1. Introduction
At present, colorectal cancer is the third most popular cancer and the third most frequent cause of cancer-related mortality in the USA. About nine out of ten people with colorectal cancer are diagnosed when they are aged fifty or older. The mortality and incidence of colorectal cancer decrease for people older than fifty but increase for those younger than fifty [
1]. Surgery is the first line of treatment for most patients with colorectal cancer [
2], whereas chemotherapy is primarily used in adjuvant therapy. Despite the progresses made in colorectal cancer treatment, postoperative mortality represents poor prognosis in the cure of colorectal cancer [
3]. The main factor resulting in the mortality of colorectal cancer is metastasis [
4]. Liver metastasis is the chief event of mortality in individuals with colorectal cancer [
5]. Almost 25% of patients with colorectal cancer are clinically identified with liver metastasis during the early stage of the disease, and about 50% of patients with colorectal cancer develop liver metastasis symptoms during disease development [
6]. About 30%-50% of patients with colorectal cancer undergo recurring liver metastases following radical resection, and 50% eventually succumb to the disease [
7]. However, the current therapeutic strategies including surgery, radiotherapy, and chemotherapy for patients with colorectal cancer and distant metastasis remain poor because of drug resistance.
5-Fluorouracil (5-FU) is an old clinical chemotherapeutic agent. It has been employed since its invention 70 years ago. 5-FU-established chemotherapy was created to treat a variety of cancers including colorectal cancer [
8,
9]. With the foremost development in novel cancer therapies, 5-FU remains the primary choice in colorectal cancer treatment [
9,
10,
11]. Unfortunately, the efficiency of 5-FU diminishes when patients develop tolerance, which results mostly from the development of resistance in cancer cells [
12,
13,
14]. However, with the absence of feasible therapeutic formulas for the resistance of colorectal cells to 5-FU, the increase in dose and application of other therapies are the only clinical approaches to prolong a patient’s life by several months [
15]. Therefore, novel therapies or agents for 5-FU-resistant cancers are important.
Parecoxib is a selective cyclooxygenase (COX)-2 inhibitor for parenteral administration in clinical settings [
16]. A previous study showed that parecoxib can remarkably increase radiation sensitivity in colorectal cells by directly influencing cancer cells and indirectly influencing the tumor vascular system [
17]. Parecoxib can also reduce esophageal squamous cell carcinoma progression (namely, cell cycle, proliferation, invasion, and migration) through the PDK1-AKT signaling route [
18]. Our recent study demonstrated that parecoxib can inhibit metastasis in human colorectal cancers. Our findings showed a novel mechanism underlying the antimetastatic activity of parecoxib in colorectal cancer by inhibiting p-Akt, p-ERK, p-GSK3β, MACC1, and cMet to induce the inhibition of epithelial–mesenchymal transition (EMT) and β-catenin [
19]. These results illustrated that parecoxib may have novel potential activity to enhance clinical chemotherapeutic drugs for colorectal cancer.
Reactive oxygen species (ROS) is an important factor in cancer metastasis. High amounts of ROS provoke cancer metastasis by stimulating the PI3K/Akt/mTOR and MAPK signaling pathways. These signaling pathways activate downstream MMP-9, thereby initiating EMT and resulting in metastasis [
20]. EMT plays a major role in metastasis; the polarity of epithelial cells leads to the disconnection of cell–cell adhesion and enhanced mobility. Some studies have demonstrated that ROS is a critical factor that induces EMT. These findings suggested that a certain generation of intracellular ROS can induce metastasis in cancer cells. Conversely, reducing ROS in cancer cells may inhibit metastasis.
Given that parecoxib is known to have potential anticancer activity, we wondered whether parecoxib may enhance 5-FU to inhibit metastasis in human colorectal cancer. To explore this possibility, we investigated whether the parecoxib/5-FU combination exhibits superior ability to modulate the PI3K/Akt/NF-κB signaling pathways for antimetastatic activity in human colorectal cancer. Moreover, we aimed to determine if the combination of parecoxib/5-FU can regulate intracellular ROS in human colorectal cancer cells. The relevant mechanisms of the parecoxib/5-FU combination on antimetastatic effects are discussed in this study.
2. Materials and Methods
2.1. Cell Line, Reagents, and Chemicals
The human colon cancer DLD-1 cell line (RRID: CVCL_0248) and SW480 cell line (RRID: CVCL_0546) were bought from the Bioresource Collection and Research Center (Hsinchu, Taiwan). RPMI-1640 medium was purchased from Hyclone (South Logan, UT, USA). Fetal bovine serum (FBS) was obtained from Gibco Inc. (Freehold, NJ, USA). The protein assay Bio-Rad kit was from Bio-Rad Laboratories (Richmond, CA, USA). Parecoxib was obtained from Pfizer (Australia). Dimethyl sulfoxide (DMSO), 5-FU, trypan blue solution, crystal violet, 2',7'-dichlorofluorescin diacetate (DCFH-DA), and other substances were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
2.2. Cell Culture and Drug Treatment
DLD-1 cells were incubated in RPMI-1640 (containing 10% FBS, 2 mM L-glutamine, 100 units/mL penicillin G, and 100 μg/mL streptomycin) and placed in an incubator below 5% CO2 at 37 °C. The stock solutions of parecoxib and 5-FU were made in DMSO, and total treatment concentrations were diluted in the incubated medium. The concentration of DMSO did not exceed 0.05%.
2.3. Cell Viability Assay
Cell viability was examined by the MTT assay. DLD-1 cancer cells (1 × 105) were cultured in 12-well culture plates for 24 h. The incubation medium was substituted with a fresh preparation, and the cells were incubated with 0, 1, 3, 5, 7.5, and 10 μM parecoxib or 0, 10, 15, 20, 25, and 30 μM 5-FU for 48 h. After incubation, cells were exposed to 0.5 mg/mL MTT solution for 2 h and dissolved with DMSO. An aliquot (200 μL) of the DMSO-dissolved solution was obtained from 12-well culture plates and transported to 96-well ELISA plates. The absorbance of the DMSO-dissolved suspension in the 96-well ELISA plates was detected at 595 nm in a microplate reader (Bio-Rad, Richmond, CA, USA).
2.4. Transwell Migration and Transwell Invasion Assay
Assays were implemented by 24-well Millicell® chamber transwell plates for migration assay and 24-well Matrigel invasion chambers for invasion assay with a polyethylene terephthalate membrane. The cells (1 × 105 cells/well) were placed in upper chamber inserts with serum-free medium, and the bottom chamber included whole medium containing 10% FBS. After 48 h of drug treatment, the upper chamber inserts were swabbed to eliminate the non-travelling cells, and the travelling cells that were distributed across the polyethylene terephthalate film were fixed with 4% formaldehyde and stained with 0.05% crystal violet solution. Cells that had passed through the pores were calculated in three randomly selected areas under a phase-contrast microscope. Each chamber insert was transferred to an unused well including 200 μL of the dissolving solution (33% acetic acid) to dissolve the cells, and 100 μL of each dissolving solution was transferred and assessed at 560 nm using an EnSpire® multimode plate reader (PerkinElmer).
2.5. Isobologram Analysis
Isobologram analysis was conducted to identify the synergistic antimetastatic effects following the treatment of parecoxib and 5-FU. When two drugs are treated concomitantly, the antimetastatic effect of the combination must be distinguished from the antimetastatic effect of each drug. Treatment of more than one drug may lead to effects that are less than, or greater than, the additive effect of each drug given alone. A non-mechanistic method of explaining the effect resulting from the treatment of two drugs is the isobologram. The isobologram offers a suitable graphical demonstration of individual concentrations through rectangular coordinates (x, y) to illustrate equieffective pairs of dosages of drugs X and Y, respectively. Therefore, the isobologram involves a set of noticeable, experimentally defined points whose position allows a category of the drug–drug interaction. For drug alone treatment, parecoxib alone (1, 2, and 3 μM) and 5-FU alone (5, 10, and 20 μM) were selected. For drug combination, 1 μM parecoxib + 5 μM5-FU, 2 μM parecoxib + 10 μM 5-FU, and 3 μM parecoxib + 20 μM 5-FU were selected. After 48 h of drug treatment, transwell migration was evaluated, and isobologram analysis of parecoxib combined with 5-FU in the DLD-1 cell line was determined by CalcuSyn software. The combination index (CI) was then assessed.
