Submitted:
28 May 2024
Posted:
29 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Indicators and Methods
2.2.1. Body Weight and Visceral Development
2.2.2. Fur Quality
2.2.3. RNA Isolation and Real-Time PCR Analysis
2.2.4. Western Blotting
2.3. Data Processing
3. Results
3.1. Growth and Development of Internal Organs
3.2. Fur Quality
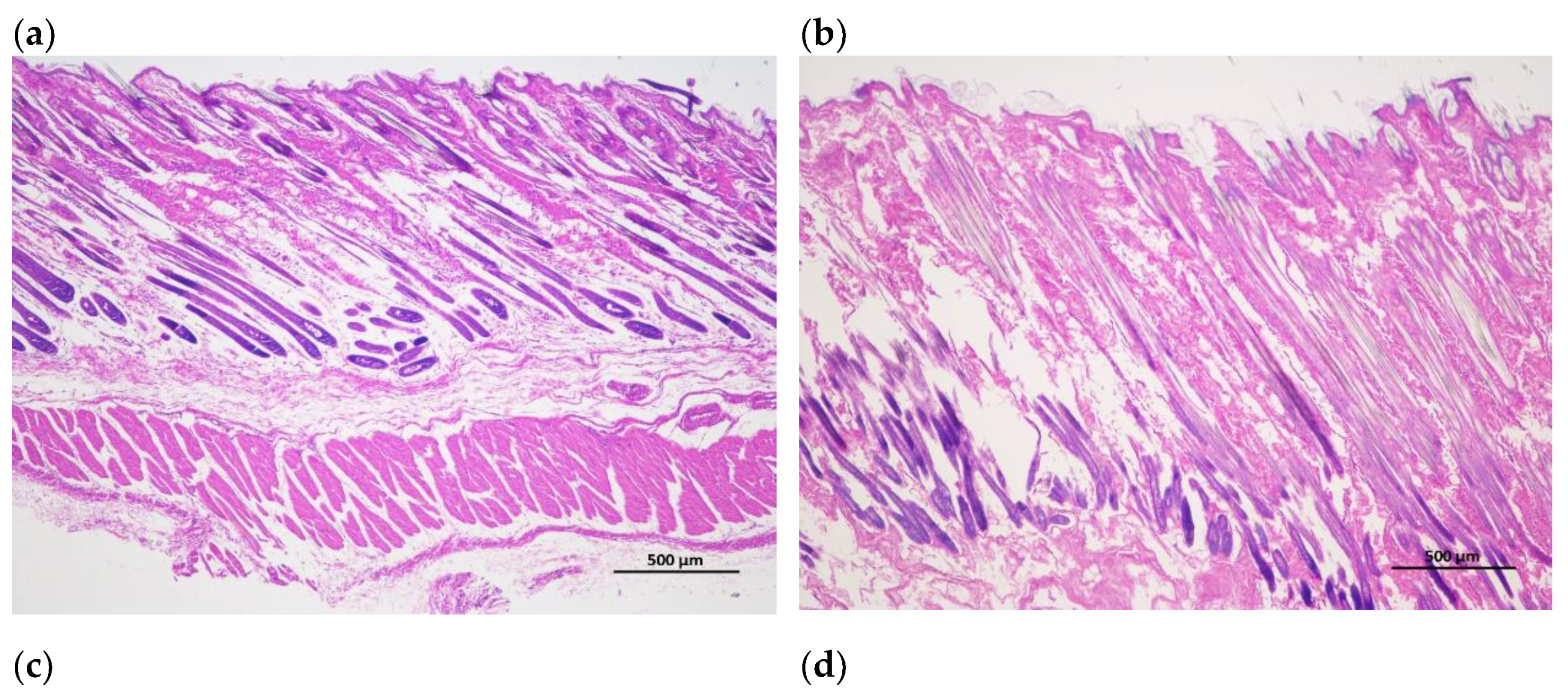
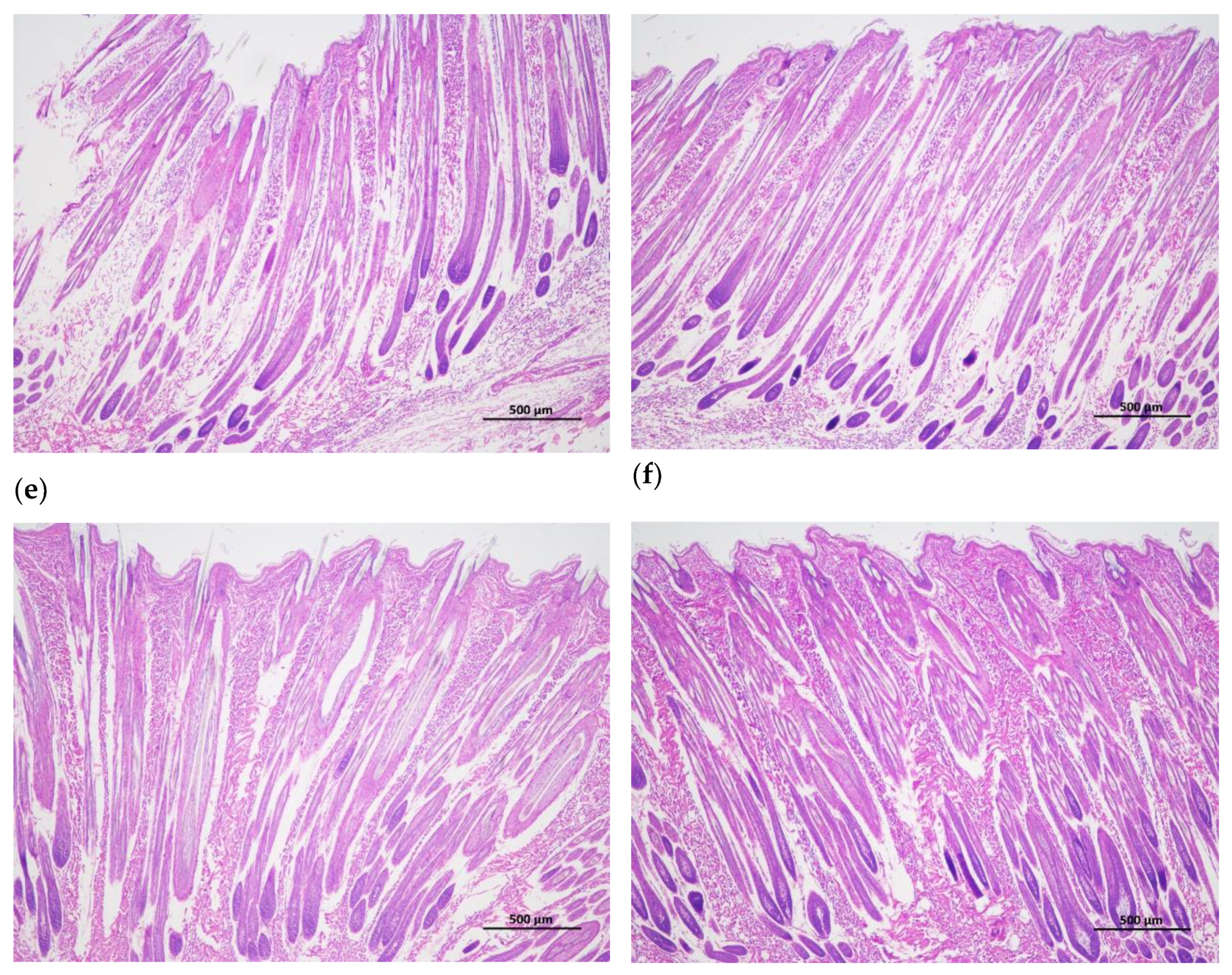
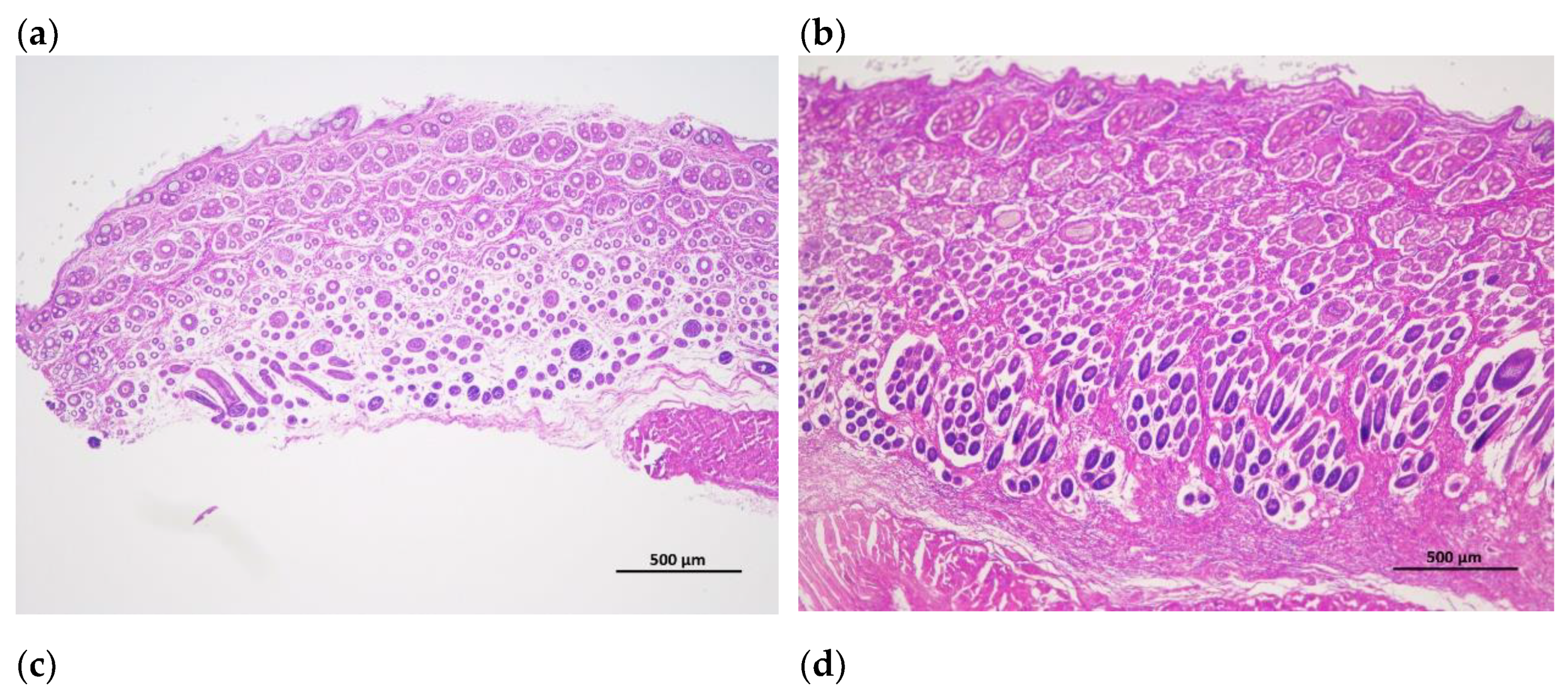
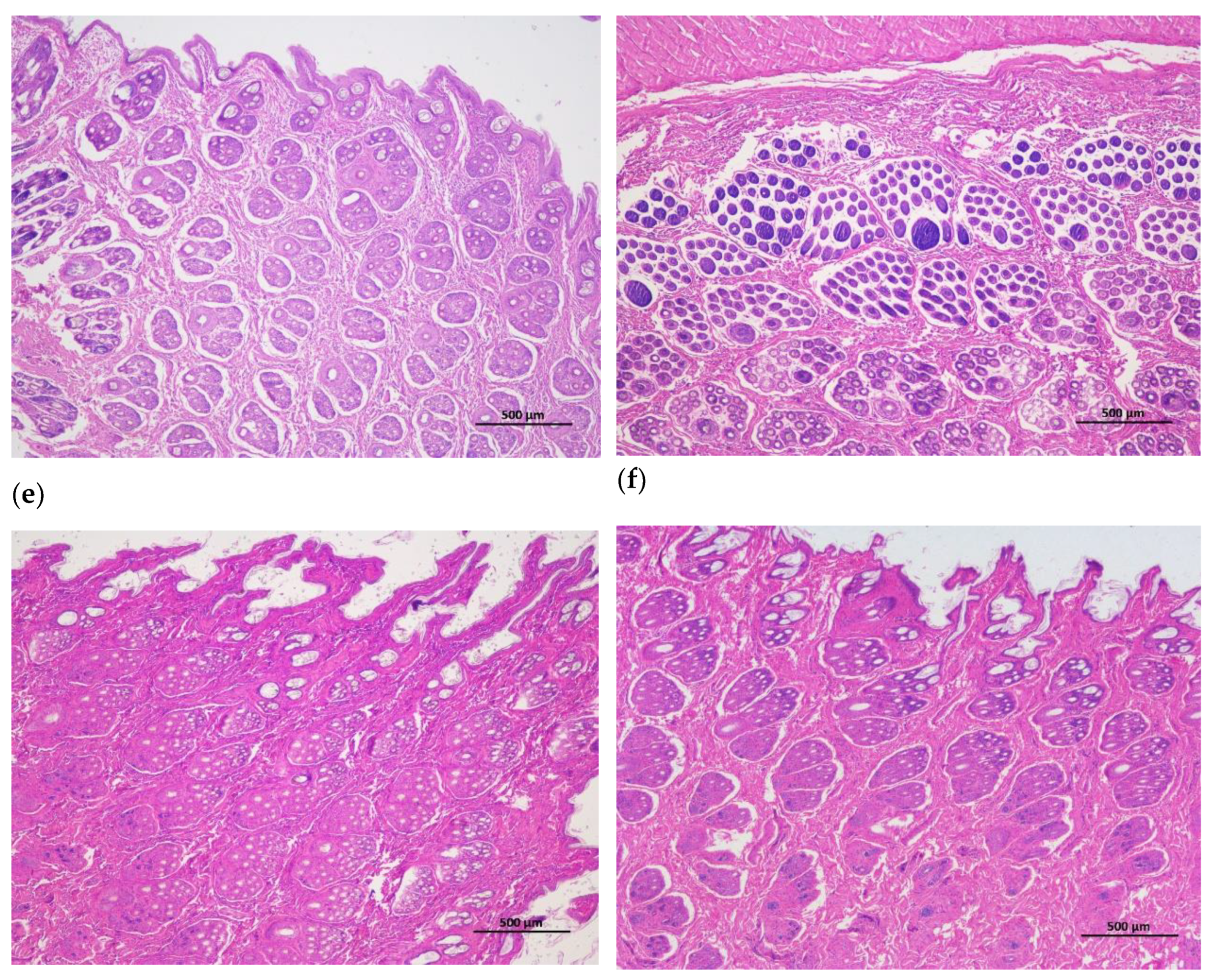
3.3. Hair Follicle Development and Density
3.4. Gene Expression of Hair Follicle Development
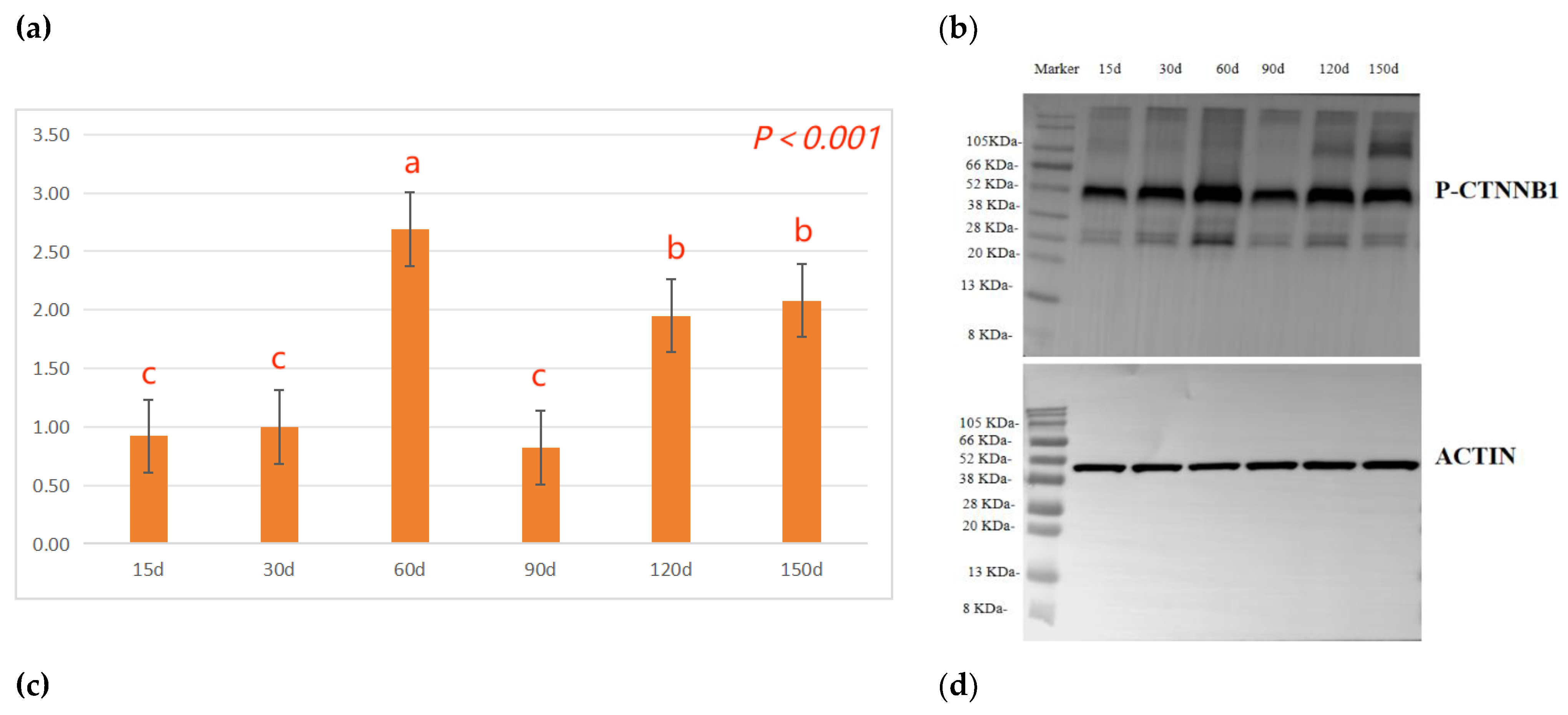
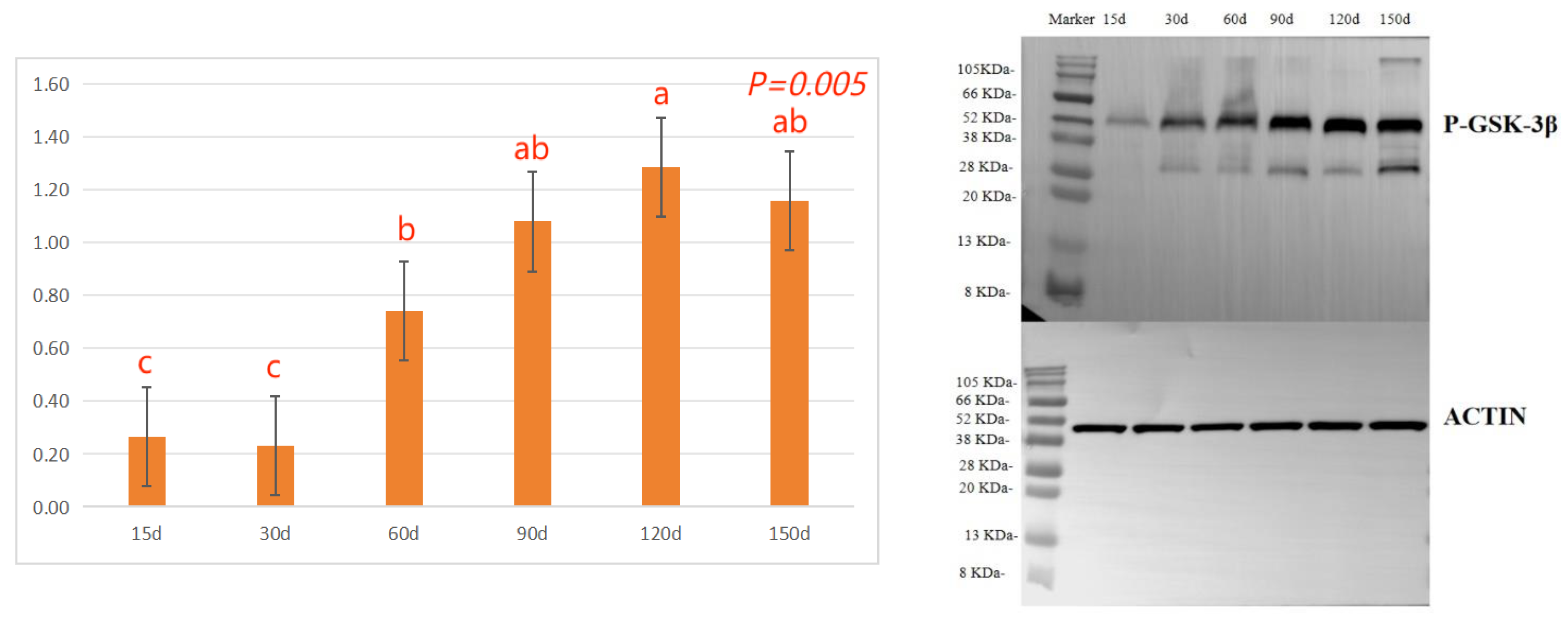
3.5. Protein Phosphorylation Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Li, L.; Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014, 157, 935–949. [Google Scholar] [CrossRef]
- Millar, S. E. Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef]
- Lee, J.; Tumbar, T. Hairy tale of signaling in hair follicle development and cycling. Semin. Cell Dev. Biol. 2012, 23, 906–916. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
- Xiang, M. Gene regulation by Wnt signaling pathway in the oriented differentiation of hair follicle stem cells. J Tissue Eng. Reconstr. Surg. 2011, 7, 290–294. [Google Scholar]
- Lin, C.; Yuan, Y.; Chen, X.; Li, H.; Cai, B.; Liu, Y.; Zhang, H.; Li, Y.; Huang, K. Expression of Wnt/β-catenin signaling, stem-cell markers and proliferating cell markers in rat whisker hair follicles. J. Mol. Histol. 2015, 46, 233–240. [Google Scholar] [CrossRef]
