Submitted:
30 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
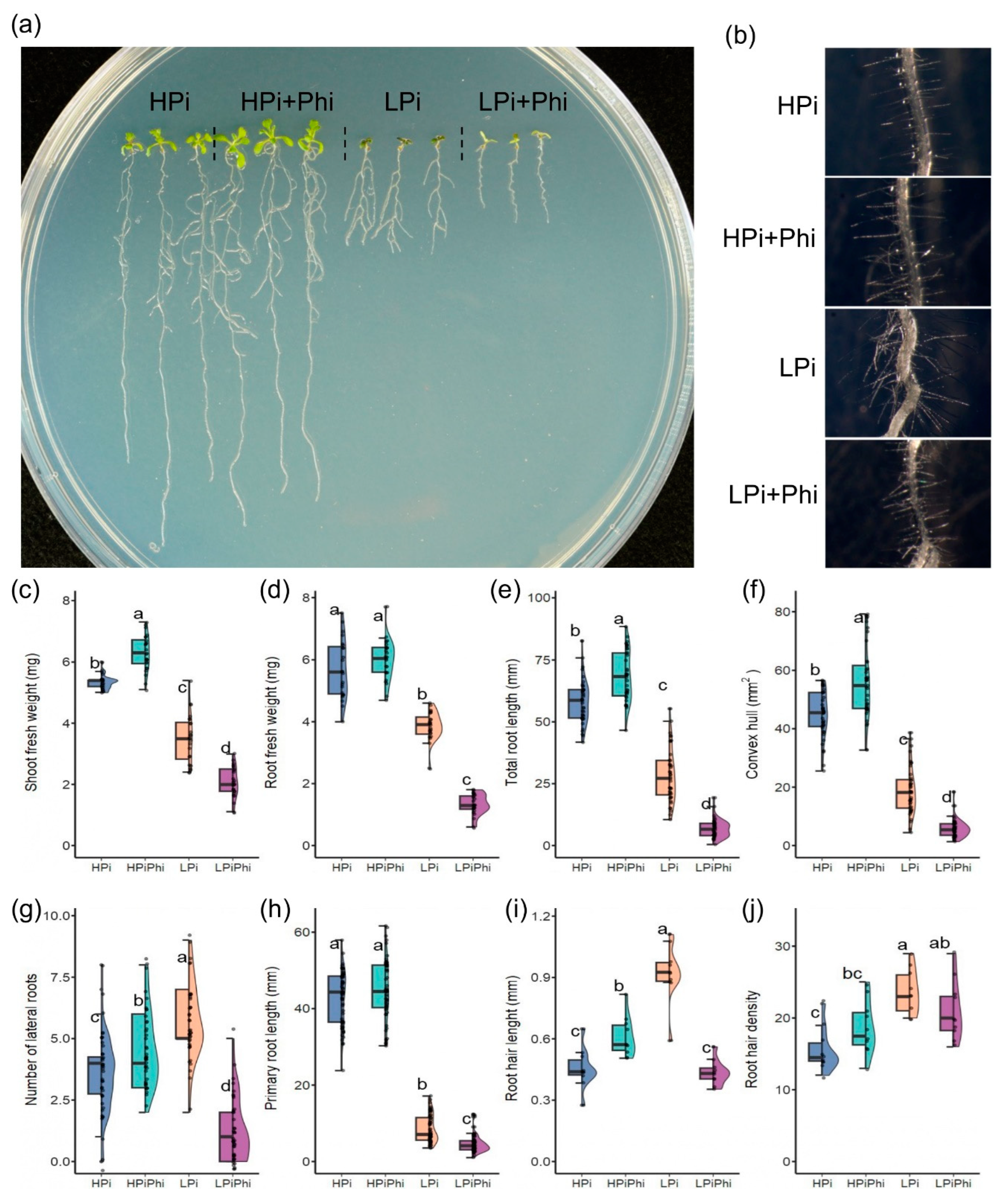
2.1. Phi Suppresses Pi Starvation Responses and Positively Affects Arabidopsis Growth under Optimal Pi Conditions
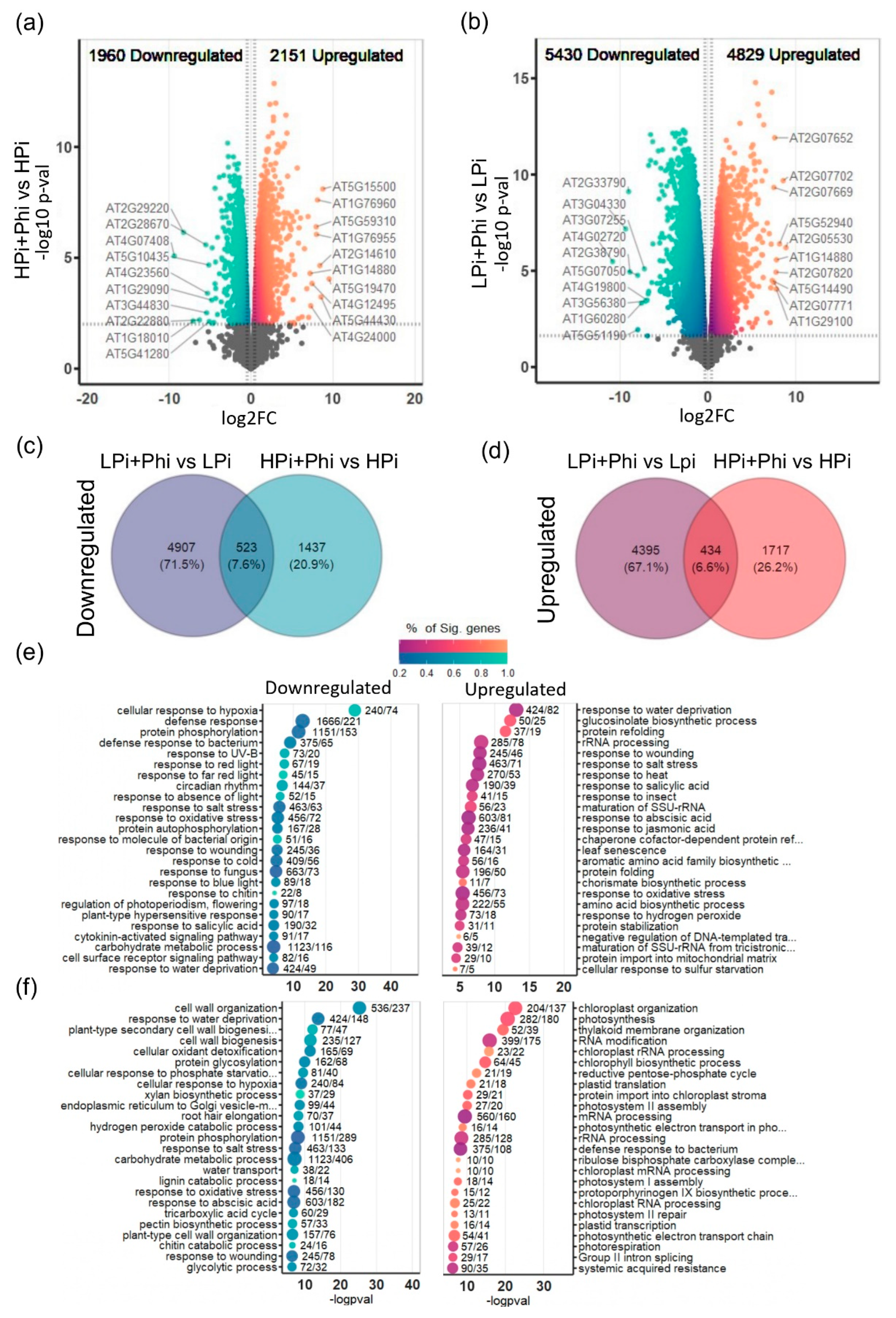
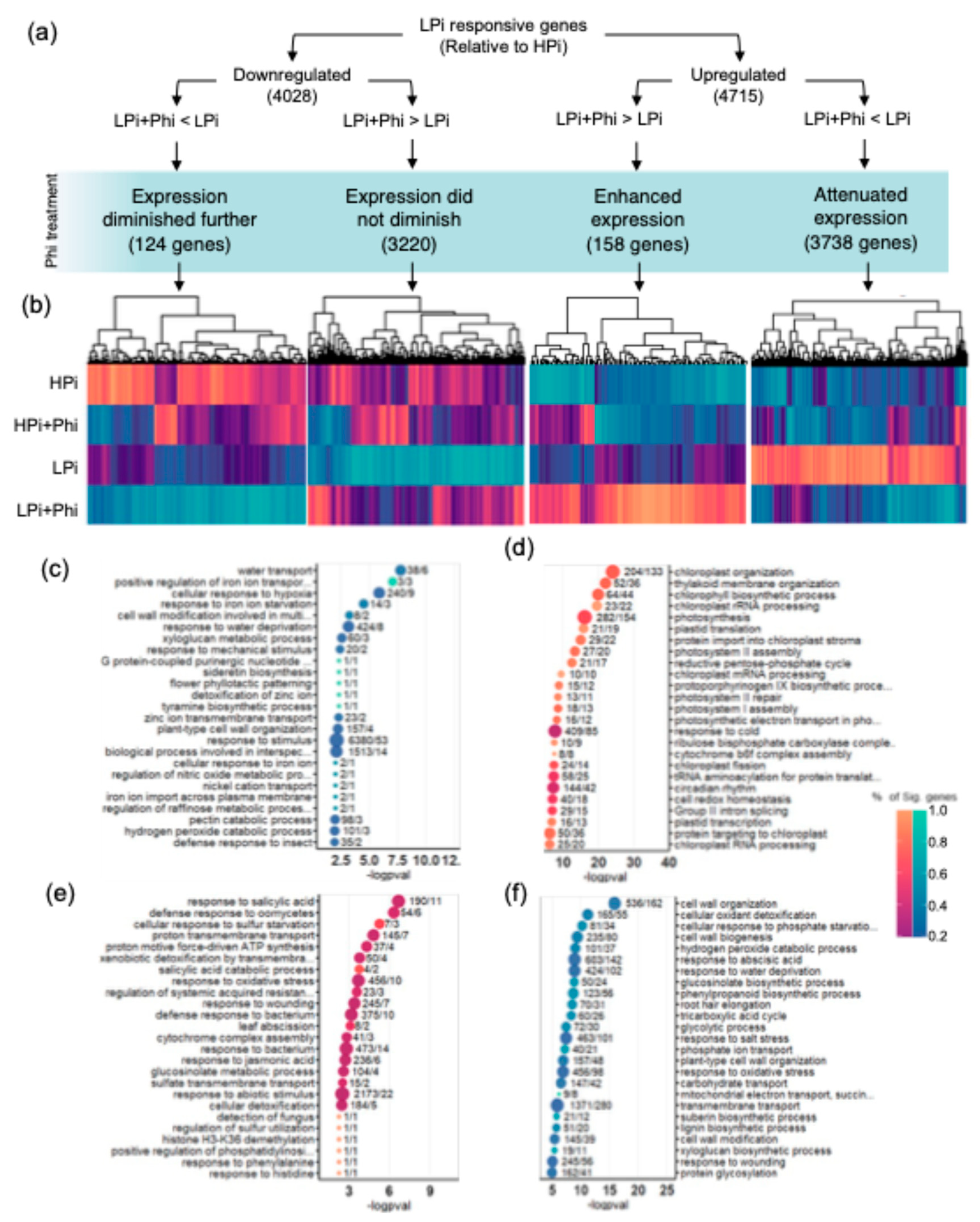
2.2. Phi Induces Transcriptional Changes in Arabidopsis under Both Low and Optimal Pi Levels
2.3. Phi Activates Plant Defense Responses under Pi Sufficiency
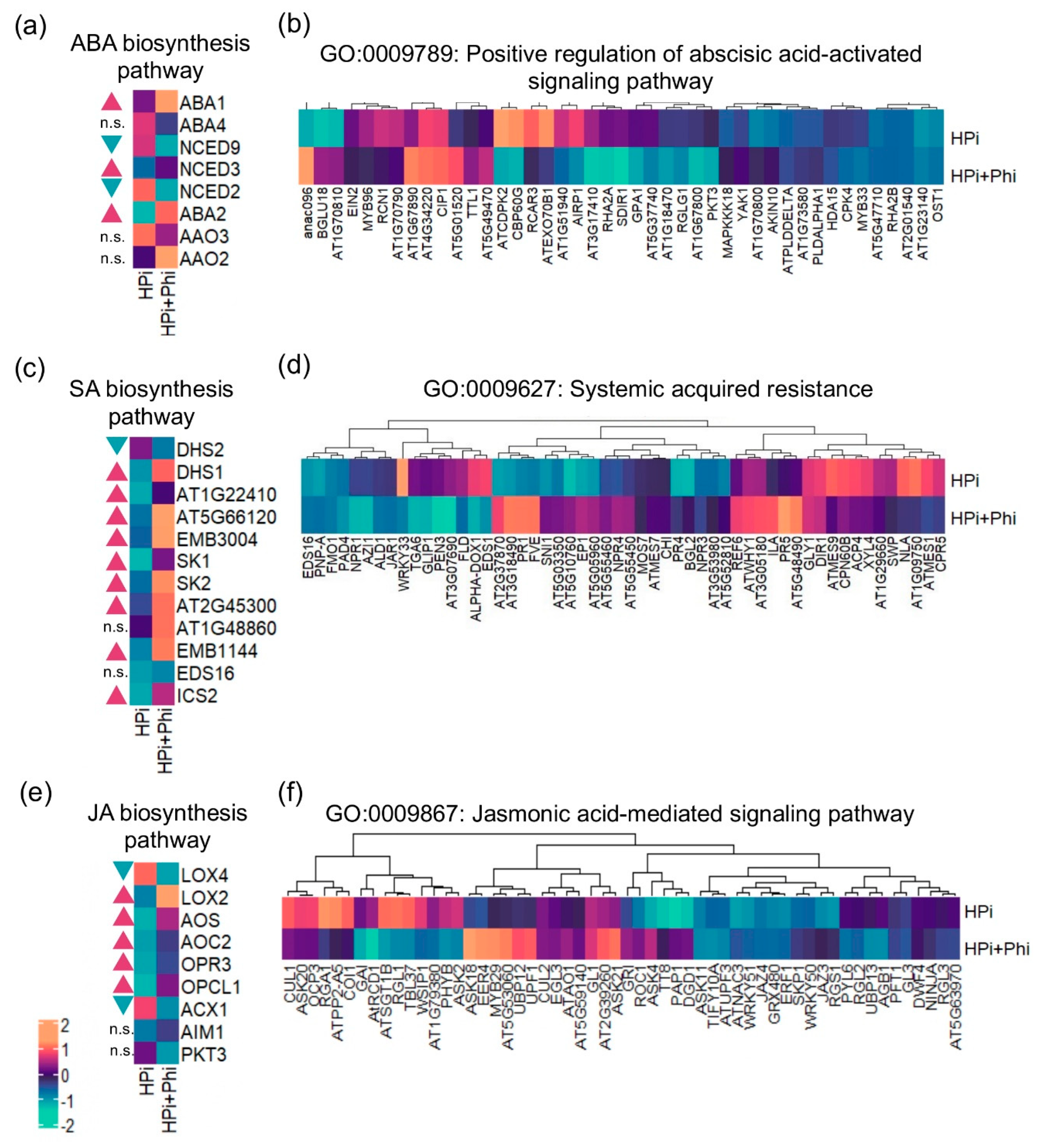
2.4. Phi Enhances the Biosynthesis of ABA, JA, and SA, and Shows a Priming Effect on the Associated Signaling Pathways
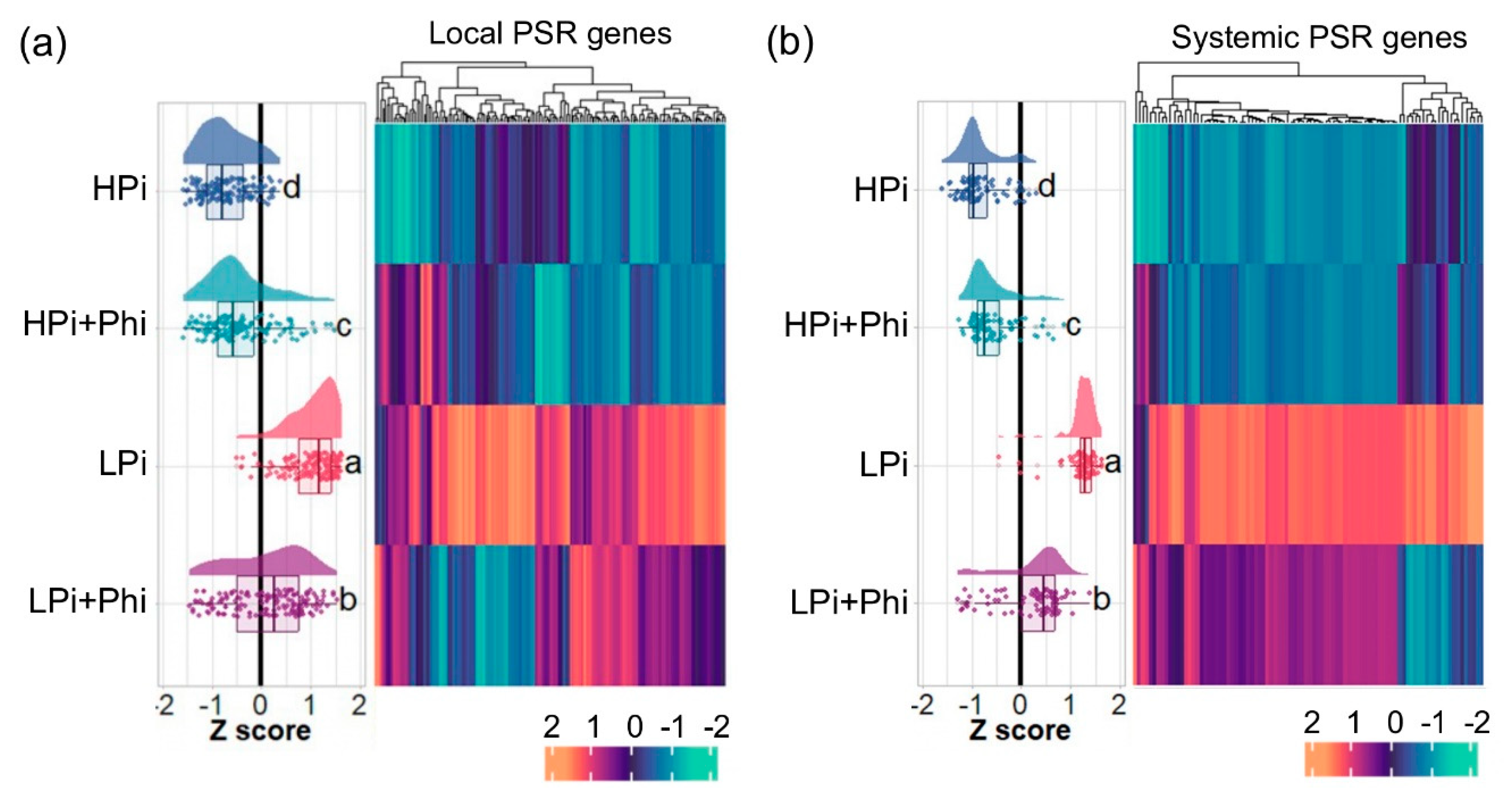
2.5. Phi Suppresses Local and Systemic Responses to Pi Starvation
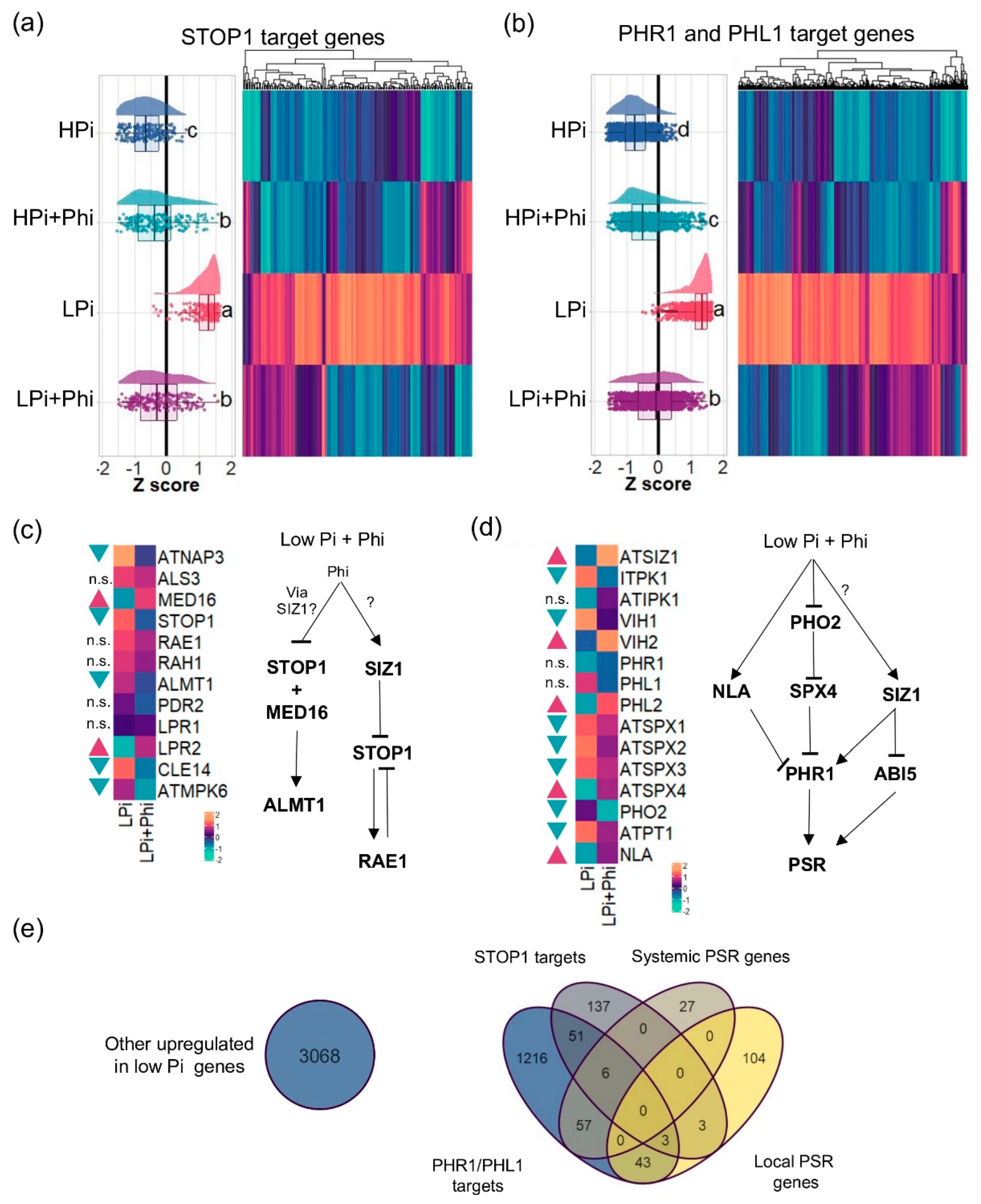
2.6. Phi-Dependent Shutdown of STOP1 and PHR1 Signaling Pathways
2.7. Phi-Dependent Shutdown of STOP1 and PHR1 Signaling Pathways
3. Discussion
3.1. Phi Has a Positive Effect on Plant Growth
3.2. Phi Activates Defense Responses against Multiple Biotic and Abiotic Stresses Using ABA, SA, and JA Signaling Pathways
3.3. Phi Effects on Low Pi Conditions Are Mainly Caused by Suppressing the Activation of the PSRs
3.4. Phi Modulates the Local and Systemic Responses to Low Pi
Conclusions
4. Materials and Methods
Biological Material and Plant Growth
Evaluation of Phi Effect on Arabidopsis Shoot and Root Growth
Experiments for RNA-Seq Studies
Data Checks and RNA-Seq Data Analysis
Histochemical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogaard, A.; Fraser, R.; Heaton, T.H.E.; Wallace, M.; Vaiglova, P.; Charles, M.; Jones, G.; Evershed, R.P.; Styring, A.K.; Andersen, N.H.; et al. Crop Manuring and Intensive Land Management by Europe’s First Farmers. Proc Natl Acad Sci U S A 2013, 110, 12589–12594. [Google Scholar] [CrossRef] [PubMed]