2.6. Gap closure Assay
The migration capacity of colon cancer cells was determined by gap closure assay. Culture-insert 4-wells were fixed onto 6-well plates. DLD-1 cells (4.4×104) were seeded onto each culture-insert 4-well for 24 h, and all culture-insert 4-wells were removed. Subsequently, the plates were replenished with fresh culture medium containing concentrations of parecoxib (3 μM) alone, 5-FU (20 μM), and parecoxib (3 μM)/5-FU (20 μM) combination and incubated for 24 and 48 h. The number of migrating cells was detected and counted by a light microscope. The Image J software was used to analyze cells migration into the cross-shaped areas.
2.7. Western Blot Analysis
After incubation with the indicated drugs for various time points, all cells were cleaned with PBS, refloated in a protein extraction solution for 10 min, and centrifuged at 12,000 ×g for 10 min at 4 °C to gain the extracted proteins. The protein concentrations were quantified with a Bio-Rad protein analysis kit. The cellular extracted proteins were heated in loading buffer, and an equal quantity of protein (30–50 μg) was divided on 12% SDS–polyacrylamide gel and then transferred to PVDF membranes. The membranes were immersed with a number of primary antibodies overnight and washed with PBST solution containing 0.05% Tween 20 PBS/PBS. After cleaning, the suitable secondary antibodies with horseradish peroxidase were mixed to the membrane for 1 h and cleaned with PBST solution containing 0.05% Tween 20/PBS. The antigen–antibody complexes were recognized by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) with a chemiluminescence analyzer.
2.8. Antibodies
Antibodies against E-cadherin (cat. no. sc-7870), β-catenin (cat. no. sc-7963), MMP-9 (cat. no. sc-21733), p-Akt (Ser473; cat. no. sc7985-R), p-IκB (cat. no. sc-8404), p-Akt (cat. no. sc-7985-R), tubulin (cat. no. sc-5286), vimentin (cat. no. sc-6260), actin (cat. no. sc-1616-R) total-Akt (cat. no. sc-8312), total-IKK (cat. no. sc-7606), total-IκB (cat. no. sc-371), total-p65 (cat. no. sc-372), and GAPDH (cat. no. sc-47724) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). p-IKK (cat. no. ab138426), Histone H3 (cat. no. ab1791), and p-p65 (cat. no. ab28856) were bought from Abcam (Waltham, MA, USA). The anti-rabbit IgG (cat. no. ab150077) and anti-mouse IgG (cat. no. ab6708) secondary antibodies were purchased from Abcam (Waltham, MA, USA).
2.9. Gelatin Zymography Analysis
Gelatin zymography was carried out to verify the activities of MMP-9 in the media. After incubation with drugs for 24 and 48 h, the medium containing 40 μg of protein was made by SDS sample solution without heating or reducing agent and exposed to 0.2% gelatin with 10% SDS–PAGE electrophoresis. After electrophoresis, the gels were cleaned with 2.5% Triton X-100 and immersed in a reaction solution (5 mM CaCl2; 1 μM ZnCl2; 0.02% NaN3 and 50 mM Tris–HCl, pH 7.4) at 37 °C for 20 h. The gel was dyed with Coomassie brilliant blue R-250 for imaging. Data normalization was presented via Image J v1.46.
2.10. Effect of p-Akt Overexpression on the Parecoxib/5-FU Combination
About 6 μg of myc-tagged myristoylated Akt expression vector (Myr-Akt, Addgene, Cambridge, MA, USA) or empty vector (pUSEamp, Upstate Technology, Skaneateles, NY, USA) was transfected to DLD-1 cells by Lipofectamine (Invitrogen Taiwan Ltd., Taiwan) following the manufacturer’s method. The G418 selection solution (400 μg/mL) was used to maintain a stable cell line for 14 days. The stable cell line was incubated in RPMI-1640 medium including 10% FBS and then subjected to the parecoxib (3 μM)/5-FU (20μM) combination for 48 h. After drug incubation, the cells were used for migration examination.
2.11. Intracellular ROS Analysis
The generation of intracellular ROS was assessed by flow cytometry by DCFH–DA. After drug incubation, the cells were dyed with 20 μM DCFH–DA for 30 min at 37 °C and then cleaned with 1 × PBS twice to eliminate DCFH–DA. All cells were trypsinized to gain a single cell suspension. Intracellular ROS amounts, which were detected by the fluorescence of dichlorofluorescein (DCF), were measured through excitation/emission: 485 nm/535 nm by a BD FACSCantoTM II flow cytometer (San Jose, CA, USA). H2O2 (800 μM) was used as an intracellular ROS positive control. About 10,000 cells were collected and analyzed per experimental conditions via mean fluorescent intensity.
2.12. Statistical Analysis
Data are presented as the mean ± standard deviation from at least three independent experiments and analyzed using Student’s t tests. A P value of less than 0.05 was considered statistically significant.
4. Discussion
Our previous reports demonstrated that parecoxib can significantly repress cell migration of DLD-1 and SW480 cells [
19]. The mechanisms include inhibition of metastasis-associated in colon cancer 1 (MACC1), Akt phosphorylation, β-catenin expression, and EMT [
19]. Other authors also revealed that parecoxib can suppress metastasis by reducing the expression of engulfment and cell motility 3 (ELMO3) in lung cancer [
21]. These results illustrated that parecoxib may offer anti-metastatic potential in combination with chemotherapeutic agents in cancer. MACC1 has been demonstrated to play an important role in metastasis formation in colorectal cancer [
22]. Knockdown of MACC1 can facilitate cell death via the PI3K/Akt pathway in 5-FU-resistant colorectal cancer [
23]. Many studies have demonstrated that MACC1 inhibition can enhance antimetastatic activity. Curcumin reduces MACC1 expression and inhibits wound healing in colorectal cancer [
22]. Selumetinib treatment induces a significant repression in metastasis only in MACC1-positive xenografts [
24]. These results indicated that MACC1 is an antimetastatic target in colorectal cancer. Recently, Kortüm et al. demonstrated that the combination of statins and niclosamide prevents colorectal cancer propagation by opening the MACC1-β-catenin-S100A4 axis of metastasis [
25]. In our previous study, we demonstrated that parecoxib inhibits the expression levels of MACC1. Thus, we speculated that the antimetastatic effect of 5-FU enhanced by parecoxib may partly result from the inhibition of MACC1.
Our present study revealed that 5-FU alone and parecoxib alone did not affect the intracellular ROS levels. Notably, the present study found that the 5-FU/parecoxib combination could significantly inhibit ROS in colorectal cancer cells for 48 h. Some reports indicated that ROS often plays a dual role in regulating various types of cellular metabolism [
26,
27]. The concentration of ROS in the cell determines the role of regulation. In general, cancer cells show higher basic concentrations of ROS compared with normal cells. At low to moderate concentrations, ROS function as signal transducers that trigger cell migration, invasion, proliferation, and angiogenesis. By contrast, high concentrations of ROS result in damage to membranes, lipids, proteins, nucleic acids, and organelles, causing cell death. ROS provoke tumor metastasis and invasion by prompting EMT in cancer cells. Notably, a large accumulation of ROS represses tumor growth through two mechanisms: (1) by blocking the signaling pathway responsible for cancer cell proliferation, as well as the cell cycle and the biosynthesis of ATP and nucleotides and (2) by promoting tumor cell death via activating the p53-independent, mitochondrial, and endoplasmic reticulum stress–apoptotic pathways and the ferroptosis pathway [
26]. As a result of the elevated levels of ROS in cancer cells, the administration of medications that further increase ROS concentrations can effectively impede the metastasis of cancer cells and even trigger apoptosis in these cells. These phenomena are consistent with the findings of Piskounova et al., who reported that ROS inhibit the spread of melanoma cancer in vivo [
28]. However, some studies showed that reducing the ROS status in cancer cells will inhibit various activities of cancer cells, including the mechanism of regulating metastasis [
29,
30]. Our previous studies also demonstrated that inhibition of intracellular ROS can repress cancer metastasis [
31]. We found that ROS inhibition by propyl gallate treatment can significantly inhibit metastasis in glioblastoma cells [
31]. Another study illustrated that mitoQ, a mitochondria-targeted antioxidant, decreases mitochondrial superoxide production and inhibits metastasis in human pancreatic cancer [
32]. Febuxostat, a xanthine oxidase inhibitor, alleviates breast cancer cell metastasis related to the inhibition of ROS [
33]. We speculate that this negative regulation of ROS may be related to the inhibition of metastasis. However, the relationship between ROS inhibition induced by the 5-FU/parecoxib combination and metastasis in vivo must be further explored in the future.
