1. Introduction
Lung Cancer (LC) is the second most common malignancy in the U.S. in both men and women and the leading cause of cancer mortality in both sexes[
1] and strategies for controlling modifiable risk factors are critically needed. Insulin therapy remains a cornerstone in Type 2 Diabetes Mellitus (T2DM) management, prescribed to millions worldwide, however, recent studies have suggested a potential connection between insulin therapy and increased incidence of LC[
2,
3,
4]. This association may stem from mechanisms such as insulin resistance, hyperinsulinemia, insulin promotion of LC growth, and the promotion of an inflammatory microenvironment[
5]. In preclinical models, insulin has been shown to foster LC growth through pathways related to these mechanisms, and clinical outcomes in LC patients receiving insulin therapy have been observed to be worse compared to those using other antidiabetic interventions[
5,
6].
Non-insulin antidiabetics have demonstrated a differential risk of LC in retrospective studies[
7,
8,
9], though the evidence remains limited and sometimes controversial[
10]. Metformin, the most widely prescribed antidiabetic drug, has been widely explored for its potential anticancer properties[
5,
11], while SGLT2 inhibitors are being explored for their potential in LC prevention[
12]. Other antidiabetics, such as sulfonylureas, alpha-glucosidase inhibitors (AGIs), thiazolidinediones (TZDs), and dipeptidyl peptidase-4 (DPP-4) inhibitors, have been associated with varying levels of cancer risk in LC studies[
13]. Among these, glucagon-like peptide 1 receptor agonists (GLP-1RAs), stand out for their wide-ranging benefits, including glycemic control, weight reduction, and immune modulation[
14,
15,
16]. Recent findings indicate that GLP-1RA use is linked to a decreased colorectal cancer risk[
17].
Despite these insights, the differential effects of antidiabetic treatments on LC risk in patients with T2DM have yet to be systematically examined and compared with insulins within a single clinical dataset, utilizing unified methods. The absence of research comparing the impact of different antidiabetic medications on LC risk prevents the development of clear guidelines for optimal treatment of T2DM with comorbidities such as tobacco use which may place them at increased risk for the development of LC. Addressing this gap, we conducted a nationwide, retrospective cohort study among drug-naive T2DM patients. Our research compares LC risk among users of GLP-1RAs and six other non-GLP-1RA antidiabetics with insulin users.
2. Methods
We used the TriNetX platform to access deidentified electronic health records of 105.9 million patients, including 7.7 million with T2DM from 61 health care organizations across 50 states, covering diverse geographical regions, age, ethnicity, income and insurance groups, and clinical settings.[
18] TriNetX built-in analytic functions allow for patient-level analyses while only reporting population-level data. The MetroHealth System institutional review board determined that using data from TriNetX is not human subject research and therefore exempt from approval. The TriNetX platform has recently been shown to be useful for multiple population based cohort studies including cancer[
17,
19,
20,
21].
The study population comprised 1,040,341 patients with T2DM who had medical encounters for T2DM and were subsequently prescribed antidiabetic medications from 2005 to 2019, no prior antidiabetic medication use (drug naive), and no prior LC diagnosis. GLP-1RAs, metformin, alpha-glucosidase inhibitors, DPP-4 inhibitors, SGLT2 inhibitors, sulfonylureas, and thiazolidinediones were all compared against insulin users. The study period of 2005 to 2019 (except for a starting year of 2013 for SGLT2 inhibitors and 2006 for DPP-4 inhibitors) was chosen based on the year drugs were first approved. The study population was divided into exposure cohorts and comparison cohorts for each comparison.
To mitigate confounding, cohorts were propensity score matched (1:1, using nearest neighbor greedy matching) for demographics, BMI, A1c, pre-existing medical conditions, family and personal history of cancers and lung nodules, lifestyle factors (e.g., smoking, and alcohol use), environmental exposures, and procedures such as LC screening, which were determined via ICD-10 codes (
Table 1). The outcome was the first diagnosis of LC that occurred within 15 years starting from the index event, also determined via ICD-10 diagnostic codes. The follow-up period for each patient began with the index event, which was the first prescription of insulins or the comparison medications, and ended with the occurrence of the outcome, death, end of patient medical record, or the end of the 15 year period. With censoring applied, hazard ratios (HRs) and 95% Confidence Intervals (CI) were calculated using Cox Proportional Hazards models. Secondary analyses were also conducted for the GLP-1RA versus insulin comparison, stratifying LC risk by histological subtype: Squamous Cell Carcinoma, Adenocarcinoma of the Lung), Large Cell Carcinoma, and Small Cell LC. Further subgroup analyses for the GLP-1RA vs insulin comparison were performed, stratifying by race, ethnicity, sex assigned at birth, and smoking status determined by the presence or absence of relevant ICD-10 codes before the index event.
