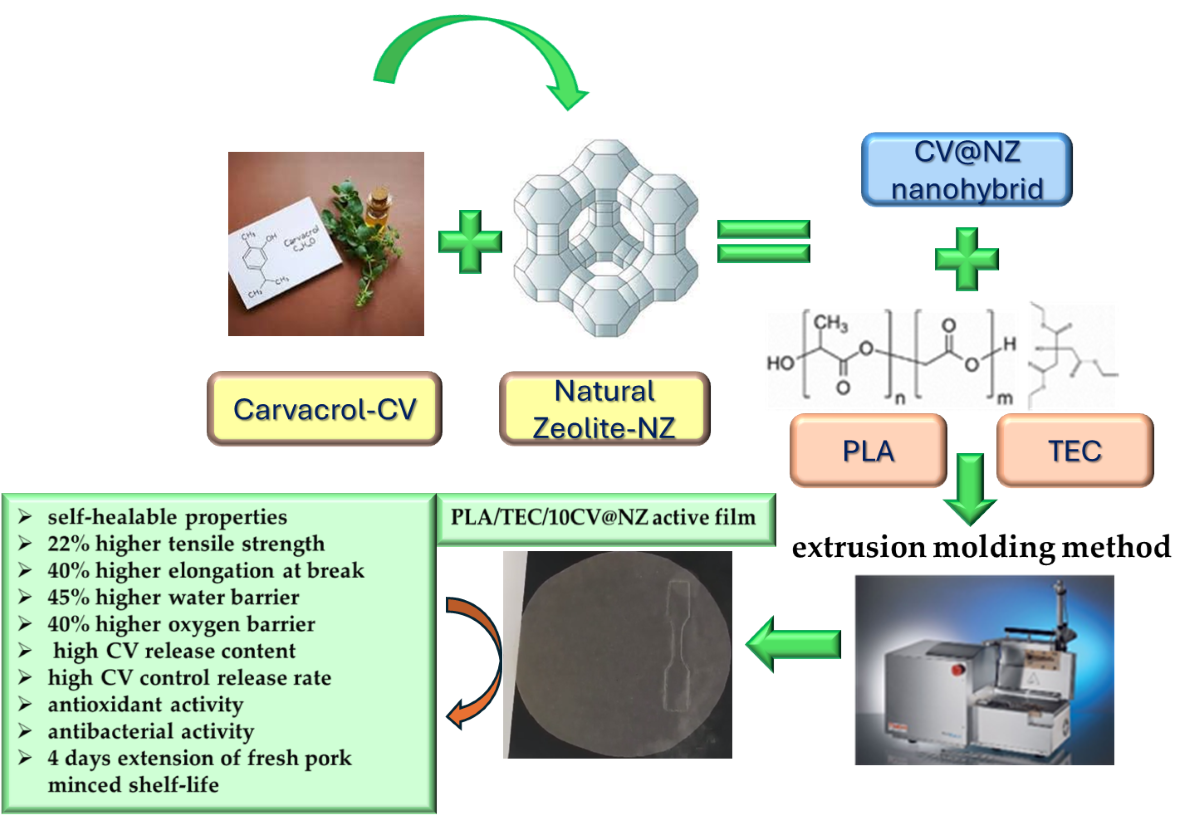
1. Introduction
Increasingly in recent years, the goal of reducing global emissions sufficiently to meet global climate goals and avoid severe and catastrophic impacts of climate change has assumed significant importance. This makes the necessity for replacement of plastics derived from fossil fuels imperative. Plastics can be replaced by natural biodegradable biopolymers such as cellulose, starch, and chitosan or by biodegradable polymers which can be developed from waste and by-products such as poly-lactic acid (PLA) [
1,
2]. Lactic acid (LA) can be separated and purified from various food and agriculture byproducts. Then LA can be converted to PLA through various polymerization processes, including direct polycondensation (DPC), reactive oxygen polymerization (ROP), direct azeotropic dehydration, and enzymatic polymerization. Polycondensation is one of the most common polymerization processes used to manufacture PLA [
3]. Thus, the low-cost accessible preparation of PLA makes it one of the most favorable biopolyesters for packaging and medical applications [
4,
5,
6]. In the packaging field it can act as a viable substitute for traditional plastics for packaging uses such as trays, bottles, and cups [
7]. However, its poor viscoelastic behavior and extremely low melting strength are inhibiting factors for film packaging production. To overcome these disadvantages and increase its ductility and flexibility PLA is blended with plasticizers. To maintain PLA’s environmentally friendly profile plasticizers such as Epoxidized soybean oil, Cardanol, Triethyl Citrate (TEC), and Acetyl Tributyl Citrate (ATBC) have been successfully used [
8,
9,
10,
11,
12,
13]. Recently, TEC has been used successfully to plasticize PLA and has been shown for first time that it also has self-healing, antioxidant, and antibacterial properties [
14]. These innovative properties of TEC make obtained PLA/TEC composite films favorable for active antibacterial flexible food packaging films [
15].
On the other hand, under the spirit of bioeconomy and sustainability trend Food Technology sector tries to: (i) replace synthetic food additives preservatives by natural abundant antioxidant/antibacterial compounds such as phytochemicals, natural extracts, and essential oils (EOs) and (ii) incorporate such novel-green natural preservatives in packaging to control their release in food. EOs have been shown to be the most favorable candidates among natural preservatives and phytochemicals to be used in antimicrobial active food packaging applications [
16,
17,
18,
19,
20,
21]. EOs are considered as safe by FDA (Food and Drug Administration) and are known as GRAS (General Recognize as Safe) [
22]. To overcome their direct loss trough evaporation process new technologies have been proposed such as their adsorption on natural adsorbents such as nanoclays, natural zeolites and activated carbons followed by their incorporation in polymer matrix to develop an active packaging film with controlled release of EOs in food [
23,
24,
25,
26,
27,
28]. Natural zeolite (NZ) is more favorable for use as a nanocarrier host of EOs than nanoclays due to its higher surface area. Unlike activated carbons, NZ is more favorable for use as a host for EOs due to its edible properties [
29]. Carvacrol (CV) is the main component of thyme and oregano oil and it is well known for its antioxidant, antibacterial, antitumor, antimutagenic, antigenotoxic, analgesic, antispasmodic, anti-inflammatory, angiogenic, antiparasitic, and antiplatelet activity [
30]. In the last few years various studies refer to the application of CV in active food packaging [
31,
32,
33,
34,
35]. Recently CV was loaded on halloysite nanoclays and was then homogeneously distributed as an active coating on the polyethylene surface [
35]. The as-prepared coatings present strong antibacterial activity against
Aeromonas hydrophila and reduce bacterial growth on packaged chicken surfaces [
35].
A novel green vacuum-adsorption method for loading high CV contents into edible NZ is presented here. To characterize the obtained CV@NZ nanohybrids desorption studies are performed for the first time. Then the CV@NZ nanohybrid was loaded in a PLA/TEC matrix at 5, 10, and 15 %wt. contents to obtain novel PLA/TEC/xCV@NZ (x=5, 10 and 15) active packaging films via a melt extrusion method. For comparison PLA/TEC based films were prepared via melt extrusion process by adding 5, 10 and 15 %wt. pure NZ. All as-prepared PLA/TEC/xNZ and PLA/TEC/xCV@NZ films were characterized by X-Ray Diffraction (XRD) analysis, Fourier Transform Infrared (FTIR) spectroscopy and Scanning Electron Microscopy (SEM). Their tensile and thermomechanical properties and water/oxygen barrier properties were also studied. Additionally, CV release experiment, antioxidant activity test via 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay method and antibacterial activity test against food pathogens were performed. The most promising active packaging film was used to wrap fresh minced pork and a shelf-life experiment was performed by monitoring lipid oxidation, heme iron content, total viable count (TVC) and sensory analysis scores. Overall, the innovative points of the current study are: (i) the novel vacuum adsorption method employed to obtain CV@NZ nanohybrid, (ii) the innovative low-cost method based on desorption kinetic experiments to determine the %wt. loading content as well as the desorption energy of CV on such CV@NZ nanohybrid (iii) the preparation and characterization of such PLA/TEC/xCV@NZ active films, and (iv) a shelf-life study of the optimum PLA/TEC/xCV@NZ film for the preservation of fresh minced pork.
2. Materials and Methods
2.1. Materials
PLA with the trade name Ingeo™ Biopolymer 3052D, crystalline melt temperature at 145-160 °C and glass transition temperature at 55-60 oC was purchased from NatureWorks LLC (Minnetonka, MN, USA). Liquid triethyl citrate (TEC) with a Mw of 276.3 g/mol was purchased from Alfa Aesar GmbH & Co KG, (Karlsruhe, Germany). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich (Darmstadt, Germany). Ethanol absolute for analysis, acetate buffer (CH3COONa·3H2O) was purchased from Merck. Edible Natural Zeolite was purchased by a local pharmacy market. Fresh minced pork was provided by the local meat processing plant “Aifantis Company” within one hour after the slaughter.
2.1. Preparation of CV@NZ Nanohybrid
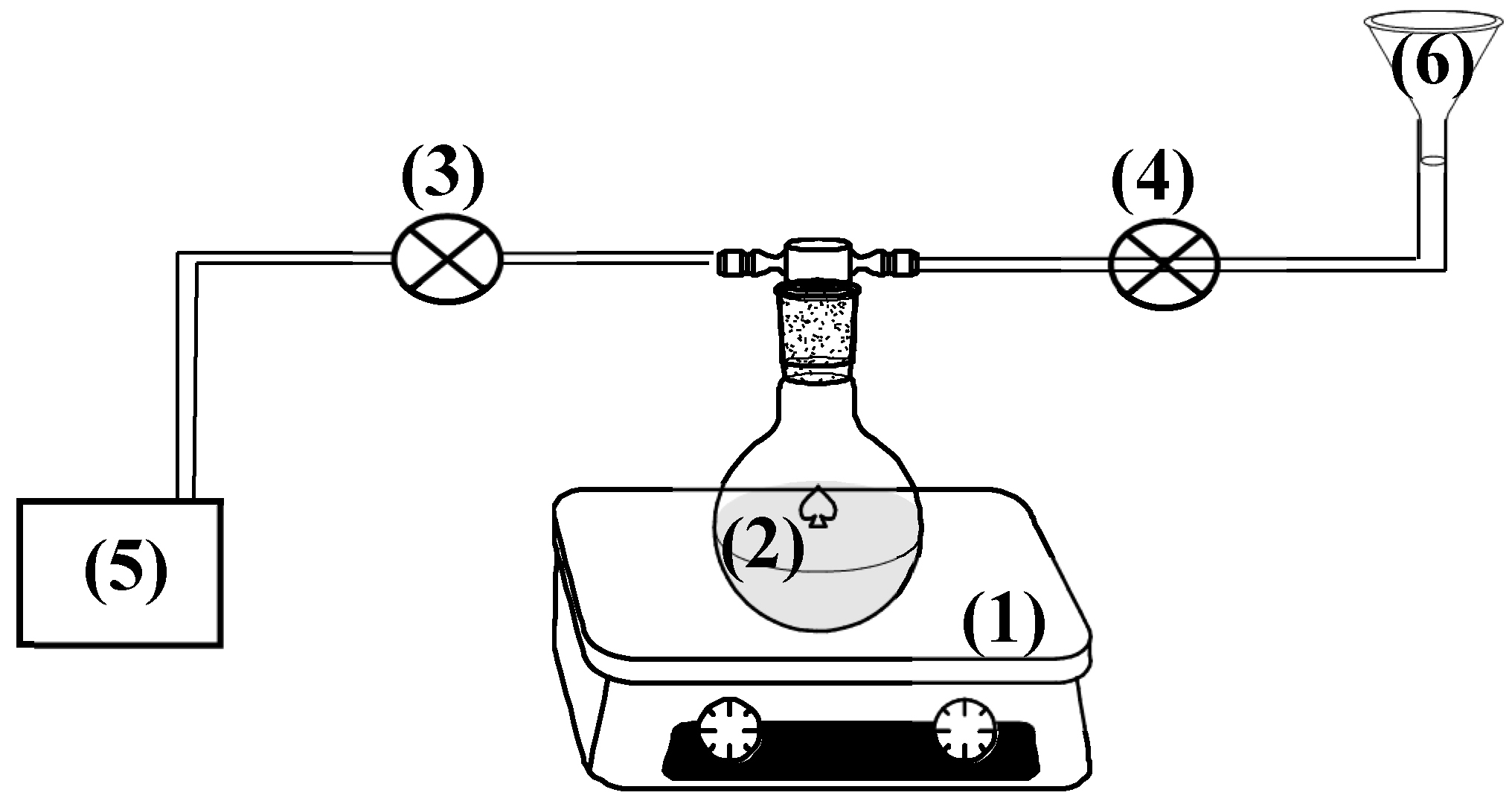
The preparation of CV@NZ nanohybrid was made as follows: First, 5 g of as received NZ were placed in a spherical glass flask and heated for 30 min at 100
oC under 3 bar vacuum (see
Figure 1). Under these conditions all the adsorbed water was removed from NZ and the initial light beige color of NZ was turned to dark beige. After the end of the dry-cleaning process, the security valve of pump was closed, and the safety valve of the CV tank was opened to allow the carvacrol to be incorporated dropwise and under stirring into the glass spherical flask (see
Figure 1). The obtained CV@NZ nanohybrid was removed and kept for further characterization and use.
2.2. CV@NZ Nanohybrid Characterization
2.2.1. XRD and FTIR Characterization of CV@NZ Nanohybrids
The obtained CV@NZ as well as NZ nanohybrids as received were physiochemically characterized by XRD analysis and FTIR spectrometry. The XRD measurements were made in the range of 0.5-30 2 theta for both CV@NZ and NZ powders and a Brüker XRD D8 Advance diffractometer (Brüker, Analytical Instruments, S.A., Athens, Greece) was employed. FTIR analysis measurements were carried out in the range of 400 to 4000 cm-1 with an FT/IR-6000 JASCO Fourier-transform spectrometer (JASCO, Interlab, S.A., Athens, Greece).
