Submitted:
28 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
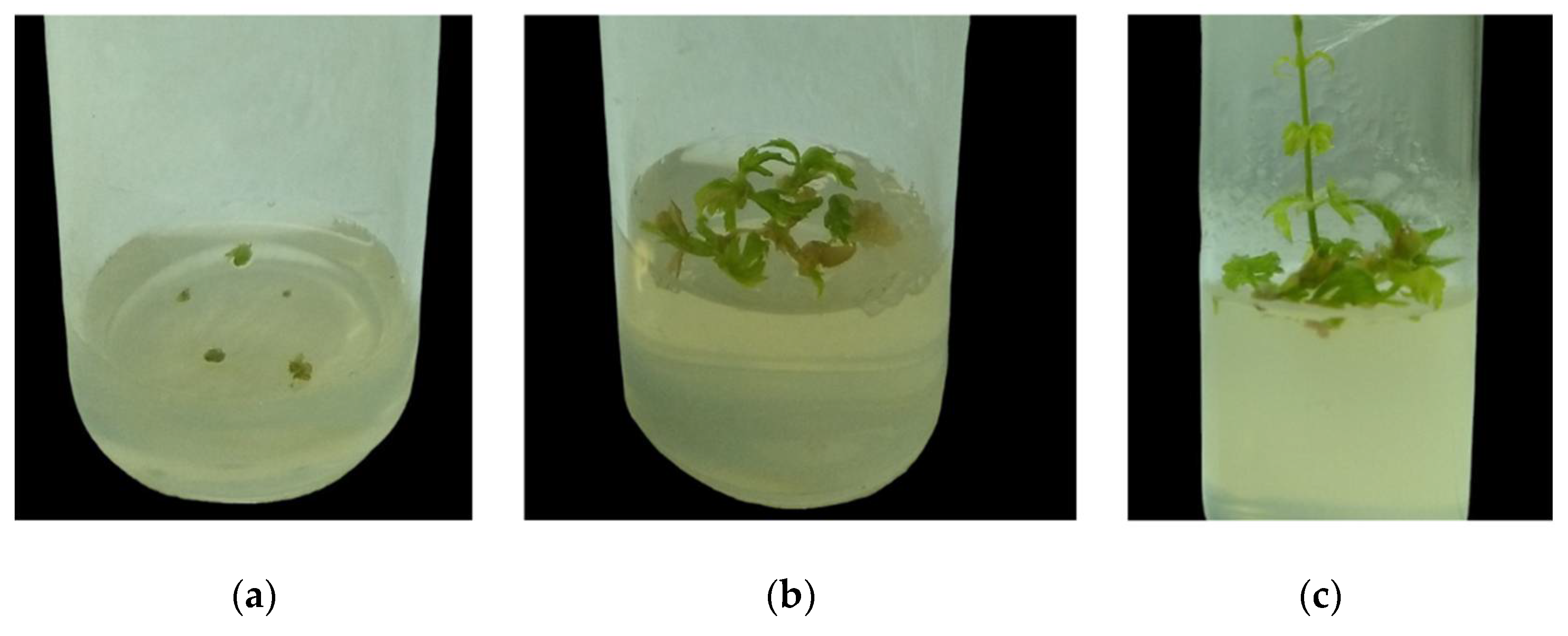
2.1. Effect of Plants Growth Regulators on Shoot Tip Cultivation
2.2. Rooting of Uninodal Segments
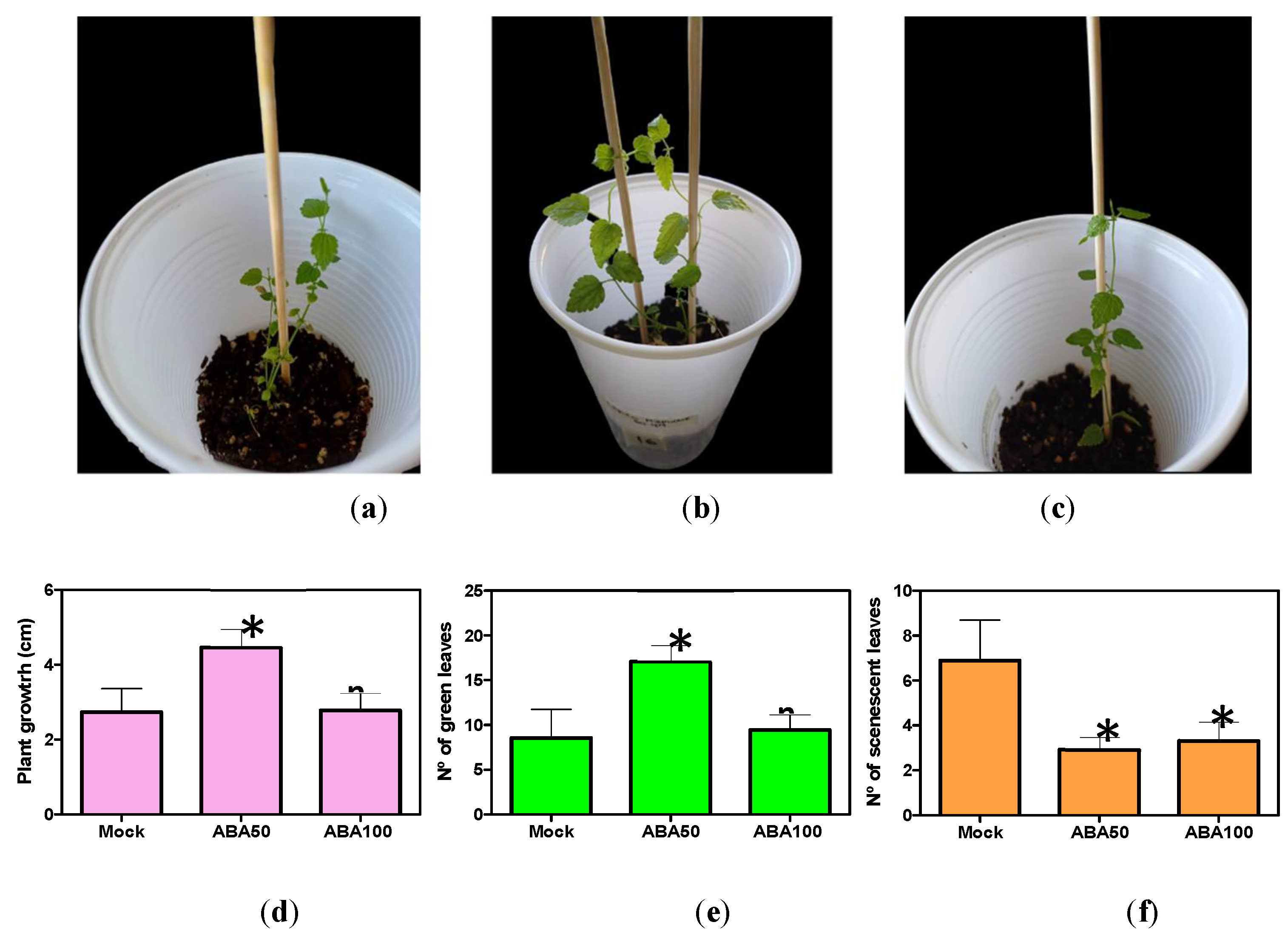
2.3. ABA Effects in the Acclimatization Process
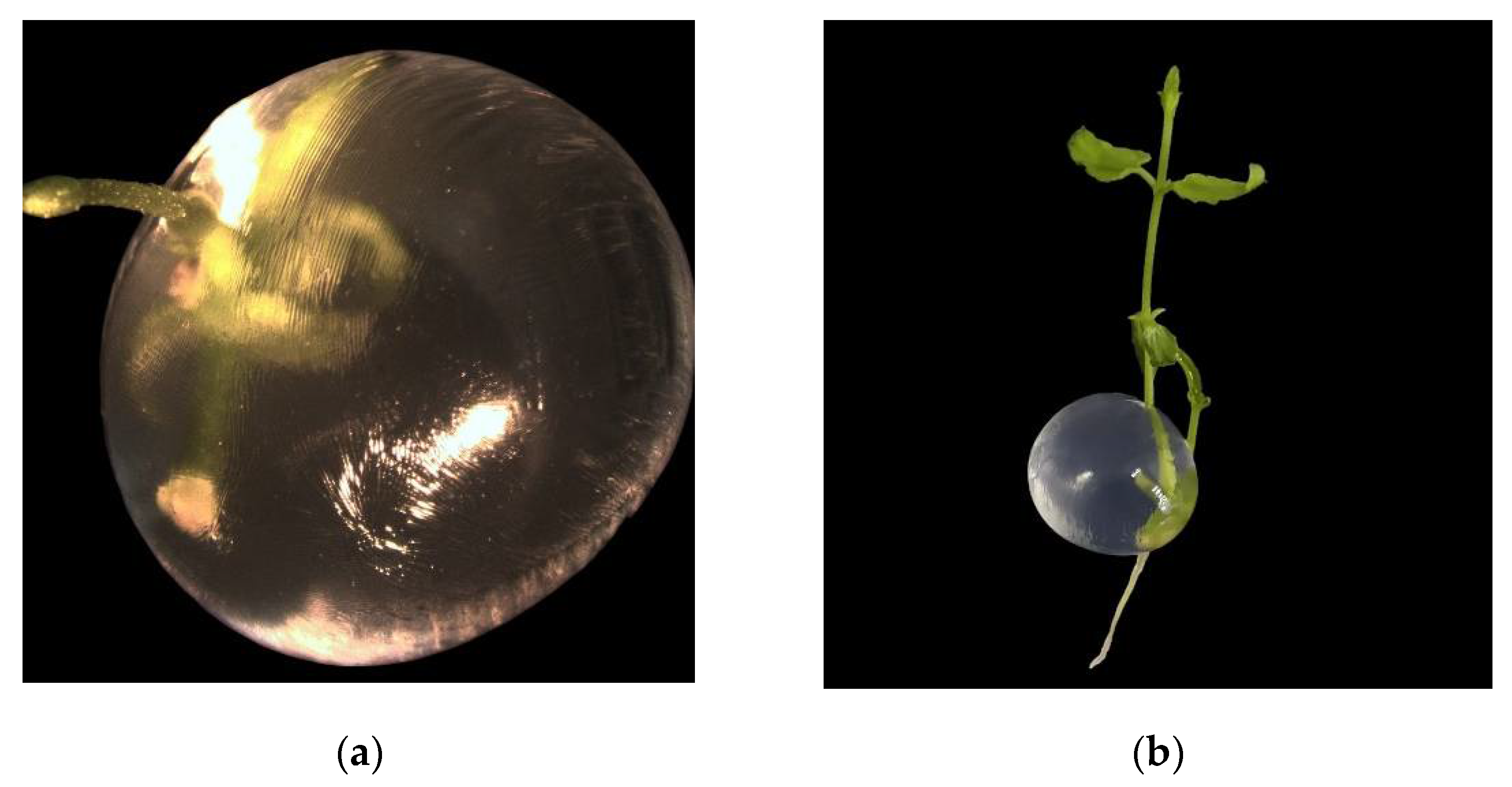
2.4. Synthetic Seeds
3. Discussion
4. Materials and Methods
4.1. Plant Material and Explant Conditioning
4.1.1. Culture Conditions
4.2. Induction of Morphogenic Responses
4.2.1. Shoot Tip Cultivation
4.2.2. Rooting Unimodal Segments
4.3. Acclimatization to Ex Vitro Conditions and ABA Treatment
4.4. Synthetic Seeds
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Dodds, K. Hops—A Guide for New Growers; NSW Department of Primary Industries: Orange, NSW, Australia, 2017; ISBN 978-1-76058-007-0. [Google Scholar]
- Mozny, M.; Trnka, M.; Vlach, V.; Zalud, Z.; Cejka, T.; Hajkova, L. ... Büntgen, U. Climate-induced decline in the quality and quantity of European hops calls for immediate adaptation measures. Nat. Commun. 2023, 14, 6028. [Google Scholar] [CrossRef]
- El cambio climático podría duplicar el coste de una cerveza. Available online: https://ee-ip.org/es/article/el-cambio-climatico-podria-duplicar-el-coste-de-una-cerveza-1187 (accesses on 25 February 2024).
