1. Introduction
Liver cancer is the fourth leading cause of cancer-related death worldwide.[
1] Traditional risk factors for hepatocellular carcinoma (HCC), the most common type of liver cancer, include hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, excessive alcohol consumption, and aflatoxin B1 (AFB1) exposure.[
2] In Mexico, HBV and HCV seroprevalence is low and the proportion of the population reporting excessive alcohol consumption is moderate.[
3,
4,
5] Recently, AFB1 exposure was shown to be highly prevalent (>90% detection) at moderate levels of exposure[
6]. Finally, in Latin America, there has been an increase in HCC cases related to metabolic dysfunction-associated fatty liver disease (MAFLD).[
7]
HCC in Mexico has a unique epidemiological pattern because HCC mortality affects women and men in a 1:1 male-to-female ratio, as opposed to the global 2.7:1 ratio. [
8,
9,
10] Also, a higher disease burden has been observed in rural areas.[
8] There is limited information regarding the distribution of risk factors and clinical characteristics of women and men in Mexico. Thus, this study explored the sex differences in the risk factors and clinical characteristics of men and women diagnosed with HCC in Mexico.
2. Materials and Methods
Mexican Interdisciplinary Network for Hepatocellular Cancer Research (RIMICH)
In November 2021, a multidisciplinary and multi-institutional consortium to study hepatocellular cancer (RIMICH, Red Interdisciplinaria Mexicana para la Investigación en Cáncer Hepatocelular) was established. This collaboration convenes various specialists, including gastroenterologists, hepatologists, radiologists, epidemiologists, public health personnel, surgical oncologists, transplant surgeons, hepatopancreatobiliary surgeons, and pathologists across the country. The Research, Ethics, and Biosecurity Committees at the National Institute of Public Health (INSP), National Cancer Institute (INCan), Naval Medical Center (CEMENAV), Mexican Institute of Social Security (IMSS), and the Institute of Social Security of the State of Mexico and its Municipalities (ISSEMYM) evaluated and approved the study protocol (INSP Study Protocol 1700).
Medical Record Review and Data Extraction
In November 2021, an invitation was made to members of RIMICH and members of the Mexican Hepato-Pancreato-Biliary Association, Mexican Hepatology Association, and Mexican Gastroenterology Association. Physicians from 12 different centers responded to the survey. Demographic and clinical information from medical records was extracted from patients diagnosed with HCC initially treated at participating institutions between 2015 and 2022. Study data were collected and managed using REDCap electronic data capture tools hosted at the Asociación Mexicana Hepatopancreatobiliar (Redcap).[
11] Data were centrally reviewed (JMR, LSR, and AM), and inconsistencies were resolved with treating facility personnel. Age at diagnosis in years was categorized (˂50, 50-59, 60-69, ≥70 years). Participants were categorized according to their sex at birth as female or male. The state of residence was extracted and participants were categorized according to eight regions in Mexico: Northwest (Sonora, Sinaloa, and Durango), northeast (Coahuila, Nuevo León, and Tamaulipas), west (Michoacán and Jalisco), east (Veracruz, Puebla, Tlaxcala, and Hidalgo), center-north (Zacatecas, San Luis Potosí, Querétaro, and Guanajuato), central-south (Mexico City, State of Mexico, and Morelos), southwest (Guerrero, Oaxaca, and Chiapas), and southeast (Yucatán, Campeche, Quintana Roo, and Tabasco). Height and weight were measured at diagnosis. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2) and categorized as overweight/obese when BMI was ≥25 kg/m2. Participants with a clinical diagnosis of cirrhosis were identified along with the etiology presumed by the treating physician [alcohol, HBV virus, HCV virus, MAFLD, autoimmune hepatitis, other etiologies, or unknown]. The liver function Child-Pugh score and clinical stage using the Barcelona Clinic Liver Cancer (BCLC) staging system were also extracted.[
12,
13,
14] Patients were categorized according to alpha-fetoprotein (AFP) levels of <20, 20-399, and ≥400 ng/mL, representing normal, moderately elevated, and markedly elevated AFP levels, respectively.[
15] The tumor types [ a) classic types: trabecular, trabecular acinar, acinar, pseudoglandular; b) fibrolamellar; c) clear cell; d) other; and e) undetermined] and tumor differentiation (well, moderate, poor to undifferentiated, unspecified) were obtained from histopathology reports. Data on imaging reports included information regarding the number of nodules (1, 2-3, ≥4, unspecified), size of the largest nodule, presence of a nodule >5 cm, metastases, and/or extrahepatic disease. Information on the different treatment modalities was also obtained and categorized as a) curative/response-intent: ablation, surgery, TAE/TACE, other/combined, b) palliative or systemic therapy, and c) no treatment.