- Russel, D.A.; Williams, G.G. History of Chemical Fertilizer Development. Soil Science Society of America Journal 1977, 41, 260–265. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front Plant Sci 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Merino, F.C.; Gómez-Trejo, L.F.; Ruvalcaba-Ramírez, R.; Trejo-Téllez, L.I. Application of Phosphite as a Biostimulant in Agriculture. New and Future Developments in Microbial Biotechnology and Bioengineering: Sustainable Agriculture: Revisiting Green Chemicals 2022, 135–153. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. Marschner’s Mineral Nutrition of Higher Plants: Third Edition 2011, 135–189. [Google Scholar] [CrossRef]
- Hoang, T.; Bich, T.; Yamakawa, T.; Thi, H.; Thao, B. Phosphite (Phosphorous Acid): Fungicide, Fertilizer or Bio-Stimulator? Soil Sci Plant Nutr 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Guest, D.; Grant, B. THE COMPLEX ACTION OF PHOSPHONATES AS ANTIFUNGAL AGENTS. Biological Reviews 1991, 66, 159–187. [Google Scholar] [CrossRef]
- Yáñez-Juárez, M.G.; López-Orona, C.A.; Ayala-Tafoya, F.; Partida-Ruvalcaba, L.; Velázquez-Alcaraz, T.d.J.; Medina-López, R.; Yáñez-Juárez, M.G.; López-Orona, C.A.; Ayala-Tafoya, F.; Partida-Ruvalcaba, L.; et al. Phosphites as Alternative for the Management of Phytopathological Problems. Revista mexicana de fitopatología 2018, 36, 79–94. [Google Scholar] [CrossRef]
- Graham, J.H. Phosphite for Control of Phytophthora Diseases in Citrus: Model for Management of Phytophthora Species on Forest Trees? †. N Z J For Sci 2011, 41, 49–56. [Google Scholar]
- Cerioni, L.; Rapisarda, V.A.; Doctor, J.; Fikker, S.; Ruiz, T.; Fassel, R.; Smilanick, J.L. Use of Phosphite Salts in Laboratory and Semicommercial Tests to Control Citrus Postharvest Decay. Plant Dis 2013, 97, 201–212. [Google Scholar] [CrossRef]