- Potopová, V.; Lhotka, O.; Možný, M.; Musiolková, M. Vulnerability of hop-yields due to compound drought and heat events over European key-hop regions. Int. J. Climatol. 2021, 41, E2136–E2158. [Google Scholar] [CrossRef]
- Agehara, S.; Acosta-Rangel, A.; Gallardo, M.; Vallad, G. Selection and Preparation of Planting Material for Successful Hop Production in Florida: HS1381, 9/2020. EDIS: Gainesville, FL, 2020; Volume 2020 (5). [CrossRef]
- Eastwell, K.C.; Barbara, D.J. Apple Mosaic Virus. In Field Guide for Integrated Pest Management in Hops, 3rd ed.; O‘Neal, S.D., Walsh, D.B., Gent, D.H., Eds.; U.S. Hop Industry Plant Protection Committee: Pullman, WA, 2015; Volume 1, p. 39. [Google Scholar]
- 7. Eastwell, K. C.; Barbara, D.J. Carlavirus Complex: American Hop Latent Virus, Hop Latent Virus, and Hop Mosaic Virus. In Field Guide for Integrated Pest Management in Hops, 3rd ed.; O‘Neal, S.D., Walsh, D.B., Gent, D.H., Eds.; U.S. Hop Industry Plant Protection Committee: Pullman, WA, 2015; Volume 1, p. 38. [Google Scholar]
- Gent, D.H.; Johnson, D.A.; Gevens, A.J.; Hausbeck, M.K. Downy Mildew. In Field Guide for Integrated Pest Management in Hops, 3rd ed.; O‘Neal, S.D., Walsh, D.B., Gent, D.H., Eds.; U.S. Hop Industry Plant Protection Committee: Pullman, WA, 2015; Volume 1, pp. 15–21. [Google Scholar]
- Gent, D.H.; Nelson, M.E.; Gadoury, D.M.; Gevens, A.J.; Hausbeck, M.K. Powdery Mildew. In Field Guide for Integrated Pest Management in Hops, 3rd ed.; O‘Neal, S.D., Walsh, D.B., Gent, D.H., Eds.; U.S. Hop Industry Plant Protection Committee: Pullman, WA, 2015; Volume 1, pp. 25–29. [Google Scholar]
- Levitus, G.; Echenique, V.; Rubinstein, C.; Hopp, E.; Mroginski, L. Biotecnología y mejoramiento vegetal II, 2nd ed.; Ediciones Instituto Nacional de Tecnología Agropecuaria (INTA): Buenos Aires, Argentina, 2010; p. 650. [Google Scholar]
- Gent, D.H.; Nelson, M.E.; George, A.E.; Grove, G.G.; Mahaffee, W.F.; Ocamb, C.M. . Turechek, W.W. A decade of hop powdery mildew in the Pacific Northwest. Plant health prog. 2008, 9, 33. [Google Scholar] [CrossRef]
- Faragó, J.; Psenácová, I.; Faragová, N. The use of biotechnology in hop (Humulus lupulus L.) improvement. Nova Biotech. 2009, 9, 279–293. [Google Scholar] [CrossRef]
- Osório, M.L.; Gonçalves, S.; Coelho, N.; Osório, J.; Romano, A. Morphological, physiological and oxidative stress markers during acclimatization and field transfer of micropropagated Tuberaria major plants. PCTOC 2013, 115, 85–97. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, G.; Santos, C. Acclimatization of micropropagated plantlets induces an antioxidative burst: a case study with Ulmus minor Mill. Photosynthetica, 2011, 49, 259–266. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Synková, H.; Haisel, D.; Baťková, P. Effect of abscisic acid on photosynthetic parameters during ex vitro transfer of micropropagated tobacco plantlets. Biol. Plant. 2009, 53, 11–20. [Google Scholar] [CrossRef]
- Aguilar, M.L.; Espadas, F.L.; Coello, J.; Maust, B.E.; Trejo, C.; Robert, M.L.; Santamaria, J.M. The role of abscisic acid in controlling leaf water loss, survival and growth of micropropagated Tagetes erecta plants when transferred directly to the field. J. Exp. Bot. 2000, 51, 1861–1866. [Google Scholar] [CrossRef]
- Santamaria, J.M.; Davies, W.J.; Atkinson, C.J. Stomata of micropropagated Delphinium plants respond to ABA, CO2, light and water potential, but fail to close fully. J. Exp. Bot. 1993, 44, 99–107. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C. ... Leung, J. An update on abscisic acid signaling in plants and more…. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef]