Statistical Analyses
Continuous and categorical variables were summarized as mean ± standard deviation (SD) or median (quartile 1-quartile 4 range) and percentages. The male-to-female ratio was calculated at national and regional levels. In addition, all results were stratified by sex. The percentage of missing information for all analyzed characteristics was reported. Statistical differences were not tested for, and rather explored to determine whether the magnitude of the observed difference was clinically meaningful.[
16] Data management and analyses were performed using the Statistical Analysis Systems statistical software package (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
Data were obtained for 784 HCC patients, and 87 patients were excluded because their diagnosis was made prior to 2015. Of the remaining 697 patients, the mean (±SD) age at diagnosis was 65.4 (±11.9) and 20% were diagnosed at ≥75 years of age (Mexico’s life expectancy).[
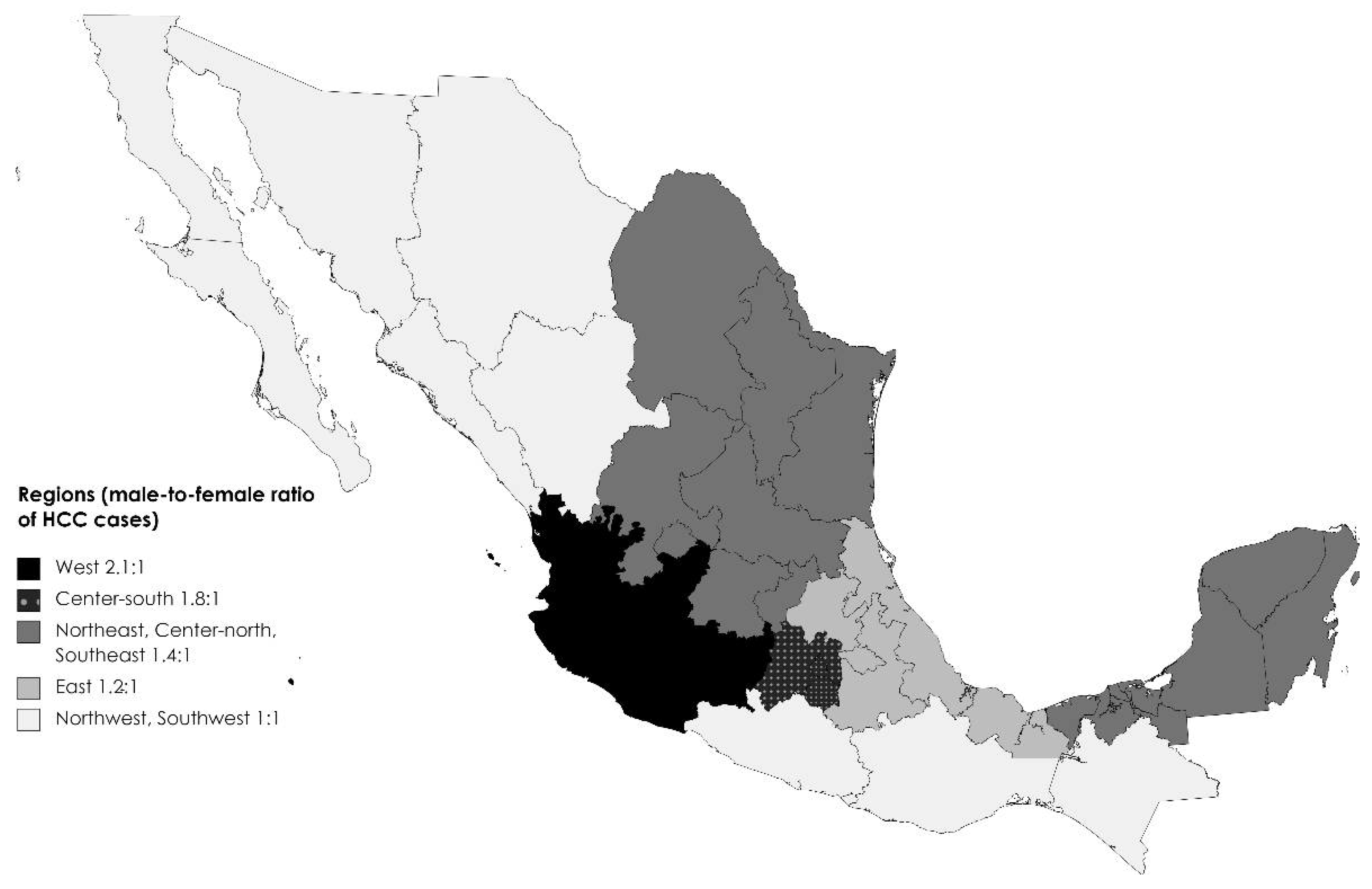
17] Male participants was 57.8% (n=403), and the male-to-female ratio was 1.4:1. However, this ratio differed across regions, from 1:1 in the northwest and southwest to 2.1:1 in the west (
