Submitted:
30 May 2024
Posted:
30 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
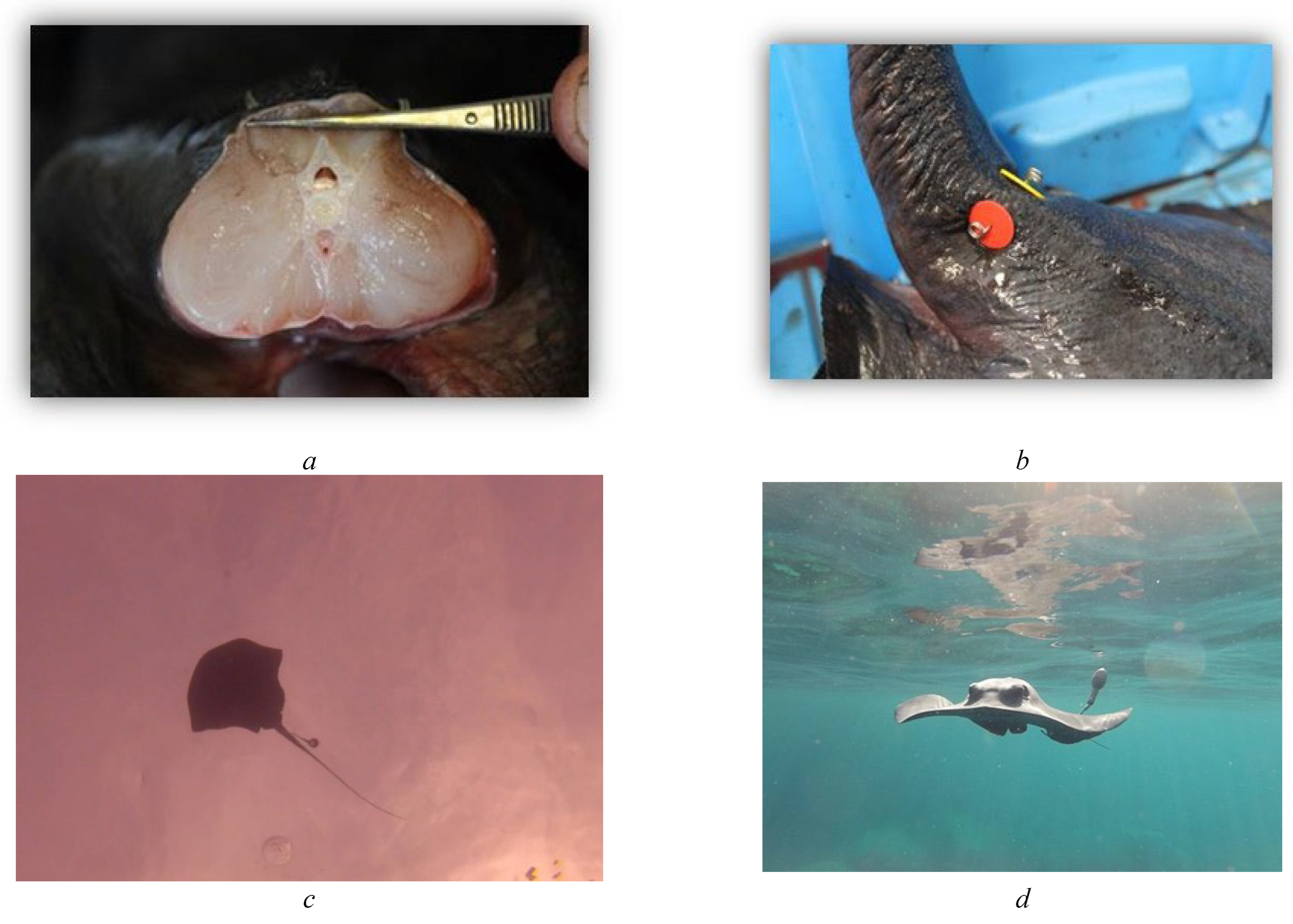
2.1. Tagging
2.2. Tag Attachment
2.3. Fishery Data
2.4. Data Analysis
3. Results