2.2.2. Desorption Kinetic Studies of CV@NZ Nanohybrid
Desorption kinetics were performed to determine the %w/w total amount of CV adsorbed on NZ, the type of adsorption and the adsorption enthalpy (ΔH
ads,CV). Desorption of CV from the obtained CV@NZ nanohybrids was studied by using a moisture analyzer AXIS AS-60 (AXIS Sp. z o.o. ul. Kartuska 375b, 80-125 Gdańsk) as follows: Approximately 150 mg (m
0) of the CV@NZ nanohybrid was spread in the inner disk of the moisture analyzer and its weight (m
t) was recorded as a function of time at 223, 243, 263 and 283
oK. The desorption experiment was repeated three times for each of the above temperatures. Then by using the recorded m
t and t values the desorption isotherms were constructed by plotting the values of (1-m
0/m
t) as a function of time and the obtained plots were fitted by using the well-known pseudo-second order adsorption-desorption equation [
36]:
where: k
2 is the rate constant of the pseudo-second order kinetic model (s
−1), q
e is the desorption capacity at equilibrium (mg desorbate/g desorbent), q
t is the desorption capacities at time t (mg desorbate/g desorbent).
By fitting the acquired experimental adsorption data into the pseudo-second order kinetic equation (1), the k
2, and q
e mean values were calculated for all selected temperatures (223, 243, 263 and 283
oK). Subsequently, by plotting the obtained ln(1/k
2) values with (1/T) we calculate the desorption energy (Ε
0des) according to the Frenkel equation [
37,
38,
39]:
and its linear transformed type:
2.2. Experimental Design - Preparation of Extruded PLA/TEC/xNZ and PLA/TEC/xCV@NZ Nanocomposite Pellets
The obtained CV@NZ was used as nanofiller at 5, 10, and 15 %wt. content in PLA/TEC matrix to obtain PLA/TEC/xCV@NZ (x=5,10,15) nanocomposite pellets. For comparison pure NZ at 5, 10, and 15 %wt. was used as nanofiller in PLA/TEC matrix to obtain PLA/TEC/xNZ (x=5,10,15) nanocomposite pellets. The PLA/TEC matrix used was obtained by blending PLA with 15 %v/w TEC content. The content of TEC chosen was the optimum one according to a recent publication [
14].
For the development of all PLA/TEC/xNZ and PLA/TEC/xCV@NZ nanocomposite pellets a Mini Lab twin-screw extruder (Haake Mini Lab II, Thermo Scientific, ANTISEL, S.A., Athens, Greece) was employed under the following operating conditions: speed 120 rpm and temperature 180
oC. The sample code names, the contents of PLA, TEC, pure NZ and CV@NZ nanohybrid and the twin extruder operation conditions (temperature, speed) used for the development of all PLA/TECx composite blends are listed in
Table 1 for comparison.
2.3. PLA/TEC/xNZ and PLA/TEC/xCV@NZ Films Formation
All the obtained, after the extrusion process, PLA/TEC/xNZ and PLA/TEC/xCV@NZ nanocomposite pellets as well as pure PLA/TEC pellets were thermomechanically formed into films through a heat-pressing process by using a hydraulic press with heated platens. Approximately, 1.0 g of pellets at a constant pressure of 0.5 MPa, and temperature of 180 °C, was applied to obtain films with an average diameter of 11 cm and a thickness of 0.05 – 0.11 mm.
2.4. Physicochemical Characterization of PLA/TEC/xNZ and PLA/TEC/xCV@NZ Films
All the obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film were characterized by XRD analysis measurements employing a Brüker XRD D8 Advance diffractometer (Brüker, Analytical Instruments, S.A., Athens, Greece). Films with 2 cm diameter were oriented on the sample chamber space and were measured at 0.5o-40o 2 theta. FTIR analysis measurements were carried out in the range of 4000–400 cm-1 with an FT/IR-6000 JASCO Fourier-transform spectrometer (JASCO, Interlab, S.A., Athens, Greece). For the FTIR measurements, 300 mg of KBr granules were mixed with 30 mg of granulated PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film and pressed in a hydraulic press to obtain the samples for FTIR measurements. Scanning electron microscopy (SEM) images were carried out using a JEOL JSM-6510 LV SEM Microscope (JEOL Ltd., Tokyo, Japan). To enhance the electrical conductivity of samples before SEM analysis, all samples were coated under vacuum with a gold/palladium (Au/Pd) thin layer (4–8 nm) in a sputtering machine (SC7620, Quorum Technologies, Lewes, UK). The tensile properties measurements were carried out according to ASTM D638 method by using a Simantzü AX-G 5kNt instrument (Simantzü. Asteriadis, S.A., Athens, Greece). Three to five dog-bone shape samples of all PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film were tensed, and the stress-strain values were recorded with the applicable software (TrapeziumX version 1.5.6, Simantzü, Asteriadis, S.A., Athens, Greece). The films’ dynamic mechanical behaviors were examined using a dynamic mechanical analyzer (DMA Q800, TA Instruments, 159 Lukens Drive New Castle, DE, USA) in film tension mode. To evaluate the storage modulus (E′), a temperature range from −20 ◦C to 60 ◦C at a rate of 5 K/min, along with a frequency of 1 Hz, was applied. Three to five rectangular-shaped films were tensed for all PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film.
2.5. Characterization of Active Packaging Properties of Obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ Films
2.5.1. Water Barrier Properties
Water barrier properties were determined at 38 °C and 95% RH according to the ASTM E96/E 96M-05 method for three to five samples of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film. A handmade apparatus was used according to the methodology described previously [
40,
41]. The obtained Water Vapor Transmission Rate (WVTR) values were transformed to water vapor diffusion coefficient values (D
wv) by using Fick’s low theory and the following equation:
where WVTR [g/(cm
2.s)] is the water-vapor transmission rate, Dx (cm) is the film thickness, and DC (g/cm
3) is the humidity concentration gradient on the two opposite sides of the film.
2.5.2. Oxygen Barrier Properties
The oxygen transmission rate (OTR) values of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film were measured by using an oxygen permeation analyzer (O.P.A., 8001, Systech Illinois Instruments Co., Johnsburg, IL, USA). Three films of each sample with an average diameter of 11 cm and a thickness of 0.05 – 0.11 mm were placed in the oxygen analyzer chamber and measured at 23
oC and 0% RH according to the ASTM D 3985 method. After the end of each measurement each sample was cut into three pieces and the thickness was measured at four points for each piece. In this way, the thickness of each film was determined by twelve different points and the average value of thickness was calculated. This mean value was used to transform the obtained OTR values to oxygen diffusion coefficient (P
O2) values using the methodology previously described [
40].
2.5.3. CV Release Kinetics - Calculation of Released CV %wt. Total Content and CV Released Diffusion Coefficient (DCV)
The kinetics of CV release from all the obtained PLA/TEC/xCV@NZ active films were carried out by using a moisture analyzer AXIS AS-60 (AXIS Sp. z o.o. ul. Kartuska 375b, 80-125 Gdańsk) and the methodology described previously [
42]. For the release kinetic experiments 600 to 900 mg of each film, in triplicates, were used. For each film at least three samples were measured. Each film was placed inside the moisture analyzer and its mass was monitored by heating at 70
oC for 1h. By the obtained film mass values (m
t) as a function of time (t), for each film, the mean values of CV release rate (RR
CV) as well as the mean values of %wt. CV released content (%RC
CV) were determined.
Next, by employing the pseudo-second order sorption mechanism model described here above the constant k2 of the CV desorption rate and the maximum CV desorbed amount qe were calculated according to the equation (1).
2.5.4. In Vitro Antioxidant Activity Determination of the obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films
For the preparation of [DPPH•] free radical standard solutions0.0212 g of [DPPH•] free radical was dissolved in 250 mL of methanol to obtain a 2.16 mM (mmol/L) methanolic solution. Next, the flask was vortexed under dark condition and its pH was measured to ensure its neutrality (7.02± 0.01). Finally, the solution was placed in a refrigerator at 4±1°C, under dark conditions, for stabilization
For the preparation of [DPPH
•] free radical calibration curve 2.16 mM (mmol/L) methanolic solution of [DPPH
•] free radical was diluted by adding appropriate volumes of methanol to obtain solutions with 10, 20, 30, 40 and 50 mg/L concentration and their absorbance was measured with a SHIMADZU UV-1280 UV/VIS Spectrometer at 517 nm. The calibration curve of absorbance (y) versus concentration (x) of [DPPH
•] free radical was expressed by the following equation:
For the determination of concentration required to obtain 50% antioxidant effect (EC
50) of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film 10, 20, 30, 40 and 50 mg of granule film were placed, in dark vials and three replicates were performed for each sample. Thereafter, 3 mL of [DPPH
•] free radical methanolic solution and 2 mL of acetate buffer 100 mM (pH = 7.10) were added in each vial and the absorbance of the reaction mixture was measured at 517 nm after 24h. As a blank sample used a vial where 3 mL of [DPPH
•] free radical methanolic solution and 2 mL of acetate buffer was added without the addition of any granule film. The % inhibition of [DPPH
•] was calculated using the following equation:
2.5.5. Antibacterial Activity of Obtained PLA/TEC/xCV@NZ Films
The antimicrobial activity of all obtained PLA/TEC/xCV@NZ nanocomposite films as well as PLA/TEC composite film was tested against one Gram-positive
Staphylococcus aureus (NCTC 6571) food pathogen and one Gram-negative
Salmonella enterica subspecies enterica serovar
Typhimurium (NCTC 12023) food pathogen [
14]. The bacteria were supplied by Supelco
® Analytical Products, a subsidiary of Merck (Darmstadt, Germany) as microbiological certified reference materials in the form of easy-tab™ pellets (
S. aureus) by LGC Standards Proficiency Testing (Chamberhall Green Bury, Lancashire, UK) and in the form of disc-shaped Vitroids™ (
S. Typhimurium).
The experimental procedure followed was based on the agar diffusion method described recently [
43]. Briefly, a concentration of approx. 1.5 × 10
8 CFU/mL (i.e., 0.5 McFarland standard) per bacterial strain was achieved in 3 mL of 0.85% peptone salt solution or maximum recovery diluent (MRD; Merck). The suspended bacteria in MRD were inoculated on Müeller-Hinton (MH) agar (Oxoid, Basingstoke, UK) plates with the help of sterile cotton applicators (Jiangsu Kangjin Medical Instrument Co., Taizhou, China) and samples with a diameter of 6 mm of all PLA/TEC/xCV@NZ nanocomposite films as well as PLA/TEC composite film were placed onto the surface of MH agar and the incubation took place at 37 °C for 18–24 h. The diameters of inhibition zones in the contact area of the films and around them, were measured by using a Vernier caliper at a 0.1 mm accuracy. The experimental procedure was repeated twice while films were measured in triplicate in each repetition.
2.6. Packaging Test of Fresh Minced Pork Wrapped with PLA/TEC and PLA/TEC/10CV@NZ Films
2.6.1. Packaging Preservation Test of Minced Pork Meat
Minced pork, in portions of approximately 70-80 g each, were aseptically wrapped between two films of PLA/TEC and PLA/TEC10CV@NZ samples, 11 cm in diameter, and placed inside the company's commercial wrapping paper, without the inner film Aifantis. As a control sample 80-90 g of minced pork were aseptically wrapped with the commercial packaging paper of Ayfantis company. For all tested packaging systems samples for the 2nd, 4th, 6th, 8th and 10th day of preservation were prepared and stored under refrigerator conditions at 4±1°C. Every two days until the 10th day of storage lipid oxidation, heme iron content, total viable count, as well as sensory analysis scores were determined for every packaging treatment.
2.6.2. Lipid Oxidation of Minced Pork Meat with Thiobarbituric-Acid-Reactive Substances
The rates of lipid oxidation of all samples of minced pork during the 10 days of storage were calculated with the thiobarbituric acid reactive substances (TBARS) method according to Tarladgis et al. [
44]. In brief, 2 g of meat sample was placed in a vial along with 5 ml of a 10% (w/v) trichloroacetic acid (TCA) solution and vortexed for 5 min. Then 5 ml of 0.02 M 2 -thiobarbituric acid aqueous solution was added to it and vortexed a second time for 5 min. After that the obtained mixture was left in the dark for 18-24 h for color development. Finally, the obtained colored mixture was centrifuged and the absorbance (D) in the resultant supernatant was measured at λ = 538 nm. As a blank sample, 5 ml of the 10% (w/v) TCA solution and 5 ml of a 0.02 M aqueous solution was mixed, and the absorbance was measured at λ = 538 nm too.
TBARS was expressed as mg of malondialdehyde (MDA)/kg of sample according to the equation:
2.6.3. Heme Iron Content
The values of heme iron content of all samples of minced pork during the 10 days of storage was determined according to the method reported by Clark et al. [
45]. 4 g of pork mined meat were dispersed in 18 ml of acidified acetone and were homogenized with a mixer (Vicko S.A, Athens, Greece). The solution was then filtered after standing at 25 ºC, under dark conditions for 1 hour. Finally, in the filtrated solution the absorbance was measured at 640 nm by using a uv-vis spectrophotometer (SHIMADZU UV-1280) and the heme iron content in minced pork was calculated using the equation (8):
where A
640 is the absorbance measured at 640 nm; 680 and 0.0882 are constant values in the equation.
2.6.4. Total Viable Count (TVC) of Minced Pork Meat
For the estimation of total viable count (TVC) for all samples of minced pork during the 10 days of storage a recently described methodology was followed [
43]. Briefly, 10 g of pork fillets was aseptically removed from each packaging system and homogenized with 90 mL of sterile buffered peptone water (ΒPW, NCM0015A, Heywood, BL97JJ, UK) at room temperature. For the microbial enumeration, 0.1 mL of serial dilutions (1:10 diluents, buffered peptone water) of homogenized minced pork products were spread on the surface of plate count agar (PCA, NCM0010A, Heywood UK).
2.6.5. Sensory Analysis of Minced Pork Meat
Sensory properties like color, odor and texture were evaluated during the 10 days of storage. At each sampling day (2nd, 4th, 6th, 8th and 10th day of storage) all the above properties, for each packaging treatment, were ranked from 0 (least liked sample) to 5 (most liked sample) by seven experienced members of the Department of Food Science and Technology [
40].
2.7. Statistical Analysis
All data acquired from structural and mechanical properties measurements, along with, antioxidant activity, thiobarbituric acid reactive substances, heme iron content, total viable count and sensory analysis scores were subjected to statistical analysis to indicate any statistical differences. Mood’s median test method, a non-parametric statistical procedure was chosen. All measurements were conducted on a minimum of three to five separate samples and the least significance difference was that of p < 0.05. Among the three packaging treatments (commercial paper (Contol), PLA/TEC, and PLA/TEC/10CV@NZ) a correlation between TBARS and heme iron content values was estimated using Pearson’s bivariate correlation (−1 to +1) at the confidence level p < 0.05, with respect to storage time (see
Table S10). Statistical analysis was done using SPSS software (v. 28.0, IBM, Armonk, NY, USA).