Figure 1).
Age at diagnosis was slightly higher in women than in men (>60 years: 78% women vs. 73% men;
Table 1). While the proportion of cirrhosis was similar across sexes (overall 67%), the proportion of women with an unknown etiology for cirrhosis was much higher than that of men (51.1% vs. 20.7%). In addition, HCV and MAFLD levels were much higher in women than in men, while almost 60% of cirrhosis in men was attributed to alcohol consumption. However, information on cirrhosis etiology was missing for 35% of the patients. Men had a higher proportion of advanced HCC (BCLC: C-D) than women. Liver dysfunction (Child-Pugh B/C), AFP levels at diagnosis, and tumor types were similar between men and women. Approximately 60% of the patients were biopsied to complete the diagnostic process, and more than 70% received some type of treatment.
Men had a higher proportion of poor/undifferentiated HCC histologic grade tumors (women: 2.2% vs. men: 16.5%) (
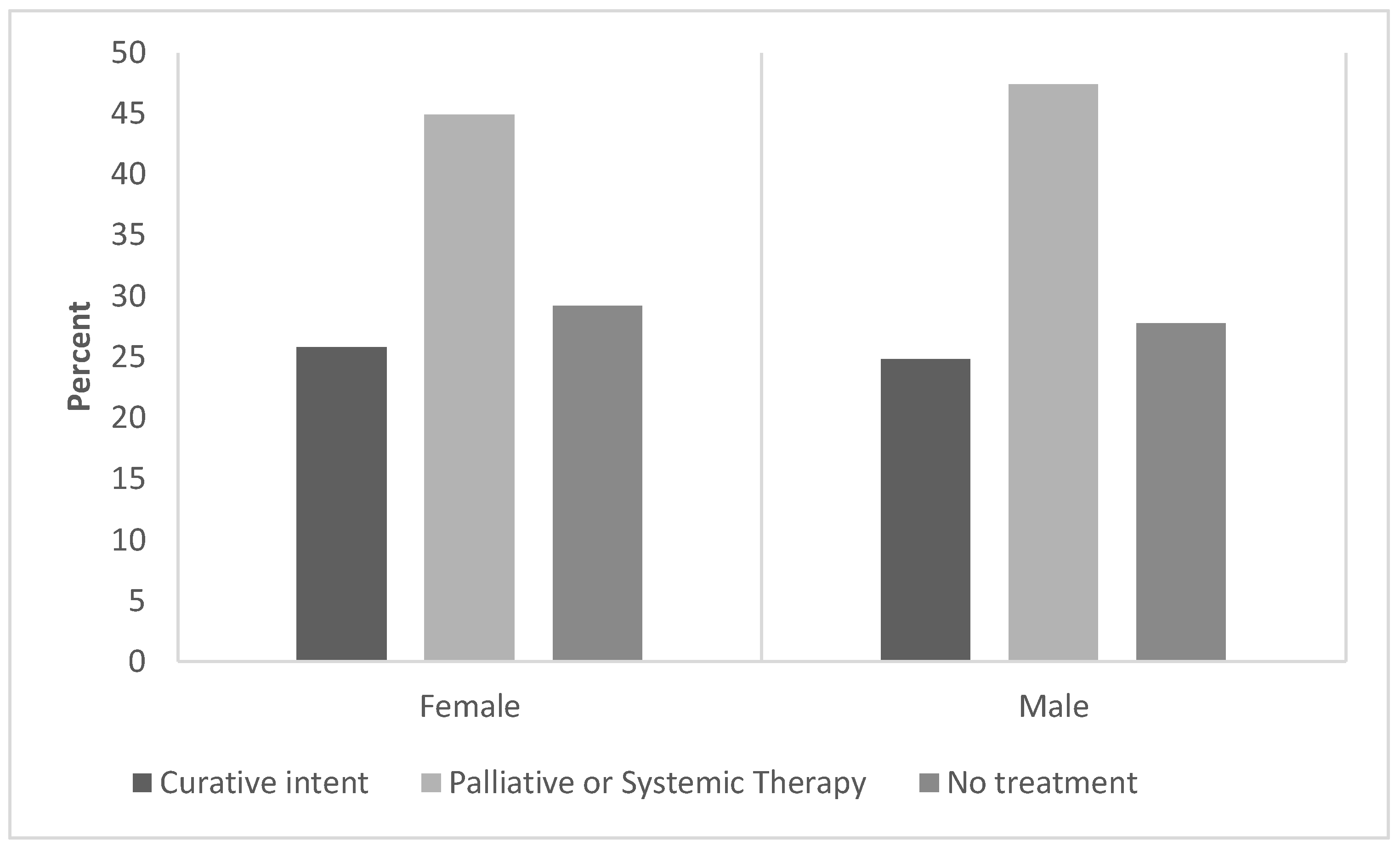
Table 2). Interestingly, the incidence of multinodular disease (≥4 nodules) was twice as high in men than in women (women: 7.5% vs. men: 14.9%). However, the frequency of metastases and/or extrahepatic disease at diagnosis was similar between women and men. The type of treatment was similar across sexes (
Figure 2). We found that curative/response-intended care was sought by 26% of female HCC patients and 25% of male HCC patients. Approximately 50% of patients with HCC (both women and men) received systemic therapy or palliative care only.
4. Discussion
In this large series study of hepatocellular carcinoma in Mexico, the male-to-female overall ratio was 1.4:1, with several important clinical differences across sex. The numerous clinical differences might explain the unique epidemiological pattern of hepatocellular carcinoma in Mexico (male-to-female ratio, 1.4:1). Globally, HCC affects men more than women.[
18] In contrast to a 2.7:1 world-average male-to-female ratio, in our study, the ratio ranged from 1:1 to 2.1:1, which is consistent with previous reports on liver cancer mortality in Mexico.[
8,
9,
10] This is significantly different from the ratio in high-income countries (2.8:1 on average).[
18]
The reason why HCC affects men and women across the country in such a different ratio might be explained by the differences in the prevalence of risk factors in women compared to men.
