1. Introduction
Idiopathic intracranial hypertension (IIH) is characterized by increased intracranial pressure as indicated by papilledema and imaging findings, with no identifiable extrinsic causes [
1]. The main symptoms are headache, vision loss, and pulsatile tinnitus, which are known to significantly affect quality of life and visual function [
1]. The cause is unknown but is thought to involve overproduction of cerebrospinal fluid (CSF), absorption disorders, impaired venous return, and metabolic disorders [
2]. The incidence is known to be higher in obese women of reproductive age [
3] at approximately 5.5 per 100,000 per year [
4], while the reported overall incidence is approximately 1 to 2 per 100,000 per year [4-6]. The overarching goal of treatment is to alleviate symptoms and prevent permanent vision loss. It has been reported that IIH can also be treated by treating obesity [
7]. On the other hand, superficial siderosis (SS) is a disorder in which hemosiderin is deposited on the cortical surface of the cerebrum or cerebellum, and although hemorrhagic tendency, tumors, trauma, and spinal disease are classic known hemorrhagic factors, the cause of SS is also frequently unknown [
8]. Persistent hemorrhage owing to spinal cord dural defects resulting from injury has also been implicated [
9] and categorized as “duropathy” [
10,
11]. An association between spontaneous intracranial hypotension and SS has also been reported [
12,
13]. However, so far, no cases of IIH with infratentorial and supratentorial cortical SS have been reported.
We encountered a very rare case of IIH complicated by infratentorial and supratentorial cortical SS. This was institutional review board approved study as Clinical trial registration number: Mito Kyodo General Hospital IRB NO 23-09, and we had obtained written consent to participate in this study from the participants. We aimed to report this case because of its clinical significance, as it presents novel findings in IIH that could aid in elucidating its pathology.
2. Case Description
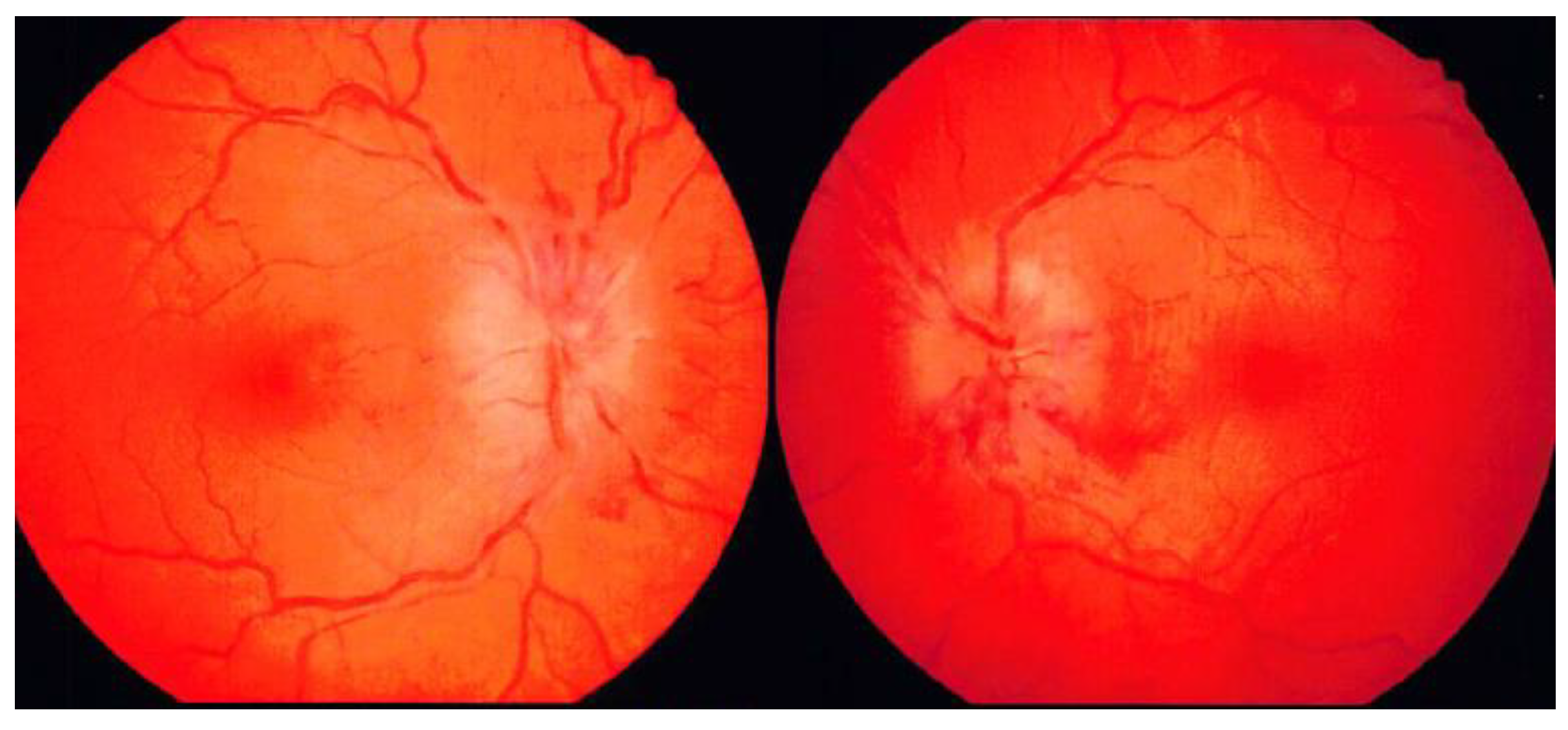