3. Results
3.1. Physicochemical Characterization of Obtained CV@NZ Nanohybrids
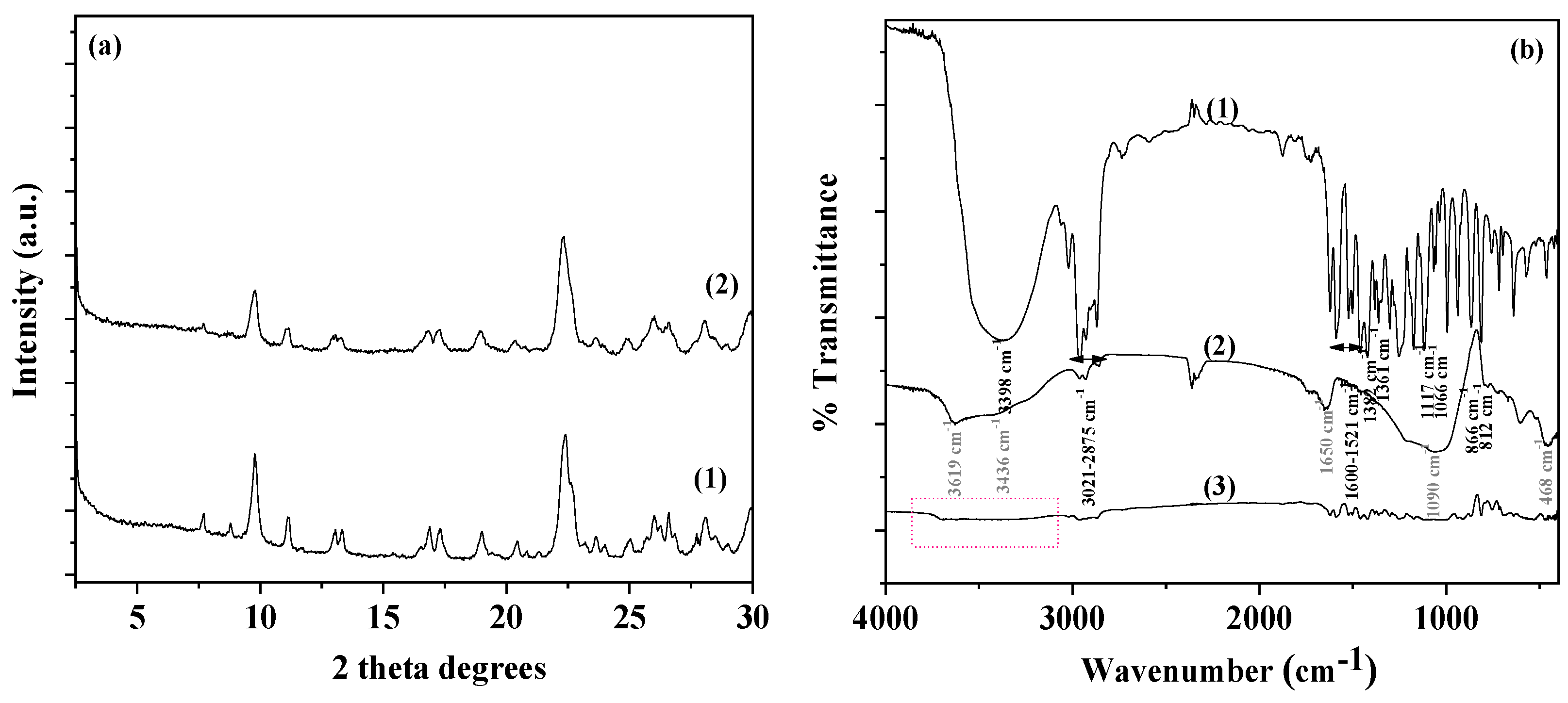
In
Figure 2a the XRD plots of pure NZ and the modified CV@NZ nanohybrid are shown for comparison.
The observed reflections in patterns of both pure NZ and TO @NZ nanohybrid are assigned to Heulandite Ca(Si
7Al
2)O
16 × 6H
2O monoclinic crystal phase (PDF-41-1357). Thus in accordance with a previous report, the adsorption of CV into NZ did not affect the crystallinity of pure NZ [
46].
Figure 2b provides a comparative presentation of FTIR plots of CV as received (plot line 1), pure NZ (plot line 2) and CV@NZ nanohybrid (plot line 3).
In the FTIR spectrum of CV (see plot line (1) in
Figure 2b) the absorption bands in the range of 1521–1600 cm
−1 are assigned to C=C bond stretching vibrations of the aromatic ring [
47,
48]. Furthermore, the bands at 1361 and 1382 cm
−1 are assigned to the symmetric and asymmetric isopropyl methyl group vibrations [
47,
48]. The broadband at 3398 cm
−1 can be attributed to the stretching vibration of the O–H functional group of CV molecule. The bands appearing at 812 and 866 cm
−1 are the characteristic bands of C–H out-of-plane wagging vibrations [
47,
48]. The absorption bands at 2875–3021 cm
−1 are assigned to the stretching vibrations of aliphatic C–H groups and the bands in the range of 1066–1117 cm
-1 are attributed to the ortho-substituted phenyl group [
47,
48].
In the FTIR plot of pure NZ see plot line (2) in
Figure 2b) the bands at 3619 and 3436 cm
-1 are assigned to the OH group stretching mode, the band at 1650 cm
-1 to the OH group bending mode, the band at 1090 cm
-1 to the Si-O stretching vibration and the band at 468 cm
-1 to the -SiO- bending mode [
46,
49,
50]. As it is shown in
Figure 2b the obtained FTIR plot of CV@NZ is a combination of pure CV and pure NZ reflections. This suggests the effective adsorption of CV molecules on NZ pores. Moreover, in the obtained FTIR plot of CV@NZ nanohybrid there are indications of chemisorption of CV on NZ. These are: (i) the band's widening at 3436 cm
-1 of NZ hydroxyl group along with the band at 3398 cm
-1 hydroxyl groups of CV and the red shift of the band at 3619 cm-1 of hydroxyl group of NZ.
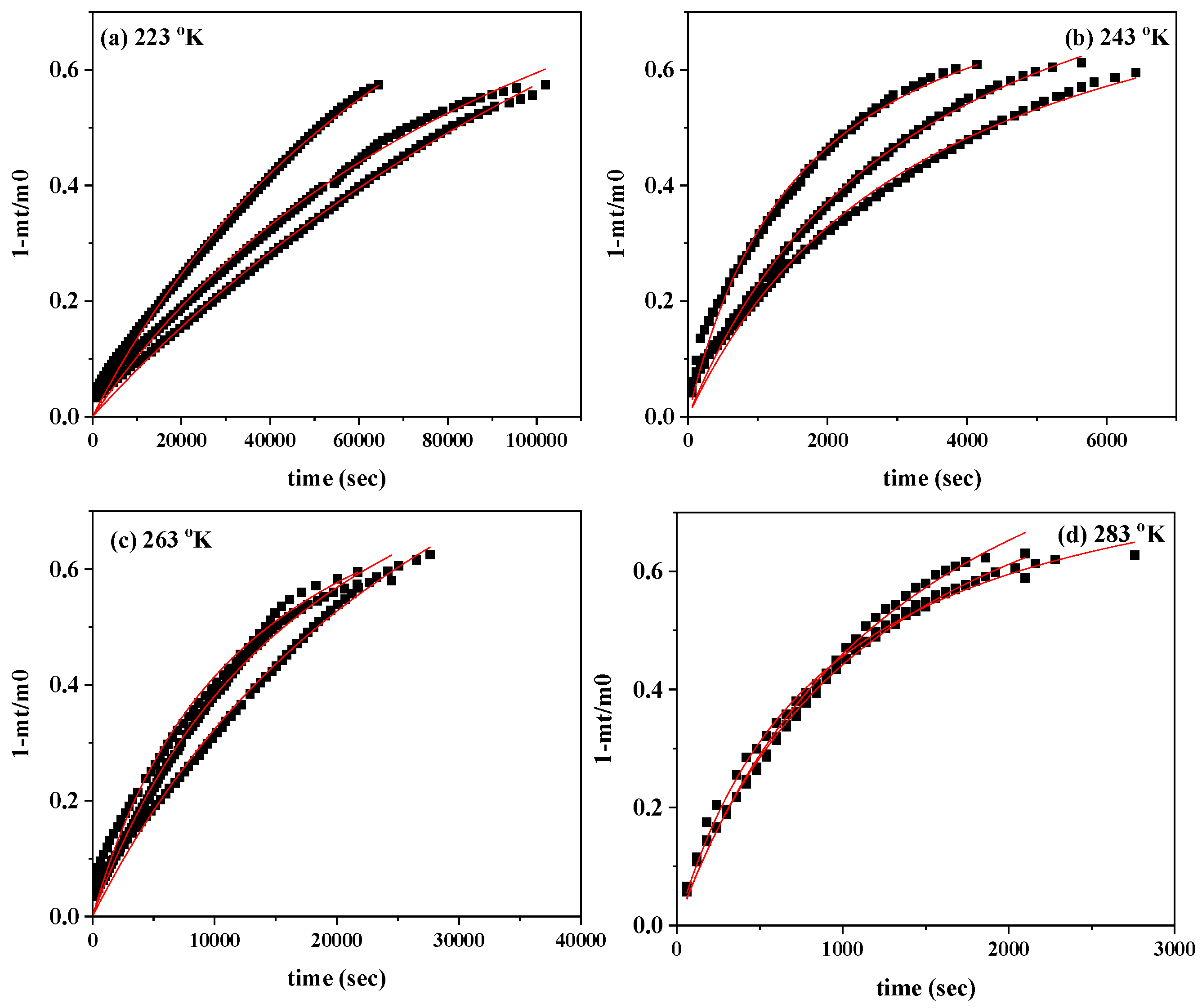
In
Figure 3, the CV desorption isotherm kinetic plots (scatter plots) and the simulation plot lines (red line plots) are compared.
The plots in
Figure 3 were simulated with a pseudo-second order kinetic model according to equation (1) and the calculated mean values of k
2, pseudo-second order constant k
2, and the desorption capacity at equilibrium q
e are all shown in
Table 2 for comparison.
Table 2 also includes the calculated mean values of %wt. CV desorbed at 223
oK, 243
oK, 263
oK and 283
oK.
As it is obtained in
Table 2 the total adsorbed amount of CV on CV@NZ nanohybrid is equal to 61.7±0.23 %wt. This amount is much higher than the value of 35.5 %wt. recently reported as the adsorbed amount of thymol on NZ [
42]. This increment must be attributed to the vacuum adsorption method employed here. The clean-vacuum process of pure NZ led to the desorption of all adsorbed water molecules of NZ and increased the available NZ adsorption active sites for CV.
Observing the average values of k
2 and q
e reported in
Table 2, it follows that with increasing temperature the value of k
2 increases while the value of q
e decreases.
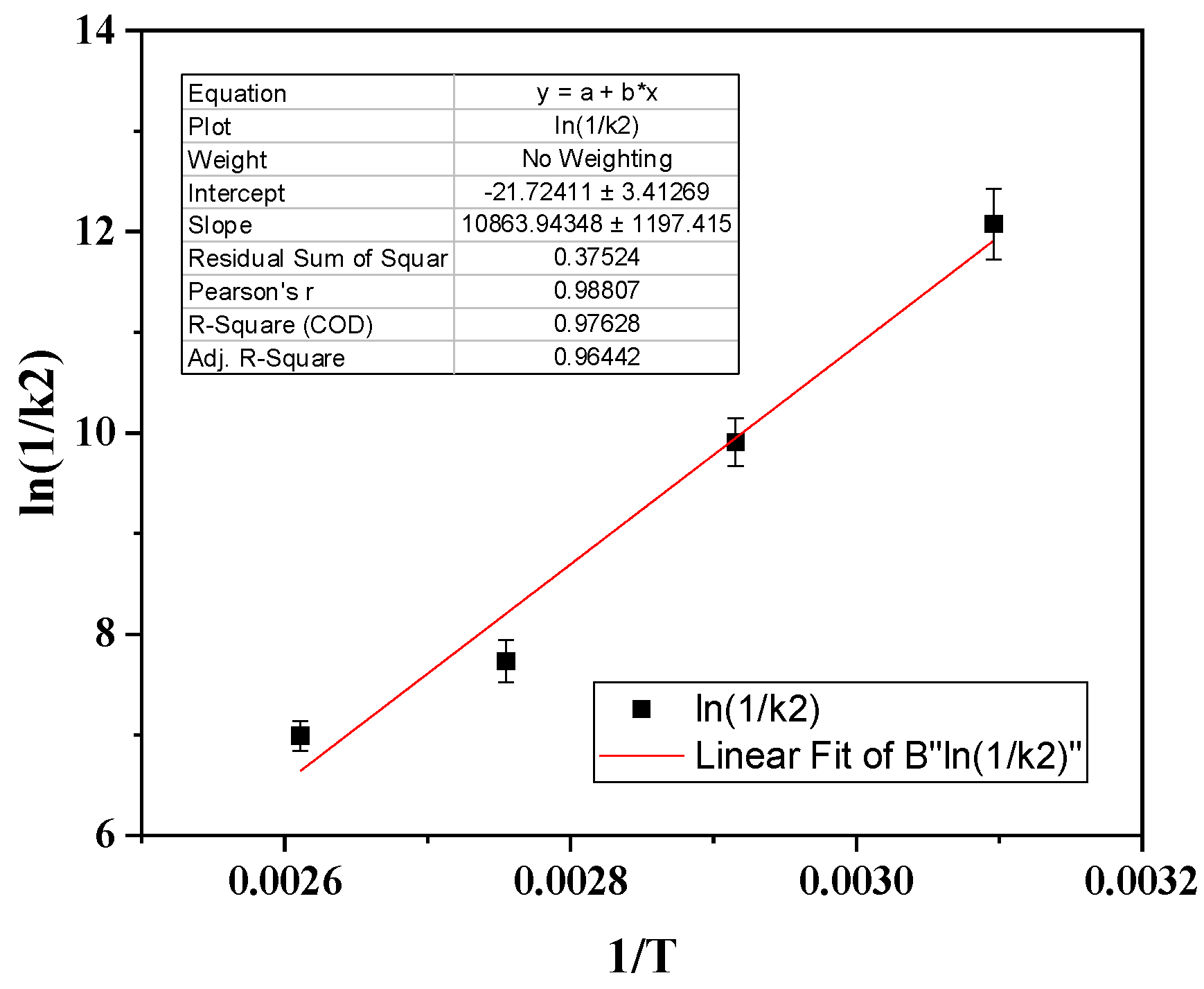
By plotting the obtained ln(1/k
2) values with (1/T) in
Figure 4 we calculated the desorption energy (Ε
0des) according to the Frenkel equation (2) and the obtained in
Figure 4 linear equation.