- González-Sancho, J.M.; Brennan, K.R.; Castelo-Soccio, L.A.; Brown, A.M.C. Wnt proteins induce dishevelled phosphorylation via an LRP5/6-independent mechanism: irrespective of their ability to stabilize b-catenin. Mol. Cell. Biol. 2004, 24, 4757–4768. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Develop. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Majidinia, M.; Aghazadeh, J.; Jahanban-Esfahlani, R.; Yousefi, B. The roles of Wnt/beta-catenin pathway in tissue development and regenerative medicine. J. Cell Physiol. 2018, 233, 5598–5612. [Google Scholar] [CrossRef]
- Tian, H. , Wang, X.; Lu, J.; Tian, W.; Chen, P. MicroRNA-621 inhibits cell proliferation and metastasis in bladder cancer by suppressing Wnt/beta-catenin signaling. Chem. Biol. Interact. 2019, 308, 244–251. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Liu, H.; Liu, G.; Li, F. Wnt10b promotes hair follicles growth and dermal papilla cells proliferation via Wnt/β-Catenin signaling pathway in Rex rabbits. Bioscience Rep. 2020, 40, BSR20191248. [Google Scholar] [CrossRef]
- Liu, G.; Liu, C.; Zhang, Y.; Sun, H.; Yang, L.; Bai, L.; Gao, S. Hair follicle development of Rex rabbits is regulated seasonally by Wnt10b/β-Catenin, TGFβ-BMP, IGF1, and EGF signaling pathways. Animals 2023, 13, 3742. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wu, Z.Y.; Zhu, Y.L.; Liu, L.; Li, F.C. Effects of dietary vitamin B6 on the skeletal muscle protein metabolism of growing rabbits. Anim. Prod. Sci. 2017, 57, 2007–2015. [Google Scholar] [CrossRef]
- LiLivak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Liu, H.; Zhu, Y.; Bai, L.; Sun, H.; Gao, S.; Jiang, W.; Li, F. The functions of ocu-miR-205 in regulating hair follicle development in Rex rabbits. BMC Dev. Biol. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Lai, S.; Yang, G.; Li, Y. A comparison between Tianfu black rabbit and New Zealand rabbit in growth rate and feed conversion efficiency. J. Sichuan Agricul. University 2002, 20, 362–363+366. [Google Scholar]