Data were collected and analyzed on Feb 1, 2024, within the TriNetX Analytics Platform using built-in functions (R, version 4.0.2 [R Project for Statistical Computing]), with statistical significance set at a 2-sided
P < .05.[
18] This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines[
22].
3. Results
In the study cohort of 1,040,341 drug-naive individuals with (T2DM) without prior LC diagnosis, significant differences were observed between the exposure and comparison cohorts (non-insulin antidiabetic drugs versus insulin) across several parameters. These included differences in average glycated hemoglobin (A1c) levels, body mass index (BMI), demographic profiles, prevalence of preexisting medical conditions, exposure to adverse factors, and personal and family history of LC, in addition to variations in alcohol and tobacco use rates. Notably, the cohort receiving glucagon-like peptide 1 receptor agonists (GLP-1RAs) in comparison to those on insulin therapy, was characterized by a younger demographic, higher average BMI, a greater proportion of females and Caucasians, and lower rates of tobacco and alcohol usage. Baseline characteristics for selected groups before and after matching are detailed in
Table 1. Following the application of propensity score matching, a balance was achieved between each treatment and comparison group for all measured covariates, as evidenced by a standardized mean difference of less than 0.1. Complete baseline characteristics for all cohorts before and after matching are available in
Supplementary Tables S1 and S2.
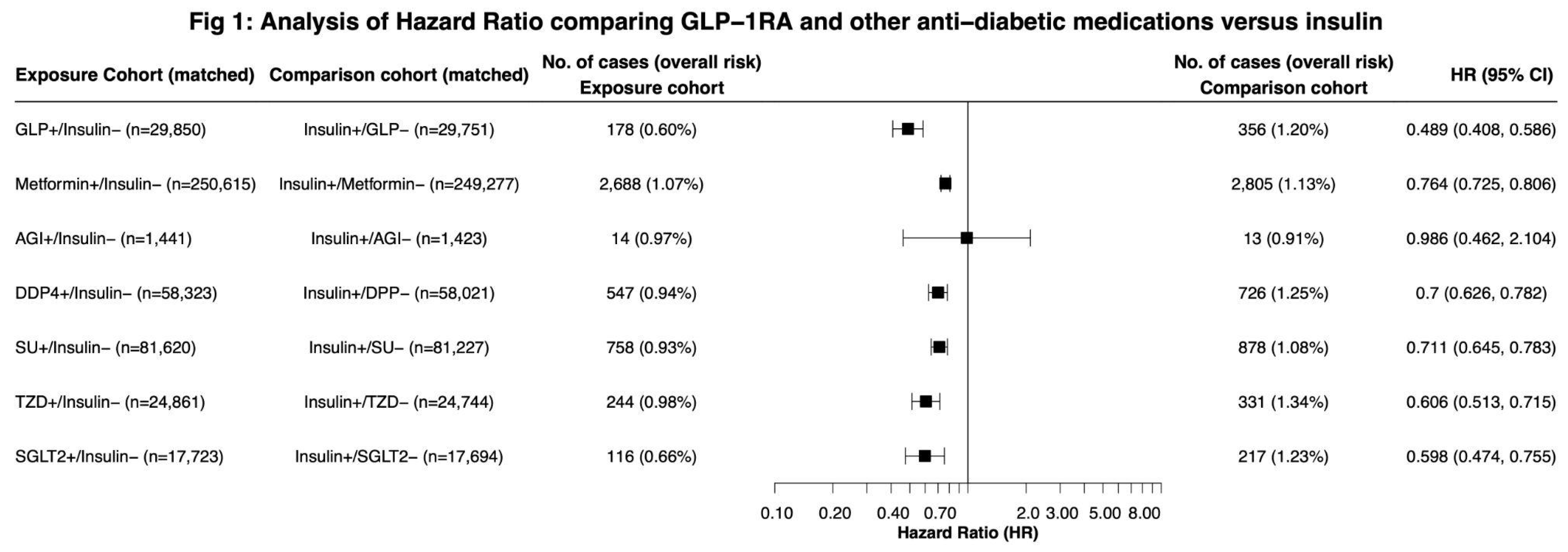
During a 15-year follow-up in 1,040,341 drug-naive patients with T2DM, we observed a decreased risk for LC among all non-insulin antidiabetic drugs when compared with insulin except for alpha-glucosidase inhibitors. GLP-1RAs were associated with the largest reduction in LC risk compared to insulin (HR, 0.489, 95% CI, 0.408,0.586), SGLT2 inhibitors (HR 0.598, 95% CI: 0.474,0.755) and TZD (HR: 0.606, 95% CI: 0.513, 0.715). (
Figure 1).