A 31-year-old woman with obesity developed sudden headache and dizziness 13 days before her hospital admission, following which vomiting continued for 3 days. Next, she lost strength in her legs and had difficulty walking. Subsequently, her headache, nausea, and dizziness, which peaked during the aforementioned 3 days, gradually subsided. Seven days before her admission, she became aware of diplopia when she turned her head to the right side. On day of emergent admission, she visited an ophthalmologist at a nearby clinic and was referred to our hospital because of congestive papillae in both eyes and marked abduction disorder in the right eye. She also experienced dizziness during eye movements. On visiting our hospital, the patient was unable to walk independently. She did not smoke, drank alcohol only once per week, and had no history of allergies. She was Irish, had never been married or given birth, and was a middle school English teacher. She had a medical history of migraine with aura since puberty. Her most recent oral medications included eletriptan hydrobromide, lomerizine hydrochloride, domperidone, and herbal remedies for migraine. She had been on the pill until approximately 1.5 months ago but had stopped taking it on the advice of her family doctor due to an increased risk of stroke. She had no notable family history. Physical examination revealed obesity, with a height of 162 cm, weight of 80 kg, and body mass index of 30.5. Her awareness score was 15 (4-5-6) points on the Glasgow Coma Scale. On ophthalmologic examination, corrected visual acuity was 1.5 in both the right and left eyes, and intraocular pressure was not elevated (right eye: 13.5 mmHg, left eye: 13.0 mmHg); however, fundus findings showed significant swelling of both optic nerve papillae, which were stasis-like (
Figure 1).
The cranial nervous system assessment revealed the following: prompt pupillary counter-reflexes, pupillary size of 3.0 mm, abducent nerve disturbances in the right eye, and absence of facial paralysis and dysarthria. An assessment of the motor system revealed a negative Barre’s sign and grade of 5/Ⅴ on the Manual Motor Test in both the upper and lower limbs. No obvious sensory disturbances were observed in the whole body. The coordination of hand rotation was smooth. Her blood data showed no abnormalities in blood counts or general biochemistry and no hyperlipidemia or diabetes mellitus, and D-dimer levels were not elevated in the coagulation system.