3.1. Tag Deployments
3.2. Movements
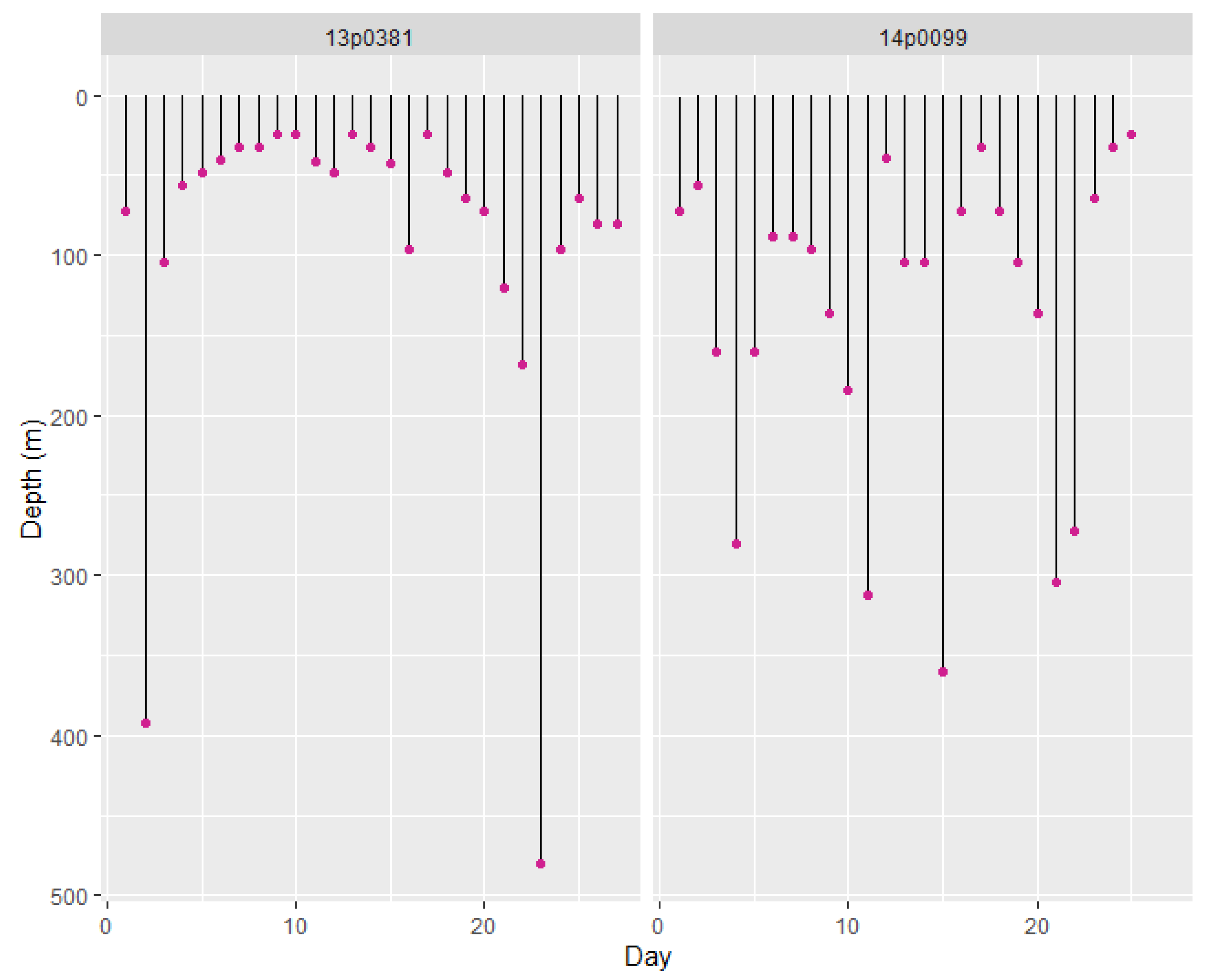
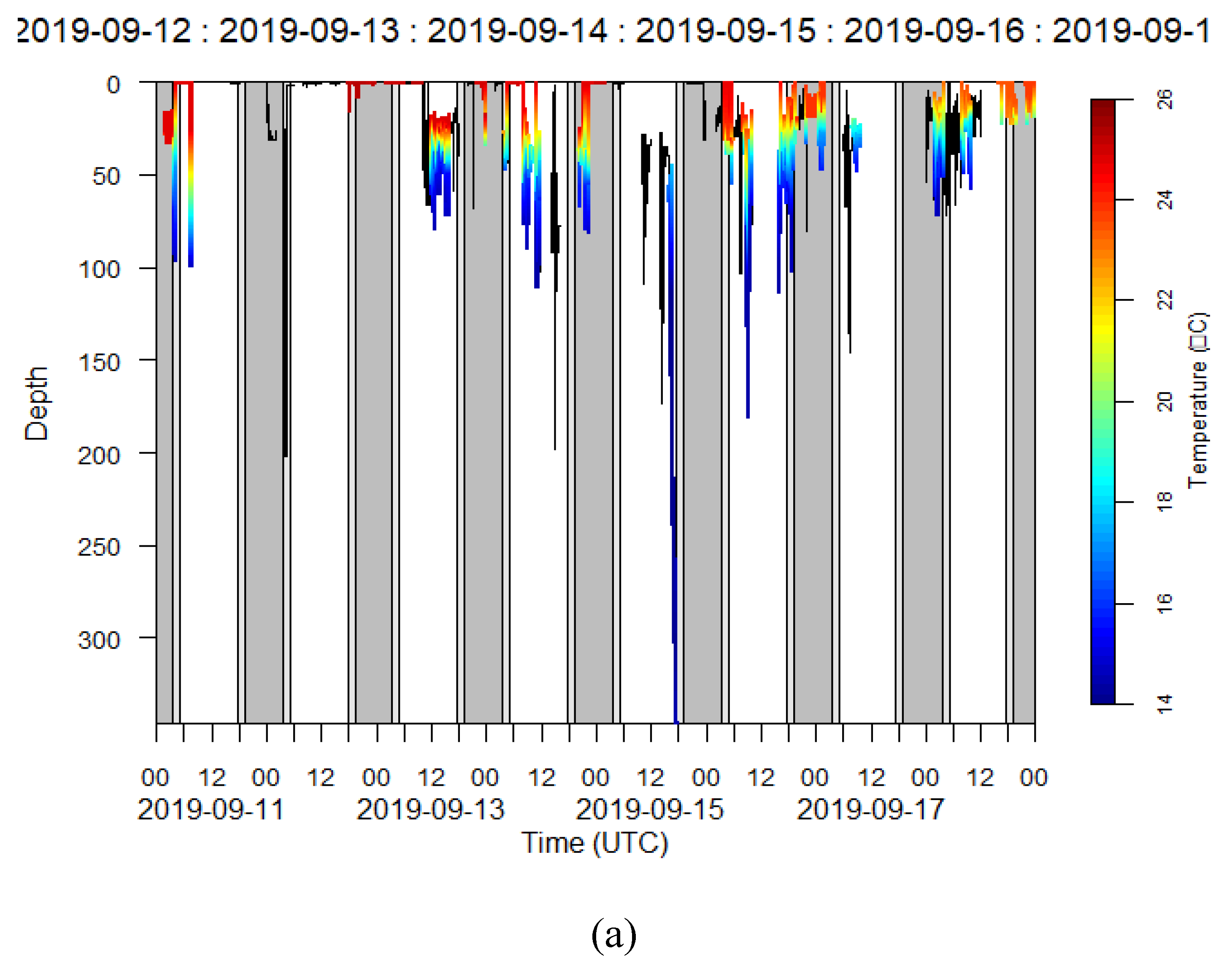
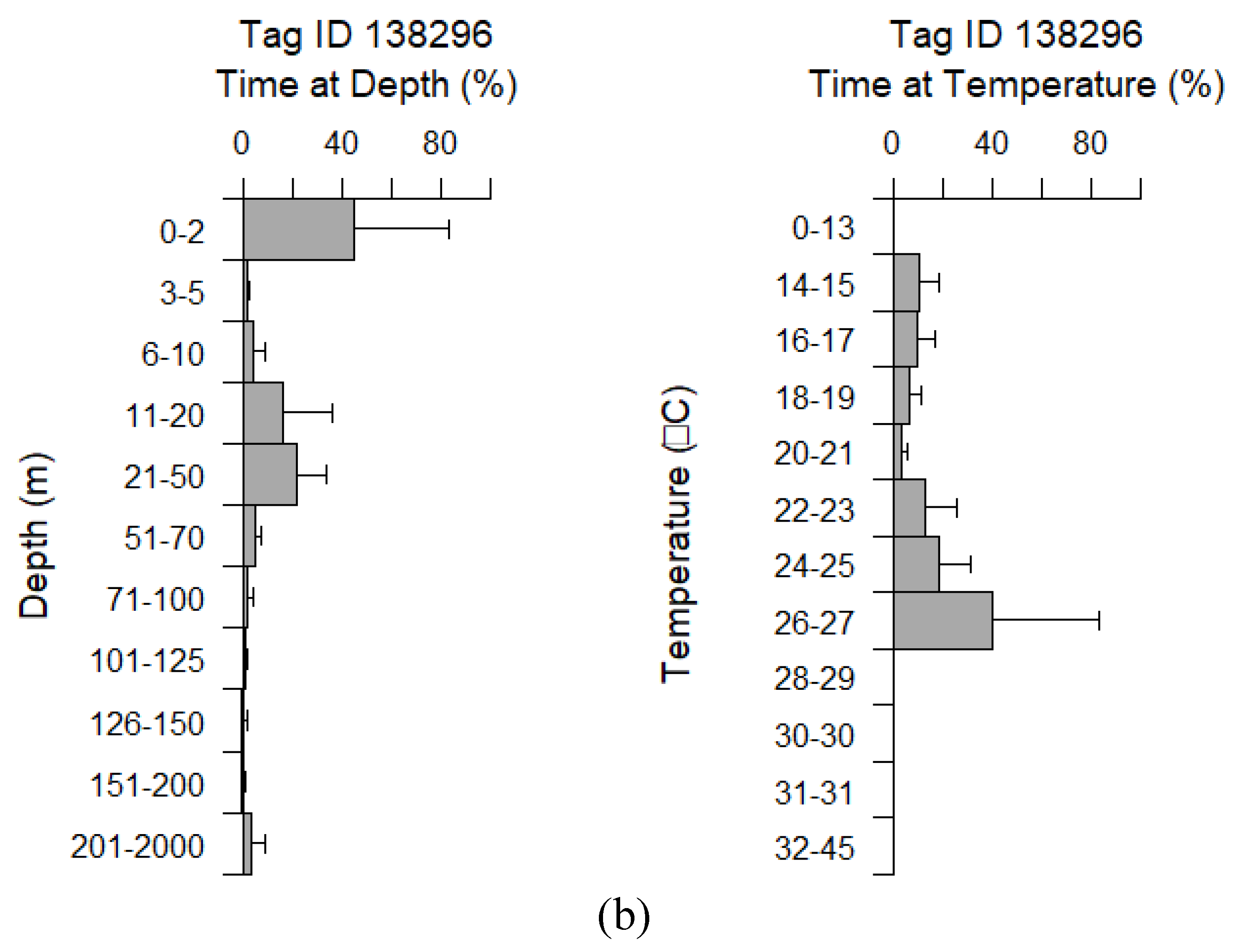
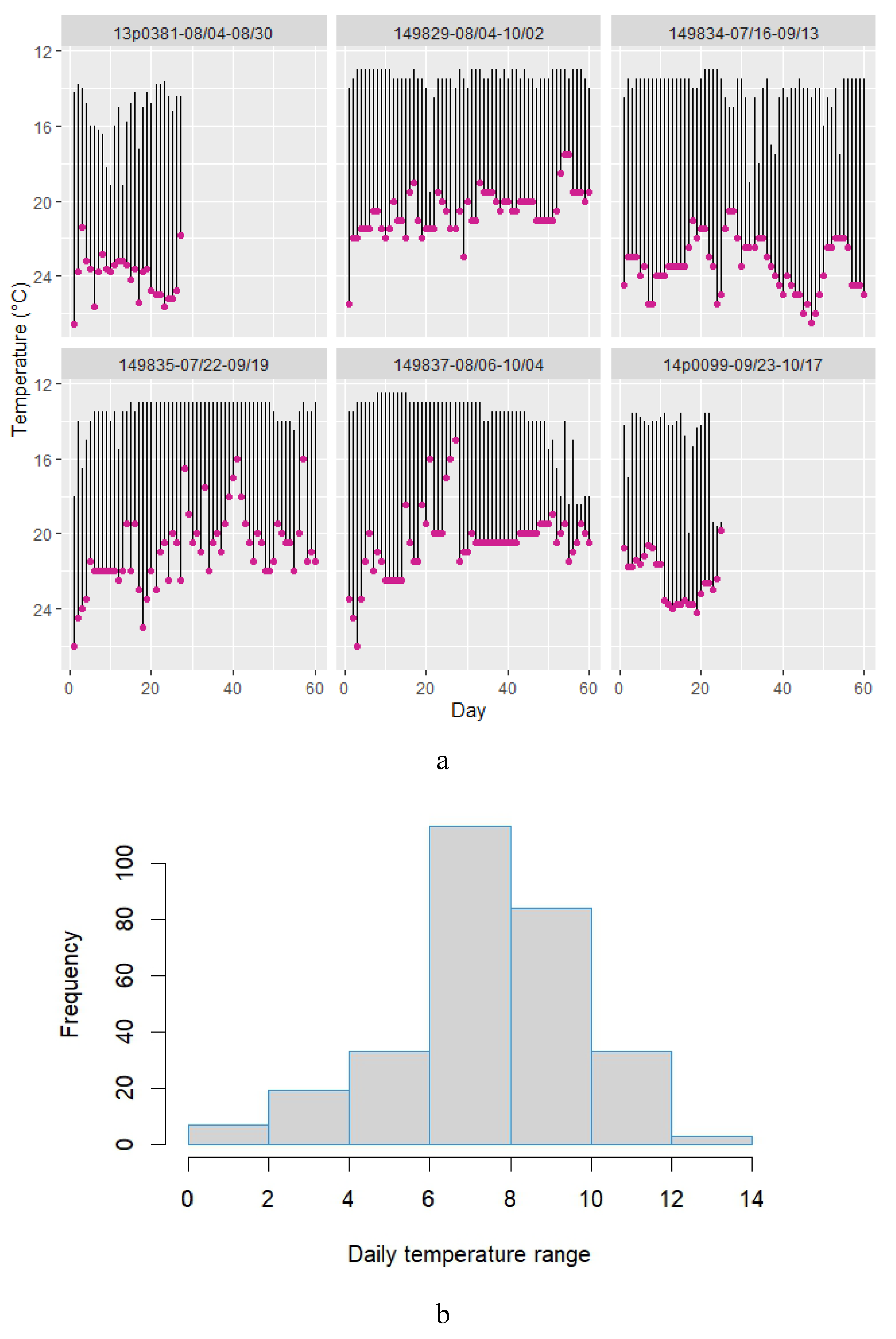