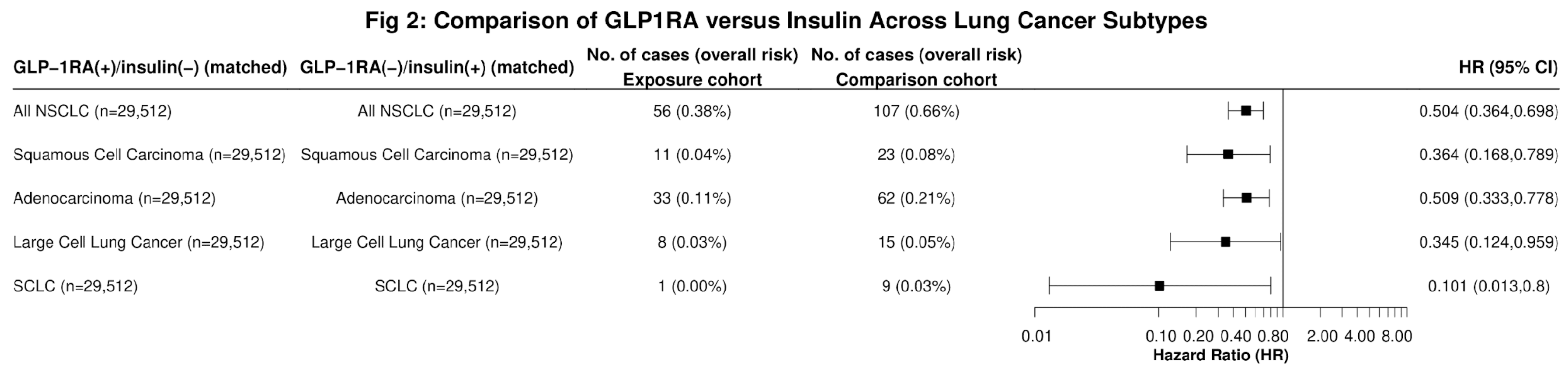
In further sub-group analysis, GLP-1RA users had significantly lower risk of incident LC when compared to insulin users across all histological types, including Squamous Cell Carcinoma (HR: 0.364, 95% CI: 0.168,0.789), Adenocarcinoma of the Lung (HR: 0.509, 95%CI: 0.333,0.778), Large Cell Carcinoma (HR: 0.345, 95% CI: 0.124,0.959), and Small Cell LC (SCLCL) (HR: 0.101, 95% CI: 0.013,0.8) (
Figure 2). Significant reductions in LC risk were observed among Black (HR: 0.382, 95% CI: 0.206, 0.71) and White (HR: 0.511, 95% CI: 0.41,0.638) patients, but not in individuals of other races or in Hispanic individuals (
Figure 3). Similar results were observed between males and females, as well as smokers and non-smokers(
Figure 3).
4. Discussion
This study aimed to explore the association between antidiabetic drug use and differential LC risk among T2DM patients. On a worldwide basis, it is the leading cause of cancer related mortality, contributing to 1.8 million deaths annually[
23]. While tobacco use control has significantly led to decreased incidence of LC in the U.S.[
1,
24], further methods for mitigating LC risk are needed. Given the rising prevalence of T2DM globally[
25], understanding the potential effects of antidiabetic medications on LC risk is of great clinical relevance.