- Qin, Y.; Li, L.; Du, Y. Comparison of early growth and feed conversion efficiency between German White Rabbit and New Zealand Rabbit. Chinese Rabbit Breeding 2000, 2, 18–20. [Google Scholar]
- Liu, G.; Li, M.; Liu, M.; Sun, H.; Bai, L.; Liu, C.; Yang, L.; Jiang, W.; Wang, P.; Gao, S. Effects of different slaughter ages on slaughter performance, fat deposition and muscle quality of Minxinan black rabbit. Chinese J. Anim. Husbandry 2022, 58, 224–228. [Google Scholar]
- Zhou, B.; Zhang, Y.; Yang, L.; Gao, S.; Liu, G.; Zhang, Y.; Zhang, H.; Bai, L. Comparative analysis of production performance and muscle quality of Laiwu black rabbits at different slaughter ages. Chinese J. Anim. Nutr. 2023, 35, 5322–5332. [Google Scholar]
- Yurniati, Y.; Raharjo, Y.; Kusmajadi, S. The effect of restricted feeding and different of slaughtering age on production of Rex rabbit pelt. J. Indonesian Trop. Anim. Agric. 2014, 35, 192–196. [Google Scholar] [CrossRef]
- Alonso, L. , Fuchs, E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003, 17, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta catenin signaling and diseease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Augustin, I. Wnt signaling in skin homeostasis and pathology. J. Dtsch. Dermatol. Ges. 2015, 13, 302–306. [Google Scholar] [CrossRef]
- Saito-Diaz, K.; Chen, T.W.; Wang, X.; Thorne, C.A; Wallace, H. A.; Page-Mc, C. A.; Lee, E. The way Wnt works: components and mechanism. Growth Factors 2013, 31, 1–31. [Google Scholar] [CrossRef]