3.2.1. Depth Profiles
3.2.2. Temperature Profiles
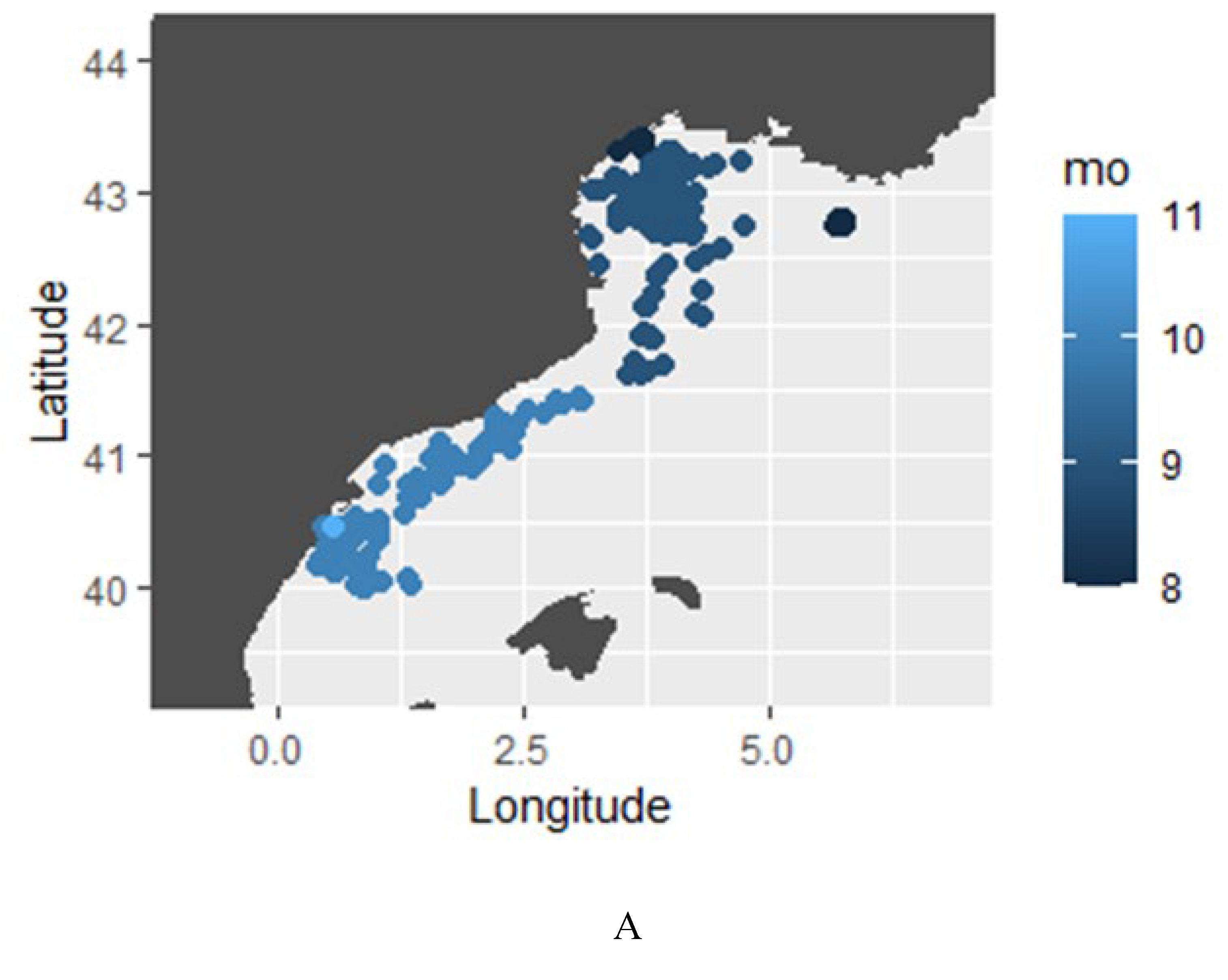
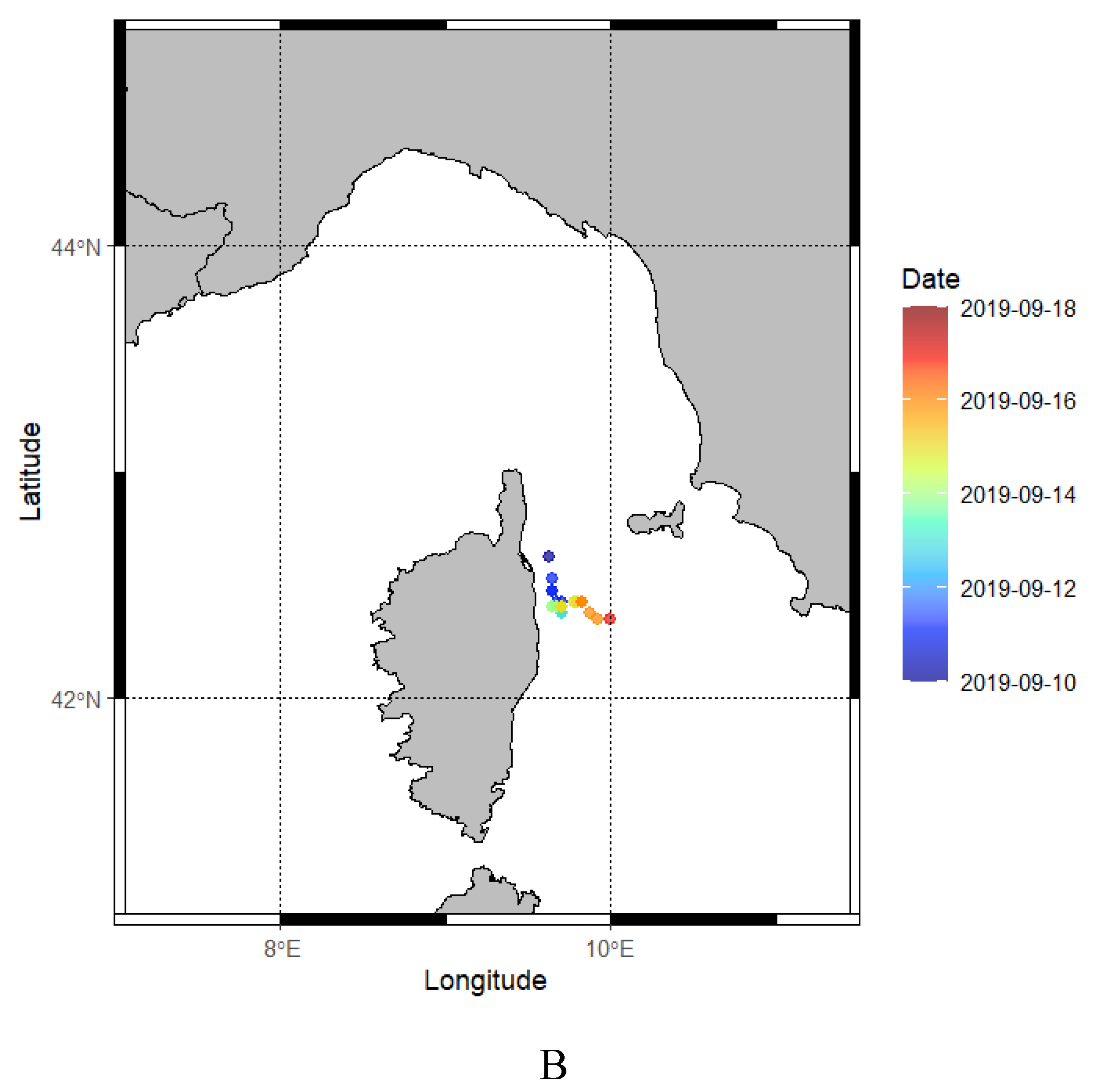
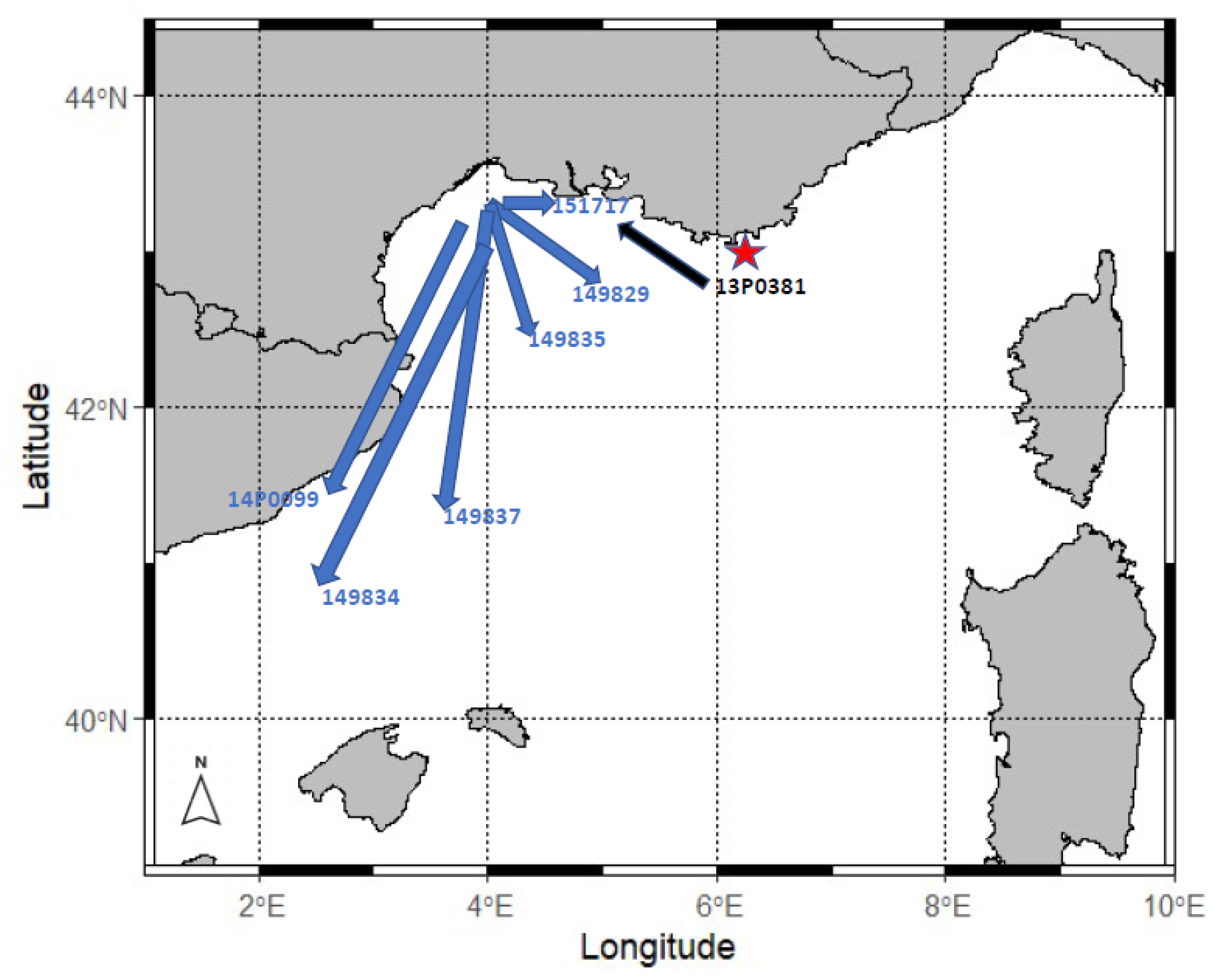
3.2.3. Horizontal Movements