- Farooq, Q.U.A.; McComb, J.; Hardy, G.E.S.J.; Burgess, T. Soil Amendments and Suppression of Phytophthora Root Rot in Avocado (Persea Americana). Australasian Plant Pathology 2023, 52, 1–11. [Google Scholar] [CrossRef]
- Pegg, K.G.; Whiley, A.W.; Saranah, J.B.; Glass, R.J. Control of Phytophthora Root Rot of Avocado with Phosphorus Acid. Australasian Plant Pathology 1985, 14, 25–29. [Google Scholar] [CrossRef]
- Da Silva, S.R.; Cantuarias-Avilés, T.; Bremer Neto, H.; Mourão Filho, F.D.A.A.; Medina, R.B. MANAGEMENT OF ROOT ROT IN AVOCADO TREES. Rev Bras Frutic 2016, 38, e175. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A Novel P Fertilizer for Weed Management and Pathogen Control. Plant Biotechnol J 2017, 15, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.L.; Schlegel, A.J.; Havlin, J.L.; Schlegel, A.J. Review of Phosphite as a Plant Nutrient and Fungicide. Soil Systems 2021, Vol. 5, Page 52 2021, 5, 52. [Google Scholar] [CrossRef]
- Market Reports World Global Potassium Phosphite Market – Market Reports World. Available online: https://www.marketreportsworld.com/global-potassium-phosphite-market-26318022 (accessed on 18 February 2024).
- Bertsch, F.; Ramírez, F.; Henríquez, C. Agronomía Costarricense. Agronomía Costarricense 2009, 33, 249–265. [Google Scholar]
- Mohammed, U.; Davis, J.; Rossall, S.; Swarup, K.; Czyzewicz, N.; Bhosale, R.; Foulkes, J.; Murchie, E.H.; Swarup, R. Phosphite Treatment Can Improve Root Biomass and Nutrition Use Efficiency in Wheat. Front Plant Sci 2022, 13, 1017048. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D.A. Review of Phosphorus Acid and Its Salts as Fertilizer Materials. J Plant Nutr 2000, 23, 161–180. [Google Scholar] [CrossRef]
- Estrada-Ortiz, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Núñez-Escobar, R.; Sandoval-Villa, M. The Effects of Phosphite on Strawberry Yield and Fruit Quality. J Soil Sci Plant Nutr 2013, 13, 612–620. [Google Scholar] [CrossRef]