A large HCC series in Brazil found that among approximately 1,400 patients, liver cirrhosis was the most common risk factor for HCC (98% cirrhosis), mainly due to HCV.[
19] Similarly, another South American study found that liver cirrhosis, mainly HCV, was the most common risk factor (85% cirrhosis).[
20] In our study, cirrhosis was an important risk factor for HCC (67%) in both women and men, mainly because of excessive alcohol consumption in men and cryptogenic cirrhosis in women. HBV and HCV seroprevalence is low in Mexico (HB surface antigens: 0.51%; anti-HCV antibodies: 0.38%), and the reported incidence is lower (0.28 and 1.06 per 100,000, respectively) [
21] than the global average.[
22] Our results show that viral-induced cirrhosis is a more common risk factor for HCC in women than in men in Mexico.
Alcohol cirrhosis was five times more prevalent in men than in women, which is consistent with alcohol consumption patterns published elsewhere.[
5] While excessive alcohol consumption in Mexico is a public health problem, its prevalence is lower than that in high-income countries such as the USA (18 vs. 29%, respectively), and differences between men and women are more pronounced (Mexico men: 30% vs. women: 6.2%; USA men: 44.7% vs. women: 13.1%).[
5]
In our study, MAFLD cirrhosis was twice as prevalent in women than in men. This is consistent with the higher prevalence of overweight/obesity among women in both our study and the national data (ENSANUT overweight/obesity 75.0% women; 69.6% men).[
23] Other countries have shown sex differences in MAFLD prevalence, but in contrast to our findings, in these countries, men had the highest prevalence.[
24]
The tumor size was similar across the sexes. According to histological reports, women and men mainly have one nodule.[
19] Other important characteristics differed between the women and men. Women were less likely to be diagnosed at advanced BCLC stages, have poorly undifferentiated tumors, and have ≥4 nodules.[
25] The most common therapies for HCC were similar across sexes; most patients had palliative care/systemic therapy or no treatment. These data suggest that the diagnosis of HCC still frequently occurs at advanced stages of the disease in Mexico, where curative-intent therapy is no longer an option, particularly among men.
Our study has several strengths, to our knowledge this is the first study focusing on sex differences. It is also the largest study to date in Mexico and one of the largest studies in Latin America. It also includes the participation of 12 different institutions from different health providers available in Mexico covering a 7 year-period. However, it is a convenience sample in which only patients who seek and receive treatment have medical records. As this was a retrospective study, we were limited to the information available in the medical records. For example, in some hospitals in the country, when patients were referred to cancer referral centers, imaging studies were not repeated because of other clinical characteristics of the disease, and we had missing information on several sections of the questionnaire, potentially affecting tumor staging accuracy. Since this study was based on medical records, access to healthcare and information on the willingness to search for healthcare were not available. Although this is the largest HCC series in Mexico, the regional differences could be explained by the smaller sample size after stratification. However, on average, this study’s male-to-female ratio was 1.4:1, compared to the global ratio of 2.7:1. Additionally, not all cancer centers participated in the study; therefore, this information may not be applicable to all centers in the country, even when the major cancer referral centers participated and most states in the country were represented (
Figure 1).
5. Conclusions
HCC in this population affects men and women at a 1.4:1 male to female ratio. Mexico’s lower male-to-female ratio could be explained by sex differences in the prevalence of HCC risk factors. Men present with more advanced disease and worse prognosis. Further research is needed to explore other factors contributing to the observed sex ratios and to develop early detection strategies for HCC in the Mexican population.
Author Contributions
Conceptualization, JMR, DMV, LSR, AM and ML; Methodology, AM and ML; Software, JMR, LSR, AM; Validation, AM, DZV; Formal analysis, AM; Investigation, DZV, LSR, AM and ML; Resources, JMR, LSR, BOMO, DSG, JAVRV, CFT, ARM, LCG, GMM, ERG, MPLL,MSGH, RAC, JUR, JMGC, JS, SRR, PG, LCM, JMRT, KGOE, YALH, RGG, DZV, AM, ML; Data curation, JMR; Writing - original draft, JMR, LSR, AM; Writing- Reviewing and Editing, JMR, LSR, BOMO, DSG, JAVRV, CFT, ARM, LCG, GMM, ERG, MPLL,MSGH, RAC, JUR, JMGC, JS, SRR, PG, LCM, JMRT, KGOE, YALH, RGG, DZV, AM, ML; Supervision, DZV; Project administration, AM and JMR; Funding acquisition, ML. All the authors have read and agreed to the published version of the manuscript.