- Dias, M.C.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Santos, C. Study of the effects of foliar application of ABA during acclimatization. PCTOC 2014, 117, 213–224. [Google Scholar] [CrossRef]
- Roy, A.T.; Leggett, G.; Koutoulis, A. Development of a shoot multiplication system for hop (Humulus lupulus L.). In Vitro Cell. Dev. Biol.-Plant 2001, 37, 79–83. [Google Scholar] [CrossRef]
- Smýkalová, I.; Ortová, M.; Lipavská, H.; Patzak, J. Efficient in vitro micropropagation and regeneration of Humulus lupulus on low sugar, starch-Gelrite media. Biol. Plant. 2001, 44, 7–12. [Google Scholar] [CrossRef]
- Mafakheri, M.; Hamidoghli, Y. Micropropagation of hop (Humulus lupulus L.) via shoot tip and node culture. In IV International Humulus Symposium 1236, 1st. ed.; Matthews, P., Stevens, F., Eds.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2019; Volume 1, pp. 154–196. [Google Scholar]
- Trojak-Goluch, A.; Kawka, M.; Czarnecka, D. The effects of explant source and hormone content on plant regeneration and induction of tetraploids in Humulus lupulus L. In Vitro Cell. Dev. Biol.-Plant, 2015, 51, 152-159. [CrossRef]
- Liberatore, C.M.; Rodolfi, M.; Beghè, D.; Fabbri, A.; Ganino, T.; Chiancone, B. Adventitious shoot organogenesis and encapsulation technology in hop (Humulus lupulus L.). Sci. Hortic. 2020, 270, 109416. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M. Establishment of an efficient micropropagation system for Humulus lupulus L. cv. cascade and confirmation of genetic uniformity of the regenerated plants through dna markers. Agronomy. 2021, 11, 2268. [Google Scholar] [CrossRef]
- Hirakawa, T.; Tanno, S.; Tanno, S. In vitro propagation of Humulus lupulus through the induction of axillary bud development. Plants 2022, 11, 1066. [Google Scholar] [CrossRef]
- Lagos, F.S.; Zuffellato-Ribas, K.C.; Deschamps, C. Vegetative propagation of hops (Humulus lupulus L.): historical approach and perspectives. Semina: Cien. Agra. 2022, 43, 1373–1394. [Google Scholar] [CrossRef]
- Di Sario, L. Evaluación del cultivo in vitro de variedades nacionales de lúpulo (Humulus lupulus) como una alternativa a la propagación agámica tradicional. Tesis de grado, Universidad Nacional de Río Negro, Villa Regina (Río Negro, Argentina), 2023. http://rid.unrn.edu.ar/handle/20.500.12049/10188. 1204. [Google Scholar]
- Ha, T.H.; Lyu, J.I.; Lee, J.H.; Ryu, J.; Park, S.H.; Kang, S.Y. Studies on growth characteristics and propagation method of introduced hop (Humulus lupulus L.) cultivars. Korean J. Plant Res. 2023, 36, 181–190. [Google Scholar] [CrossRef]
- Iacuzzi, N.; Salamone, F.; Farruggia, D.; Tortorici, N.; Vultaggio, L.; Tuttolomondo, T. Development of a New Micropropagation Protocol and Transfer of In Vitro Plants to In Vivo Conditions for Cascade Hop. Plants 2023, 12, 2877. [Google Scholar] [CrossRef]
- Batista, D.; Sousa, M.J.; Pais, M.S. Plant regeneration from stem and petiole-derived callus of Humulus lupulus L.(Hop) clone Braganca and var. Brewer’s Gold. In Vitro–Plant 1996, 32, 37–41. [Google Scholar] [CrossRef]
- Gurriarán, M.J.; Revilla, M.A.; Tamés, R.S. Adventitious shoot regeneration in cultures of Humulus lupulus L.(hop) cvs. Brewers Gold and Nugget. Plant Cell Rep. 1999, 18, 1007–1011. [Google Scholar] [CrossRef]
- Peredo, E.L.; Revilla, M.Á.; Arroyo-García, R. Assessment of genetic and epigenetic variation in hop plants regenerated from sequential subcultures of organogenic calli. J. Plant Physiol. 2006, 163, 1071–1079. [Google Scholar] [CrossRef]
- Adams, A.N. Elimination of viruses from the hop (Humulus lupulus) by heat therapy and meristem culture. J. Hortic. Sci. 1975, 50, 151–160. [Google Scholar] [CrossRef]
- Martinez, D.; Tamés, R.S.; Angeles Revilla, M. Cryopreservation of in vitro-grown shoot-tips of hop (Humulus lupulus L.) using encapsulation/dehydration. Plant Cell Rep. 1999, 19, 59–63. [Google Scholar] [CrossRef]