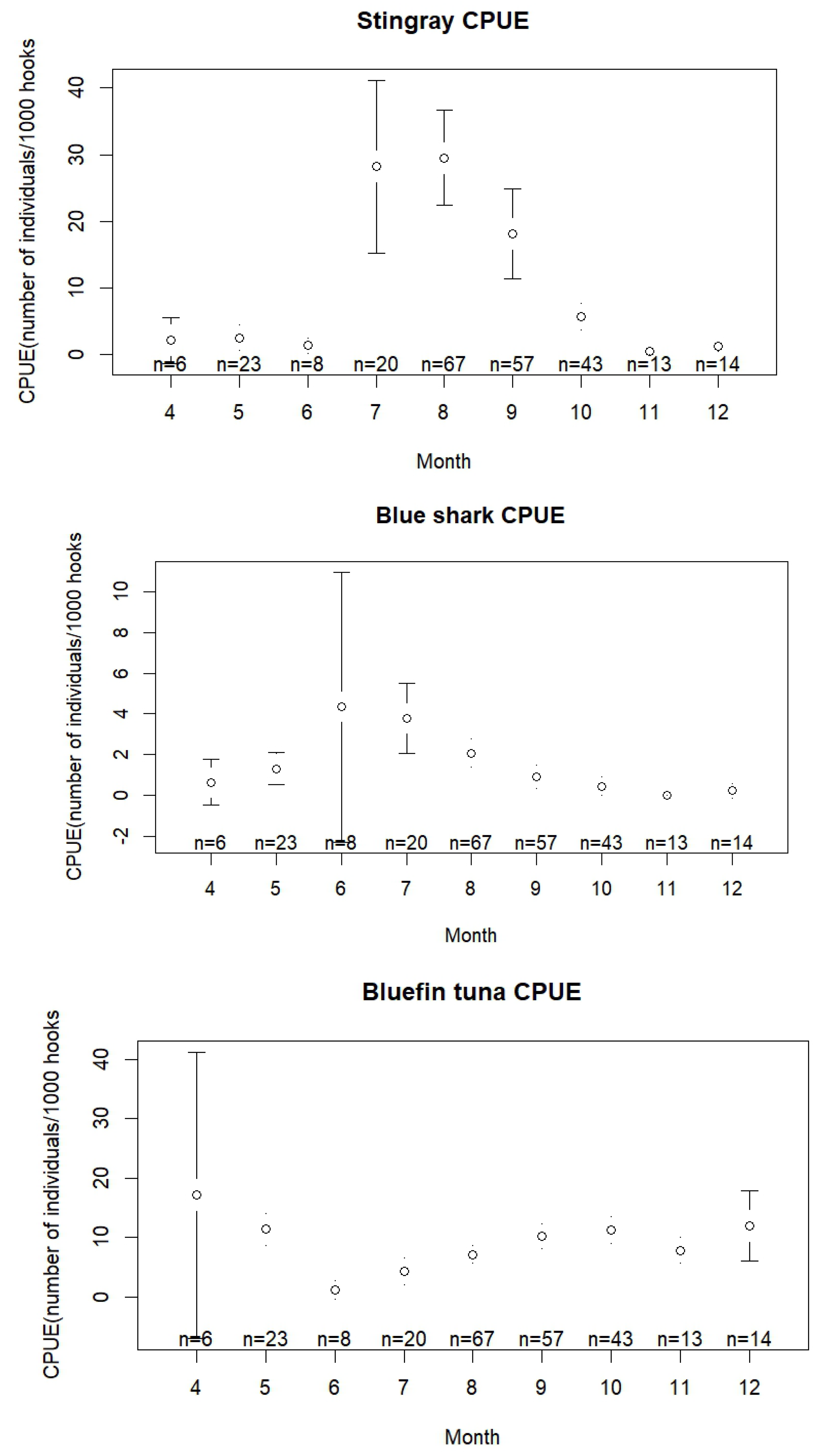
3.3. Interactions with the Longline Fishery
4. Discussion
4.1. Satellite Tag Deployment
4.2. Vertical Movements
4.3. Horizontal and Seasonal Movements
4.4. Interactions with the Longline Fishery
4.5. Mitigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Nakaya, N. Fishes of the Kyushu–Palau Ridge and Tosa Bay. Japan Fisheries Resource Conservation Association, Tokyo, Japan. 1982.