Our results indicate that all non-insulin antidiabetic drugs, excluding AGIs, are associated with a decreased risk of LC compared to insulins in antidiabetic-naive T2DM patients. GLP-1RAs exhibited the most significant reduction in LC risk (HR: 0.489), though the 95% confidence interval overlapped with that of SGLT2 inhibitors and TZDs. AGIs did not meet the threshold for significance, which may be attributed to their small sample size. Subgroup analysis highlighted GLP-1RAs’ efficacy across different LC histologies and demonstrated significant risk reductions in Black and White patients, with limitations in other racial and Hispanic groups likely due to insufficient sample sizes. These findings suggest a substantial association between non-insulin antidiabetic medications, especially GLP-1RAs, and decreased LC risk compared to insulin therapy. These results may guide clinicians in selecting appropriate medications for diabetes management while mitigating the risk of developing LC. Our findings highlight that GLP-1RAs are associated with a significant reduction in LC risk compared with insulins, aligning with existing research suggesting that GLP-1RAs may be associated with reduced risk of colorectal cancer[
17]. A study comparing GLP-1RAs to metformin, but not insulin, showed an adjusted odds ratio of 0.81 of LC among GLP-1RA users[
9]. Similarly, analysis of the SEER database indicates that DPP-4 inhibitors, which prevent the inactivation of endogenous GLP-1, are linked to improved survival in colon and lung cancers[
26]. Despite these associations, there remains a scarcity of pre-clinical evidence directly elucidating the mechanisms by which GLP-1RA therapy influences LC risk.
Several studies have suggested that insulin therapy could elevate cancer risk due to its mitogenic effects, potentially fostering the growth of certain cancer types[
13,
27,
28,
29,
30]. Potential confounders to these findings have been widely discussed, namely that insulin is often prescribed to patients with advanced T2DM or those with comorbid conditions, complicating direct comparisons with other medications[
31]. To date, 2 large clinical trials have been conducted, which did not find significant increases in overall cancer risk with glargine insulin[
32,
33]. However, these trials were powered to assess cardiovascular outcomes, precluding definitive conclusions about the risk of specific malignancies[
5].
In addition to studies suggesting a correlation between insulin therapy and an elevated risk of LC, several studies suggest worsened survival outcomes[
2,
3,
4,
6]. Hyperinsulinemia, which may occur in the context of insulin therapy, has been associated with increased LC development[
34]. Insulin can stimulate the proliferation, migration, and drug resistance of NSCLC cells via the PI3K/Akt pathway[
35]. Additionally, insulin has been shown to enhance LC survival by inhibiting proapoptotic cytokines[
36]. Our study’s findings that non-insulin antidiabetic drugs except for AGIs were associated with significantly lower LC risk than insulin therapy align with previous research evidence supporting that insulin therapy may promote a higher risk of LC.
One potential mechanism through which GLP-1RAs reduce LC risk include the enhancement of innate immunity, which plays a crucial role in preventing tumorigenesis[
16]. Specifically, GLP-1RA therapy has been observed to boost the functionality of natural killer (NK) cells, vital components of anti-tumor immune surveillance[
37]. Additionally, GLP-1RAs have been shown to significantly improve biomarkers of inflammation and oxidative stress more effectively than standard antidiabetic treatments[
38]. Given the susceptibility of both NSCLC and SCLC to immune-modulating therapies[
39], these immunological effects may be particularly relevant in reducing LC risk.
Another possible explanation to consider is the pronounced weight loss facilitated by GLP-1RAs. Many cancers, such as colon, pancreatic, and renal cancers are associated with obesity[
40]. However, the relationship between BMI and LC risk is debated, with several meta-analyses suggesting an inverse relationship, though these results are not universally accepted[
41]. Our study accounted for BMI variations through propensity score matching, and the observed reduction in LC risk associated with GLP-1RAs persisted regardless of BMI. This indicates that the beneficial impact of GLP-1RAs on LC risk may extend beyond their weight loss effects alone.