- Batista, P.F.; da Costa, A.C.; da Silva, A.A.; Almeida, G.M.; Rodrigues, M.F.M.; Santos, E.C.D.; Rodrigues, A.A.; Müller, C. Potassium Phosphite Induces Tolerance to Water Deficit Combined with High Irradiance in Soybean Plants. Agronomy 2023, 13, 382. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Lin, M.; Chen, G.; Ding, X.; Weng, Q.; Chen, Q. Phosphite-Induced Reactive Oxygen Species Production and Ethylene and ABA Biosynthesis, Mediate the Control of Phytophthora Capsici in Pepper (Capsicum Annuum). Funct Plant Biol 2016, 43, 563–574. [Google Scholar] [CrossRef]
- Lim, S.; Borza, T.; Peters, R.D.; Coffin, R.H.; Al-Mughrabi, K.I.; Pinto, D.M.; Wang-Pruski, G. Proteomics Analysis Suggests Broad Functional Changes in Potato Leaves Triggered by Phosphites and a Complex Indirect Mode of Action against Phytophthora Infestans. J Proteomics 2013, 93, 207–223. [Google Scholar] [CrossRef]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an Analog of Phosphate, Suppresses the Coordinated Expression of Genes under Phosphate Starvation. Plant Physiol 2002, 129, 1232–1240. [Google Scholar] [CrossRef]
- McDonald, A.E.; Grant, B.R.; Plaxton, W.C. PHOSPHITE (PHOSPHOROUS ACID): ITS RELEVANCE IN THE ENVIRONMENT AND AGRICULTURE AND INFLUENCE ON PLANT PHOSPHATE STARVATION RESPONSE. J Plant Nutr 2001, 24, 1505–1519. [Google Scholar] [CrossRef]
- Lee, T.M.; Tsai, P.F.; Shyu, Y.T.; Sheu, F. THE EFFECTS OF PHOSPHITE ON PHOSPHATE STARVATION RESPONSES OF ULVA LACTUCA (ULVALES, CHLOROPHYTA)1. J Phycol 2005, 41, 975–982. [Google Scholar] [CrossRef]
