Privilege to Inflammation. Int. J. Mol. Sci. 2023, 24, 12090. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.A.; Ibrahim, W.W.; Mohamed, A.F.; Abdelkader, N.F. Microglia polarization in nociplastic pain: mechanisms and perspectives. Inflammopharmacology 2023, 31, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, J.; Liang, X.; Li, Y.; Zhu, P.; Zhou, M.; Qin, L.; Deng, Y.; Li, J.; Wang, Y.; Jiang, L.; Huang, D.; Zhou, Y.; Wang, S.; Xiao, Q.; Luo, Y.; Tang, Y. , Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress. Mol Psychiatry 2024. [CrossRef] [PubMed]
- Wang, G.; Li, X.; Li, N.; Wang, X.; He, S.; Li, W.; Fan, W.; Li, R.; Liu, J.; Hou, S. , Icariin alleviates uveitis by targeting peroxiredoxin 3 to modulate retinal microglia M1/M2 phenotypic polarization. Redox Biol 2022, 52, 102297. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Wang, L.-L.; Abdelmaksoud, S.; Aboelgheit, A.; Saeed, S.; Zhang, C.-L. Minocycline modulates microglia polarization in ischemia-reperfusion model of retinal degeneration and induces neuroprotection. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J. A. , The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Qi, X.-M.; Chen, G. p38γ MAPK Inflammatory and Metabolic Signaling in Physiology and Disease. Cells 2023, 12, 1674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; Peng, S.; Yuan, W.; Zhao, R.; Zhang, Z. , Reactive oxygen species-responsive mitochondria-targeted liposomal quercetin attenuates retinal ischemia-reperfusion injury via regulating SIRT1/FOXO3A and p38 MAPK signaling pathways. Bioeng Transl Med 2023, 8, e10460. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Compromised MAPK signaling in human diseases: an update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, C.; Meng, J.; Li, N.; Xu, Z.; Liu, X.; Hou, S. , Galectin-3 regulates microglial activation and promotes inflammation through TLR4/MyD88/NF-kB in experimental autoimmune uveitis. Clin Immunol 2022, 236, 108939. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, L.; Zhu, T.; Xu, S.; He, J.; Mao, N.; Liu, Z.; Wang, D. , Neuroprotective effects of Lycium barbarum polysaccharide on light-induced oxidative stress and mitochondrial damage via the Nrf2/HO-1 pathway in mouse hippocampal neurons. Int J Biol Macromol 2023, 251, 126315. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Guo, J.; Zhang, B.; Chen, J.; Ou, H.; He, R.-R.; So, K.-F.; Zhang, L. Lycium barbarum (Wolfberry) glycopeptide prevents stress-induced anxiety disorders by regulating oxidative stress and ferroptosis in the medial prefrontal cortex. Phytomedicine 2023, 116, 154864. [Google Scholar] [CrossRef]
- Kong, Q.; Han, X.; Cheng, H.; Liu, J.; Zhang, H.; Dong, T.; Chen, J.; So, K.-F.; Mi, X.; Xu, Y.; et al. Lycium barbarum glycopeptide (wolfberry extract) slows N-methyl-N-nitrosourea-induced degradation of photoreceptors. Neural Regen. Res. 2023, 19, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, G.; Chen, M.; Muckersie, E.; Xu, H. Culture and Characterization of Microglia from the Adult Murine Retina. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saddala, M.S.; Yang, X.; Tang, S.; Huang, H. Transcriptome-wide analysis reveals core sets of transcriptional regulators of sensome and inflammation genes in retinal microglia. Genomics 2021, 113, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Saura, J.; Tusell, J.M.; Serratosa, J. High-yield isolation of murine microglia by mild trypsinization. Glia 2003, 44, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Wu, H.