In our study, both SGLT2 inhibitors and TZDs demonstrated significant reductions in LC risk compared with insulins, with hazard ratios overlapping the 95% confidence intervals of GLP-1RAs. SGLT2 inhibitors, highlighted in research for their expression in metastatic LC tissues, have been associated with improved LC patient survival, though their anticancer mechanisms are still being defined[
42]. These may include effects on glucose transport, mitochondrial function, and key signaling pathways, alongside enhancing immune surveillance through PD-L1 suppression and increased T cell cytotoxicity [
12].
Regarding TZDs, there have been reports of a reduced risk of LC among users, although the evidence remains mixed[
43]. While some studies suggest a protective effect of TZDs against LC, meta-analyses have yet to confirm a significant difference in LC risk with TZD use[
44]. The clinical implications of these findings for the management of LC risk in patients with diabetes necessitate cautious interpretation and further research to validate these associations and understand the underlying mechanisms.
Our study identifies several limitations that must be acknowledged to contextualize our findings on the decreased LC risk associated with non-insulin antidiabetic use compared to insulin. Firstly, our study’s observational nature is inherently susceptible to biases typical of such research methodologies. In all EHR databases, there is the possibility of inaccurate documentation of outcome diagnoses. As data from TriNetX is population-level, we were not able to independently verify individual cancer diagnoses through patient-level data points. The validity and completeness of cancer incidence ascertainment in TriNetX EHRs is unknown. However as our study focuses on relative risk comparison between groups on the same platform, these limitations do not have a substantial impact upon our interpretation. While propensity score matching was employed to align cohorts on a range of factors—including demographics, BMI, A1c levels, medical history, and lifestyle factors—the reliance on EHR accuracy introduces the potential for unmeasured or uncontrolled confounders. These confounders could skew the observed associations, suggesting a need for cautious interpretation of the decreased LC risk findings. Of note, detailed information regarding the extent, duration, and amount of tobacco use is unavailable on the TriNetX platform.
Additionally, the limitations of EMR/EHR database research limit some aspects of our analysis. Incomplete data on medication dosing and duration of use in the TriNetX database prevented a comprehensive assessment of the effects of these variables on LC risk. As the TriNetX database does not allow for controlling for events that occur after the index event, we are unable to account for the possibility that some individuals may have switched to another medication after their initial prescription. Similarly, incomplete data from ICD-O coding regarding histological subtypes limited the statistical power of our conclusions in these areas. The study’s exclusion of patients with prior antidiabetic medication use narrows the generalizability of our findings across the broader T2DM patient population. Additionally, many patients may receive multiple combinations of antidiabetic medications, which may alter their risk profile further. Nonetheless, the observed associations between antidiabetic medication use, particularly GLP-1RA, and LC risk reduction necessitates further investigation to confirm these findings and the more fully understand the underlying mechanisms.
5. Conclusion
In conclusion, our study finds that GLP-1RAs and other non-insulin antidiabetic medications are associated with a lower risk of LC in patients with T2DM compared with insulins, providing information to guide clinicians in the selection of appropriate therapies for individuals with T2DM while mitigating the risk of developing LC. Investigating the underlying mechanisms that contribute to the reduced LC risk observed with GLP-1RAs and other non-insulin antidiabetics is essential for a deeper understanding of these associations. Expanding the evidence base will be vital for refining treatment decisions and developing comprehensive care strategies for this patient population. Future studies should aim to elucidate the biological pathways involved, assess the impact of combination therapies, and validate these findings across diverse populations to ensure the broad applicability and effectiveness of these treatment modalities in reducing LC risk among T2DM patients.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Table S1: Demographic Characteristics of All Cohorts Before Propensity Score Matching; Supplementary Table S2: Demographic Characteristics of the Cohorts After Propensity Score Matching.
Author Contributions
Conceptualization, T.T, R.X., N.A.B; Methodology, T.T, R.X., N.A.B ; Software, X.X.; Validation, T.T, R.X., N.A.B ; Formal Analysis, T.T, R.X., N.A.B.; Investigation, T.T, R.X., N.A.B; Resources, R.X., D.K., N.A.B; Data Curation, T.T,.; Writing—Original Draft Preparation, T.T, R.X., N.A.B; Writing—Review & Editing, T.T, R.X., N.A.B; Visualization, L.W..; Supervision, R.X., N.A.B; Project Administration, R.X., N.A.B; Funding Acquisition, R.X., N.A.B.