- Yuan, Y.P.; Huang, K.; Xu, Y.M.; Chen, X.C.; Li, H.H.; Zhang, H.; Li, Y.; Lin, C.M. Canonical and non-canonical Wnt signaling control the regeration of amputated rodent vibrissae follicles. J. Mol. Histol. 2016, 47, 47,1–8. [Google Scholar] [CrossRef]
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.L.; Fuentealba, L.C. , Vorwald, P.P., Gumper, I., Sabatini, D.D.; Robertis, E.M. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010, 143, 1136–1148. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008029. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 promotes regression of hair follicles. J. Invest. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, B.; Zhu, Y.; Wang, C.; Li, F. Differential gene expression profiles in foetal skin of Rex rabbits with different wool density. World Rabbit Sci. 2016, 24, 223–231. [Google Scholar] [CrossRef]
- Fu, J.; Hsu, W. Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. J. Invest. Dermatol. 2013, 133, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Chen D, Jarrell A, Guo, C. Dermal beta catenin activity in response to epidermal Wntligands is required for fibroblast proliferation and hair follicle initiation. Development 2012, 139, 1522–1533. [Google Scholar]
- Wu, Z.; Sun, L.; Liu, G.; Liu, H.; Liu, H.; Yu, Z.; Xu, S.; Li, F.; Qin, Y. Hair follicle development and related gene and protein expression of skins in Rex rabbits during the first 8 weeks of life. Asian Austral. J. Anim. 2019, 32, 477–484. [Google Scholar] [CrossRef]
- Woo, C.; Eleanor, W.; Bruce, A. Morgan dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Develop. Stem Cells 2013, 140, 1676–1683. [Google Scholar]