Τhe CV desorption energy (E
0des) was calculated from the calculated slope of
Figure 4 and found to be 90.32 KJ/mol which is equal to 21.58 Kcal/mol. According to Arrhenius theory E
0des values higher than 20 Kcal/mol correspond to chemisorption. In our case the calculated value of 21.58 Kcal/mol is close to the threshold of 20 Kcal/mol which corresponds to mixed chemisorbed and physiosorbed adsorption of CV on NZ. This mixed chemisorbed and physiosorbed adsorption of CV on NZ is in accordance with the pseudo-second order of desorption kinetic followed and in accordance with the FTIR results discussed hereabove and suggest chemisorption of CV molecules via an interaction of CV’s OH groups with NZ’s OH groups.
3.2. Physicochemical Characterization of PLA/TEC/xNZ and PLA/TEC/xCV@NZ Films
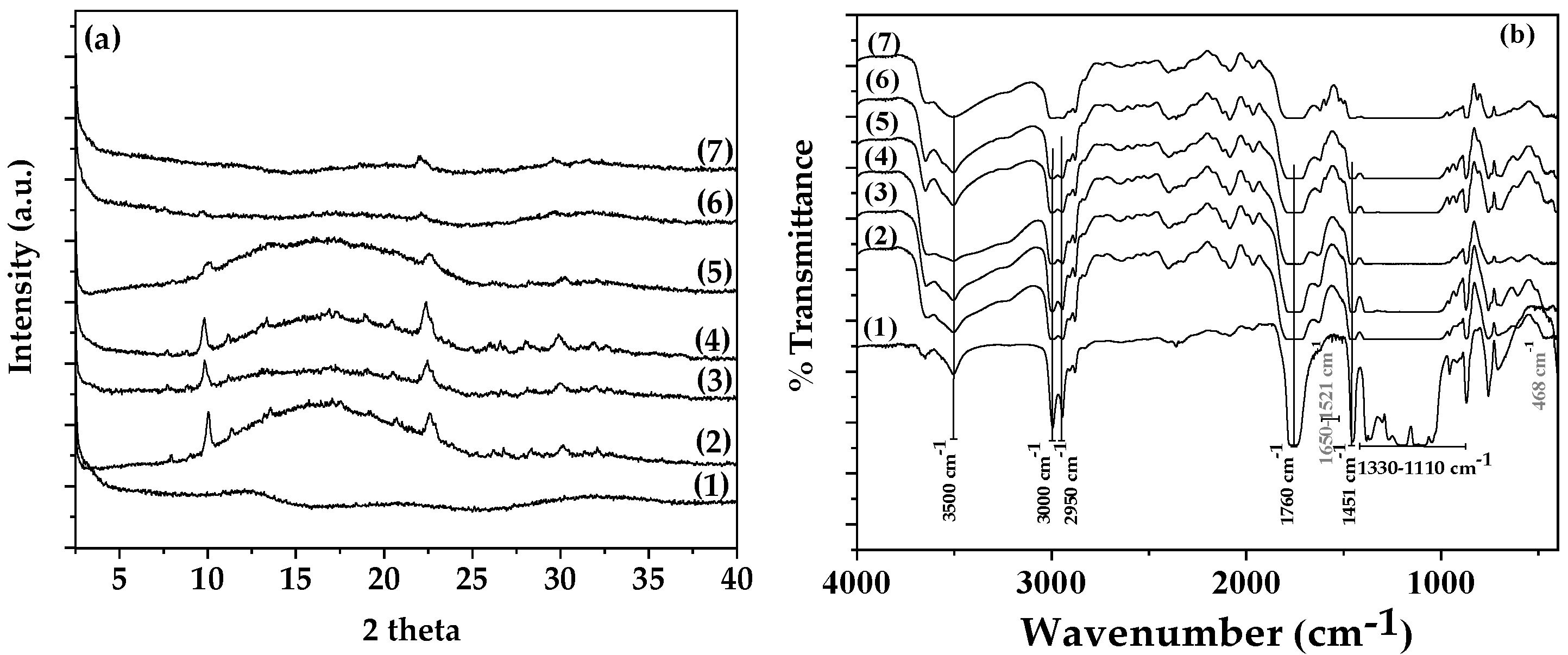
In
Figure 5a there are shown the XRD plots of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as PLA/TEC film in the range of 0.5
o to 40
o 2theta for comparison.
As it is observed in
Figure 5a the addition of both pure NZ and CV@NZ nanohybrid in PLA/TEC matrix results in the appearance of small peaks in the amorphous crystal phase plot of PLA/TEC. These reflections correspond to the reflections of NZ. At a first glance such NZ reflections are more intensive in the case of all PLA/TEC/xNZ films than in the case of PLA/TEC/xCV@NZ films and following recent reports suggest the highest dispersion of CV@NZ nanohybrid in PLA/TEC matrix than pure NZ [
42,
46].
In plot line (1) of
Figure 5b where the FTIR spectrum of PLA/TEC is shown, the following characteristic absorption bands are observed: the O–H stretching vibration at 3450 cm
−1, the asymmetrical and symmetrical –C–H stretching vibrations of the methyl group in the side chains at 2950, 3000, and 3050 cm
−1, the C = O stretching vibration carboxyl group from the repeated ester unit at 1760 cm
−1, the–C–O– stretching vibrations in the range of 1110–1330 cm
−1 and the corresponding bending vibrations of –CH
3 group at 1451 cm
−1 [
14].
When both pure NZ and CV@NZ nanohybrid added in the PLA/TEC matrix the characteristic bands of NZ are added in the FTIR plots of PLA/TEC/xNZ and PLA/TEC/xCV@NZ films. More specifically, there are observed the band at 468 cm
-1 which corresponds to the -SiO- bending mode and the band at 1650 cm
-1 which corresponds to the OH group bending mode [
42,
46]. At the same time, it is observed that all the characteristic reflections of PLA/TEC FTIR are increased. This increment is more observable for the reflection of the O–H stretching vibration of PLA/TEC at 3450 cm
−1. Overall FTIR plots show that both NZ and CV@NZ are effectively mixed with PLA/TEC matrix concluded in interactions with both hydroxyl and aliphatic chain of PLA/TEC matrix. This result follows previous reports and suggests NZ and modified CV@NZ as an excellent reinforcement material for both polymer and biopolymer-based matrixes [
42,
46].
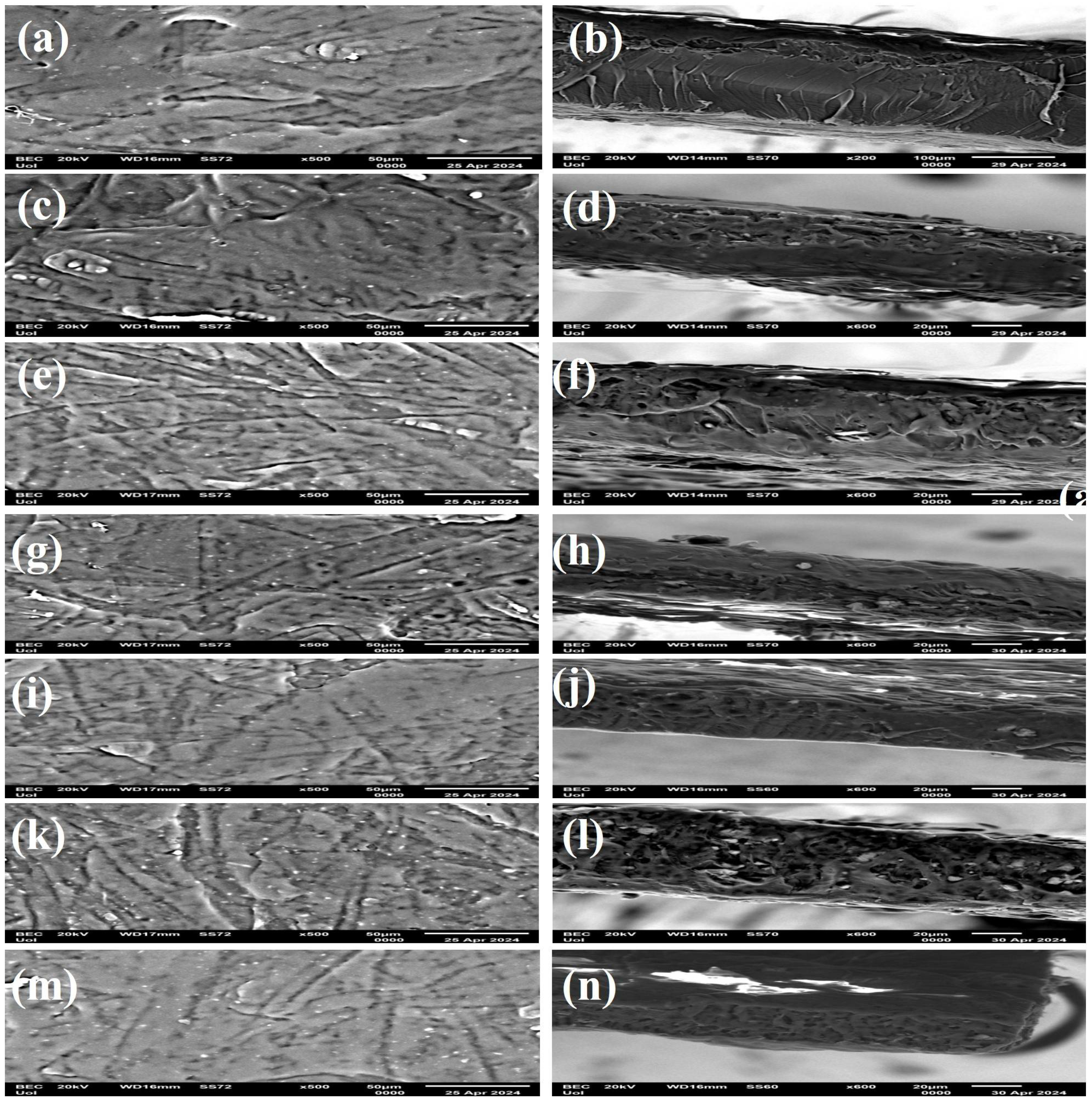
Scanning electron microscopy (SEM) was used to investigate the material’s behavior based on the effect of nanohybrid level on films and the surface features. Thus, morphological characteristics of the surface and cross-sections of film samples were evaluated with SEM and are presented in
Figure 6.
The surface and cross-section images of polymer matrix PLA/TEC and hybrid nanocomposite films of PLA/TEC/NZ and PLA/TEC/CV@NZ with different concentrations (5, 10, 15) of CV@NZ hybrid nanostructure and pure NZ (white dots can be clearly identified on the surface of PLA/TEC matrix) are shown in
Figure 6. All obtained films were clear and uniform in thickness. The surface/cross section of the polymer matrix PLA/TEC film (
Figure 6a,b) revealed smooth and clear surface morphology with a continuous phase without heterogeneities.
Surface, relative cross-section images of PLA/TEC/xNZ and PLA/xCV@NZ with different ratios of NZ and CV@NZ are presented in Figures 6c-n. It is evident that increasing the contents (after incorporation of NZ and CV@NZ) in the nanocomposites increases the degree of dispersion accordingly. Nevertheless, the results of SEM studies of the final nanocomposite films confirmed that the nanohybrids were homogeneously dispersed, indicating their enhanced compatibility with the polymer matrix, which was the major parameter that improved the behavior of the films.
It should be mentioned, based on the SEM studies (surface and cross-section), that a significant difference is observed when CV@NZ hybrid nanostructure is incorporated into the amorphous parts of the PLA/TEC polymer matrix as better interfacial adhesion and homogenous dispersion are evident compared to the corresponding nanocomposite film with pure NZ.
3.3. Mechanical and Thermomechanical Properties of PLA/TEC/xNZ and PLA/TEC/xCV@NZ Films
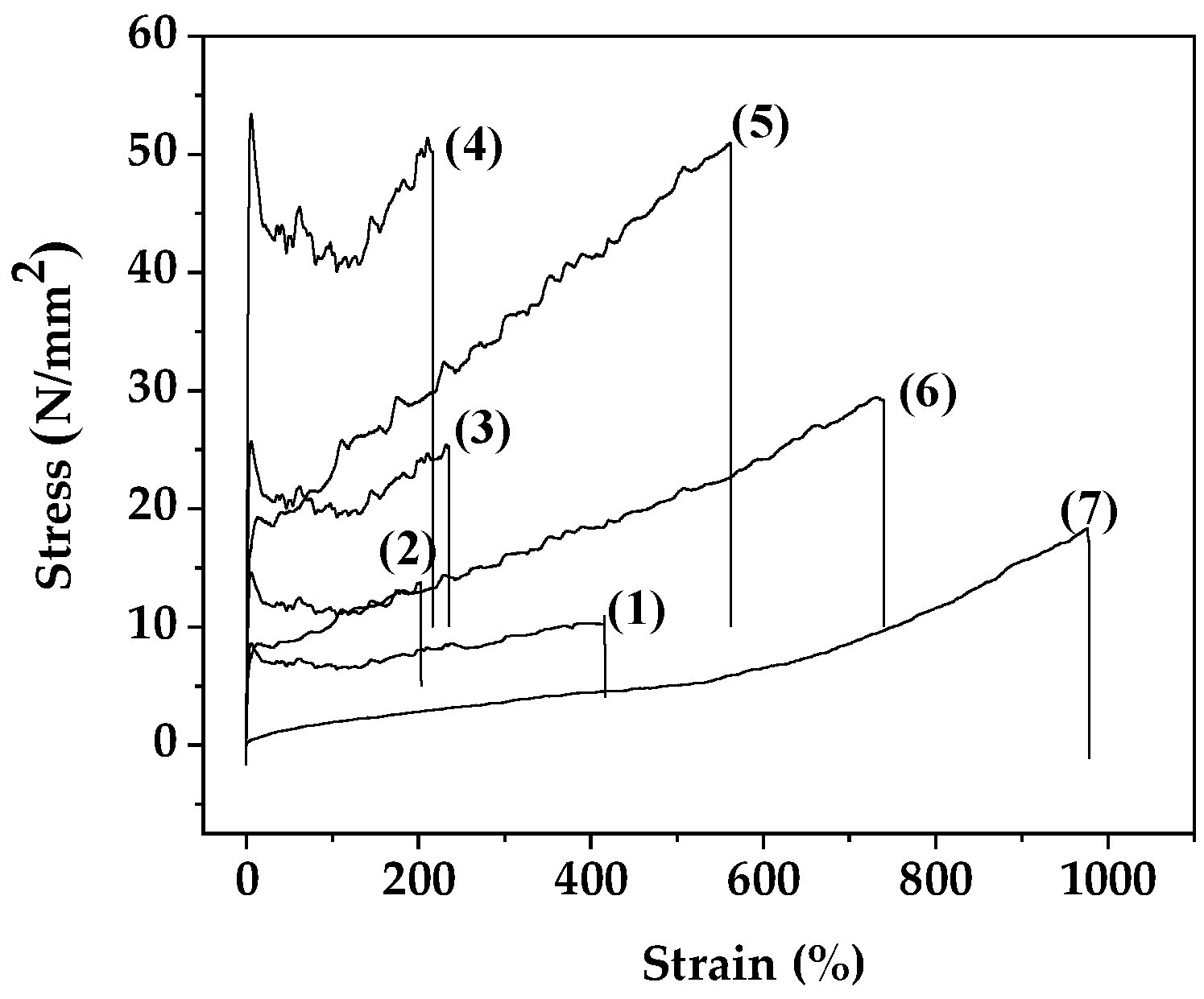
The stress-strain curves of all PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film are plotted in
Figure 7. From these stress-strain curves the Elastic Modulus (E) values, the ultimate strength In the
Figure 5 (σ
uts) values, and the % elongation at break (%ε) values were calculated and listed in
Table 3 for comparison. Independent-sample Median Test and pairwise comparisons of the different treatments according to the mean values of E, σ
uts, %ε are shown in
Figure S1 and
Table S1, respectively.