- Neer, J.A. The biology and ecology of the pelagic stingray, Pteroplatytrygon violacea (Bonaparte, 1832). Sharks of the open sea. Biology, fisheries & conservation. Oxford: Blackwell Publishers Ltd 2008, 152-159.
- Weidner, T.; Cotton, C.F.; Schieber, J.J.; Collatos, C.; Kerstetter, D.W. Short-term habitat use and vertical movements of the pelagic stingray Pteroplatytrygon violacea in the western North Atlantic Ocean determined by pop-up archival satellite tags. Bulletin of Marine Science 2023. [CrossRef]
- Bonanomi, S.; Pulcinella, J.; Fortuna, C.M.; Moro, F.; Sala, A. Elasmobranch bycatch in the Italian Adriatic pelagic trawl fishery. PloS one 2018, 13, e0191647. [CrossRef]
- Mollet, H.F. Distribution of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832), off California, Central America, and worldwide. Marine and Freshwater Research 2002, 53, 525-530, . [CrossRef]
- Forselledo, R.; Pons, M.; Miller, P.; Domingo, A. Distribution and population structure of the pelagic stingray, Pteroplatytrygon violacea (Dasyatidae), in the south-western Atlantic. Aquatic Living Resources 2008, 21, 357-363. [CrossRef]
- Poisson, F.; Gaertner, J.C.; Taquet, M.; Durbec, J.P.; Bigelow, K. Effects of lunar cycle and fishing operations on longline-caught pelagic fish: fishing performance, capture time, and survival of fish. Fishery Bulletin 2010, 108, 268-281.
- Wang, J.; Gao, C.; Wu, F.; Dai, L.; Ma, Q.; Tian, S. Environmental Characteristics Associated with the Presence of the Pelagic Stingray (Pteroplatytrygon violacea) in the Pacific High Sea. Fishes 2023, 8, 46. [CrossRef]
- Véras, D.P.; Vaske Júnior, T.; Hazin, F.H.V.; Lessa, R.P.; Travassos, P.E.; Tolotti, M.T.; Barbosa, T.M. Stomach contents of the pelagic stingray (Pteroplatytrygon violacea)(Elasmobranchii: Dasyatidae) from the tropical Atlantic. Brazilian Journal of Oceanography 2009, 57, 339-343. [CrossRef]
- Wilson, P.C.; Beckett, J.S. Atlantic Ocean distribution of the pelagic stingray, Dasyatis violacea. Copeia 1970, 696-707. [CrossRef]
- Orsi Relini, L.; Garibaldi, B.; Digitali, B.; Lanteri, L.; Vacchi, M.; La Mesa, G.; Serena, F.; Séret, B. Abundance of the pelagic stingray, Pteroplatytrygon (Dasyatis) violacea, in the Ligurian Sea, with preliminary notes about its feeding and growth. Proc. 4rd Europ. Elasm. Assoc. Meet., Livorno (Italy) 2000.
- Báez, J.C.; Crespo, G.O.; García-Barcelona, S.; Ortiz De Urbina, J.M.; Macías, D. Understanding pelagic stingray (Pteroplatytrygon violacea) by-catch by Spanish longliners in the Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 2015, 96, 1387-1394. [CrossRef]
- Poisson, F.; Catteau, S.; Chiera, C.; Groul, J.-M. The effect of hook type and trailing gear on hook shedding and fate of pelagic stingray (Pteroplatytrygon violacea): New insights to develop effective mitigation approaches. Marine Policy 2019, 107, 103594, . [CrossRef]
- Banaru, D.; Dekeyser, I.; Imbert, G.; Laubier, L. Non-target and released alive by-catches distributions observed during French driftnet fishery in the Northwestern Mediterranean Sea (2000-2003 database). 2010, Vol. 3, 33-45.
- Piovano, S.; Basciano, G.; Swimmer, Y.; Giacoma, C. Evaluation of a bycatch reduction technology by fishermen: A case study from Sicily. Marine Policy 2012, 36, 272-277. [CrossRef]
- Cook, M.D.; Matteucci, M.J.; Lall, R.; Ly, B.T. Stingray envenomation. Journal of Emergency Medicine 2006, 30, 345-347. [CrossRef]
- Last, P.; Seret, B.; Stehmann, M.; White, W.; Carvalho Naylor, G. Rays of the World. eds. Last, P., Seret, B., Stehmann, M., White, W., Carvalho, Naylor. Comstock. CSIRO Publishing, Comstock Publishing Associates 2016, 790.
- Ranzi, S. Le basi fi sio-moriologiche dello sviluppo embrionale dei selaci. Part 2. Pubblicazioni della Stazione Zoologica di Napoli 13, 331–437. 1934.
- Bianco, S.L. Notizie biologiche riguardanti specialmente il periodo di maturità sessuale degli animali del golfo di Napoli; 1909.
- Cortés, E.; Arocha, F.; Beerkircher, L.; Carvalho, F.; Domingo, A.; Heupel, M.; Holtzhausen, H.; Santos, M.N.; Ribera, M.; Simpfendorfer, C. Ecological risk assessment of pelagic sharks caught in Atlantic pelagic longline fisheries. Aquatic Living Resources 2010, 23, 25-34. [CrossRef]
- Walls, R.H.; Dulvy, N.K. Tracking the rising extinction risk of sharks and rays in the Northeast Atlantic Ocean and Mediterranean Sea. Scientific Reports 2021, 11, 15397. [CrossRef]
- Kyne, P.M.; Barreto, R.; Carlson, J.; Fernando, D.; Francis, M.P.; Fordham, S.; Jabado, R.W.; Liu, K.M.; Marshall, A.; Pacoureau, N.; et al. 2019. Pteroplatytrygon violacea. The IUCN Red List of Threatened Species 2019: e.T161731A896169. 2019. [CrossRef]
- Cavanagh, R.D.; Gibson, C. Overview of the conservation status of cartilaginous fishes (Chrondrichthyans) in the Mediterranean Sea; Iucn: 2007.