In our previous studies, we demonstrated that 10 μM parecoxib inhibited the metastasis of colon cancer through the PI3K/Akt signaling pathway [
19]. We hypothesized that low concentrations of parecoxib may enhance 5-FU’s ability to inhibit metastasis. As expected, the present results demonstrated that a low concentration of parecoxib (3 μM) and 5-FU synergistically inhibited the migration and invasion of DLD-1 cells. To further verify the relationship between inhibition of the PI3K/Akt pathway and metastasis in the parecoxib/5-FU combination, we used the overexpression of p-Akt in DLD-1 cells in our experiments. The results indicated that migration significantly increased in the p-Akt-overexpressing DLD-1 cells under the parecoxib/5-FU combination. On the other hand, wortmannin co-treatment with parecoxib/5-FU combination further more increase the anti-migration effect. Similarly, a previous study reported that the PI3K/Akt/NF-
κB/MMP-9 signaling pathway plays an essential role in tumor metastasis and growth [
34]. The activation of the PI3K/Akt signaling pathway can regulate cancer cell metastasis by increasing the expression of MMP-9 at the transcriptional level [
35]. MMP-9 can destroy type IV collagen, which exists in the basement membrane of the extracellular matrix and elevates cancer metastasis [
36,
37]. PI3K/Akt can regulate the downstream of the NF-κB transcription factor to transcript many metastasis-related genes including the expression of MMP-9 and EMT markers to promote the metastasis of cancer cells [
38]. Our results showed that the parecoxib/5-FU combination led to the inhibition of downstream effectors such as NF-κB transcription factor, EMT, and MMPs, indicating that the parecoxib/5-FU combination exerted antimetastatic activity by inhibiting the PI3K/Akt signaling pathway.
Accumulative reports have shown that the transcription factor Snail contributes to the conversion from epithelial-like cells into mesenchymal-like cells via repressing E-cadherin expression in many cancer cells [
39]. To discover whether Snail is included with the EMT course, we detected the expression of Snail in DLD-1 cells treated by the parecoxib/5-FU combination. We found that the parecoxib/5-FU combination resulted in the inhibition of Snail to less than half in DLD-1 cells (
Figure 4). As a transcriptional factor, Snail is triggered by the PI3K/Akt signaling pathway [
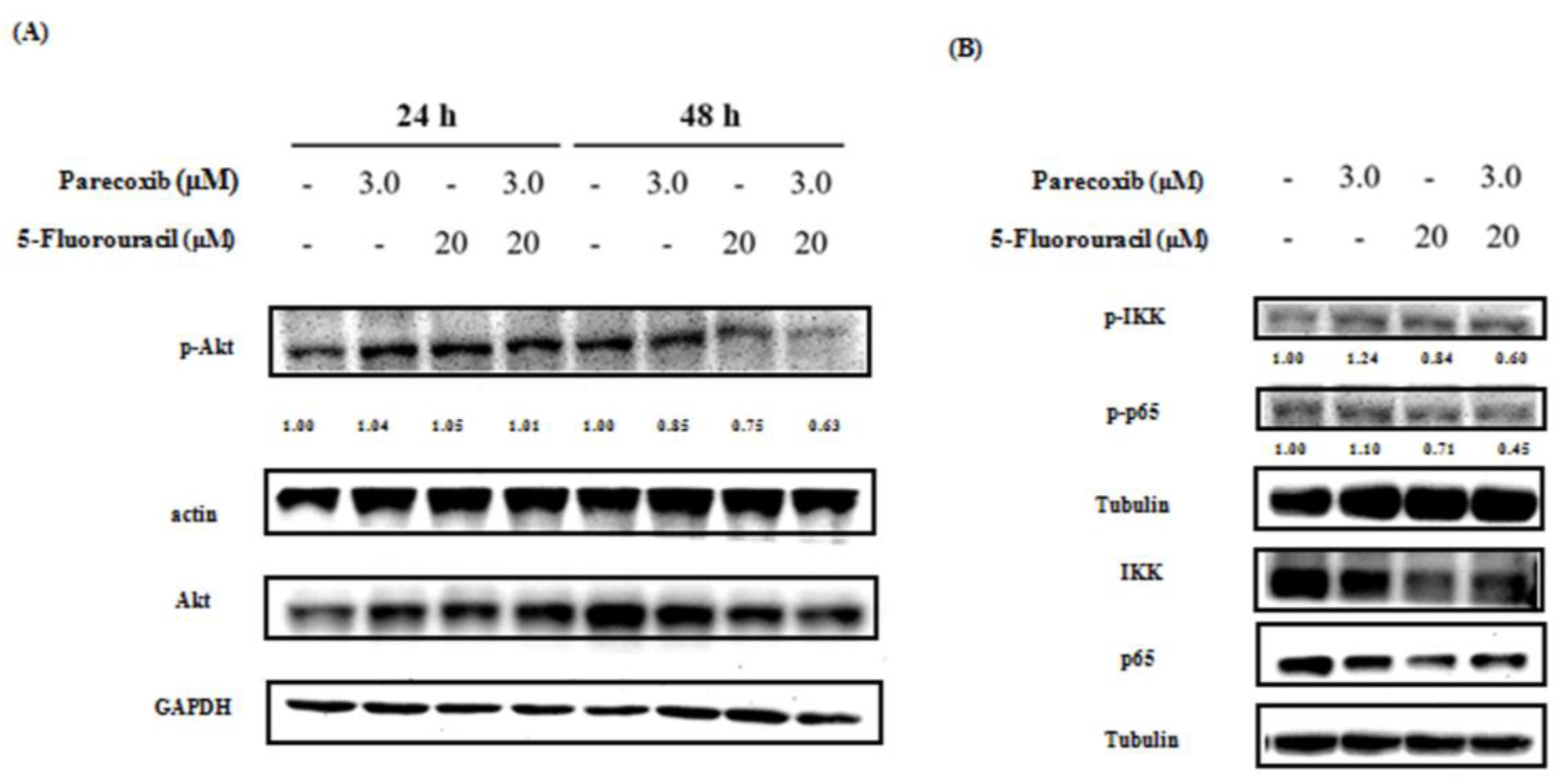
40]. Moreover, our results showed that the expression of p-Akt was inhibited by the parecoxib/5-FU combination (
Figure 7A). Thus, the EMT process, which was induced by the downstream effect of the PI3K/AKT pathway, was inhibited by the combination of parecoxib and 5-FU.
Author Contributions
Conceptualization, C.-H.C. and W.-L.C; methodology, J.-Y.P., C.-L.H., P.-C.L. and W.-L.C and C.-H.C.; software, J.-Y.P., W.-L.C. and F.-J.L; validation, C.-H.C. and W.-L.C.; resources, C.-L.H., P.-C.L. and W.-L.C; data curation, J.-Y.P., W.-L.C. and C.-H.C.; writing—original draft preparation, W.-L.C., J.-Y.P. and C.-H.C.; writing—review and editing, C.-H.C.; supervision, C.-H.C. and W.-L.C; project administration, C.-H.C. and W.-L.C; funding acquisition, W.-L.C. All authors have read and agreed to the published version of the manuscript.