- Lei, M.; Lai, X.; Bai, X.; Qiu, W.; Yang, T.; Liao, X.; Chuong, C.M.; Yang, L.; Lian, X.; Zhong J., L. Prolonged overexpression of Wnt10b induces epidermal keratinocyte transformation through activating EGF pathway. Histochem. Cell Biol. 2015, 144, 209–221. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, K.; Yang, K.; Ye, J.X.; Xing, Y.Z.; Guo, H.Y.; Deng, F.; Lian, X.H.; Yang, T. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J. Invest. Dermatol. 2013, 133, 42–48. [Google Scholar] [CrossRef]
- Lei, M.; Guo, H.; Qiu, W.; Lai, X.; Yang, T.; Widelitz R., B.; Chuong, C.M.; Lian, X.; Yang, L. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp. Dermatol. 2014, 23, 407–413. [Google Scholar] [CrossRef]
- Lichtenberger, B.M.; Mastrogiannaki, M.; Watt, F.M. Epidermal beta-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat. Commun. 2016, 7, 10537. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. Beta catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Dasgupta, R.; Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Develop. 1999, 126, 4557–4568. [Google Scholar] [CrossRef]
- Niezgoda, A.; Niezgoda, P.; Nowowiejska, L.; Białecka, A.; Męcińska-Jundziłł, K.; Adamska, U.; Czajkowski, R. Properties of skin stem cells and their potential clinical applications in modern dermatology. Eur. J. Dermatol. 2017, 27, 227–236. [Google Scholar] [CrossRef]
- Mastrogiannaki M, Lichtenberger B M, Reimer A, Collins, C. A.; Driskell, R. R.; Watt, F. M. Beta-catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J. Invest. Dermatol. 2016, 136, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, C.; Gilmore, A.C. What lies beneath: Wnt/beta-catenin signaling and cell fate in the lower dermis. J. Invest. Dermatol. 