- Carruthers, E.H.; Neis, B. Bycatch mitigation in context: using qualitative interview data to improve assessment and mitigation in a data-rich fishery. Biological Conservation 2011, 144, 2289-2299. [CrossRef]
- Poisson, F.; Budan, P.; Coudray, S.; Gilman, E.; Kojima, T.; Musyl, M.; Takagi, T. New technologies to improve bycatch mitigation in industrial tuna fisheries. Fish and Fisheries 2022, 23, 545-563. [CrossRef]
- Poisson, F.; Métral, L.; Brisset, B.; Wendling, B.; Cornella, D.; Segorb, C.; Marchand, M.; Cuvilliers P.; Guilbert, G.; Bailleul, D.; et al. Rapport de fin de projet. Projet SELPAL.125p. 2016.
- Renshaw, S.; Hammerschlag, N.; Gallagher, A.J.; Lubitz, N.; Sims, D.W. Global tracking of shark movements, behaviour and ecology: A review of the renaissance years of satellite tagging studies, 2010–2020. Journal of Experimental Marine Biology and Ecology 2023, 560, 151841. [CrossRef]
- Wearmouth, V.J.; Sims, D.W. Movement and behaviour patterns of the critically endangered common skate Dipturus batis revealed by electronic tagging. Journal of Experimental Marine Biology and Ecology 2009, 380, 77-87. [CrossRef]
- Branco-Nunes, I.; Veras, D.; Oliveira, P.; Hazin, F. Vertical movements of the southern stingray, Dasyatis americana (Hildebrand & Schroeder, 1928) in the Biological Reserve of the Rocas Atoll, Brazil. Latin American Journal of Aquatic Research 2016, 44, 216-227.
- Le Port, A.; Sippel, T.; Montgomery, J.C. Observations of mesoscale movements in the short-tailed stingray, Dasyatis brevicaudata from New Zealand using a novel PSAT tag attachment method. Journal of Experimental Marine Biology and Ecology 2008, 359, 110-117. [CrossRef]
- Boggs, C.H. Depth, capture time and hooked longevityof longline-caught pelagic fish- Timing bites of fish with chips. Fish. bull. 1992, 90, 642-658.
- Luo, J.; PRINCE, E.D.; GOODYEAR, C.P.; LUCKHURST, B.E.; SERAFY, J.E. Vertical habitat utilization by large pelagic animals: a quantitative framework and numerical method for use with pop-up satellite tag data. Fisheries Oceanography 2006, 15, 208-229, . [CrossRef]
- Lam, C.H.; Nielsen, A.; Sibert, J.R. Improving light and temperature based geolocation by unscented Kalman filtering. Fisheries Research 2008, 91, 15-25, . [CrossRef]
- Bauer, R. Rchivaltag: Analyzing archival tagging data. R package version 0.1 2020, 2.
- Bauer, R. Oceanmap: a plotting toolbox for 2D oceanographic data. R Package Version 2020.
- Poisson, F.; Demarcq, H.; Coudray, S.; Bohn, J.; Camiñas, J.A.; Groul, J.-M.; March, D. Movement pathways and habitat use of blue sharks (Prionace glauca) in the Western Mediterranean Sea: Distribution in relation to environmental factors, reproductive biology, and conservation issues. Fisheries Research 2024, 270, 106900, . [CrossRef]
- Hussey, N.E.; Orr, J.; Fisk, A.T.; Hedges, K.J.; Ferguson, S.H.; Barkley, A.N. Mark report satellite tags (mrPATs) to detail large-scale horizontal movements of deep water species: First results for the Greenland shark (Somniosus microcephalus). Deep Sea Research Part I: Oceanographic Research Papers 2018, 134, 32-40. [CrossRef]
- Kneebone, J.; Sulikowski, J.; Knotek, R.; McElroy, W.D.; Gervelis, B.; Curtis, T.; Jurek, J.; Mandelman, J. Using conventional and pop-up satellite transmitting tags to assess the horizontal movements and habitat use of thorny skate (Amblyraja radiata) in the Gulf of Maine. ICES Journal of Marine Science 2020, 77, 2790-2803. [CrossRef]
- Chen, S.-C.; Chang, C.-R.; Han, Y.-S. Seaward migration routes of indigenous eels, Anguilla japonica, A. marmorata, and A. bicolor pacifica, via satellite tags. Zoological Studies 2018, 57.
- Lipej, L.; Mavrič, B.; Paliska, D.; Capapé, C. Feeding habits of the pelagic stingray Pteroplatytrygon violacea (Chondrichthyes: Dasyatidae) in the Adriatic Sea. Journal of the Marine Biological Association of the United Kingdom 2013, 93, 285-290. [CrossRef]
- Elliott, R.G.; Montgomery, J.C.; Della Penna, A.; Radford, C.A. Satellite tags describe movement and diving behaviour of blue sharks Prionace glauca in the southwest Pacific. Marine Ecology Progress Series 2022, 689, 77-94. [CrossRef]
- Queiroz, N.; Vila-Pouca, C.; Couto, A.; Southall, E.J.; Mucientes, G.; Humphries, N.E.; Sims, D.W. Convergent foraging tactics of marine predators with different feeding strategies across heterogeneous ocean environments. Frontiers in Marine Science 2017, 4, 239. [CrossRef]
- Wilson, S.G.; Block, B.A. Habitat use in Atlantic bluefin tuna Thunnus thynnus inferred from diving behavior. Endangered Species Research 2009, 10, 355-367. [CrossRef]
- Matern, S.A.; Cech, J.J.; Hopkins, T.E. Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environmental Biology of Fishes 2000, 58, 173-182. [CrossRef]
- Rouyer, T.; Bonhommeau, S.; Bal, G.; Derridj, O.; Fromentin, J.-M. The environment drives Atlantic bluefin tuna availability in the Gulf of Lions. Fisheries Oceanography 2021, 30, 490-498, . [CrossRef]
- Bauer, R.K.; Forget, F.; Fromentin, J.-M.; Capello, M. Surfacing and vertical behaviour of Atlantic bluefin tuna (Thunnus thynnus) in the Mediterranean Sea: implications for aerial surveys. ICES Journal of Marine Science 2020, 77, 1979-1991. [CrossRef]
- Bauer, R.K.; Fromentin, J.-M.; Demarcq, H.; Bonhommeau, S. Habitat use, vertical and horizontal behaviour of Atlantic bluefin tuna (Thunnus thynnus) in the Northwestern Mediterranean Sea in relation to oceanographic conditions. Deep Sea Research Part II: Topical Studies in Oceanography 2017, 141, 248-261. [CrossRef]
- Aldebert, Y. Demersal resources of the Gulf of Lions (NW Mediterranean). Impact of exploitation on fish diversity. Vie et Milieu/Life & Environment 1997, 275-284.
- Millot, C. Wind induced upwellings in the Gulf of Lions. Oceanologica Acta 1979, 2, 261-274.
- Ranzi, S.; Zezza, P. Fegato, maturita sessuale e gestazione in Trygon violacea. Pubblicazioni della Stazione Zoologica di Napoli 1936, 15, 355-367.