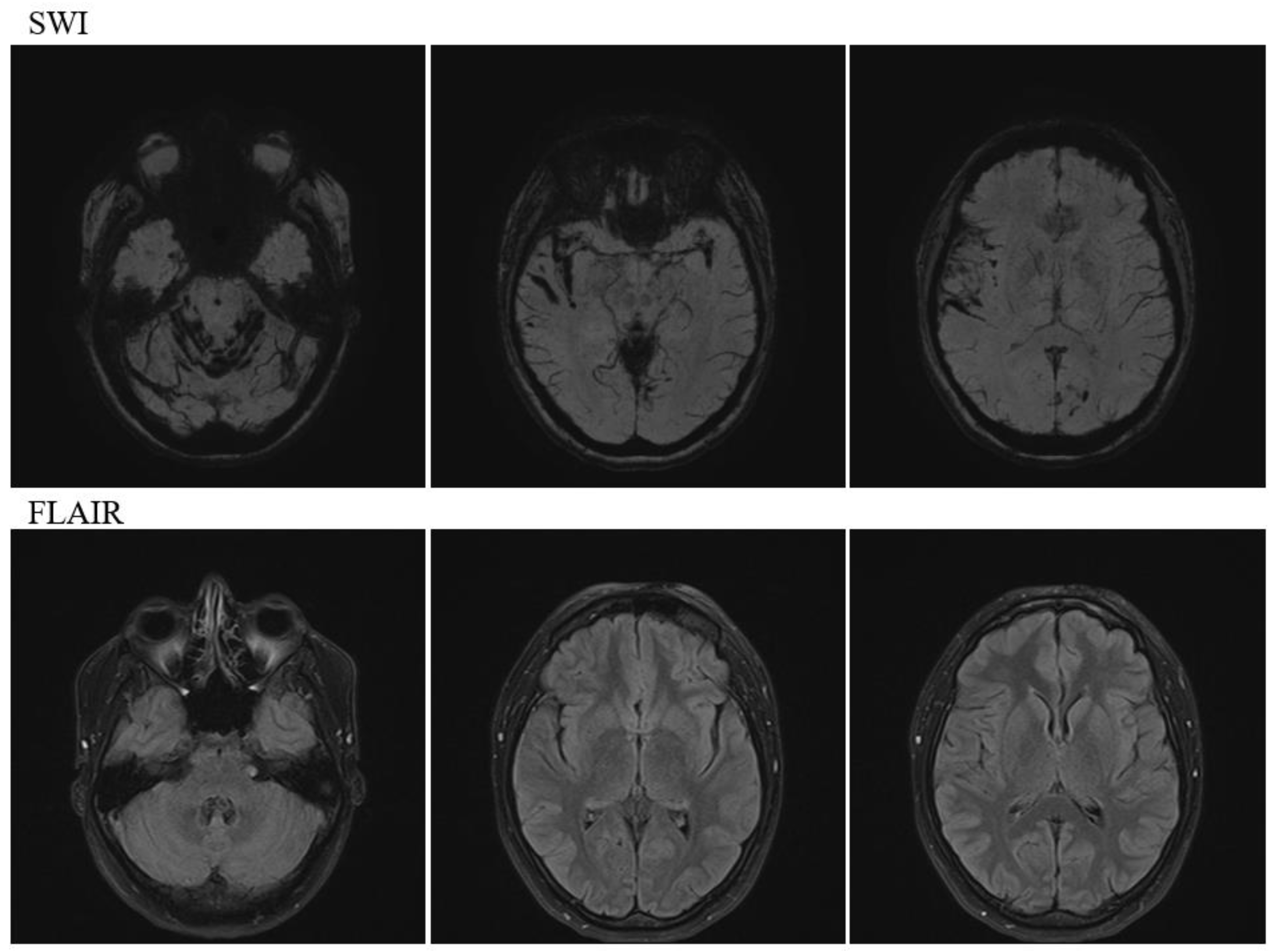
Head magnetic resonance imaging (MRI) (
Figure 2) revealed multiple prominent hemosiderin deposits on the cerebellar surface and similar hemosiderin deposits in the temporal lobe and fissure sylviae, indicating infratentorial and cortical SS.