- Jost, R.; Pharmawati, M.; Lapis-Gaza, H.R.; Rossig, C.; Berkowitz, O.; Lambers, H.; Finnegan, P.M. Differentiating Phosphate-Dependent and Phosphate-Independent Systemic Phosphate-Starvation Response Networks in Arabidopsis Thaliana through the Application of Phosphite. J Exp Bot 2015, 66, 2501–2514. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Delatorre, C.A.; Abel, S. Attenuation of Phosphate Starvation Responses by Phosphite in Arabidopsis. Plant Physiol 2001, 127, 963–972. [Google Scholar] [CrossRef]
- Ojeda-Rivera, J.O.; Alejo-Jacuinde, G.; Nájera-González, H.R.; López-Arredondo, D. Prospects of Genetics and Breeding for Low-Phosphate Tolerance: An Integrated Approach from Soil to Cell. Theoretical and Applied Genetics 2022, 135, 4125–4150. [Google Scholar] [CrossRef]
- Madison, I.; Gillan, L.; Peace, J.; Gabrieli, F.; Van den Broeck, L.; Jones, J.L.; Sozzani, R. Phosphate Starvation: Response Mechanisms and Solutions. J Exp Bot 2023, 74, 6417–6430. [Google Scholar] [CrossRef]
- Mccarthy, D.J.; Smyth, G.K. Testing Significance Relative to a Fold-Change Threshold Is a TREAT. Bioinformatics 2009, 25, 765. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Elucidation of the Indirect Pathway of Abscisic Acid Biosynthesis by Mutants, Genes, and Enzymes. Plant Physiol 2003, 131, 1591–1601. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. ABSCISIC ACID BIOSYNTHESIS AND CATABOLISM. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Xiong, L.; Lee, H.; Ishitani, M.; Zhu, J.K. Regulation of Osmotic Stress-Responsive Gene Expression by TheLOS6/ABA1 Locus InArabidopsis. Journal of Biological Chemistry 2002, 277, 8588–8596. [Google Scholar] [CrossRef] [PubMed]
- Thibaud, M.C.; Arrighi, J.F.; Bayle, V.; Chiarenza, S.; Creff, A.; Bustos, R.; Paz-Ares, J.; Poirier, Y.; Nussaume, L. Dissection of Local and Systemic Transcriptional Responses to Phosphate Starvation in Arabidopsis. The Plant Journal 2010, 64, 775–789. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, R.C.; Huang, S.S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; De Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root Microbiota Drive Direct Integration of Phosphate Stress and Immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.T.; Wang, L.F.; Guo, J.X.; Lu, K.K.; Song, R.F.; Zuo, J.X.; Chen, H.H.; Liu, W.C. Abscisic Acid Facilitates Phosphate Acquisition through the Transcription Factor ABA INSENSITIVE5 in Arabidopsis. The Plant Journal 2022, 111, 269–281. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, J.; Han, X.; Li, H.; Kui, M.; Zhang, J.; Huang, Z.; Fu, Q.; Jiang, Y.; Hu, Y. PHOSPHATE STARVATION RESPONSE1 (PHR1) Interacts with JASMONATE ZIM-DOMAIN (JAZ) and MYC2 to Modulate Phosphate Deficiency-Induced Jasmonate Signaling in Arabidopsis. Plant Cell 2023, 35, 2132–2156. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, D. Ethylene and Plant Responses to Phosphate Deficiency. Front Plant Sci 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Herrera-Estrella, L. Engineering Phosphorus Metabolism in Plants to Produce a Dual Fertilization and Weed Control System. Nature Biotechnology 2012, 30, 889–893. [Google Scholar] [CrossRef]
- Dormatey, R.; Sun, C.; Ali, K.; Fiaz, S.; Xu, D.; Calderón-Urrea, A.; Bi, Z.; Zhang, J.; Bai, J. PtxD/Phi as Alternative Selectable Marker System for Genetic Transformation for Bio-Safety Concerns: A Review. PeerJ 2021, 9, e11809. [Google Scholar] [CrossRef]
- Eshraghi, L.; Anderson, J.; Aryamanesh, N.; Shearer, B.; Mccomb, J.; Hardy, G.E.S.J.; O’Brien, P.A. Phosphite Primed Defence Responses and Enhanced Expression of Defence Genes in Arabidopsis Thaliana Infected with Phytophthora Cinnamomi. Plant Pathol 2011, 60, 1086–1095. [Google Scholar] [CrossRef]