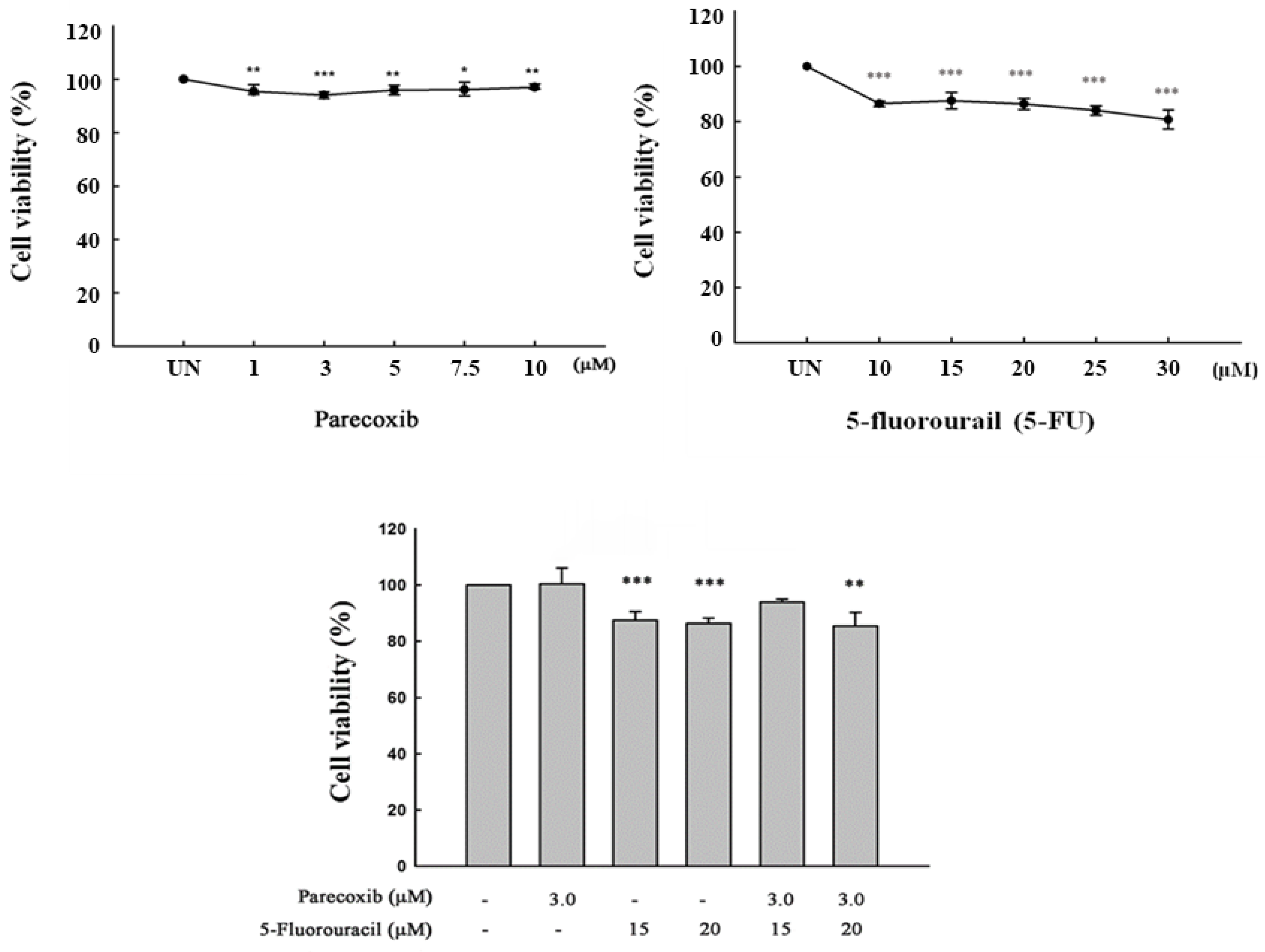
Figure 1.
Effect of parecoxib and 5-FU on cell viability by MTT assay. DLD-1 cells were plated on a 24-well culture plate with a density of 1× 105 cells/well. DLD-1 cells were treated with various concentrations of parecoxib (0, 1, 3, 5, 7.5, and 10 μM), 5-FU (0, 10 15, 20, 25, and 30 μM), and parecoxib (3 μM) combined with 5-FU (15 and 20 μM) for 48 h. After incubation, cell viability was assessed by MTT analysis. Significant differences in the untreated group (UN) are shown as follows: P<0.05 (*), P<0.01 (**), and P<0.001 (***).
Figure 1.
Effect of parecoxib and 5-FU on cell viability by MTT assay. DLD-1 cells were plated on a 24-well culture plate with a density of 1× 105 cells/well. DLD-1 cells were treated with various concentrations of parecoxib (0, 1, 3, 5, 7.5, and 10 μM), 5-FU (0, 10 15, 20, 25, and 30 μM), and parecoxib (3 μM) combined with 5-FU (15 and 20 μM) for 48 h. After incubation, cell viability was assessed by MTT analysis. Significant differences in the untreated group (UN) are shown as follows: P<0.05 (*), P<0.01 (**), and P<0.001 (***).
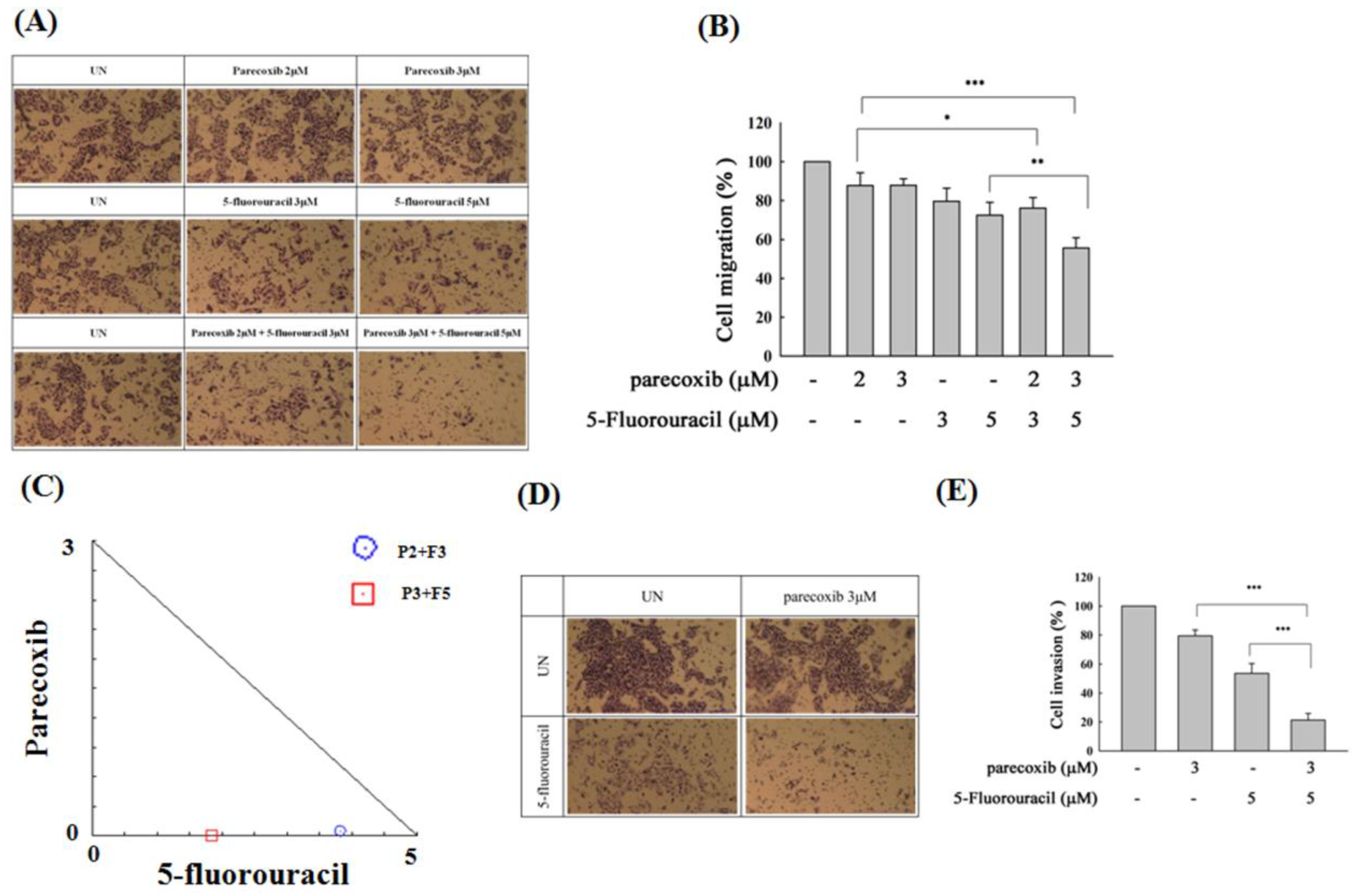
Figure 2.