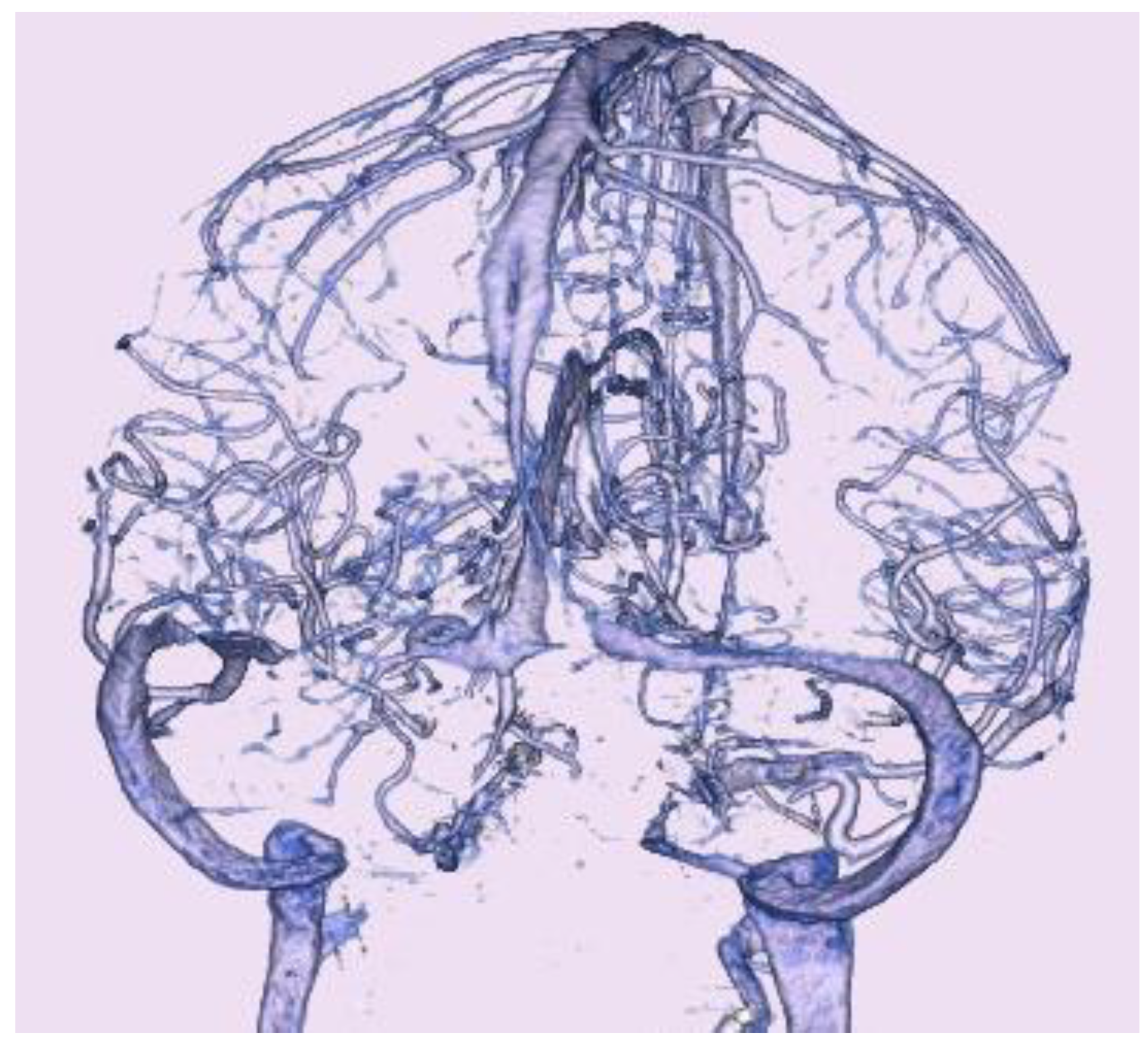
Computed tomography (CT) showed no acute hemorrhage. CT venography (
Figure 3) showed bilateral stenosis of the transverse sinus, or bilateral dilatation of the adjoining vein of Labbe.