For PLA/TEC/xNZ films as the NZ %wt. content increases the ultimate strength values increase and the %ε decreases compared to PLA/TEC film as shown by the values reported in
Table 3. In the case of PLA/TEC/xCV@NZ films the addition of CV@NZ results in an increase of obtained %ε values as compared to pure PLA/TEC. For 5 %wt. CV@NZ nanohybrid addition, the obtained PLA/TEC/5CV@NZ film has higher ultimate strength values than both pure PLA/TEC and PLA/TEC/5NZ films. For 10 %wt. CV@NZ nanohybrid addition, the obtained PLA/TEC/10CV@NZ film has ultimate strength value approximately equal to pure PLA/TEC film and lower than PLA/TEC/10NZ film. For 15 %wt. CV@NZ nanohybrid addition, the obtained PLA/TEC/15CV@NZ film has much lower ultimate strength values than PLA/TEC film. Overall, it is concluded that pure NZ acts as a reinforcement agent and reinforces the PLA/TEC matrix when it is added in low, medium, and higher contents. On the other hand, the role of CV@NZ nanohybrid appears to be more of a plasticizer than a reinforcement agent. Considering that the aim of adding CV@NZ nanohybrid to the PLA/TEC matrix is to load as much as CV %wt. as we can without destroying its tensile properties, we concluded that 10%wt. addition CV@NZ is optimal.
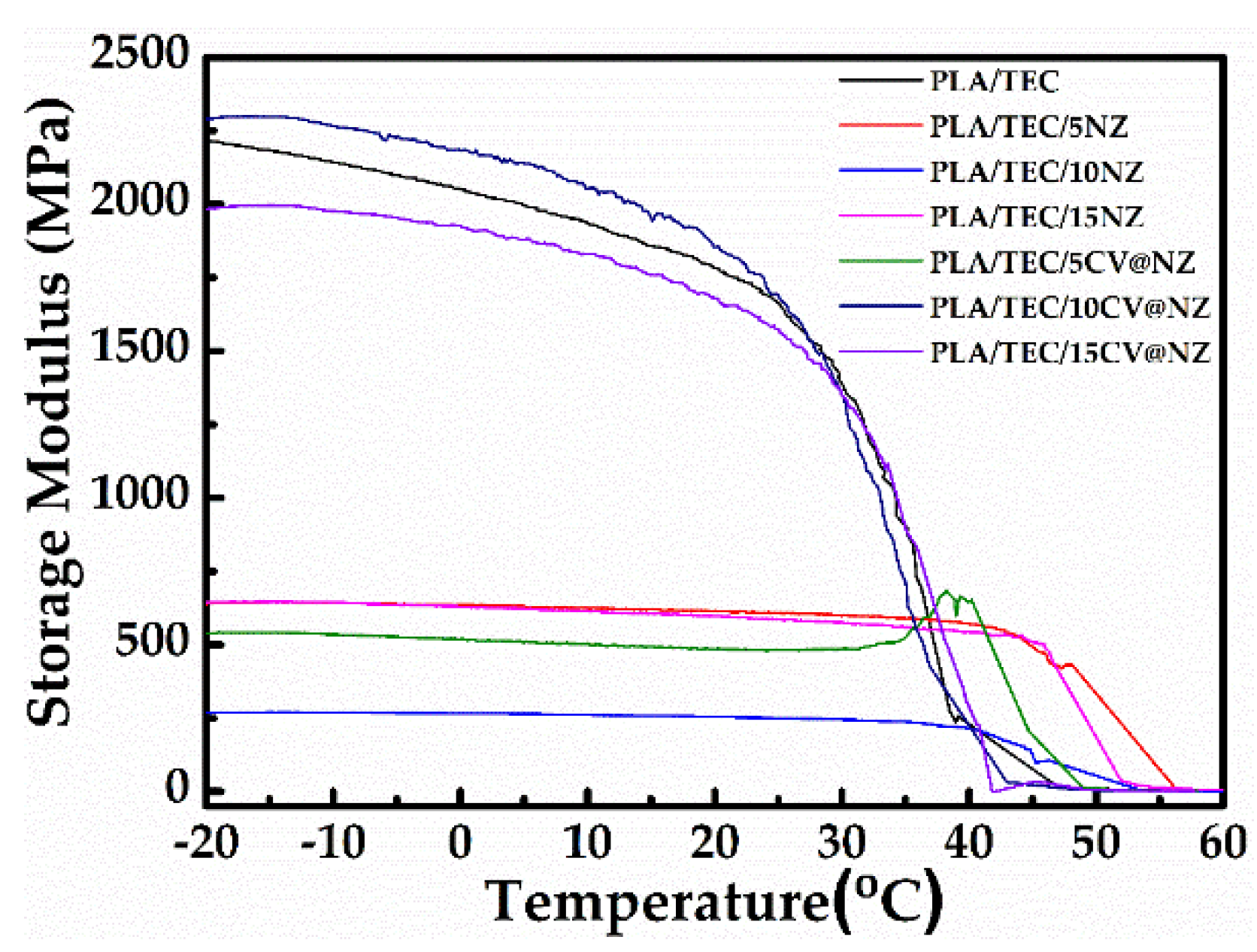
The dynamic mechanical analysis (DMA) measurements of storage modulus for all tested films of polymer matrix PLA/TEC and hybrid nanocomposite films of PLA/TEC/NZ and PLA/TEC/CV@NZ with different concentrations of NZ and hybrid nanostructure CV@NZ are summarized in
Figure 8.
As shown in
Figure 8, the integration of NZ in the PLA/TEC matrix decreased the storage modulus at all concentrations used (5, 10 and 15%). The incorporation of the hybrid nanocomposite CV@NZ, and especially the concentrations 10 % and 15 %, improved the thermomechanical behavior of the samples perhaps due to the better dispersion of the hybrid nanostructure in the PLA/TEC matrix.
3.6. Water/Oxygen Barrier Properties of PLA/TEC/xNZ and PLA/TEC/xCV@NZ Films
In
Table S2 the obtained water vapor transmission rate (WVTR), and oxygen transmission rate (OTR) mean values of all tested PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as for pure PLA/TEC film are listed for comparison. From these values, the water vapor diffusion coefficient (D
wv) values and the oxygen permeability P
eO2 values were calculated and listed in
Table S2 for comparison too. In
Figure S2 and
Table S3 the independent-sample Median Test and pairwise comparisons of all the obtained films according to the mean values of Dwv and P
eO2 are shown. In
Figure 9 the obtained D
wv and P
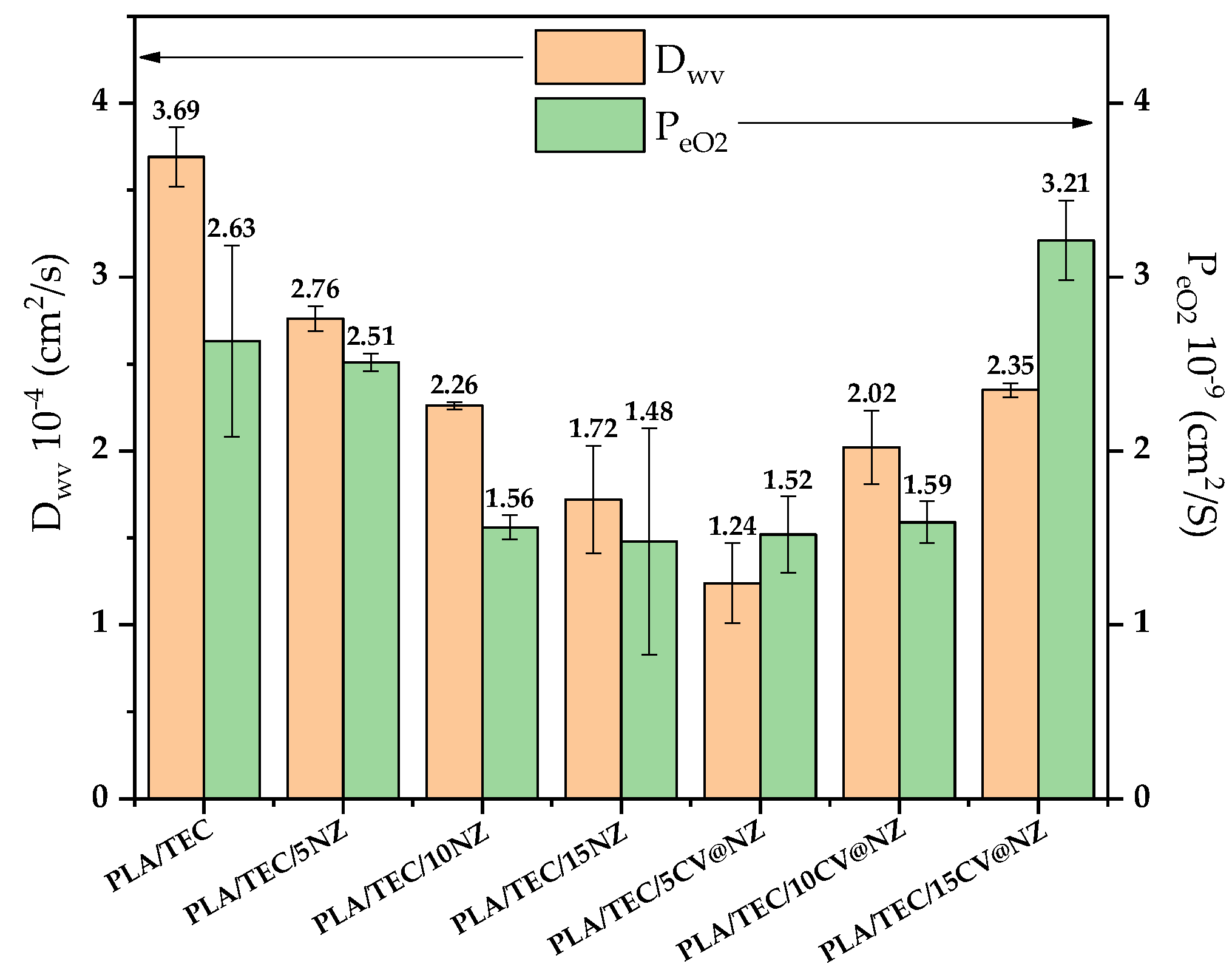
eO2 mean values of all tested PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as for pure PLA/TEC film are plotted for comparison.
As it is obtained in
Figure 9 the addition of both pure NZ and CV@NZ nanohybrid led to the increase of both water/oxygen barrier properties of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films compared to the water/oxygen barrier of pure PLA/TEC film. This result agrees with the high dispersity of both pure NZ and CV@NZ hybrid in obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films correspondingly obtained from the above SEM images. The high dispersity of a nanofiller is known to be beneficial for the water/oxygen barrier properties of polymer nanocomposite because because it reduces the free paths available for water/oxygen to permeate through the polymer matrix [
51,
52]. In the case of PLA/TEC/xNZ films water/oxygen barrier increase as the NZ %wt. content increases. So, the lowest D
wv and Pe
O2 values are obtained for PLA/TEC/15NZ film. In the case of PLA/TEC/xCV@NZ films water/oxygen barrier decrease as the CV@NZ %wt. content increases. Thus, the lowest D
wv and Pe
O2 values are obtained for PLA/TEC/5CV@NZ film.
3.7. CV Release Kinetics from PLA/TEC/xCV@NZ Films
In
Figure S3 there are plotted the obtained (1-m
t/m
o) values as a function of time used for each film for the simulation with pseudo-second order equation (1). From the simulation of experimental values with equation (1) the mean values of CV desorption equilibrium constant (q
e), and the desorption rate constant mean values (k
2) were calculated and listed in
Table 4 for each film for comparison. In the same
Table 4, there are listed the calculated mean values of CV release rate (RR
CV) as well as the mean values of %wt. Values of CV released content (%RC
CV) for each film are listed for comparison too.
Data in
Table 4 show that both the k
2 mean values and RR
cv mean values decrease as the CV@NZ content increases. This means that as the CV@NZ content increases the CV release rate decreases. On the other hand, both the CV desorption equilibrium constant (q
e) mean values and the %RC
CV mean values increase as the CV@NZ content increases. This means that as the CV@NZ content increases the CV released amount increases too. In other words, by increasing CV@NZ content total amount of CV release content increases but the CV release rate decreases. This means that medium CV@NZ content (10CV@NZ) is optimal for obtaining high CV release content and CV control release rate.
3.8. Antioxidant Activity of PLA/TEC/xCV@NZ Films
In
Table 5 there are listed the calculated EC50 mean values for all obtained PLA/TEC/xCV@NZ active films. In
Figures S4-S6 the plots and the linear equations used to calculate the mean EC50 values for each PLA/TEC/xCV@NZ film are presented. In
Figure S7 and
Table S4 the independent-samples Median Test and pairwise comparisons of all the obtained films according to the mean values of EC50 are listed.