Effect of parecoxib and 5-FU on cell migration and invasion by transwell and matrix gel assays in DLD-1 cells. DLD-1 cells (1×105/well) were seeded onto the upper chambers without matrix gel for migration assay (A and B) and with matrix gel for invasion assay (D and E) and then incubated with parecoxib (3 μM) alone, 5-FU (15 and 20 μM) alone, and parecoxib (3 μM) combined with 5-FU (15 and 20 μM) for 48 h. (A and D) Arbitrary fields from each of the triplicate migration assays were calculated by a phase-contrast microscope (magnification 200×). (B and E) The pigment was eluted with 33% acetic acid, and the absorbance of crystal violet was determined at 570 nm by a microplate reader. The values are displayed as the mean ± SD of separate trials. Significant differences are set at p<0.001 (***). (C) Isobologram analysis of the parecoxib and 5-FU combination in DLD-1 cells. Combination index data were computed by CalcuSyn software. Points below the backslash line indicate the synergistic outcome. The trials were conducted at least three times. A descriptive trial is shown.
Figure 2.
Effect of parecoxib and 5-FU on cell migration and invasion by transwell and matrix gel assays in DLD-1 cells. DLD-1 cells (1×105/well) were seeded onto the upper chambers without matrix gel for migration assay (A and B) and with matrix gel for invasion assay (D and E) and then incubated with parecoxib (3 μM) alone, 5-FU (15 and 20 μM) alone, and parecoxib (3 μM) combined with 5-FU (15 and 20 μM) for 48 h. (A and D) Arbitrary fields from each of the triplicate migration assays were calculated by a phase-contrast microscope (magnification 200×). (B and E) The pigment was eluted with 33% acetic acid, and the absorbance of crystal violet was determined at 570 nm by a microplate reader. The values are displayed as the mean ± SD of separate trials. Significant differences are set at p<0.001 (***). (C) Isobologram analysis of the parecoxib and 5-FU combination in DLD-1 cells. Combination index data were computed by CalcuSyn software. Points below the backslash line indicate the synergistic outcome. The trials were conducted at least three times. A descriptive trial is shown.
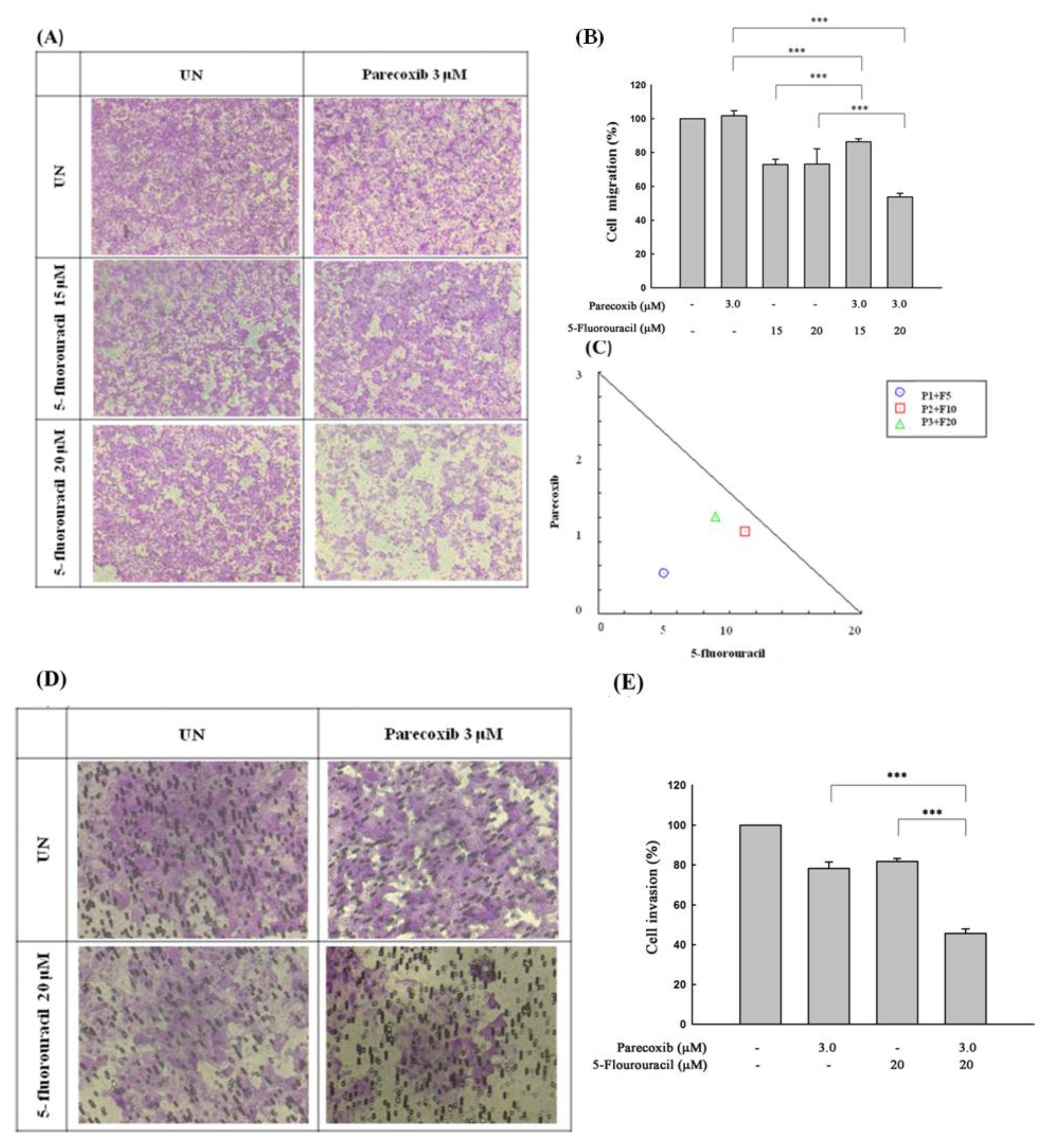
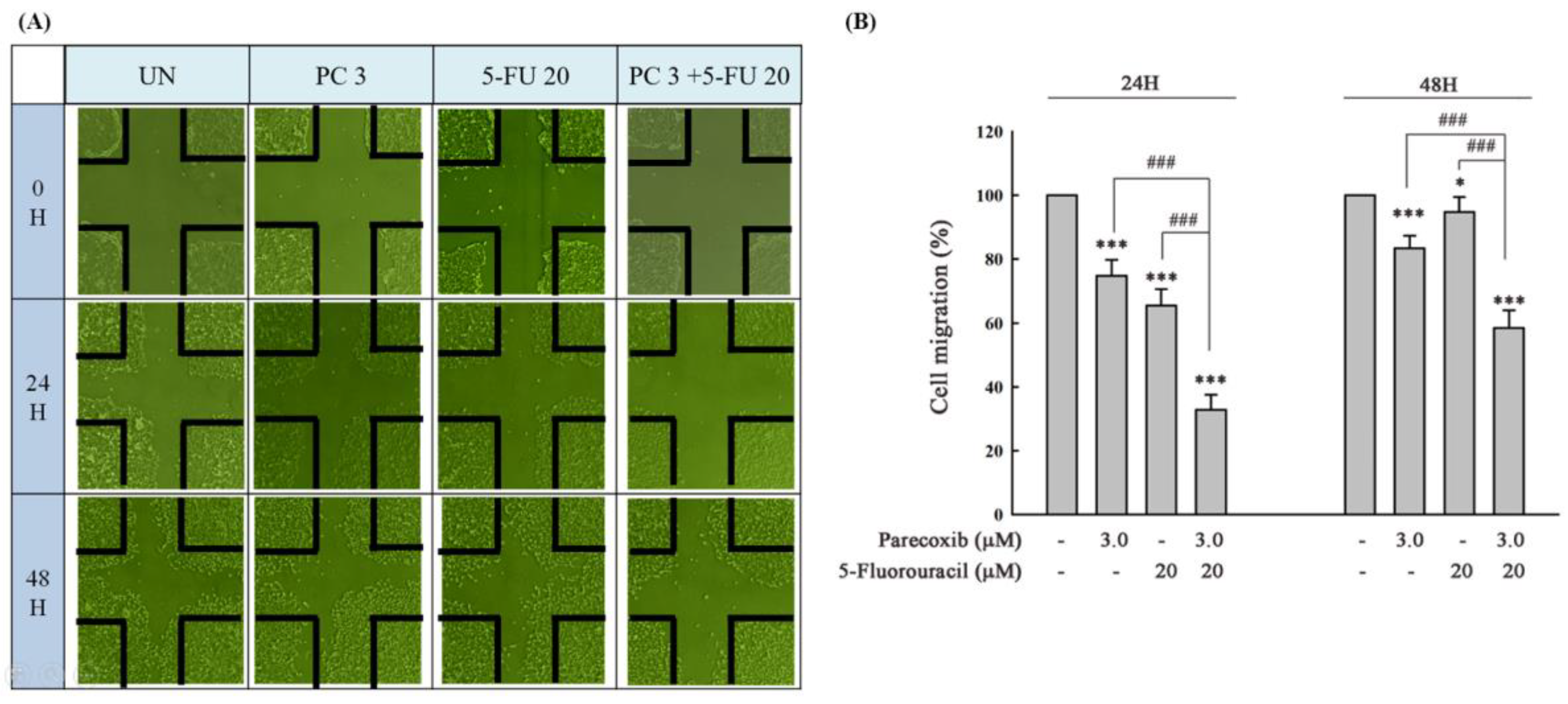
Figure 3.