Lumbar puncture on day 1 after her admission showed an increased intracranial pressure of 32 cmH
2O, and the following were the laboratory findings in the CSF sample: colorless and transparent CSF, specific gravity of 1.006 (normal range: 1.005-1.007), glucose level of 78 mg/dL (normal range: 40-75), protein level of 25 mg/dL (normal range: 10-40), and cell count of 4/3. Additional laboratory tests showed no notable findings. Therefore, she was diagnosed with IIH because no other disease that could cause her symptoms was apparent, and there was no obvious cause of intracranial hypertension, such as an occupying lesion, which aligned with the modified Dandy criteria [
14] for the diagnosis of IIH. Treatment with oral acetazolamide (750 mg/day) was started. Approximately 3 days after treatment initiation, the abduction disorder in the patient’s right eye almost disappeared, her dizziness disappeared, and she was able to walk unassisted. Ten days later from her admission, the patient was discharged on her own. Twenty-two days later from her admission, a follow-up lumbar puncture showed a decreased intracranial pressure of 20 cmH
2O, and the following were the laboratory findings in the CSF sample: colorless and transparent CSF, specific gravity of 1.006 (normal range: 1.005-1.007), glucose level of 68 mg/dL (normal range: 40-75), protein level of 40 mg/dL (normal range: 10-40), and cell count of 5/3. Sixty-three days later from her admission, on examination by the ophthalmologist, all findings had improved. Dynamic visual field testing showed that although the enlargement of Mariotte’s blind spot was still present and not yet normalized, it had shrunk. Ocular position testing showed that the strabismus and eye movement disorder had disappeared. Fundus examination showed that the swelling of the optic nerve papilla had improved, with only slight residual swelling left, and the subjective complaints of diplopia were no longer present. Seventy-one days later from her admission, head MRI showed no change in intracranial SS, and spinal MRI showed no CSF leakage that could be a cause of infratentorial siderosis. All of her aftereffects had disappeared, as evidenced by a modified Rankin scale score of 0, and she returned to her pre-onset life as a teacher.
3. Discussion
In this case, a young woman with obesity and IIH developed sudden onset headache and nausea, followed by right eye abduction disorder and papilledema. The patient had marked SS in both the infratent and supratent. There have been no previous reports of IIH patients with both infratentorial and supratentorial SS. Intravoxel incoherent motion (IVIM) MRI was performed to visualize the movement of the CSF (Clinical trial registration number: Mito Kyodo General Hospital IRB No. 23-09), suggesting a causal relationship between IIH and SS. Therefore, this report is highly novel and of high clinical significance, as it provides insights into the relationship between IIH and SS and has revealed novel imaging findings, which could be clues regarding the pathophysiology.
Causal Relationship between IIH and SS
SS of the central nervous system is characterized by linear hemosiderin deposition in the superficial layers of the leptomeninges, cerebrum, and spinal cord [
15]. Subarachnoid space infratentorial SS is thought to be caused by recurrent or continuous slight hemorrhage into the subarachnoid space, with the most important cause being spinal dural lesions that cause CSF leakage [
15]. The classical types of infratentorial SS have been reported to involve dural abnormalities, spinal CSF leakage, craniocervical surgery, spinal trauma, head trauma, arteriovenous malformation (AVM), and tumors, while the secondary types involve subarachnoid hemorrhage (SAH), AVM, and cerebral venous embolism due to ruptured cerebral aneurysm [
15]. The other types of SS have been classified into SS confined to the supratentorial compartment or cortical SS. Supratentorial cortical SS involves cerebral amyloid angiopathy in the elderly, ruptured cerebral aneurysmal SAH, AVM, reversible cerebral vasoconstriction syndrome, amyloid-related imaging abnormalities due to hemosiderin deposition, vasculitis, severe stenosis of the cerebral vessels, sinus occlusion, head trauma, and tumors [
15].