Funding
We acknowledge support from the National Cancer Institute Case Comprehensive Cancer Center (nos. CA221718, CA043703 and CA2332216), National Institute on Aging (AG057557, AG061388, AG062272, AG07664), National Institute on Alcohol Abuse and Alcoholism (AA029831). The funding sources had no role in the design, execution, analysis, data interpretation or decision to submit the results of this study.
Institutional Review Board Statement
The MetroHealth System institutional review board determined that using data from TriNetX is not human subject research and therefore exempt from approval.
Informed Consent Statement
Informed consent not applicable for this study, as the MetroHealth System institutional review board determined that using data from TriNetX is not human subject research and therefore exempt from approval.
Data Availability Statement
This study used population-level aggregate and de-identified data collected by the TriNetX Platform, which are available from TriNetX (
https://trinetx.com/); however, third-party restrictions apply to the availability of these data. The data were used under license for this study with restrictions that do not allow for data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs might be incurred and a data-sharing agreement would be necessary. Data specific to this study, including diagnosis codes and group characteristics in aggregated format, are included in the paper as tables, figures and supplementary files.
Conflicts of Interest
The authors declare no conflict of interest. We acknowledge support from the National Cancer Institute Case Comprehensive Cancer Center (nos. CA221718, CA043703 and CA2332216). The funding sources had no role in the design, execution, analysis, data interpretation or decision to submit the results of this study.
Conflicts of Interest Statement
The authors declare no potential conflicts of interest.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: A Cancer Journal for Clinicians. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Tseng, CH. Human Insulin Therapy Is Associated With an Increased Risk of Lung Cancer: A Population-Based Retrospective Cohort Study. Front Endocrinol (Lausanne), 4: 10. [CrossRef]
- Luo J, Chlebowski R, Wactawski-Wende J, Schlecht NF, Tinker L, Margolis KL. Diabetes and Lung Cancer Among Postmenopausal Women. Diabetes Care. 2012, 35, 1485–1491. [Google Scholar] [CrossRef]
- Wu Y, Liu HB, Shi XF, Song Y. Conventional Hypoglycaemic Agents and the Risk of Lung Cancer in Patients with Diabetes: A Meta-Analysis. PLoS One. 2014, 9, e99577. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and Cancer: A Consensus Report. CA: A Cancer Journal for Clinicians. 2010, 60, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Farhan SY, Jankowski M, Hanbali A, Wang D. The use of insulin and the effect on survival of non-small cell lung cancer patients. JCO. 2009, 27, e22073–e22073. [Google Scholar] [CrossRef]
- Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DPH, Chen CC. Antidiabetes Drugs Correlate With Decreased Risk of Lung Cancer: A Population-Based Observation in Taiwan. Clinical Lung Cancer. 2012, 13, 143–148. [Google Scholar] [CrossRef]
- Wang J, Kim CH. Malignancies associated with DPP4 inhibitors and GLP1 receptor agonists: Data from a large real-world database. JCO. 2020, 38, 1567–1567. [Google Scholar] [CrossRef]
- Wang J, Kim CH. Differential Risk of Cancer Associated with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Real-world Databases. Endocrine Research. 2022, 47, 18–25. [Google Scholar] [CrossRef]
- Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol. 2017, 14, 85–99. [Google Scholar] [CrossRef]
- Yao L, Liu M, Huang Y, et al. Metformin Use and Lung Cancer Risk in Diabetic Patients: A Systematic Review and Meta-Analysis. Dis Markers. 2019, 2019, 6230162. [Google Scholar] [CrossRef]
- Basak D, Gamez D, Deb S. SGLT2 Inhibitors as Potential Anticancer Agents. Biomedicines. 2023, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Liu YC, Nguyen PA, Humayun A, et al. Does long-term use of antidiabetic drugs changes cancer risk? Medicine (Baltimore). 2019, 98, e17461. [Google Scholar] [CrossRef] [PubMed]
- Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Molecular Metabolism. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Yao H, Zhang A, Li D, et al. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024, 384, e076410. [Google Scholar] [CrossRef]
- Chen J, Mei A, Wei Y, et al. GLP-1 receptor agonist as a modulator of innate immunity. Front Immunol. 2022, 13, 997578. [Google Scholar] [CrossRef]
- Wang L, Wang W, Kaelber DC, Xu R, Berger NA. GLP-1 Receptor Agonists and Colorectal Cancer Risk in Drug-Naive Patients With Type 2 Diabetes, With and Without Overweight/Obesity. <italic>JAMA Oncology</italic>. Published online December 7, 2023. [CrossRef]
- TriNetX. Accessed February 2, 2024. https://trinetx.com/.
- Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 Breakthrough Infection and Hospitalization in Fully Vaccinated Patients With Multiple Myeloma. JAMA Netw Open. 2021, 4, e2137575. [Google Scholar] [CrossRef] [PubMed]
- Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Mortality in Vaccinated Patients With Cancer in the US Between December 2020 and November 2021. JAMA Oncol. 2022, 8, 1027–1034. [Google Scholar] [CrossRef]
- Wang W, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med. 2024, 30, 168–176. [Google Scholar] [CrossRef]
- Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of Clinical Epidemiology. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Warren GW, Cummings KM. Tobacco and lung cancer: risks, trends, and outcomes in patients with cancer. <italic>Am Soc Clin Oncol Educ Book</italic>. Published online 2013, 359–364. [CrossRef]
- Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes – Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Bishnoi R, Hong YR, Shah C, et al. Dipeptidyl peptidase 4 inhibitors as novel agents in improving survival in diabetic patients with colorectal cancer and lung cancer: A Surveillance Epidemiology and Endpoint Research Medicare study. Cancer Medicine. 2019, 8, 3918–3927. [Google Scholar] [CrossRef]
- Currie CJ, Poole CD, Gale EAM. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009, 52, 1766–1777. [Google Scholar] [CrossRef]
- Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia. 2009, 52, 1745–1754. [Google Scholar] [CrossRef]
- Colhoun HM, SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009, 52, 1755–1765. [Google Scholar] [CrossRef]
- Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009, 52, 1732–1744. [Google Scholar] [CrossRef]
- Gerstein, HC. Does Insulin Therapy Promote, Reduce, or Have a Neutral Effect on Cancers? JAMA. 2010, 303, 446–447. [Google Scholar] [CrossRef]
- Rosenstock J, Fonseca V, McGill JB, et al. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia. 2009, 52, 1971–1973. [Google Scholar] [CrossRef]
- Bordeleau L, Yakubovich N, Dagenais GR, et al. The Association of Basal Insulin Glargine and/or n-3 Fatty Acids With Incident Cancers in Patients With Dysglycemia. Diabetes Care. 2014, 37, 1360–1366. [Google Scholar] [CrossRef]
- Argirion I, Weinstein SJ, Männistö S, Albanes D, Mondul AM. Serum Insulin, Glucose, Indices of Insulin Resistance, and Risk of Lung Cancer. Cancer Epidemiol Biomarkers Prev. 2017, 26, 1519–1524. [Google Scholar] [CrossRef]
- Jiang J, Ren HY, Geng GJ, et al. Oncogenic activity of insulin in the development of non-small cell lung carcinoma. Oncology Letters. 2018, 15, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Frisch CM, Zimmermann K, Zilleßen P, Pfeifer A, Racké K, Mayer P. Non-small cell lung cancer cell survival crucially depends on functional insulin receptors. Endocrine-Related Cancer. 2015, 22, 609–621. [Google Scholar] [CrossRef] [PubMed]
- De Barra C, Khalil M, Mat A, et al. Glucagon-like peptide-1 therapy in people with obesity restores natural killer cell metabolism and effector function. Obesity. 2023, 31, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Bray JJH, Foster-Davies H, Salem A, et al. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes, Obesity and Metabolism. 2021, 23, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- <bold>39. </bold>Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer, 2023; 22, 40. [CrossRef]
- Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers (Basel). 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Vedire Y, Kalvapudi S, Yendamuri S. Obesity and lung cancer—a narrative review. J Thorac Dis. 2023, 15, 2806–2823. [Google Scholar] [CrossRef] [PubMed]
- Luo J, Hendryx M, Dong Y. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and non-small cell lung cancer survival. Br J Cancer. 2023, 128, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007, 25, 1476–1481. [Google Scholar] [CrossRef]
- Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer Risk for Patients Using Thiazolidinediones for Type 2 Diabetes: A Meta-Analysis. Oncologist. 2013, 18, 148–156. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).