DLD-1 cells were seeded onto each culture-insert 4-well in 6-well plates for 24 h, and all culture-insert 4-wells were removed to create wounded regions. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were incubated for 48 h. (A) After treatment, the cells that migrated to the wounded regions were calculated by a phase-contrast microscope (magnification 200×). (B) Percentages of DLD-1 cells that migrated to the wound area following treatment with parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were evaluated. Significant differences in the untreated group (UN) are denoted as P<0.001 (***). Significant differences in parecoxib (3 μM) alone or 5-FU (20 μM) alone are indicated as P<0.001 (###).
Figure 3.
DLD-1 cells were seeded onto each culture-insert 4-well in 6-well plates for 24 h, and all culture-insert 4-wells were removed to create wounded regions. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were incubated for 48 h. (A) After treatment, the cells that migrated to the wounded regions were calculated by a phase-contrast microscope (magnification 200×). (B) Percentages of DLD-1 cells that migrated to the wound area following treatment with parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were evaluated. Significant differences in the untreated group (UN) are denoted as P<0.001 (***). Significant differences in parecoxib (3 μM) alone or 5-FU (20 μM) alone are indicated as P<0.001 (###).
Figure 4.
Effect of parecoxib and 5-FU on EMT. DLD-1 cells were seeded in 6 cm dishes with a density of 4× 105 cells/well. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were incubated for 24 and 48 h. After treatment, the levels of protein expression were evaluated using the extracted proteins. About 30–50 μg of protein was loaded onto a 12% SDS–polyacrylamide gel and assessed by Western blot. Actin or tubulin was used as internal control.
Figure 4.
Effect of parecoxib and 5-FU on EMT. DLD-1 cells were seeded in 6 cm dishes with a density of 4× 105 cells/well. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were incubated for 24 and 48 h. After treatment, the levels of protein expression were evaluated using the extracted proteins. About 30–50 μg of protein was loaded onto a 12% SDS–polyacrylamide gel and assessed by Western blot. Actin or tubulin was used as internal control.
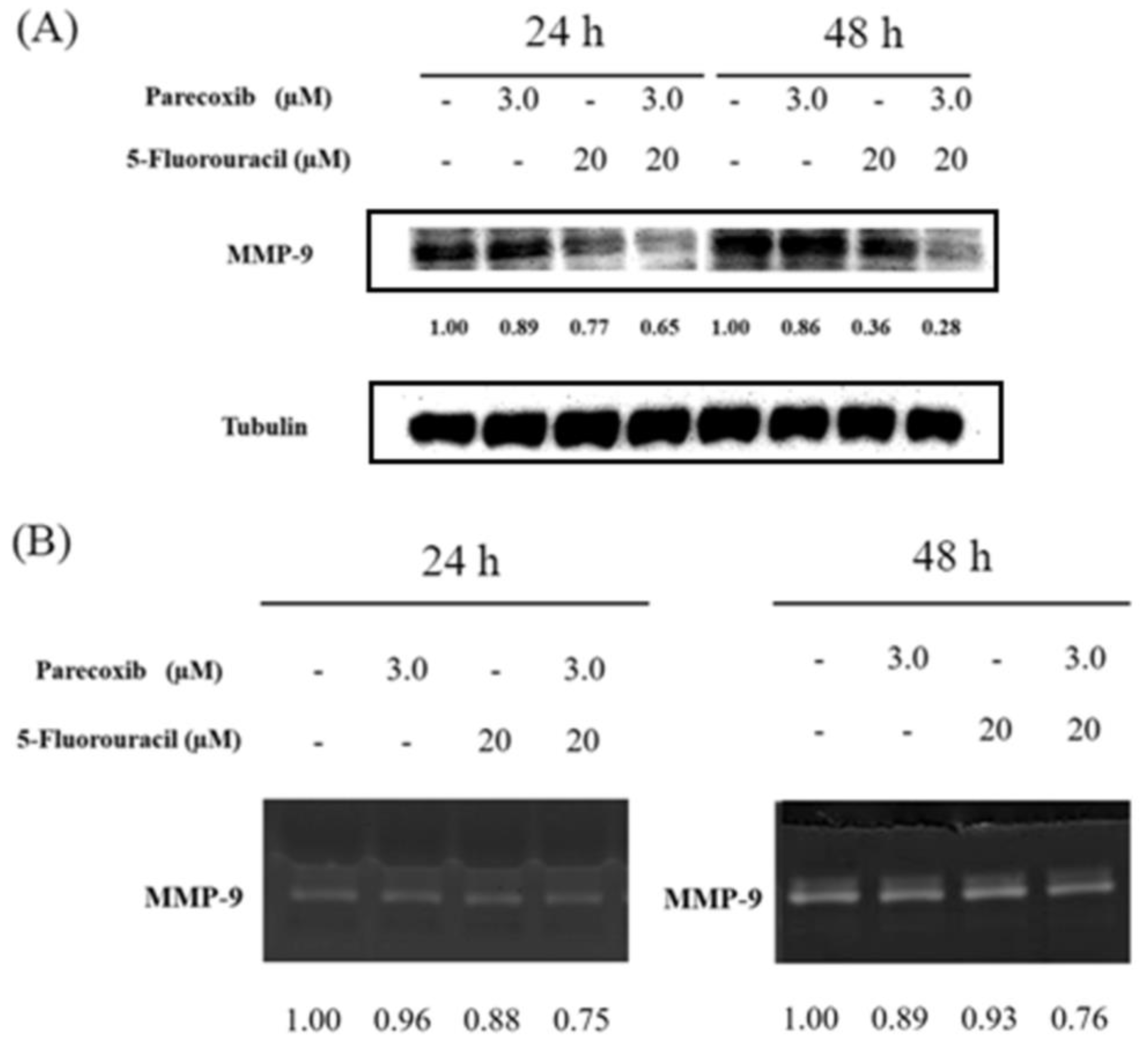
Figure 5.