- Burra, D.D.; Berkowitz, O.; Hedley, P.E.; Morris, J.; Resjö, S.; Levander, F.; Liljeroth, E.; Andreasson, E.; Alexandersson, E. Phosphite-Induced Changes of the Transcriptome and Secretome in Solanum Tuberosum Leading to Resistance against Phytophthora Infestans. BMC Plant Biol 2014, 14, 1–17. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Lin, M.; Chen, G.; Ding, X.; Weng, Q.; Chen, Q. Phosphite-Induced Reactive Oxygen Species Production and Ethylene and ABA Biosynthesis, Mediate the Control of Phytophthora Capsici in Pepper (Capsicum Annuum). Functional Plant Biology 2016, 43, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.; da Silva, A.A.; Batista, P.F.; Moura, L.M. de F.; Vital, R.G.; Costa, A.C. Hydrogen Sulfide, Potassium Phosphite and Zinc Sulfate as Alleviators of Drought Stress in Sunflower Plants. Ciência e Agrotecnologia 2020, 44, e006320. [Google Scholar] [CrossRef]
- Glinicki, R.; Sas-Paszt, L.; Jadczuk-Tobjasz, E. THE EFFECT OF PLANT STIMULANT/FERTILIZER “RESISTIM” ON GROWTH AND DEVELOPMENT OF STRAWBERRY PLANTS. Journal of Fruit and Ornamental Plant Research 2010, 18, 111–124. [Google Scholar]
- William Ávila, F.; Faquin, V.; Ramos, S.J.; Pinheiro, G.L.; Marques, D.J.; Klynger, A.; Lobato, S.; Ferreira De Oliveira Neto, C.; Ávila, P.A. Effects of Phosphite and Phosphate Supply in a Weathered Tropical Soil on Biomass Yield, Phosphorus Status and Nutrient Concentrations in Common Bean. J Food Agric Environ 2012, 10, 312–317. [Google Scholar]
- Bachiega Zambrosi, F.C. Phosphite and Phosphate Have Contrasting Effects on Nutrient Status of Plants. J Crop Improv 2016, 30, 421–432. [Google Scholar] [CrossRef]
- Thao, H.T.B.; Yamakawa, T.; Shibata, K. Effect of Phosphite-Phosphate Interaction on Growth and Quality of Hydroponic Lettuce (Lactuca Sativa). Journal of Plant Nutrition and Soil Science 2009, 172, 378–384. [Google Scholar] [CrossRef]
- Ali, M.S.; Sutradhar, A.; Edano, M.L.; Edwards, J.T.; Girma, K. Response of Winter Wheat Grain Yield and Phosphorus Uptake to Foliar Phosphite Fertilization. International Journal of Agronomy 2014, 2014. [Google Scholar] [CrossRef]