2016, 136, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.J.; Lin, G.Y.; Zhu, J.Y. , Yin, G.Q.; Huang, D.; Yan Y.Y. LncRNA-PCAT1 maintains characteristics of dermal papilla cells and promotes hair follicle regeneration by regulating miR-329/Wnt10b axis. Exp. Cell Res. 2020, 394, 112031. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.S.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Paus, R.; Doetschman, T. The TGF-beta 2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Deve.Biol. 1999, 212, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Fuchs, E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012, 10, 63–75. [Google Scholar] [CrossRef]
- Calvo-Sanchez, M.I.; Fernandez-Martos, S.; Carrasco, E.; Bernabéu, C.; Quintanilla, M.; Jesús, E.J. A role for the Tgf-beta/bmp co-receptor endoglin in the molecular oscillator that regulates the hair follicle cycle. J. Mol. Cell Biol. 2019, 11, 39–52. [Google Scholar] [CrossRef]
| Gene | Accession number | Primer sequence (5′-3′) | Product length, bp |
|---|---|---|---|
| GAPDH | NM_001082253.1 | F: TTCCAGTATGATTCCACCCACG | 232 |
| R: GGGCTGAGATGATGACCCTTTT | |||
| Wnt10b | XM_002711076.4 | F: GGCGAGAATGAGAATCCATAACAA | 196 |
| R: GTTGTGGGTGTCAATGAAGATGG | |||
| Fzd4 | XM_002708648 | F: AAGTGGGTCAGATGGTCCTG | 117 |
| R: CCCGATGAAGTGAAACTGGT | |||
| CTNNB1 | XM_051852655.1 | F: TGGATACCTCCCAAGTCCTGTA | 207 |
| R: CCAGACGCTGAACATTAGTAGGAT | |||
| APC | XM_008248401 | F: GACTCCAGGCTTCTGGTTTG | 121 |
| R: TAGTGCTCTGGTGGGCTCTT | |||
| DVL2 | XM_008270807 | F: ACTCCACCATGTCCCTCAAC | 117 |
| R: CGATGTAGATGCCTCCGTCT | |||
| GSK-3β | XM_017347066.1 | F: TGAGGTCTATCTTAATCTGGTGCTG | 183 |
| R: TGTGGTTTAATATCCCGATGGC | |||