- Di Sario, L.; Zubillaga, M.F.; Sharry, S.E.; Boeri, P.A. Semillas sintéticas: una estrategia para la conservación de lúpulo (Humulus lupulus L.). In Biotecnología productiva y sostenible, 1st ed; Gutiérrez Mora, A., Ed.; Editorial CIATEJ: Guadalajara, México, 2023; Volume 1, pp. 101–112. [Google Scholar]
- Danso, K.E.; Ford-Lloyd, B.V. Encapsulation of nodal cuttings and shoot tips for storage and exchange of cassava germplasm. Plant Cell Rep. 2003, 21, 718–725. [Google Scholar] [CrossRef]
- Naik, S.K.; Chand, P.K. Nutrient-alginate encapsulation of in vitro nodal segments of pomegranate (Punica granatum L.) for germplasm distribution and exchange. Sci. Hortic., 2006, 108, 247–252. [Google Scholar] [CrossRef]
- Rai, M.K.; Jaiswal, V.S.; Jaiswal, U. Alginate-encapsulation of nodal segments of guava (Psidium guajava L.) for germplasm exchange and distribution. J. Hortic. Sci. Biotechnol. 2008, 83, 569–573. [Google Scholar] [CrossRef]
- Ray, A.; Bhattacharya, S. Storage and plant regeneration from encapsulated shoot tips of Rauvolfia serpentina—an effective way of conservation and mass propagation. S AFR J BOT 2008, 74, 776–779. [Google Scholar] [CrossRef]
- Rai, M.K.; Asthana, P.; Singh, S.K.; Jaiswal, V.S.; Jaiswal, U. The encapsulation technology in fruit plants—a review. Biotechnol. Adv. 2009, 27, 671–679. [Google Scholar] [CrossRef]
- Parveen, S.; Shahzad, A. Encapsulation of nodal segments of Cassia angustifolia Vahl. for short-term storage and germplasm exchange. Acta Physiol. Plant. 2014, 36, 635–640. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Anis, M.; Al-Etta, H.A. Encapsulation technology for short-term storage and germplasm exchange of Vitex trifolia L. Rend. Fis. Acc. Lincei 2015, 26, 133–139. [Google Scholar] [CrossRef]
- Gantait, S.; Kundu, S.; Ali, N.; Sahu, N.C. Synthetic seed production of medicinal plants: a review on influence of explants, encapsulation agent and matrix. Acta Physiol. Plant. 2015, 37, 98. [Google Scholar] [CrossRef]
- Alatar, A.A.; Ahmad, N.; Javed, S.B.; Abdel-Salam, E.M.; Basahi, R.; Faisal, M. Two-way germination system of encapsulated clonal propagules of Vitex trifolia L.: an important medicinal plant. J. Hortic. Sci. Biotechnol. 2017, 92, 175–182. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial seeds (principle, aspects and applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, R.; Sharma, N. In vitro morphogenic response of different explants of Gentiana kurroo Royle from Western Himalayas—an endangered medicinal plant. Physiol. Mol. Biol. Plants 2014, 20, 249–256. [Google Scholar] [CrossRef]
- Hasan, S.M.Z.; Takagi, H. Alginate-coated nodal segments of yam (Dioscorea spp.) for germplasm exchange and distribution. Plant Genet. Resour. Newsl. 1995, 103, 32–35. [Google Scholar]
- Pattnaik, S.; Chand, P.K. Morphogenic response of the alginate-encapsulated axillary buds from in vitro shoot cultures of six mulberries. PCTOC 2000, 60, 177–185. [Google Scholar] [CrossRef]
- Soneji, J.; Rao, P.; Mhatre, M. Germination of synthetic seeds of pineapple (Ananas comosus L. Merr.). Plant Cell Rep. 2002, 20, 891–894. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin–cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Fortes, A.M.; Pais, M.S. Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am. J. Bot. 2000, 87, 971–979. [Google Scholar] [CrossRef]
- Villarreal, B. ¿Cómo se forman las nuevas plantas in vitro? - Morfogénesis in vitro. Organogénesis. Embriogénesis somática. In Plantas de probeta, 1st ed.; Sharry, S., Adema, M., Abedini, W., Eds.; Editorial de la Universidad de La Plata: La Plata, Argentina, 2015; Volume 1, pp. 92-97.