-J.; Li, H.-Q.; Qin, S.; Wang, Y.-E.; Li, J.; Lou, H.-F.; Chen, Z.; Li, X.-M.; Luo, Q.-M.; et al. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012, 22, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Mesquida, M.; Drawnel, F.; Fauser, S. The role of inflammation in diabetic eye disease. Semin. Immunopathol. 2019, 41, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-T.; Zhang, J.-F.; Tang, L. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Langmann, T. Indole-3-carbinol regulates microglia homeostasis and protects the retina from degeneration. J. Neuroinflammation 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, X.; Zhao, M.; Bao, Y. The Changes of Irisin and Inflammatory Cytokines in the Age-Related Macular Degeneration and Retinal Vein Occlusion. Front. Endocrinol. 2022, 13, 861757. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.-A.; Kaur, C. Retinal microglia – A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C. A.; Harry, G. J. , Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Q.; Yang, J.; Wang, G.; Zhao, F.; Chen, Y.; Jin, Y. , Reactive astrocytes induced by 2-chloroethanol modulate microglia polarization through IL-1β, TNF-α, and iNOS upregulation. Food Chem Toxicol 2021, 157, 112550. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, L.; Li, X.; Wang, J.; Li, Y.; Huang, Z. , Ferulic acid alleviates retinal neovascularization by modulating microglia/macrophage polarization through the ROS/NF-κB axis. Front Immunol 2022, 13, 976729. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, S.; Xu, T.; Liu, J.; Zhao, L.; Xu, H.; Zhang, W.; Zhu, Y.; Yang, Z. , Retinal Microenvironment-Protected Rhein-GFFYE Nanofibers Attenuate Retinal Ischemia-Reperfusion Injury via Inhibiting Oxidative Stress and Regulating Microglial/Macrophage M1/M2 Polarization. Adv Sci (Weinh) 2023, 10, e2302909. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.-J.; Park, S.K.; Park, J.-H.; Jeong, H.-R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.-S. Donepezil Regulates LPS and Aβ-Stimulated Neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, J.; Du, M.; Liu, X.; Zhang, S.; Zhang, D.; Qin, W.; Xu, X.; Li, X.; Su, R.; et al. Chitosan-Rapamycin Carbon Dots Alleviate Glaucomatous Retinal Injury by Inducing Autophagy to Promote M2 Microglial Polarization. Int. J. Nanomed. 2024, ume 19, 2265–2284. [Google Scholar] [CrossRef]
- Zou, Y.; Jiang, J.; Li, Y.; Ding, X.; Fang, F.; Chen, L. , Quercetin Regulates Microglia M1/M2 Polarization and Alleviates Retinal Inflammation via ERK/STAT3 Pathway. Inflammation 2024. [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. , Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther 2020, 5, 209. [Google Scholar] [CrossRef]
- Han, Y.; Guo, S.; Li, Y.; Li, J.; Zhu, L.; Liu, Y.; Lv, Y.; Yu, D.; Zheng, L.; Huang, C.; et al. Berberine ameliorate inflammation and apoptosis via modulating PI3K/AKT/NFκB and MAPK pathway on dry eye. Phytomedicine 2023, 121, 155081. [Google Scholar] [CrossRef]