Funding
Research reported in This study was supported by the Fogarty International Center of the National Institutes of Health (award number 1R21TW011720-01). The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The Research, Ethics, and Biosecurity Committees at the National Institute of Public Health (INSP), National Cancer Institute (INCan), Naval Medical Center (CEMENAV), Mexican Institute of Social Security (IMSS), and the Institute of Social Security of the State of Mexico and its Municipalities (ISSEMYM) evaluated and approved the study protocol (INSP Study Protocol 1700, approved may 13, 2021).
Informed Consent Statement
Patient consent was waived for the extraction of unidentifiable data from medical records.
Data Availability Statement
Data described in the manuscript, codebook, and analytic code will be made available upon reasonable request, pending application and approval.
Acknowledgments
We would like to thank Dr. Ángel Molina and Dr. César Godoy Valdez for retrospectively tracking patients in Chiapas.
Conflicts of Interest
David Suarez reports a relationship with Bayer Mexico that includes consulting or advisory. Javier Melchor-Ruan reported a relationship with F Hoffmann-La Roche Ltd. and AstraZeneca Pharmaceuticals LP, which includes speaking and lecture fees. Erika Ruiz-Garcia reported a relationship with Hoffmann-La Roche Ltd., which includes speaking and lecture fees. Ruiz-Garcia reports a relationship with AstraZeneca Pharmaceuticals LP, which includes consulting or advisory. Martin Lajous reports financial support provided by the National Institutes of Health Fogarty International Center. Martin Lajous reports a relationship with Roche that includes funding grants. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results. All authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349. e315. [Google Scholar] [CrossRef]
- El, H. Serag,“Hepatocellular carcinoma,”. N Engl J Med 2011, 365, 1118–1127. [Google Scholar]
- Carnalla, M.; Vidaña-Pérez, D.; Alpuche-Aranda, C.; Chávez-Tapia, N.C.; Romero-Martínez, M.; Shamah-Levy, T.; Barrientos-Gutiérrez, T. Hepatitis B infection in Mexican adults: Results of a nationally representative survey. Annals of Hepatology 2022, 27, 100583. [Google Scholar] [CrossRef]
- Carnalla, M.; Barrientos-Gutiérrez, T.; Vidaña-Perez, D.; Romero-Martínez, M.; Martínez-Bohorquez, M.C.; González-Pier, E.; Fagundo-Sierra, R.; Kershenobich, D.; Alpuche-Aranda, C.; Lazcano-Ponce, E.; et al. Prevalence of hepatitis C in the adult Mexican population: National Survey of Health and Nutrition 2018. The Lancet Regional Health - Americas 2022, 8, 100165. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. World Health Organization global health observatory. 2023.