Effect of parecoxib and 5-FU on MMP-9 expression and activity. DLD-1 cells were seeded in 6 cm dishes with a density of 4× 105 cells/well. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were cultured for 24 and 48 h. After treatment, the total proteins were extracted, and conditional media were collected to evaluate protein expression and enzyme activity. (A) About 30–50 μg of protein was loaded onto 12% SDS–polyacrylamide gel and assessed by Western blot. Tubulin was selected as loading control. (B) About 40 μg of conditional media was run over non-reduced denatured 12% polyacrylamide gel containing gelatin and stained with Coomassie Blue.
Figure 5.
Effect of parecoxib and 5-FU on MMP-9 expression and activity. DLD-1 cells were seeded in 6 cm dishes with a density of 4× 105 cells/well. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were cultured for 24 and 48 h. After treatment, the total proteins were extracted, and conditional media were collected to evaluate protein expression and enzyme activity. (A) About 30–50 μg of protein was loaded onto 12% SDS–polyacrylamide gel and assessed by Western blot. Tubulin was selected as loading control. (B) About 40 μg of conditional media was run over non-reduced denatured 12% polyacrylamide gel containing gelatin and stained with Coomassie Blue.
Figure 6.
Effect of parecoxib and 5-FU on the p-Akt and NF-κB pathway. DLD-1 cells were cultured in 6 cm dishes with a density of 4× 105 cells/well. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were treated for (A) 24 and 48, (B and D) 1, and (C) 48 h. After incubation, levels of protein expression were evaluated using the proteins. About 30–50 μg of protein was loaded onto a 12% SDS–polyacrylamide gel and assessed by Western blot. Actin, tubulin, GAPDH, or histone H3 were selected as loading control.
Figure 6.
Effect of parecoxib and 5-FU on the p-Akt and NF-κB pathway. DLD-1 cells were cultured in 6 cm dishes with a density of 4× 105 cells/well. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were treated for (A) 24 and 48, (B and D) 1, and (C) 48 h. After incubation, levels of protein expression were evaluated using the proteins. About 30–50 μg of protein was loaded onto a 12% SDS–polyacrylamide gel and assessed by Western blot. Actin, tubulin, GAPDH, or histone H3 were selected as loading control.
Figure 7.
Effect of p-Akt overexpression and inhibition on the migration in parecoxib- and 5-FU-treated DLD1 cells. (A) Akt overexpression (pcDNA-Myr-Akt1) and pcDNA3 (empty) plasmids were transfected in DLD1 cells. Overexpression of Akt phosphorylation increased in the pcDNA3-Akt-transfected DLD-1 cells compared with the empty control. The density of the relative p-Akt (S473) protein band was normalized to actin. (B and E) LDL-1 cells (1×105/well) were cultured onto the higher chambers for migration assay and then treated with parecoxib (3 μM) alone, 5-FU (20 μM) alone, parecoxib (3 μM) combined with 5-FU (20 μM), and parecoxib (3 μM) combined with 5-FU (20 μM) co-treated with wortmannin (20 μM) for 48 h. Random areas from each of the triplicate migration assays were calculated by a phase-contrast microscope (magnification 200×). (C and F) The stain was eluted with 33% acetic acid, and the absorbance of crystal violet was detected at 570 nm by using a microplate reader. (D) Expression of Akt phosphorylation in wortmannin treatment. The density of the relative p-Akt (S473) protein band was normalized to GAPDH. The data are shown as the mean ± SD of separate tests. Significant differences are expressed as p<0.001 (***).
Figure 7.
Effect of p-Akt overexpression and inhibition on the migration in parecoxib- and 5-FU-treated DLD1 cells. (A) Akt overexpression (pcDNA-Myr-Akt1) and pcDNA3 (empty) plasmids were transfected in DLD1 cells. Overexpression of Akt phosphorylation increased in the pcDNA3-Akt-transfected DLD-1 cells compared with the empty control. The density of the relative p-Akt (S473) protein band was normalized to actin. (B and E) LDL-1 cells (1×105/well) were cultured onto the higher chambers for migration assay and then treated with parecoxib (3 μM) alone, 5-FU (20 μM) alone, parecoxib (3 μM) combined with 5-FU (20 μM), and parecoxib (3 μM) combined with 5-FU (20 μM) co-treated with wortmannin (20 μM) for 48 h. Random areas from each of the triplicate migration assays were calculated by a phase-contrast microscope (magnification 200×). (C and F) The stain was eluted with 33% acetic acid, and the absorbance of crystal violet was detected at 570 nm by using a microplate reader. (D) Expression of Akt phosphorylation in wortmannin treatment. The density of the relative p-Akt (S473) protein band was normalized to GAPDH. The data are shown as the mean ± SD of separate tests. Significant differences are expressed as p<0.001 (***).