As it is obtained from
Table 5 all PLA/TEC/xCV@NZ active films exhibited low EC50 value in the range of 2.85 to 4.65 mg/L. These EC50 values are much lower than 212.6 mg/L which is the EC50 value of pure PLA/TEC recently reported [
14]. In addition, it is observed that all PLA/TEC/xCV@NZ films exhibited low and equal EC50 values no statistically significant difference (see
Table S4). This result agrees with the CV release kinetics discussed here above. It was shown that by increasing CV@NZ content total amount of CV release content increases but the CV release rate decreases. So, it seems that all PLA/TEC/xCV@NZ films release equal amounts of CV and reach equal EC50 values during the time of incubation for the antioxidant activity experiment.
3.9. Antibacterial Activity of PLA/TEC/xCV@NZ Films
The results of the antibacterial activity of all PLA/TEC/xCV@NZ films against
S. aureus and
S. Typhimurium are presented in
Table 6.
The values in
Table 6 show that all PLA/TEC/xCV@NZ films exhibited significant antibacterial activity against one Gram-positive,
Staphylococcus aureus, and one Gram-negative,
Salmonella enterica spp.
enterica serovar Typhimurium, pathogenic bacteria. All tested films had strong antibacterial activity against both pathogens
S. aureus and
S. Typhimurium in the contact area but also created a significant inhibition zone. As recently shown, pure PLA/TEC film exhibited antibacterial activity against the same pathogens only in the contact area [
14]. Thus, it is concluded that the incorporation of CV@NZ nanohybrid to obtain PLA/TEC/xCV@NZ films not only has a positive effect on the antibacterial activity but also, by the inhibition zones generated from all films, it is stronger . As it is shown in
Table 6 no significant differences can be observed for the antibacterial activity of films loaded with different CV@NZ nanohybrid contents. This result is in accordance with the antioxidant activity results of PLA/TEC/xCV@NZ films.
3.10. Preservation of Fresh Minced Pork Meat Wrapped with PLA/TEC and PLA/TEC/10CV@NZ Films
3.10.1. Microbiological Evaluation of Minced Pork Wrapped with PLA/TEC and PLA/TEC/10CV@NZ Films
The obtained mean TVC values as a function of the kind of film used and the storage time are listed in Table S5. The independent-samples Median Test and pairwise comparisons of all obtained films according to the mean values of TVC are listed in
Figure S8 and
Table S6. For better comparison,
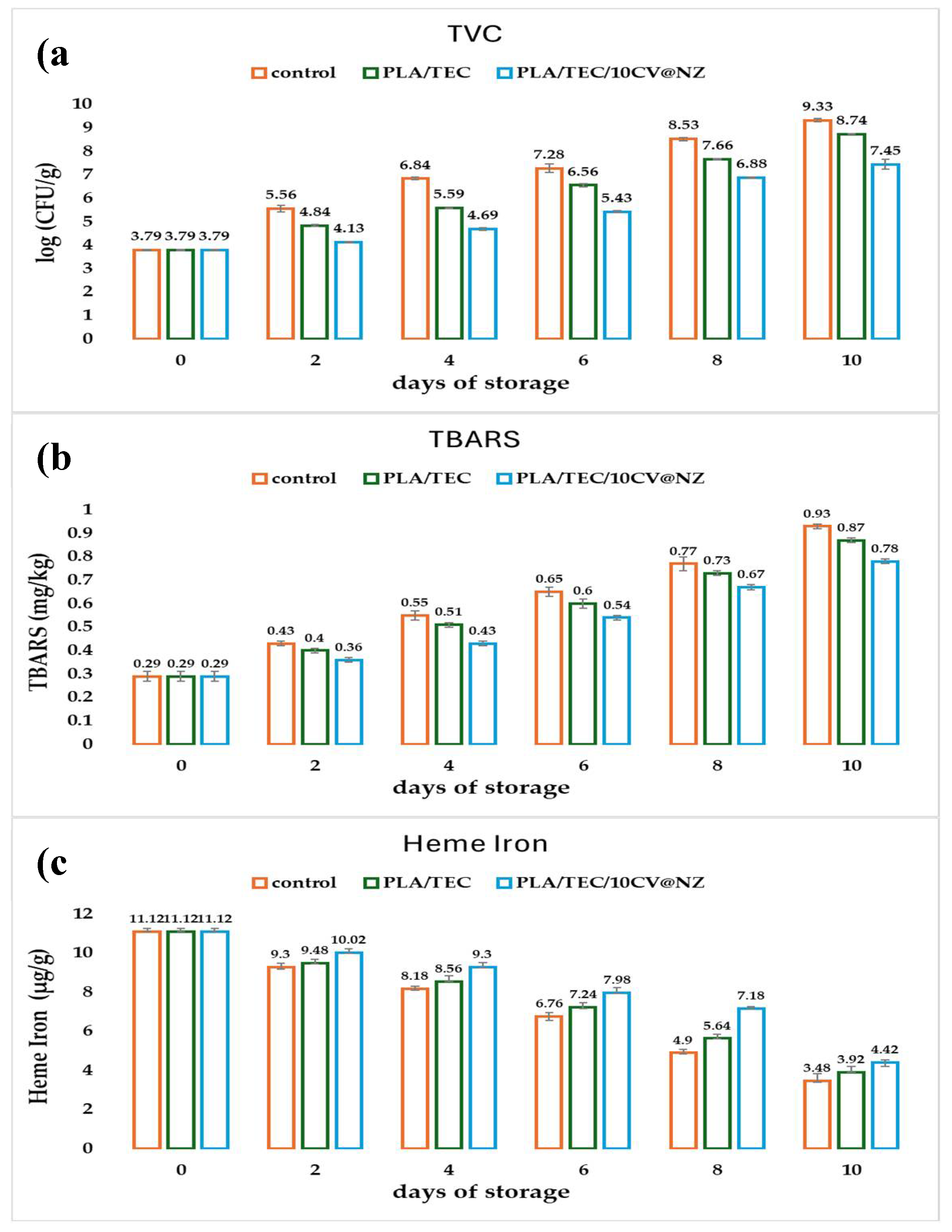
TVC values are plotted in the column bar diagram in Figure 10a as a function of film used and days of storage.
Total viable count (TVC) of bacteria is an important microbiology indicator for the quality and safety of meat[
53,
54]. The TVC limit of acceptance for fresh pork meat is 7 log CFU/g according to International Commission on Microbiological Specifications for Foods (ICMFS) [
55]
. When pork
meat TVC exceeds this standard value of acceptance could greatly endanger consumers’ health [
54]
. As it is obtained in
Figure 10a and
Table S5 minced pork wrapped with the commercial packaging of Aifantis Company and PLA/TEC film almost exceeded the limit of acceptance after the fourth and sixth day of storage, respectively. The minced pork wrapped with PLA/TEC/10CV@NZ film almost exceeded that value after the eighth day of storage. According to the obtained values, for total viable count, both PLA/TEC and PLA/TEC/10CV@NZ films succeed to extend the microbiological shelf life of fresh minced pork for two and four days, compered to the commercial packaging paper (control) , respectively. The two-day shelf life extension of fresh minced pork using the PLA/TEC film is in agreement with a previous report where it was shown that the incorporation of TEC into the PLA matrix not only extends the self life of pork meat for two days but also improves the antioxidant and antibacterial activity of the obtained PLA/TEC film [
14]. Extending the shelf life of fresh minced pork for four days using PLA/TEC/10CV@NZ film is reported for the first time, making it very promising for active packaging. It should be stresed that PLA/TEC/10CV@NZ recorded the lowest and statistically significant different values throughout storage time (see
Table S6).This result is supported by the characterization results of the PLA/TEC/10CV@NZ film where a high dispersion of CV@NZ nanohybrid in the PLA/TEC matrix was shown, improving the antibacterial activity of the film and the obtained CV’s control release rates.
3.10.2. Lipid Oxidation of Minced Pork Wrapped with PLA/TEC and PLA/TEC/10CV@NZ Films
2-Thiobarbituric acid reactive substances (TBARS) is an important quality index for pork meat preservation [
56]. TBARS value reflects the content of malonaldehyde, one of the degradation products of lipid hydro peroxides and peroxides formed during the oxidation of polyunsaturated fatty acids and thus TBARS value is widely used as an indicator of the degree of lipid oxidation during the deterioration of pork meat [
57].The obtained mean TBARS values as a function of the kind of film used and the storage time are listed in
Table S7. The independent-sample Median Test and pairwise comparisons of all the obtained films according to the mean values of TBARS are listed in
Figure S9 and
Table S8. For better comparison TBARS values are plotted in the column bar diagram in
Figure 10b as a function of film used and days of storage. As it is obtained from TBARS plots in
Figure 10b and
Table S3 all packaging systems had statistically significant differences throughout storage time expept for the 8th day which control and PLA/TEC samples had similar values. Minced pork wrapped with both PLA/TEC and PLA/TEC/10CV@NZ films exhibited lower TBARS increment rates than minced pork wrapped with the commercial film (control sample). Minced pork wrapped with PLA/TEC/10CV@NZ film recorded the lowest TBARS increment rate. Thus, on the 8th day when minced pork wrapped with PLA/TEC/10CV@NZ film almost exceed the limit of acceptance for TVC, 7 logCFU/g, minced pork meat had a TBARS value 17% lower than minced pork meat wrapped with commercial film. In other words, PLA/TEC/10CV@NZ film succeeds in preventing minced pork from lipid oxidation deterioration. This result is in accordance with the improved water/oxygen barrier properties and enhanced antioxidant activity of this film discussed here above.
3.10.3. Heme Iron Content of Minced Pork Wrapped with PLA/TEC and PLA/TEC/10CV@NZ Films
Heme iron content is an important nutritional index for pork and other meats. The obtained mean heme iron content values as a function of the kind of film used and the storage time are listed in
Table S7. The independent-sample Median Test and pairwise comparisons of all the obtained films according to the mean values of heme iron are listed in
Figure S10 and
Table S9. For better comparison, heme iron content values are plotted in the column bar diagram in
Figure 10c as a function of film used and days of storage. As it is obtained in
Figure 10c in all samples heme iron content decreases as the days of storage increase. According to
Table S3 control and PLA/TEC samples seemed to have the same trend in the first two days of storage, while PLA/TEC/10CV@NZ differ statistically significant. On the 4th, 6th and 8th day of storage all packaging treatments recorded statistically significant differences. On 10th day of storage only minced pork wrapped with PLA/TEC/10CV@NZ films recorded lower and statistically significant different values for Heme iron content. Both PLA/TEC and PLA/TEC/10CV@NZ films suceeds to keep wrapped minced pork with higher heme iron contents during the ten days of storage compared to minced pork wrapped with commercial paper (control sample). The highest heme iron content values, during the ten days of storage, were obtained for minced pork wrapped with PLA/TEC/10CV@NZ film. This result is in accordance with the low TBARS values obtained for the minced pork wrapped with the same packaging system. In other words, PLA/TEC/10CV@NZ prevents minced pork from lipid oxidation deterioration and thus keeps minced pork in higher heme iron contents. Following previous reports a linear correlation between the increasing TBARS and the decreasing heme iron content values has been recorded [
28,
42,
58]. In this study, a correlation between TBARS and heme iron content values was estimated using Pearson’s bivariate correlation (−1 to +1) at the confidence level p < 0.05, with respect to storage time. The results of the statistical analysis showed that there is a statistically significant and positive correlation between the two methods throughout the storage period (see
Table S10).
3.10.4. Sensory Analysis of Minced Pork Wrapped with PLA/TEC and PLA/TEC/10CV@NZ Films
Sensory analysis results of odor, color, and texture of minced pork wrapped with PLA/TEC and PLA/TEC/10CV@NZ films as well as with commercial film are presented in
Table 7 for comparison. The independent-sample Median Test and pairwise comparisons of all the obtained films according to the mean values of odor, color, and texture are listed in
Figures S11-S13 and
Tables S11-13.
According to statistical analysis results odor, color, and texture are significantly different for all tested samples after the 4th day of storage. For the minced pork sample wrapped with PLA/TEC/10CV@NZ film odor, color, and texture are significantly different from the other two samples from the 2nd day of storage. Overall, it is concluded that both pure PLA/TEC films and PLA/TEC/10CV@NZ films succeed in preserving wrapped minced pork in much better and acceptable sensory condition than commercial packaging paper. Minced pork wrapped with PLA/TEC/10CV@NZ film exhibited the highest sensory analysis values during the 10 days of storage. It is noteworthy to state that minced pork packaged with PLA/TEC/10CV@NZ film keeps odor, color, and texture values higher than three on the 10th day of storage.
4. Discussion
In this study a novel CV@NZ hybrid nanostructure and PLA/TEC/xNZ, PLA/TEC/xCV@NZ (x=5, 10, 15 %wt.) nanocomposite active packaging films were succefully developed. XRD analysis of CV@NZ nanohybrid showed that adsorption of CV into NZ does not affect the crystallinity of pure NZ while the FTIR analysis suggests the effective adsorption of CV molecules on NZ pores. Moreover, in the obtained FTIR plot of CV@NZ nanohybrid there are indications of chemisorption of CV on NZ. The desorption kinetic experiments of CV from CV@NZ nanohybrid revealed that the total adsorbed amount of CV on CV@NZ nanohybrid is equal to 61.7±0.23 %wt. This amount is much higher than the value of 35.5 %wt. reported recently as the adsorbed amount of thymol on NZ [
42]. This increment is attributed to the novel vacuum adsorption method employed herein. In addition, desorption kinetic experiments showed that the desorption energy of CV is 21.58 Kcal/mol which suggests mixed chemisorbed and physiosorbed adsorption of CV on NZ following the FTIR results. It must be stressed that such desorption kinetic experiments are first time reported as a novel method to determine the %wt. loading content as well as the desorption energy of CV on such CV@NZ nanohybrid. This CV desorption kinetic method is provided as a novel and low-cost method to replace high-cost methods usually used such as thermogravimetric analysis (TG) and differential scanning calorimetry (DSC) measurements [
42,
59].
For PLA/TEC/xNZ and PLA/TEC/xCV@NZ (x=5, 10, 15 %wt.) nanocomposite active packaging films both XRD and FTIR suggest the highest dispersion of CV@NZ nanohybrid in PLA/TEC matrix than pure NZ. SEM studies confirmed that both pure NZ and CV@NZ nanohybrid were homogeneously dispersed which indicates their enhanced compatibility with the PLA/TEC matrix. Tensile measurements concluded that pure NZ acts as a reinforcement agent to the PLA/TEC matrix regardless of if it is added in low, medium, or higher amounts. On the other hand, CV@NZ nanohybrid role seems to be more of a plasticizer agent than a reinforcement agent, due to the presence of CV adsorbed on NZ. Considering that the aim of this study was to add CV@NZ nanohybrid to PLA/TEC matrix to gain as much as possible higher CV content (%wt.), without worsening its tensile properties, we concluded that the addition of 10%wt CV@NZ is optimal. Both Water and oxygen barriers in the case of PLA/TEC/xNZ films were increased as the NZ (%wt.) content increased and the lowest Dwv and PeO2 values were obtained for (or from?) PLA/TEC/15NZ film. In the case of PLA/TEC/xCV@NZ films water and oxygen barriers were decreased as the CV@NZ (%wt.) content was increasing. The lowest Dwv and PeO2 values were recorded for PLA/TEC/5CV@NZ film. In general, CV release kinetics for PLA/TEC/xCV@NZ composite films showed that when the amount of CV@NZ (%wt.) was increased the CV release increased too, but the CV release rate was decreased. According to the above a medium CV@NZ content (10 %wt.) is optimal to get high CV release content and CV control release rate.