| TCF3 | NM_001171390 | F: CGGGAGATAGAGCAGGTGAA | 127 |
| R: GGTAGTCATCGCCGTAGGAG | |||
| LEF1 | XM_008267508 | F: GCGTCCACACCTGTAACCTT | 122 |
| R: CTCTTCCTCAAATCCCTCCA | |||
| DDK1 | NM_001082737.2 | F: ATGGGTATTCCCGCAGAACC | 150 |
| R: CCTTGAGGACGGGCTTACAG | |||
| TGF-β1 | XM_008249704.2 | F: CTGCTGTGGCTCCTAGTGTTGA | 134 |
| R: AGCCGCAGTTTGGACAGGAT |
| Items | Age (days) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 120 | 150 | |||
| Body weight (g) | 340.0 f | 785.0 e | 1455.0 d | 2073.3 c | 2601.7 b | 3178.3 a | 169.15 | <0.001 |
| Heart weight (g) | 1.73 e | 3.15 d | 5.48 c | 6.97 b | 7.18 b | 8.62 a | 0.441 | <0.001 |
| Lung weight (g) | 3.30 d | 7.28 c | 10.13 bc | 11.58 b | 10.27 bc | 17.20 a | 0.877 | <0.001 |
| Liver weight (g) | 11.25 d | 27.43 c | 58.28 b | 60.30 b | 66.98 b | 80.22 a | 4.174 | <0.001 |
| Kidney weight (g) | 4.27 e | 8.13 d | 11.43 cd | 13.77 bc | 17.48 ab | 18.65 a | 0.985 | <0.001 |
| Heart index (g/kg) | 5.21 a | 3.98 b | 3.79 bc | 3.37 bc | 2.75 c | 2.71 c | 0.200 | <0.001 |
| Lung index (g/kg) | 9.95 a | 9.26 ab | 6.98 abc | 5.65 bc | 3.96 c | 5.37 bc | 0.609 | 0.018 |
| Liver index (g/kg) | 33.02 b | 34.53 b | 41.62 a | 28.12 c | 25.81 c | 25.24 c | 1.129 | <0.001 |
| Kidney index (g/kg) | 12.56 a | 10.32 ab | 7.89 bc | 6.64 c | 6.81 c | 5.87 c | 0.515 | <0.001 |
| Items | Age (days) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 120 | 150 | |||
| Coat length (cm) | 1.81 b | 1.82 b | 1.88 ab | 1.86 ab | 1.87 ab | 1.95 a | 0.013 | 0.026 |
| Skin thickness (mm) | 4.67 b | 6.62 a | 6.87 a | 6.92 a | 7.25 a | 7.28 a | 0.174 | <0.001 |
| Skin area (cm2) | 367.50 f | 649.67 e | 996.50 d | 1354.75 c | 1699.00 b | 1937.80 a | 169.145 | <0.001 |