- Segura, J. Introducción al desarrollo. Concepto de hormona vegetal. In Fundamentos de fisiología vegetal, 2nd ed.; Azcon, J., Talon, M., Eds.; McGraw-Hill Interamericana de España: Barcelona, España, 2008; pp. 349–376. [Google Scholar]
- Krikorian, A.D.; Kelly, K.; Smith, D.L. Hormones in Tissue Culture and Micro-Propagation. In Plant Hormones and their Role in Plant Growth and Development; Davies, P.J., Eds.; Springer, Dordrecht, 1987; pp.
- Peredo, E.L.; Arroyo-Garcia, R.; Revilla, M.Á. Epigenetic changes detected in micropropagated hop plants. J. Plant Physiol. 2009, 166, 1101–1111. [Google Scholar] [CrossRef]
- Engelmann, F. In vitro conservation of tropical plant germplasm - a review. Euphytica 1991, 57, 227–243. [Google Scholar] [CrossRef]
- Sharma, S.; Shahzad, A.; da Silva, J.A.T. Synseed technology—a complete synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef]
- Nievas, W.E.; Villarreal, P.; Rosati, A.; Rodriguez, A.B.; Lago, J. El cultivo del lúpulo. Aspectos agroambientales y económicos para el Alto Valle del río Negro, 1st ed.; Ediciones Instituto Nacional de Tecnología Agropecuaria (INTA): Buenos Aires, Argentina, 2021; p. 82. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pizzio, G.A.; Mayordomo, C.; Lozano-Juste, J.; Garcia-Carpintero, V.; Vazquez-Vilar, M.; Nebauer, S.G. ... Rodriguez, P.L. PYL1-and PYL8-like ABA receptors of Nicotiana benthamiana play a key role in ABA response in seed and vegetative tissue. Cells 2022, 11, 795. [Google Scholar] [CrossRef]
- Micheli, M.; Hafiz, I.A.; Standardi, A. Encapsulation of in vitro-derived explants of olive (Olea europaea L. cv. Moraiolo): II. Effects of storage on capsule and derived shoots performance. Sci. Hortic. 2007, 113, 286–292. [Google Scholar] [CrossRef]


| Culture medium | BAP (µM) | GA3 (µM) | Regenerated shoot tips [%] |
Elongation [%] |
|---|---|---|---|---|
| M1 | 0 | 0 | 0 ± 0 a | 0 ± 0 a |
| M2 | 0.88 | 0 | 75.56 ± 12.2 b | 42.22 ± 9.8 b |
| M3 | 0 | 2.89 | 86.67 ± 10 b | 35.56 ± 10.6 b |
| Morphogenetic responses (%) | ||||||
|---|---|---|---|---|---|---|
| IAA (µM) | IBA (µM) | Rooting | Direct rooting | Indirect rooting | Callus | |
| T1 | 0 | 0 | 91.11 ± 8.45 b | 100 ± 0 b | 0 ± 0 a | 0 ± 0 a |
| T2 | 5.7 | 0 | 60 ± 14.14 a | 32.41 ± 14.7 a | 67.59 ± 14.7 b | 84.44 ± 8.82 b |
| T3 | 0 | 4.9 | 57.78 ± 21.08 a | 45.37 ± 27.99 a | 54.63 ± 27.99 b | 91.11 ± 8.53 bc |
| T4 | 5.7 | 4.9 | 57.78 ± 15.63 a | 22.22 ± 18.16 a | 77.78 ± 18.16 b | 100 ± 0 c |
| Evaluated responses (%) | ||||
|---|---|---|---|---|
| Week(s) at 4°C | Conversion | Rooting | Viability | |
| T0 | 0 (control) | 90 ± 10 c | 56.67 ± 12.11 c | 100 ± 0.00 b |
| TA | 1 | 76.67 ± 16.33 bc | 46.67 ± 19.66 bc | 86.67 ± 5.16 a |
| TB | 2 | 66.67 ± 15.06 ab | 26.67 ± 5.16 ab | 90.00 ± 0.00 a |
| TC | 3 | 46.67 ± 8.16 a | 33.33 ± 12.11 a | 86.67 ± 5.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