- Hemida, F.; Seridji, R.; Ennajar, S.; Bradaî, M.N.; Collier, E.; Guélorget, O.; Capapé, C. New observations on the reproductive biology of the pelagic stingray, Dasyatis violacea Bonaparte, 1832 (Chondrichthyes: Dasyatidae) from the Mediterranean Sea. Acta Adriatica 2003, 44, 193-204.
- Carruthers, E.H.; Schneider, D.C.; Neilson, J.D. Estimating the odds of survival and identifying mitigation opportunities for common bycatch in pelagic longline fisheries. Biological Conservation 2009, 142, 2620-2630. [CrossRef]
- Coelho, R.; Fernandez-Carvalho, J.; Lino, P.G.; Santos, M.N. An overview of the hooking mortality of elasmobranchs caught in a swordfish pelagic longline fishery in the Atlantic Ocean. Aquatic Living Resources 2012, 25, 311-319. [CrossRef]
- Ellis, J.R.; McCully Phillips, S.R.; Poisson, F. A review of capture and post-release mortality of elasmobranchs. Journal of Fish Biology 2017, 90, 653-722. [CrossRef]
- Hutchinson, M.; Siders, Z.; Stahl, J.; Bigelow, K. Quantitative estimates of post-release survival rates of sharks captured in Pacific tuna longline fisheries reveal handling and discard practices that improve survivorship. 2021.
- Musyl, M.K.; Gilman, E.L. Meta-analysis of post-release fishing mortality in apex predatory pelagic sharks and white marlin. Fish and Fisheries 2019, 20, 466-500, . [CrossRef]
- SPC. Report of the expert workshop on shark post-release mortality tagging studies: review of best practice and survey design. Wellington, New Zealand. 2017.
- Poisson, F.; Wendling, B.; Cornella, D.; Segorb, C. Guide du pêcheur responsable: Bonnes pratiques pour réduire la mortalité des espèces sensibles capturées accidentellement par les palangriers pélagiques français en Méditerranée. Projets SELPAL et RéPAST, Editeurs France Filière Pêche, Ifremer, AMOP 2016.
- Domingo, A.; Menni, R.C.; Forselledo, R. Bycatch of the pelagic ray Dasyatis violacea in Uruguayan longline fisheries and aspects of distribution in the southwestern Atlantic. Sci Mar 2005, 69, 161-166. [CrossRef]
- Prat-Varela, A.; Torres, A.; Cervantes, D.; Aquino-Baleytó, M.; Abril, A.-M.; Clua, E.E.G. Improved Baited Remote Underwater Video (BRUV) for 24 h Real-Time Monitoring of Pelagic and Demersal Marine Species from the Epipelagic Zone. Journal of Marine Science and Engineering 2023, 11, 1182. [CrossRef]
- Bègue, M.; Clua, E.; Siu, G.; Meyer, C. Prevalence, persistence and impacts of residual fishing hooks on tiger sharks. Fisheries Research 2020, 224, 105462. [CrossRef]
- Gilman, E.; Chaloupka, M.; Merrifield, M.; Malsol, N.D.; Cook, C. Standardized catch and survival rates, and effect of a ban on shark retention, Palau pelagic longline fishery. Aquatic Conservation: Marine and Freshwater Ecosystems 2016, 26, 1031-1062, . [CrossRef]
- Piovano, S.; Clo, S.; Giacoma, C. Reducing longline bycatch: The larger the hook, the fewer the stingrays. Biological Conservation 2010, 143, 261-264. [CrossRef]
- Gilman, E.; Chaloupka, M.; Swimmer, Y.; Piovano, S. A cross-taxa assessment of pelagic longline by-catch mitigation measures: conflicts and mutual benefits to elasmobranchs. Fish and Fisheries 2016, 17, 748-784. [CrossRef]
- Gilman, E.; Chaloupka, M.; Dagorn, L.; Hall, M.; Hobday, A.; Musyl, M.; Pitcher, T.; Poisson, F.; Restrepo, V.; Suuronen, P. Robbing Peter to pay Paul: replacing unintended cross-taxa conflicts with intentional tradeoffs by moving from piecemeal to integrated fisheries bycatch management. Reviews in Fish Biology and Fisheries 2019, 29, 93-123. [CrossRef]
- Poisson, F.; Catteau, S. No repellent effect of neodymium magnet on pelagic stingray (Pteroplatytrygon violacea): test in captivity. Ifremer. https://image.ifremer.fr/data/00888/99951/. 2017.
- Poisson, F.; Crespo, F.A.; Ellis, J.R.; Chavance, P.; Pascal, B.; Santos, M.N.; Séret, B.; Korta, M.; Coelho, R.; Ariz, J.; et al. Technical mitigation measures for sharks and rays in fisheries for tuna and tuna-like species: turning possibility into reality. Aquat. Living Resour. 2016, 29, 402. [CrossRef]
| Site | Tag type | # | Tagging date | Sex | DW (cm) |
Distance (km) |
Days at liberty (d) |
Mortality | Last tagging transmission |
Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| GoL | SeaTag-3D | 151713 | 2015-08-27 | F | 49 | 418 | 35 | - | 2015-09-26 | Short period of transmission |
| GoL | 151717 | 2015-08-03 | F | 60 | 25 | 2 | No | Captured by a French gillnetter | ||
| GoL | 151719* | 2015-08-27 | F | 53 | - | - | - | Failure | ||
| GoL | 151720* | 2015-08-05 | M | 46 | - | - | - | Failure | ||
| GoL | MrPAT | 149829 | 2015-08-03 | F | 52 | 94 | 60 | No | 2015-10-02 | |
| GoL | 149834 | 2015-07-15 | F | 47 | 303 | 60 | No | 2015-09-13 | ||
| GoL | 149835 | 2015-07-21 | F | 50 | 97 | 60 | No | 2015-09-19 | ||
| GoL | 149837 | 2015-08-05 | F | 47 | 217 | 60 | No | 2015-10-04 | ||
| GoL | 149836* | 2015-08-05 | F | 51 | - | - | - | Failure | ||
| GoL | 149833* | 2015-08-27 | M | 43 | - | - | - | Failure | ||
| GoL | 149832* | 2015-08-03 | F | 48 | - | - | - | Failure | ||
| GoL | 149828* | 2015-08-27 | F | 55 | - | - | - | Failure | ||
| GoL | 149830* | 2015-08-03 | F | 49 | - | - | - | Failure | ||
| GoL | 149831* | 2015-08-27 | M | 39 | - | - | - | Failure | ||
| GoL | sPAT | 14P0099 | 2016-09-23 | F | 55 | 245 | 25 | Unknown | 2016-10-17 | |
| GoL | 13P0381 | 2016-08-04 | F | 45 | 73 | 27 | Unknown | 2016-08-30 | Sighted and filmed close to shore 5 days after tagging | |
| Cor | miniPAT | 138296 | 2019-09-10 | F | 54 | 89 | 8 | Yes | 2016-09-17 | Caught by the wing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