| Skin weight (g) | 55.0 f | 118.8 e | 227.6 d | 322.9 c | 435.1 b | 545.4 a | 29.928 | <0.001 |
| Relative weight of skin (%) | 16.19 | 14.26 | 15.59 | 15.56 | 16.64 | 17.16 | 0.316 | 0.119 |
| Items | Age (days) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 120 | 150 | |||
| Total hair follicle density (count/mm2) | 232.8 d | 287.8 c | 308.7 bc | 334.9 b | 370.8 a | 319.4 bc | 8.508 | <0.001 |
| Primary hair follicle density (count/mm2) | 12.02 | 14.36 | 12.17 | 13.58 | 13.11 | 14.04 | 0.291 | 0.089 |
| Secondary hair follicle density (count/mm2) | 220.8 d | 273.4 c | 296.5 bc | 321.3 b | 357.7 a | 305.4 bc | 8.421 | <0.001 |
| Secondary hair follicle/ Primary hair follicle ratio | 18.47 d | 19.46 cd | 24.52 ab | 23.85 b | 27.42 a | 21.87 bc | 0.667 | <0.001 |
| Items | Age (days) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 120 | 150 | |||
| Wnt10b | 1.00 b | 2.43 b | 2.54 b | 4.24 a | 1.85 b | 1.29 b | 0.273 | 0.003 |
| Fzd4 | 1.00 | 1.12 | 2.53 | 1.30 | 2.06 | 1.69 | 0.169 | 0.052 |
| CTNNB1 | 1.00 b | 3.05 a | 1.39 b | 2.68 a | 0.59 b | 0.52 b | 0.220 | <0.001 |
| APC | 1.00 b | 1.68 b | 5.38 a | 1.89 b | 5.46 a | 3.66 a | 0.376 | <0.001 |
| DVL2 | 1.00 c | 0.87 c | 1.39 bc | 2.10 a | 1.74 ab | 1.45 bc | 0.103 | 0.001 |
| GSK-3β | 1.00 b | 2.61 a | 0.98 b | 2.21 a | 0.66 b | 0.82 b | 0.178 | 0.001 |
| TCF3 | 1.00 | 1.11 | 1.13 | 1.64 | 1.38 | 1.45 | 0.089 | 0.281 |
| LEF1 | 1.00 c | 1.64 bc | 3.93 a | 3.42 ab | 3.27 ab | 2.57 abc | 0.311 | 0.037 |
| DDK1 | 1.00 b | 0.35 c | 1.83 a | 0.43 bc | 0.45 bc | 0.56 b | 0.112 | <0.001 |
| TGF-β1 | 1.00 bc | 1.40 ab | 1.04 bc | 2.04 a | 1.47 ab | 0.53 c | 0.115 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