In the present case, hemosiderin deposition was observed above and below the tent. However, there was no obvious cause of SS. There were no findings in the CSF specimen that would suggest persistent hemorrhage due to CSF leakage, as described in classical SS; similarly, head and spinal cord MRI findings did not reveal the presence of any disease that could have caused SS. However, the possibility that IIH may have caused the onset of SS due to arachnoid damage could not be ruled out although this has not been previously reported. Therefore, it should be kept in mind that IIH may cause SS due to arachnoid membrane rupture, which is a newly identified cause of SS.
Conversely, to the best of our knowledge, there are no reports regarding the possibility of IIH caused by SS, which have documented the mechanism of IIH, such as CSF malabsorption associated with SS. However, it is important to accumulate case reports similar to ours in the future.
Was SS Asymptomatic in this Case?
Typical neurological manifestations of infratentorial SS include slowly progressive sensorineural hearing impairment and cerebellar manifestations, such as ataxia, motor tremor, nystagmus, and dysarthria [8,16-22]. There are reported cases in which hemosiderin deposition in the cranial nerves has caused the appearance of individual cranial nerve symptoms [
23].
The patient's main symptoms of congestive papillae and abducens nerve palsy disappeared due to a successful response to acetazolamide and decrease in intracranial pressure; therefore, we believe that these symptoms were not caused by SS but by IIH. However, it is undeniable that the abducens nerve palsy in our patient at the time of intracranial hypertension onset may have been influenced by hemosiderin deposition in the cranial nerves.
Did SS Affect The Clinical Course of IIH?
In cerebral surface hemosiderin deposition disease, hemosiderin in the blood is deposited on the surface of the brain and spinal cord due to persistent and repeated bleeding, causing nerve damage due to oxidative stress [
24]. At the time of admission, there were no acute hemorrhagic changes that could have resulted in a high signal on CT, and we believe that hemosiderin deposition was originally part of the patient's previous history. MRI at the time of admission also showed hemosiderin deposition in the abducens nerve; however, we cannot rule out the possibility that neuropathy caused by oxidative stress may have precipitated the appearance of abducens nerve palsy due to IICP although not to the extent that it caused continuous subjective symptoms.