Antioxidant and antibacterial activity experiments showed that PLA/TEC/xCV@NZ films had no statistically significant differences on the EC50 mean values and no significant differences on the inhibition zones created by these films, against the Gram-positive, Staphylococcus aureus, and the Gram-negative, Salmonella enterica spp. enterica serovar Typhimurium pathogens.
Considering all the parameters tested for PLA/TEC/xCV@NZ active films and emphasizing on the mechanical properties, CV release amount and CV release rate as the most critical factors for an active packaging film, we concluded that PLA/TEC10CV@NZ film is the optimal for a fresh minced pork shelf-life experiment. Both PLA/TEC and PLA/TEC/10CV@NZ films succeeded to extend the shelf life of minced pork meat for two and four days, respectively, compared to minced pork wrapped with commercial paper. In addition, PLA/TEC/10CV@NZ packaging system recorded the lowest and statistically significant different TBARS values throughout the 10 days of storage time delaying lipid oxidation deterioration of minced pork. In agreement with the above are the results obtained for the Heme Iron content determination, where the minced pork wrapped with PLA/TEC/10CV@NZ films recorded higher and statistically significant different values throughout storage time. Sensory analysis scores, for odor, color, and texture, showed that PLA/TEC/10CV@NZ films succeed in preserving wrapped minced pork in a much better and statistically significant sensory condition than commercial packaging paper. It is noteworthy to state that minced pork packaged with PLA/TEC/10CV@NZ film keeps odor, color, and texture values higher than three after 10 days of storage. Last but not least it must be noticed that this novel PLA/TEC/10CV@NZ active film keeps the self-healable property was observed also for the pure PLA/TEC composite matrix recently [
14]. As shown in video S1 this PLA/TEC/10CV@NZ active film succeeds in completely healing the dog bone-shaped initial scratch after 3 minutes.
5. Conclusion
Overall, this study provides a novel PLA/TEC/10CV@NZ biodegradable, self-healable active packaging film with improved water/oxygen barrier properties and enhanced antioxidant/antibacterial activity which succeeded in extending the shelf-life of fresh minced pork, according to total viable count values, for four days. Simultaneously this film delays the lipid oxidation, keeps fresh minced pork in higher nutritional condition, as revealed by the determination of TBARS and heme iron content, and in a much better and acceptable sensory condition than commercial packaging paper. This film has a great potential to be used as active film to extend the self-life of fresh meat products.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Independent-Samples Median Test for E, σ, %ε.; Figure S2: Independent-Samples Median Test for Dwv,PeO2.; Figure S3: Plots of (1-mt/m0) as a function of time for all PLA/TEC/xCV@NZ samples (in triplicate) simulated with the pseudo-second order kinetic equation to obtain k2, and qe mean values.; Figure S4: Equations for the determination of EC50 in PLA/TEC/5CV@NZ films; Figure S5 : Equations for the determination of EC50 in PLA/TEC/10CV@NZ films.; Figure S6 : Equations for the determination of EC50 in PLA/TEC/15CV@NZ films.; Figure S7 : Independent-Samples Median Test of EC50 values.; Figure S8: Independent-Samples Median Test of TVC durring storage time.; Figure S9: Independent-Samples Median Test of TBARS durring storage time.; Figure S10 : Independent-Samples Median Test of Heme-iron content durring storage time.; Figure S11 : Independent-Samples Median Test of Odor durring storage time.; Figure S12: Independent-Samples Median Test of Color durring storage time.; Figure S13: Independent-Samples Median Test of Texture durring storage time.; Table S1: Pairwise Comparisons of the different treatments according to the mean values of E, σ, %ε.; Table S2: WVTR, Dwv, O.T.R. and PeO2 values for all PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as for pure PLA/TEC films.; Table S3: Pairwise Comparisons of the different treatments according to the mean values of Dwv,PeO2.; Table S4 : Independent-Samples Median Test Summary of the different treatments according to the mean values of EC50.; Table S5 : TVC of Minced pork in Different Packaging Systems with Respect to Storage Time; Table S6 : Pairwise Comparisons of the different treatments according to the mean values of TVC during storage time.; Table S7: TBARS and Heme-Iron Content of Minced pork in Different Packaging Systems with Respect to Storage Time.; Table S8: Pairwise Comparisons of the different treatments according to the mean values of TBARS during storage time.; Table S9: Pairwise Comparisons of the different treatments according to the mean values of Heme-iron content during storage time.; Table S10 : Pearson’s Correlation between TBARS and Heme-iron content during storage time.; Table S11: Pairwise Comparisons of the different treatments according to the mean values of Odor during storage time.; Table S12: Pairwise Comparisons of the different treatments according to the mean values of Color during storage time.; Table S13: Pairwise Comparisons of the different treatments according to the mean values of Texture during storage time; Video S1: Self-healing test of PLA/TEC/10CV@NZ active film.
Author Contributions
Conceptualization, V.K.K., A.E.G., and C.E.S.; methodology, V.K.K., A.E.G., C.P., and C.E.S.; software, D.M. and A.K.-M.; validation, A.E.G., A.A., N.E.Z., and C.E.S.; formal analysis, N.D.A. and A.L.; investigation, V.K.K., N.D.A., D.M., and A.K.-M.; resources, A.E.G., A.A., N.E.Z., C.P., and C.E.S.; data curation, V.K.K., and A.L.; writing—original draft preparation, A.E.G. and C.E.S.; writing—review and editing, V.K.K., A.L., A.E.G. and C.E.S.; visualization, V.K.K., A.L., A.E.G., and C.E.S.; supervision, A.E.G., and C.E.S.; project administration, A.E.G. and C.E.S.; funding acquisition, A.A., N.E.Z. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Jlksadjlkjas.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jain, P.C. Greenhouse Effect and Climate Change: Scientific Basis and Overview. Renewable Energy 1993, 3, 403–420. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ Chem Lett 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. An Overview of Biodegradable Poly (Lactic Acid) Production from Fermentative Lactic Acid for Biomedical and Bioplastic Applications. Biomass Conv. Bioref. 2024, 14, 3057–3076. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. International Journal of Food Properties 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A Review of Poly(Lactic Acid)-Based Materials for Antimicrobial Packaging. Journal of Food Science 2014, 79, R1477–R1490. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann Biomed Eng 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Polymers | Free Full-Text | Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications Available online:. Available online: https://www.mdpi.com/2073-4360/13/11/1822 (accessed on 6 February 2024).
- Eco-Friendly Plasticizers for PLA Films - Italian Food Tech 2019.
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly(Lactic Acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Liu, Q.; Dong, H.; Duan, Y.; Zhang, J. Plasticization of Poly (Lactic) Acid Film as a Potential Coating Material. IOP Conf. Ser.: Earth Environ. Sci. 2018, 108, 022062. [Google Scholar] [CrossRef]
- Mele, G.; Bloise, E.; Cosentino, F.; Lomonaco, D.; Avelino, F.; Marcianò, T.; Massaro, C.; Mazzetto, S.E.; Tammaro, L.; Scalone, A.G.; et al. Influence of Cardanol Oil on the Properties of Poly(Lactic Acid) Films Produced by Melt Extrusion. ACS Omega 2019, 4, 718–726. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An Environmentally Sustainable Plasticizer Toughened Polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing Effects of Citrate Esters on Properties of Poly(Lactic Acid). Journal of Polymer Engineering 2016, 36, 371–380. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Moschovas, D.; Karydis-Messinis, A.; Leontiou, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension. Polymers 2024, 16, 1130. [Google Scholar] [CrossRef] [PubMed]
- Molecules | Free Full-Text | Recent Advances in PLA-Based Antibacterial Food Packaging and Its Applications Available online:. Available online: https://www.mdpi.com/1420-3049/27/18/5953 (accessed on 6 February 2024).
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K. Synchronous Application of Antibiotics and Essential Oils: Dual Mechanisms of Action as a Potential Solution to Antibiotic Resistance. Critical Reviews in Microbiology 2018, 44, 414–435. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential Oils as Antimicrobial Agents in Biopolymer-Based Food Packaging - A Comprehensive Review. Food Bioscience 2020, 38, 100785. [Google Scholar] [CrossRef]
- Use of Essential Oils in Bioactive Edible Coatings: A Review | SpringerLink Available online:. Available online: https://link.springer.com/article/10.1007/s12393-010-9031-3 (accessed on 8 August 2019).
- Foods | Free Full-Text | Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry Available online:. Available online: https://www.mdpi.com/2304-8158/11/3/464 (accessed on 16 December 2023).
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. Journal of Food Protection 2023, 86, 100025. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; R, S.; Pal, K.; Sarkar, P. Nanoclay-Based Active Food Packaging Systems: A Review. Food Packaging and Shelf Life 2022, 31, 100803. [Google Scholar] [CrossRef]
- Essifi, K.; Hammani, A.; Berraaouan, D.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Montmorillonite Nanoclay Based Formulation for Controlled and Selective Release of Volatile Essential Oil Compounds. Materials Chemistry and Physics 2022, 277, 125569. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Kumar, L.; Gaikwad, K.K. Halloysite Nanotubes for Food Packaging Application: A Review. Applied Clay Science 2023, 234, 106856. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Almeida-Aguiar, C.; Costa, S.P.G.; Neves, I.C. Essential Oils Encapsulated in Zeolite Structures as Delivery Systems (EODS): An Overview. Molecules 2022, 27, 8525. [Google Scholar] [CrossRef] [PubMed]
- Controlled Release of Essential Oil from a Rubberwood Box Using Activated Carbon to Extend the Shelf Life of Strawberries and Its Possible Mode of Action :: BioResources Available online:. Available online: https://bioresources.cnr.ncsu.edu/ (accessed on 6 February 2024).
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyeth-Ylene Based Active Packaging Films for Pork Fillets Shelf-Life Extension 2023.
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical Applications of Zeolite-Based Materials: A Review. Materials Science and Engineering: C 2020, 116, 111225. [Google Scholar] [CrossRef] [PubMed]
- Can Baser, K.H. Biological and Pharmacological Activities of Carvacrol and Carvacrol Bearing Essential Oils. Current Pharmaceutical Design 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O.; Shu, Z. Development of Biodegradable Composite Chitosan-Based Films Incorporated with Xylan and Carvacrol for Food Packaging Application. Food Packaging and Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Persico, P.; Ambrogi, V.; Carfagna, C.; Cerruti, P.; Ferrocino, I.; Mauriello, G. Nanocomposite Polymer Films Containing Carvacrol for Antimicrobial Active Packaging. Polymer Engineering & Science 2009, 49, 1447–1455. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P. Antimicrobial-Releasing Films and Coatings for Food Packaging Based on Carvacrol and Ethylene Copolymers. Polymer International 2015, 64, 1747–1753. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol Loaded Electrospun Fibrous Films from Zein and Poly(Lactic Acid) for Active Food Packaging. Food Hydrocolloids 2018, 81, 48–59. [Google Scholar] [CrossRef]
- Alkan Tas, B.; Sehit, E.; Erdinc Tas, C.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Carvacrol Loaded Halloysite Coatings for Antimicrobial Food Packaging Applications. Food Packaging and Shelf Life 2019, 20, 100300. [Google Scholar] [CrossRef]
- Saleh, T.A. Chapter 3 - Kinetic Models and Thermodynamics of Adsorption Processes: Classification. In Interface Science and Technology; Saleh, T.A., Ed.; Surface Science of Adsorbents and Nanoadsorbents; Elsevier, 2022; Vol. 34, pp. 65–97.
- Frenkel, J. Theorie der Adsorption und verwandter Erscheinungen. Z. Physik 1924, 26, 117–138. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Zeitschrift für Physikalische Chemie 1889, 4U, 226–248. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Zeitschrift für Physikalische Chemie 1889, 4U, 96–116. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Baikousi, M.; Karabagias, V.K.; Karageorgou, I.; Iordanidis, G.; Emmanouil-Konstantinos, C.; Leontiou, A.; Karydis-Messinis, A.; Zafeiropoulos, N.E.; Kehayias, G.; et al. Low-Density Polyethylene-Based Novel Active Packaging Film for Food Shelf-Life Extension via Thyme-Oil Control Release from SBA-15 Nanocarrier. Nanomaterials 2024, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A Distillation Method for the Quantitative Determination of Malonaldehyde in Rancid Foods. J Am Oil Chem Soc 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Clark, E.M.; Mahoney, A.W.; Carpenter, C.E. Heme and Total Iron in Ready-to-Eat Chicken. J. Agric. Food Chem. 1997, 45, 124–126. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; De, G.C.R. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. American Journal of Analytical Chemistry 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Elahi, M.G.; Hekmati, M.; Esmaeili, D.; Ziarati, P.; Yousefi, M. Evaluation and Efficacy Modified Carvacrol and Anti-Cancer Peptide Against Cell Line Gastric AGS. Int J Pept Res Ther 2022, 28, 125. [Google Scholar] [CrossRef]
- Elaiopoulos, K.; Perraki, Th.; Grigoropoulou, E. Monitoring the Effect of Hydrothermal Treatments on the Structure of a Natural Zeolite through a Combined XRD, FTIR, XRF, SEM and N2-Porosimetry Analysis. Microporous and Mesoporous Materials 2010, 134, 29–43. [Google Scholar] [CrossRef]
- Olegario, E.M.; Mark Pelicano, C.; Cosiñero, H.S.; Sayson, L.V.; Chanlek, N.; Nakajima, H.; Santos, G.N. Facile Synthesis and Electrochemical Characterization of Novel Metal Oxide/Philippine Natural Zeolite (MOPNZ) Nanocomposites. Materials Letters 2021, 294, 129799. [Google Scholar] [CrossRef]
- Espuche, E. Nanocomposites for Gas Barrier Applications: Governing Factors, Single and Coupled Effects, New Routes to Optimise the Function Properties. Polymer Testing 2023, 125, 108124. [Google Scholar] [CrossRef]
- Arunvisut, S.; Phummanee, S.; Somwangthanaroj, A. Effect of Clay on Mechanical and Gas Barrier Properties of Blown Film LDPE/Clay Nanocomposites. Journal of Applied Polymer Science 2007, 106, 2210–2217. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Rapid Detection of Total Viable Count (TVC) in Pork Meat by Hyperspectral Imaging. Food Research International 2013, 54, 821–828. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, Y.; Wang, W. A Nondestructive Real-Time Detection Method of Total Viable Count in Pork by Hyperspectral Imaging Technique. Applied Sciences 2017, 7, 213. [Google Scholar] [CrossRef]
- Stewart, G.S.A.B. Micro-Organisms in Food—2. Sampling for Microbiological Analysis: Principles and Specific Applications: ICMSF, Blackwell Scientific Publications, Oxford, 1986. 310 Pp. Price: £19·50 (Cloth). Meat Science 1987, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Wenjiao, F.; Yongkui, Z.; Yunchuan, C.; Junxiu, S.; Yuwen, Y. TBARS Predictive Models of Pork Sausages Stored at Different Temperatures. Meat Science 2014, 96, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H. de A.; Silva, E.N. da; Nascimento, M.R.L. do; Fukuma, H.T. Evaluation of the 2-Thiobarbituric Acid Method for the Measurement of Lipid Oxidation in Mechanically Deboned Gamma Irradiated Chicken Meat. Food Chemistry 2003, 80, 433–437. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Leontiou, A.; Karabagias, I.K.; Georgopoulos, S.; Karydis-Messinis, A.; Zaharioudakis, K.; Andritsos, N.; Kehayias, G.; et al. Thymol@activated Carbon Nanohybrid for Low-Density Polyethylene-Based Active Packaging Films for Pork Fillets’ Shelf-Life Extension. Foods 2023, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Schematic presentation of handmade apparatus used for the preparation of CV@NZ nanohybrid (1) stirrer with heating plate, (2) spherical glass flask, (3) security valve of pump, (4) security valve of CV tank, (5) air vacuum pump and (6) CV tank.