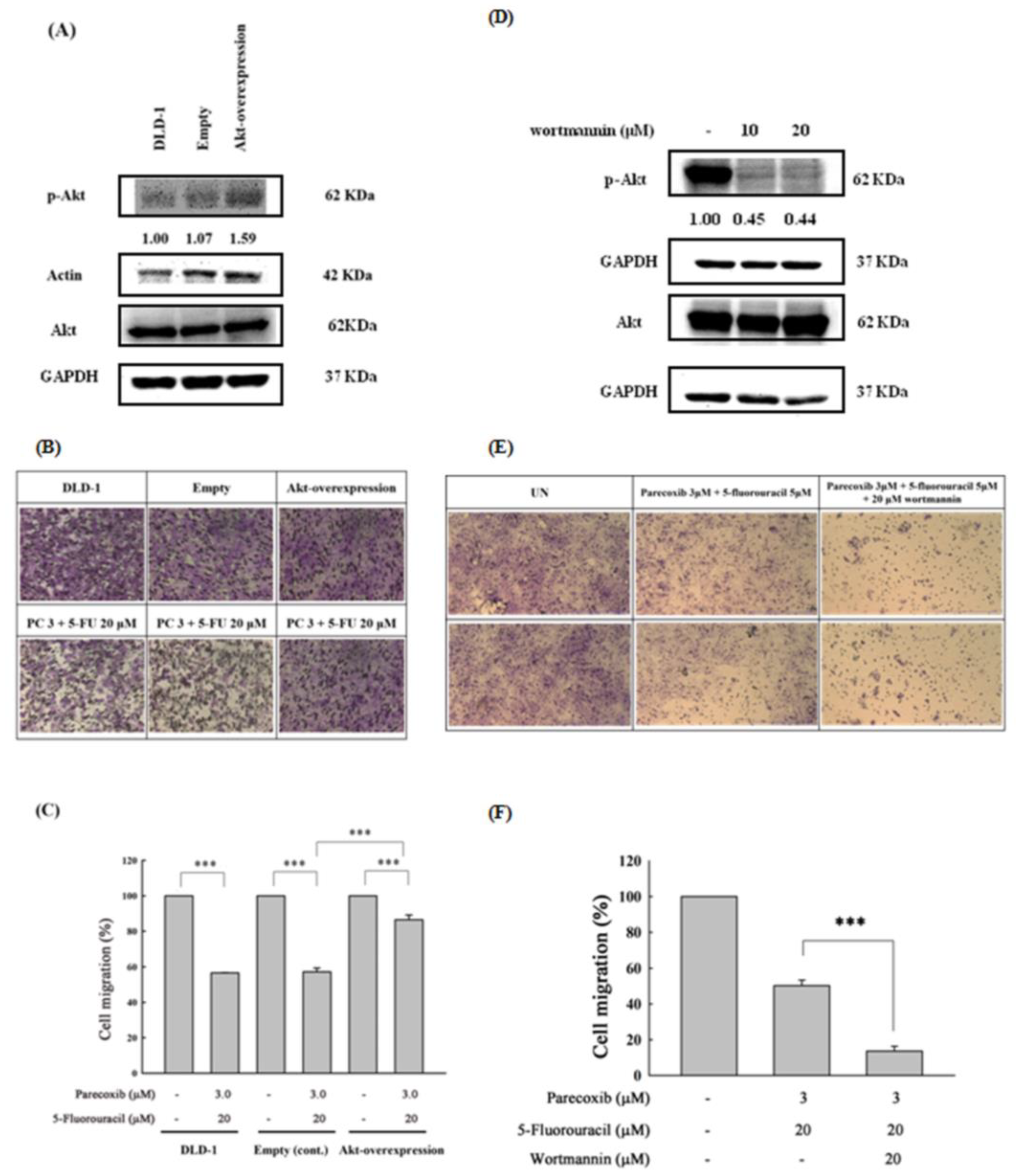
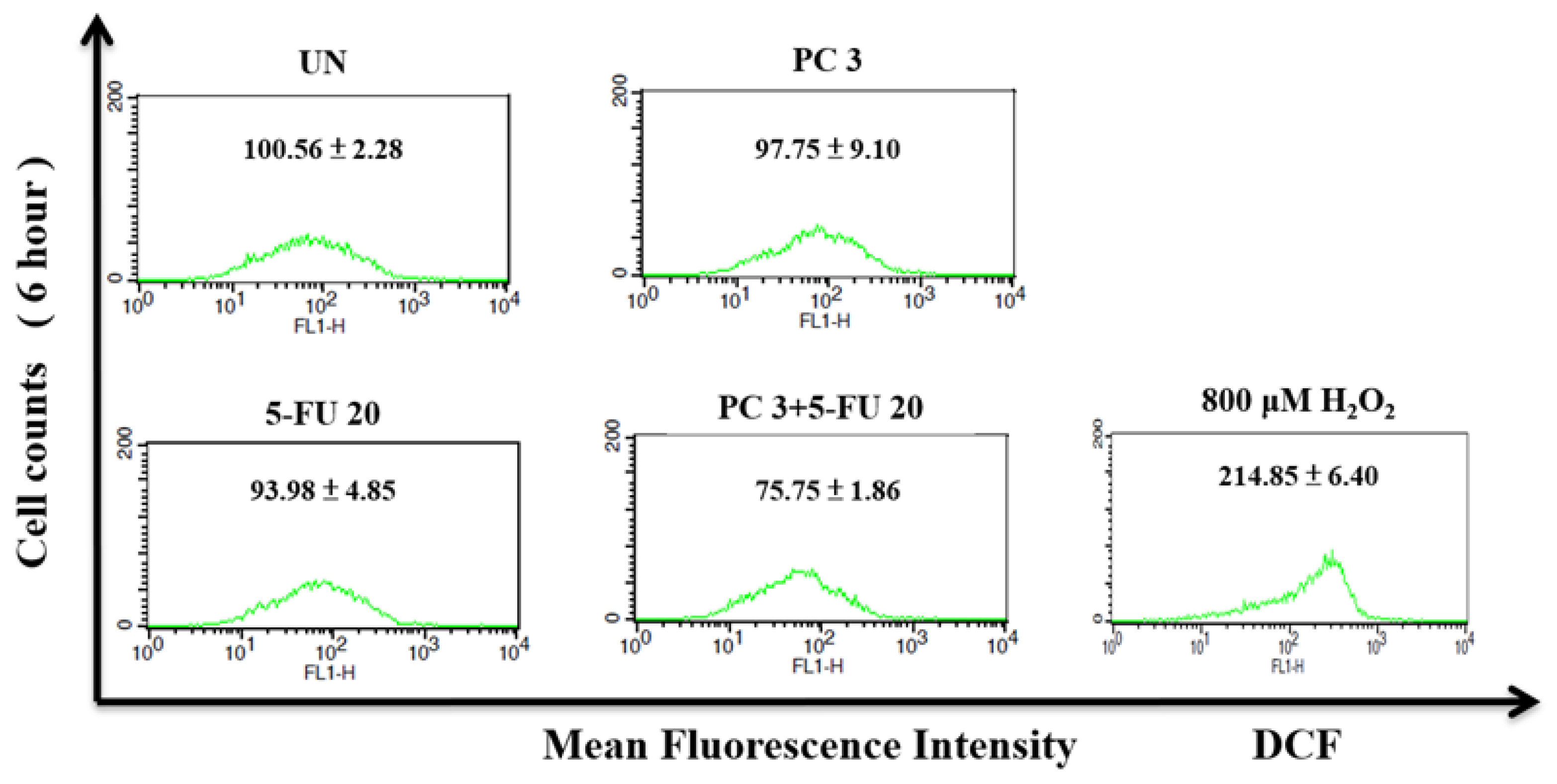
Figure 8.
Effect of parecoxib and 5-FU on intracellular ROS in DLD-1 cells. DLD-1 cells were seeded in 6 cm dishes with a density of 4× 105 cells/dish. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were treated for 6 h. After treatment, all cells were incubated with DCFH-DA for intracellular ROS detection and determined by using a flow cytometer. Cells were treated with 800 μM H2O2 as the positive control of intracellular ROS. Data in each panel show the mean fluorescence intensity of DCF inside the cells. The data are shown as the mean ± SD (n = 5–8) of individual experiments.
Figure 8.
Effect of parecoxib and 5-FU on intracellular ROS in DLD-1 cells. DLD-1 cells were seeded in 6 cm dishes with a density of 4× 105 cells/dish. Parecoxib (3 μM) alone, 5-FU (20 μM) alone, and parecoxib (3 μM) combined with 5-FU (20 μM) were added, and the cells were treated for 6 h. After treatment, all cells were incubated with DCFH-DA for intracellular ROS detection and determined by using a flow cytometer. Cells were treated with 800 μM H2O2 as the positive control of intracellular ROS. Data in each panel show the mean fluorescence intensity of DCF inside the cells. The data are shown as the mean ± SD (n = 5–8) of individual experiments.
Figure 9.
Effect of parecoxib and 5-FU on cell migration and invasion by transwell and matrix gel assays in SW480 cells. SW480 cells (7×104/well) were seeded onto the upper chambers without matrix gel for migration assay (A and B) and with matrix gel for invasion assay (D and E) and then incubated with parecoxib (2 and 3 μM) alone, 5-FU (3 and 5 μM) alone, and parecoxib (2 and 3 μM) combined with 5-FU (3 and 5 μM) for 48 h (migration assay) and 96 h (invasion assay). (A and D) Random fields from each of the triplicate migration assays were calculated by a phase-contrast microscope (magnification 200×). (B and E) The pigment was eluted with 33% acetic acid, and the absorbance of crystal violet was determined at 570 nm by using a microplate reader. The values are displayed as the mean ± SD of separate trials. Significant differences are expressed as p<0.05 (*), p<0.01 (**) and p<0.001 (***). (C) Isobologram analysis of the parecoxib and 5-FU combination in SW480 cells. Combination index data were computed by CalcuSyn software. Points below the backslash line indicate the synergistic outcome. The trials were conducted at least three times. A descriptive trial is shown.
Figure 9.
Effect of parecoxib and 5-FU on cell migration and invasion by transwell and matrix gel assays in SW480 cells. SW480 cells (7×104/well) were seeded onto the upper chambers without matrix gel for migration assay (A and B) and with matrix gel for invasion assay (D and E) and then incubated with parecoxib (2 and 3 μM) alone, 5-FU (3 and 5 μM) alone, and parecoxib (2 and 3 μM) combined with 5-FU (3 and 5 μM) for 48 h (migration assay) and 96 h (invasion assay). (A and D) Random fields from each of the triplicate migration assays were calculated by a phase-contrast microscope (magnification 200×). (B and E) The pigment was eluted with 33% acetic acid, and the absorbance of crystal violet was determined at 570 nm by using a microplate reader. The values are displayed as the mean ± SD of separate trials. Significant differences are expressed as p<0.05 (*), p<0.01 (**) and p<0.001 (***). (C) Isobologram analysis of the parecoxib and 5-FU combination in SW480 cells. Combination index data were computed by CalcuSyn software. Points below the backslash line indicate the synergistic outcome. The trials were conducted at least three times. A descriptive trial is shown.