CSF Circulation in IIH with SS
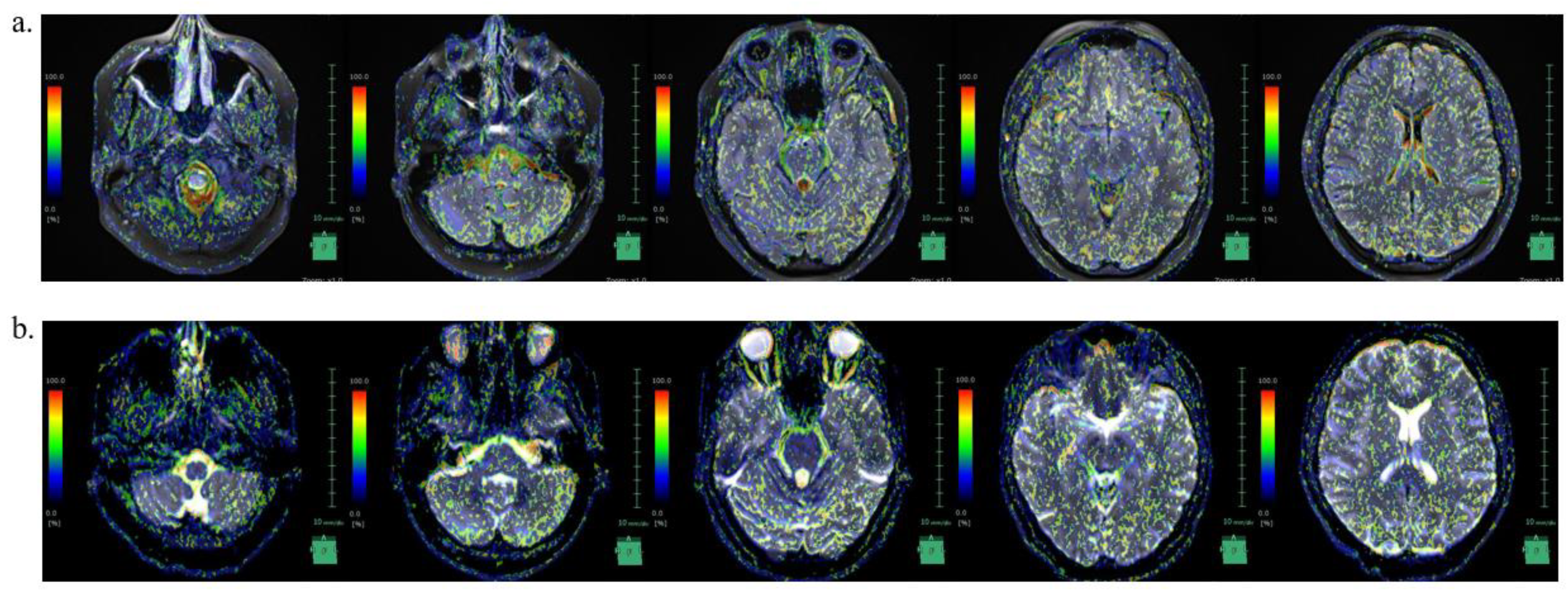
We were not able to find reports on CSF dynamics in IIH in the literature. Recently, IVIM MRI was used to visualize CSF movement [
25]. CSF movement was visualized in our case as well (Fig 4). In this case, the SS site matched the area of strong incoherent CSF motion at the time of pre-treatment.
In the IICP condition, incoherent CSF movement was pronounced in the caudal portion of the medulla oblongata, fourth ventricle, lateral ventricles, and brain surface; however, in the decreased intracranial pressure condition following acetazolamide treatment, the incoherent movement in these regions decreased. In our case, the areas of SS coincided with areas of increased incoherent CSF motion before treatment, supporting the association between IIH and SS.
4. Conclusions
This is the first reported case of IIH with underlying cerebral surface hemosiderin deposition both above and below the tent; there was no obvious condition, such as CSF leakage, which could have caused SS, suggesting a causal relationship between IIH and SS. CSF analysis using IVIM MRI may be effective for diagnosing IIH and determining treatment efficacy.
Author Contributions
Conceptualization, S.W. and Y.S.; methodology, S.W. and Y.S..; software, S.W.; validation, Y.S.; resources, S.W.; data curation, X.X.; writing—original draft preparation, S.W.; writing—review and editing, all authors.; supervision, E.I.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Mito Kyodo General Hospital (IRB number: NO 23-09 and date of approval: August 7, 2023).” for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, and written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang MTM, Bhatti MT, Danesh-Meyer HV. Idiopathic intracranial hypertension: pathophysiology, diagnosis and management. Review J Clin Neurosci. 2022;95: 172-179. [CrossRef]
- Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. 2016;15: 78-91. [CrossRef]
- Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991;114 (Pt 1A): 155-180.