Figure 1.
Schematic presentation of handmade apparatus used for the preparation of CV@NZ nanohybrid (1) stirrer with heating plate, (2) spherical glass flask, (3) security valve of pump, (4) security valve of CV tank, (5) air vacuum pump and (6) CV tank.
Figure 2.
(a) XRD plots of (1) pure NZ and (2) CV@NZ nanohybrid, and (b) FTIR plots of (1) CV as received, (2) pure NZ and (3) CV@NZ nanohybrid.
Figure 2.
(a) XRD plots of (1) pure NZ and (2) CV@NZ nanohybrid, and (b) FTIR plots of (1) CV as received, (2) pure NZ and (3) CV@NZ nanohybrid.
Figure 3.
CV desorption isotherm kinetic plots from CV@NZ nanohybrid at (a) 223 oK, (b) 243 oK, (c) 263 oK and (d) 283 oK. With a red line plot the simulation plots according to the pseudo second-order kinetic model.
Figure 3.
CV desorption isotherm kinetic plots from CV@NZ nanohybrid at (a) 223 oK, (b) 243 oK, (c) 263 oK and (d) 283 oK. With a red line plot the simulation plots according to the pseudo second-order kinetic model.
Figure 4.
Plot of ln(1/k2) values as a function of (1/T).
Figure 4.
Plot of ln(1/k2) values as a function of (1/T).
Figure 5.
(a) XRD and (b) FTIR plots of films (1) PLA/TEC, (2) PLA/TEC/5NZ, (3) PLA/TEC/10NZ, (4) PLA/TEC/15NZ, (5) PLA/TEC/5CV@NZ, (6) PLA/TEC/10CV@NZ, and (7) PLA/TEC/15CV@NZ.
Figure 5.
(a) XRD and (b) FTIR plots of films (1) PLA/TEC, (2) PLA/TEC/5NZ, (3) PLA/TEC/10NZ, (4) PLA/TEC/15NZ, (5) PLA/TEC/5CV@NZ, (6) PLA/TEC/10CV@NZ, and (7) PLA/TEC/15CV@NZ.
Figure 6.
SEM images of surface (left column) and cross-section (right column) for polymer matrix film of PLA/TEC (a,b), nanocomposite films of PLA/TEC/5NZ (c,d), PLA/TEC/5CV@NZ (e,f), PLA/TEC/10NZ (g,h), PLA/TEC/10CV@NZ (i,j), PLA/TEC/15NZ (k,l), and PLA/TEC/15CV@NZ (m,n).
Figure 6.
SEM images of surface (left column) and cross-section (right column) for polymer matrix film of PLA/TEC (a,b), nanocomposite films of PLA/TEC/5NZ (c,d), PLA/TEC/5CV@NZ (e,f), PLA/TEC/10NZ (g,h), PLA/TEC/10CV@NZ (i,j), PLA/TEC/15NZ (k,l), and PLA/TEC/15CV@NZ (m,n).
Figure 7.
Stress-strain curves of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film. (1) PLA/TEC, (2) PLA/TEC/5NZ, (3) PLA/TEC/10NZ, (4) PLA/TEC/15NZ, (5) PLA/TEC/5CV@NZ, (6) PLA/TEC/10CV@NZ, and (7) PLA/TEC/15CV@NZ.
Figure 7.
Stress-strain curves of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film. (1) PLA/TEC, (2) PLA/TEC/5NZ, (3) PLA/TEC/10NZ, (4) PLA/TEC/15NZ, (5) PLA/TEC/5CV@NZ, (6) PLA/TEC/10CV@NZ, and (7) PLA/TEC/15CV@NZ.
Figure 8.
Storage modulus plots as a function of temperature of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film.
Figure 8.
Storage modulus plots as a function of temperature of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film.
Figure 9.
Plot line diagram of mean calculated water vapor diffusion coefficient (Dwv) values and mean calculated oxygen permeability (PO2) values of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film.
Figure 9.
Plot line diagram of mean calculated water vapor diffusion coefficient (Dwv) values and mean calculated oxygen permeability (PO2) values of all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film.
Figure 10.
Column bar plots of (a) TVC values (b) TBARS values and (c) heme iron content values of minced pork wrapped with the commercial film (control), PLA/TEC film, and PLA/TEC/10CV@NZ film as a function of storage time.
Figure 10.
Column bar plots of (a) TVC values (b) TBARS values and (c) heme iron content values of minced pork wrapped with the commercial film (control), PLA/TEC film, and PLA/TEC/10CV@NZ film as a function of storage time.
Table 1.
Sample names, contents of PLA, TEC, pure NZ and CV@NZ nanohybrid and twin extruder operation conditions (temperature, speed) used for the development of all PLA/TECx composite blends.
Table 1.
Sample names, contents of PLA, TEC, pure NZ and CV@NZ nanohybrid and twin extruder operation conditions (temperature, speed) used for the development of all PLA/TECx composite blends.
| Sample name |
PLA
(g) |
TEC
(ml-%v/w) |
NZ
(g-%w/w) |
Twin extruder operated conditions |
| T (oC) |
speed (rpm) |
Time (min) |
| PLA/TEC |
4 |
0.6-15 |
- |
180 |
120 |
5 |
| PLA/TEC/5NZ |
4 |
0.6-15 |
0.2-5 |
180 |
120 |
5 |
| PLA/TEC/10NZ |
4 |
0.6-15 |
0.4-10 |
180 |
120 |
5 |
| PLA/TEC/15NZ |
4 |
0.6-15 |
0.6-15 |
180 |
120 |
5 |
| PLA/TEC/5CV@NZ |
4 |
0.6-15 |
0.2-5 |
180 |
120 |
5 |
| PLA/TEC/10CV@NZ |
4 |
0.6-15 |
0.4-10 |
180 |
120 |
5 |
| PLA/TEC/15CV@NZ |
4 |
0.6-15 |
0.6-15 |
180 |
120 |
5 |
Table 2.
Calculated mean post-simulation values for %wt. CV content release, pseudo-second order constant k2, desorption capacity at equilibrium qe and R2 at 223 oK, 243 oK, 263 oK and 283 oK.
Table 2.
Calculated mean post-simulation values for %wt. CV content release, pseudo-second order constant k2, desorption capacity at equilibrium qe and R2 at 223 oK, 243 oK, 263 oK and 283 oK.
| temperature |
%wt. CV desorbed |
k2 (s-1) |
qe (mg/g) |
R2
|
| 223 oK |
56.7±0.6 |
5.7.10-6±0.2310-6
|
1.5±0.26 |
0.994±0.002 |
| 243 oK |
60.5±1.8 |
4.98.10-5±0.31.10-5
|
1.16±0.24 |
0.989±0.008 |
| 263 oK |
60.7±0.6 |
4.39.10-40.23.10-4
|
0.92±0.07 |
0.992±0.003 |
| 283 oK |
61.7±0.23 |
9.21.10-4±0.13.10-4
|
0.99±0.13 |
0.995±0.001 |
Table 3.
Calculated values of Elastic Modulus (E), the ultimate strength (σuts) and the % elongation at break (%ε) for all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film.
Table 3.
Calculated values of Elastic Modulus (E), the ultimate strength (σuts) and the % elongation at break (%ε) for all obtained PLA/TEC/xNZ and PLA/TEC/xCV@NZ films as well as pure PLA/TEC film.
| Sample name |
Ε(MPa) |
σuts
|
%ε |
| PLA/TEC |
564.1±93.7a
|
8.0±1.9a
|
436.3±285.6a
|
| PLA/TEC/5NZ |
760.33±116.4a
|
13.6±1.4b
|
202.7±143.8a
|
| PLA/TEC/10NZ |
1536.00±852.3b,a
|
24.0±8.7c
|
247.8±114.6a
|
| PLA/TEC/15NZ |
2144.25±662.5b
|
49.7±4.1d
|
216.2±54.1a
|
| PLA/TEC/5CV@NZ |
1093.33±313.7b
|
22.5±5.8c
|
567.4±252.3b,a
|
| PLA/TEC/10CV@NZ |
404.50±282.1c,a
|
10.3±2.1a,b
|
724.6±160.0b,a
|
| PLA/TEC/15CV@NZ |
33.00±9.5d
|
1.6±0.4e
|
993.7±61.5c
|
Table 4.
Calculated mean values of the desorption rate constant mean values (k2), the CV desorption equilibrium constant (qe), the CV release rate (RRCV) as well as the %wt. CV released content (%RCCV) for all PLA/TEC/xCV@NZ films.
Table 4.
Calculated mean values of the desorption rate constant mean values (k2), the CV desorption equilibrium constant (qe), the CV release rate (RRCV) as well as the %wt. CV released content (%RCCV) for all PLA/TEC/xCV@NZ films.
| |
k2 (s-1) |
qe (μg/g) |
%RCcv
|
RRcv (μg/s) |
| PLA/TEC/5CV@NZ |
1.65±1.05 |
0.0168±0.0031 |
1.74±0.44 |
0.0082±0.0026 |
| PLA/TEC/10CV@NZ |
0.0178±0.0081 |
0.0471±0.0076 |
4.20±0.43 |
0.0049±0.0006 |
| PLA/TEC/15CV@NZ |
0.0095±0.0038 |
0.0611±0.0142 |
6.17±0.42 |
0.0039±0.0003 |
Table 5.
Calculated EC50 mean values for all PLA/TEC/xCV@NZ active films.
Table 5.
Calculated EC50 mean values for all PLA/TEC/xCV@NZ active films.
| Sample |
EC50
|
| PLA/TEC/5CV@NZ |
4.65 ± 1.99a
|
| PLA/TEC/10CV@NZ |
2.18 ± 1.15 a
|
| PLA/TEC/15CV@NZ |
2.85 ± 2.22 a
|
Table 6.
Antibacterial activity results of all PLA/TEC/xCV@NZ films against S. aureus and S. Typhimurium.
Table 6.
Antibacterial activity results of all PLA/TEC/xCV@NZ films against S. aureus and S. Typhimurium.
| |
S. aureus |
S. Typhimurium |
| Sample |
No. of 6 replicates with growth in the contact area of the sample |
Inhibition zone range (mm) |
No. of 6 replicates with growth in the contact area of the sample |
Inhibition zone range (mm) |
| PLA/TEC/5CV@NZ |
0/6a
|
0.23-0.36* |
0/6a
|
0.17-0.24** |
| PLA/TEC/10CV@NZ |
0/6a
|
0.27-0.42* |
0/6a
|
0.16-0.21** |
| PLA/TEC/15CV@NZ |
0/6a
|
0.25-0.44*** |
0/6a
|
0.18-0.23* |
Table 7.
Odor, color, and texture scores of wrapped pork fillets during storage at 4 ± 1◦C.
Table 7.
Odor, color, and texture scores of wrapped pork fillets during storage at 4 ± 1◦C.
| Sample name |
0 day |
2nd day |
4th day |
6th day |
8th day |
10th day |
| odor |
| CONTROL |
5.00±0.00a
|
4.45±0.10b
|
3.90±0.20d
|
3.20±0.10f
|
2.85±0.10i
|
2.50±0.10m
|
| PLA/TEC |
- |
4.55±0.10b
|
4.25±0.10e
|
3.60±0.20g
|
3.25±0.18j
|
2.80±0.20l
|
| PLA/TEC/10CV@NZ |
- |
4.70±0.05b.c
|
4.50±0.15e
|
4.05±0.16h
|
3.80±0.10k
|
3.20±0.16n
|
| color |
| CONTROL |
5.00±0.00a
|
4.30±0.20b
|
3.98±0.29d
|
2.85±0.15e
|
2.70±0.15h
|
2.55±0.12j
|
| PLA/TEC |
- |
4.50±0.10b
|
4.35±0.27d
|
3.45±0.16f
|
2.85±0.11h
|
2.70±0.16j
|
| PLA/TEC/10CV@NZ |
- |
4.75±0.05c
|
4.45±0.27d
|
4.02±0.19g
|
3.70±0.12i
|
3.50±0.15k
|
| texture |
| CONTROL |
5.00±0.00a
|
4.48±0.29b
|
3.80±0.20c
|
3.32±0.11e
|
2.84±0.08g
|
2.54±0.10k
|
| PLA/TEC |
- |
4.40±0.20b
|
4.22±0.15d
|
3.70±0.10f
|
3.22±0.18h
|
2.75±0.10l
|
| PLA/TEC/10CV@NZ |
- |
4.56±0.14b
|
4.32±0.11d
|
3.94±0.18f
|
3.60±0.10i
|
3.04±0.12m
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).