- Monge, A.; Romero-Martínez, M.; Groopman, J.D.; McGlynn, K.A.; Santiago, L.; Villalpando-Hernández, S.; Mannan, R.; Burke, S.M.; Remes-Troche, J.M.; Lajous, M. Aflatoxin Exposure in Adults in Southern and Eastern Mexico in 2018: A Descriptive Study. Available at SSRN 439 9084. [CrossRef]
- Farah, M.; Anugwom, C.; Ferrer, J.D.; Baca, E.L.; Mattos, A.Z.; Possebon, J.P.P.; Arrese, M.; Prieto, J.; Balderramo, D.; Carrera, E.; et al. Changing epidemiology of hepatocellular carcinoma in South America: A report from the South American liver research network. Ann Hepatol 2023, 28, 100876. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.S.; Espinosa-Tamez, P.; López-Ridaura, R.; Lamadrid-Figueroa, H.; Melchor-Ruan, J.; McGlynn, K.A.; Lajous, M. Liver cancer mortality in Mexico: trend analysis from 1998 to 2018. salud pública de méxico 2022, 64, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Huezo, M.S.G.; Ávila, J.F.S.; de Gastroenterología, A.M.; de Radiología, S.M.; de Oncología, S.M.; de Carcinoma, G.M.d.C. Consenso mexicano de diagnóstico y manejo del carcinoma hepatocelular. Revista de Gastroenterología de México 2014, 79, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Leal, Y.A.; Torres, J.; Gamboa, R.; Mantilla-Morales, A.; Piña-Sanchez, P.; Arrieta, O.; Bonifaz, L.; Meneses, A.; Duque, C.; Piñeros, M. Cancer Incidence in Merida, Mexico 2015-2018: First Report from the Population-based Cancer Registry. Archives of Medical Research 2022, 53, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Child, C.G. Surgery and portal hypertension. The liver and portal hypertension. 1964, 50–52. [Google Scholar]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. In Proceedings of the Seminars in liver disease; 1999; pp. 329–338. [Google Scholar]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Tangkijvanich, P.; Anukulkarnkusol, N.; Suwangool, P.; Lertmaharit, S.; Hanvivatvong, O.; Kullavanijaya, P.; Poovorawan, Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol 2000, 31, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hayes-Larson, E.; Kezios, K.L.; Mooney, S.J.; Lovasi, G. Who is in this study, anyway? Guidelines for a useful Table 1. Journal of clinical epidemiology 2019, 114, 125–132. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Esperanza de vida al nacimiento por entidad federativa según sexo, serie anual de 2010 a 2022. Available online: https://www.inegi.org.mx/app/tabulados/interactivos/?pxq=Mortalidad_Mortalidad_09_61312f04-e039-4659-8095-0ce2cd284415 (accessed on 11 september).
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. Journal of Hepatology 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Carrilho, F.J.; Kikuchi, L.; Branco, F.; Goncalves, C.S.; de Mattos, A.A.; Group, B.H.S. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics 2010, 65, 1285–1290. [Google Scholar] [CrossRef]
- Fassio, E.; Díaz, S.; Santa, C.; Reig, M.E.; Artola, Y.M.; de Mattos, A.A.; Míguez, C.; Galizzi, J.; Zapata, R.; Ridruejo, E. Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Annals of hepatology 2010, 9, 63–69. [Google Scholar] [CrossRef] [PubMed]
- DGE. Dirección de Vigilancia Epidemiológica de Enfermedades No Transmisibles. Informe Anual de Vigilancia Epidemiológica de Hepatitis Virales, México 2020. Available online: https://www.gob.mx/cms/uploads/attachment/file/615926/HepatitisViralesInformeAnual2020.pdf (accessed on 11 september).
- Organization, W.H. Global hepatitis report 2017; World Health Organization: 2017.
- Shamah-Levy, T.; Romero-Martínez, M.; Barrientos-Gutiérrez, T.; Cuevas-Nasu, L.; Bautista-Arredondo, S.; Colchero, M.; Gaona-Pineda, E.; Lazcano-Ponce, E.; Martínez-Barnetche, J.; Alpuche-Arana, C. Encuesta nacional de salud y nutrición 2020 sobre Covid-19. Resultados nacionales. Cuernavaca, México: Instituto Nacional de Salud Pública 2021.
- Nagral, A.; Bangar, M.; Menezes, S.; Bhatia, S.; Butt, N.; Ghosh, J.; Manchanayake, J.H.; Al Mahtab, M.; Singh, S.P. Gender Differences in Nonalcoholic Fatty Liver Disease. Euroasian Journal of Hepato-Gastroenterology 2022, 12, S19. [Google Scholar] [PubMed]
- Wu, E.M.; Wong, L.L.; Hernandez, B.Y.; Ji, J.-F.; Jia, W.; Kwee, S.A.; Kalathil, S. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma research 2018, 4. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).