- Kim, S.-R.; Seong, K.-J.; Kim, W.-J.; Jung, J.-Y. , Epigallocatechin Gallate Protects against Hypoxia-Induced Inflammation in Microglia via NF-κB Suppression and Nrf-2/HO-1 Activation. Int J Mol Sci 2022, 23, (7). [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Chu, F.; Ji, S.; Liao, K.; Cui, Z.; Chen, J.; Tang, S. Clock Gene Nr1d1 Alleviates Retinal Inflammation Through Repression of Hmga2 in Microglia. J. Inflamm. Res. 2021, ume 14, 5901–5918. [Google Scholar] [CrossRef]
- Wu, J.; Mo, J.; Xiang, W.; Shi, X.; Guo, L.; Li, Y.; Bao, Y.; Zheng, L. , Immunoregulatory effects of Tetrastigma hemsleyanum polysaccharide via TLR4-mediated NF-κB and MAPK signaling pathways in Raw264. 7 macrophages. Biomed Pharmacother 2023, 161, 114471. [Google Scholar]
- Wu, J.; Han, Y.; Xu, H.; Sun, H.; Wang, R.; Ren, H.; Wang, G. , Deficient chaperone-mediated autophagy facilitates LPS-induced microglial activation via regulation of the p300/NF-κB/NLRP3 pathway. Sci Adv 2023, 9, eadi8343. [Google Scholar] [CrossRef] [PubMed]
- Burton, J. C.; Antoniades, W.; Okalova, J.; Roos, M. M.; Grimsey, N. J. , Atypical p38 Signaling, Activation, and Implications for Disease. Int J Mol Sci 2021, 22, (8). [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Lee, Y.-S.; Choi, B.-R.; Yoon, D.; Lee, D. Y. , Anti-Neuroinflammatory Effect of the Ethanolic Extract of Black Ginseng through TLR4-MyD88-Regulated Inhibition of NF-κB and MAPK Signaling Pathways in LPS-Induced BV2 Microglial Cells. Int J Mol Sci 2023, 24, (20). [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Bo, X.; Zhang, J.; Liu, S.; Li, X.; Liao, Y.; Liu, Q.; Cheng, Y.; Cheng, J. , Bergapten alleviates depression-like behavior by inhibiting cyclooxygenase 2 activity and NF-κB/MAPK signaling pathway in microglia. Exp Neurol 2023, 365, 114426. [Google Scholar] [CrossRef]
- Srivastava, S. K.; Ramana, K. V. , Focus on molecules: nuclear factor-kappaB. Exp Eye Res 2009, 88, 2–3. [Google Scholar] [CrossRef]
- Lan, W.; Petznick, A.; Heryati, S.; Rifada, M.; Tong, L. , Nuclear Factor-κB: central regulator in ocular surface inflammation and diseases. Ocul Surf 2012, 10, 137–148. [Google Scholar] [CrossRef]
- Zheng, J.; Luo, Z.; Chiu, K.; Li, Y.; Yang, J.; Zhou, Q.; So, K.-F.; Wan, Q.-L. Lycium barbarum glycopetide prolong lifespan and alleviate Parkinson’s disease in Caenorhabditis elegans. Front. Aging Neurosci. 2023, 15, 1156265. [Google Scholar] [CrossRef]
- Yao, J.; Hui, J.-W.; Chen, Y.-J.; Luo, D.-Y.; Yan, J.-S.; Zhang, Y.-F.; Lan, Y.-X.; Yan, X.-R.; Wang, Z.-H.; Fan, H.; Xia, H.-C. , Lycium barbarum glycopeptide targets PER2 to inhibit lipogenesis in glioblastoma by downregulating SREBP1c. Cancer Gene Ther 2023, 30, 1084–1093. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Z.; He, H.; Li, M.; Lan, Y.; Hui, J.; Bie, P.; Chen, Y.; Liu, H.; Fan, H.; Xia, H. , Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways. J Transl Med 2023, 21, 770. [Google Scholar] [CrossRef]
- Lin, S.; So, K.-F.; Ren, C.-R.; Fu, Y.-W.; Peng, Y.-F.; Huang, X.-D.; Yang, Y.; Huang, L.; Xi, Y.; Hu, Z.-F. Lycium barbarum polysaccharide-glycoprotein preventative treatment ameliorates aversive. Neural Regen. Res. 2021, 16, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, Y.; Yang, F.; Feng, Y.; Xu, K.; Wu, J.; Qu, S.; Yu, Z.; Fan, F.; Huang, L.; Qin, M.; He, Z.; Nie, K.; So, K.-F. , Lycium barbarum Glycopeptide prevents the development and progression of acute colitis by regulating the composition and diversity of the gut microbiota in mice. Front Cell Infect Microbiol 2022, 12, 921075. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).