- Kesler A, Stolovic N, Bluednikov Y, Shohat T. The incidence of idiopathic intracranial hypertension in Israel from 2005 to 2007: results of a nationwide survey. Eur J Neurol. 2014;21: 1055-1059. [CrossRef]
- Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45: 875-877. [CrossRef]
- Radhakrishnan K, Ahlskog JE, Cross SA, Kurland LT, O'Fallon WM. Idiopathic intracranial hypertension (pseudotumor cerebri). Descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol. 1993;50: 78-80. [CrossRef]
- Subramaniam S, Fletcher WA. Obesity and weight loss in idiopathic intracranial hypertension: a narrative review. J Neuroophthalmol. 2017;37: 197-205. [CrossRef]
- Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol. 2007;3: 54-58; quiz 59. [CrossRef]
- Wilson D, Chatterjee F, Farmer SF, Rudge P, McCarron MO, Cowley P, Werring DJ. Infratentorial superficial siderosis: classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. 2017;81: 333-343. [CrossRef]
- Kumar, N. Beyond superficial siderosis: introducing "duropathies". Neurology. 2012;78: 1992-1999. [CrossRef]
- Kumar N, Miller GM, Piepgras DG, Mokri B. A unifying hypothesis for a patient with superficial siderosis, low-pressure headache, intraspinal cyst, back pain, and prominent vascularity. J Neurosurg. 2010;113: 97-101. [CrossRef]
- Mokri, B. Spontaneous intracranial hypotension. Continuum (Minneap Minn). 2015;21: 1086-1108. [CrossRef]
- Schievink WI, Maya M, Moser F, Nuño M. Long-term risks of persistent ventral spinal CSF leaks in SIH: superficial siderosis and bibrachial amyotrophy. Neurology. 2021;97: e1964-e1970. [CrossRef]
- Kosmorsky, GS. Idiopathic intracranial hypertension: pseudotumor cerebri. Headache. 2014;54: 389-393. [CrossRef]
- Weidauer S, Neuhaus E, Hattingen E. Cerebral superficial siderosis: etiology, neuroradiological features and clinical findings. Clin Neuroradiol. 2023;33: 293-306. [CrossRef]
- van Harskamp NJ, Rudge P, Cipolotti L. Cognitive and social impairments in patients with superficial siderosis. Brain. 2005;128(Pt 5): 1082-1092. [CrossRef]
- Uttner I, Tumani H, von Arnim C, Brettschneider J. Cognitive impairment in superficial siderosis of the central nervous system: a case report. Cerebellum. 2009;8: 61-63. [CrossRef]
- Anderson NE, Sheffield S, Hope JK. Superficial siderosis of the central nervous system: a late complication of cerebellar tumors. Neurology. 1999;52: 163-169. [CrossRef]
- Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain. 1995;118 ( Pt 4): 1051-1066. [CrossRef]
- Bürk K, Skalej M, Dichgans J. High prevalence of CSF-containing cysts in superficial hemosiderosis of the central nervous system. J Neurol. 2001;248: 1005-1006. [CrossRef]
- Kumar N, Cohen-Gadol AA, Wright RA, Miller GM, Piepgras DG, Ahlskog JE. Superficial siderosis. Neurology. 2006;66: 1144-1152. [CrossRef]
- Wilson D, Chatterjee F, Farmer SF, Rudge P, McCarron MO, Cowley P, Werring DJ. Infratentorial superficial siderosis: classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. 2017;81: 333-343. [CrossRef]
- Savoiardo M, Maccagnano E, Pareyson D, Grisoli M. Superficial siderosis. Neurology. 2007;68: 623; author reply 623-624. [CrossRef]
- Koeppen AH, Michael SC, Li D, Chen Z, Cusack MJ, Gibson WM, et al. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol. 2008;116: 371-382. [CrossRef]
- Yamada S, Hiratsuka S, Otani T, Ii S, Wada S, Oshima M, et al. Usefulness of intravoxel incoherent motion MRI for visualizing slow cerebrospinal fluid motion. Fluids Barriers CNS. 2023;20: 16. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).