- Omar, M.M.; Taha, A.A.; Shokir, S.A. Effect of Applying Potassium Phosphite with Potassium Fulvate on Plant Growth. Journal of Soil Sciences and Agricultural Engineering 2020, 11, 255–263. [Google Scholar] [CrossRef]
- Whan, A.P.; Verbyla, A.P.; Mieog, J.C.; Howitt, C.A.; Ral, J.-P. Transferring a Biomass Enhancement Biotechnology from Glasshouse to Field: A Case Study on Wheat GWD RNAi. Agronomy 2017, 7, 82. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytologist 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry 2016, 1, 105–143. [Google Scholar] [CrossRef]
- William Ávila, F.; Faquin, V.; Lopes Araújo, J.; José Marques, D.; Martins Ribeiro Júnior, P.; Klynger da Silva Lobato, A.; Junio Ramos, S.; Pereira Baliza, D. Phosphite Supply Affects Phosphorus Nutrition and Biochemical Responses in Maize Plants. Aust J Crop Sci 2011, 5, 646–653. [Google Scholar]
- Raya-González, J.; Ojeda-Rivera, J.O.; Mora-Macias, J.; Oropeza-Aburto, A.; Ruiz-Herrera, L.F.; López-Bucio, J.; Herrera-Estrella, L. MEDIATOR16 Orchestrates Local and Systemic Responses to Phosphate Scarcity in Arabidopsis Roots. New Phytologist 2021, 229, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An Auxin-Dependent Distal Organizer of Pattern and Polarity in the Arabidopsis Root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Colón-Carmona, A.; You, R.; Haimovitch-Gal, T.; Doerner, P. Spatio-Temporal Analysis of Mitotic Activity with a Labile Cyclin–GUS Fusion Protein. The Plant Journal 1999, 20, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Pound, M.P.; French, A.P.; Atkinson, J.A.; Wells, D.M.; Bennett, M.J.; Pridmore, T. RootNav: Navigating Images of Complex Root Architectures. Plant Physiol 2013, 162, 1802–1814. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J.; Lengauer, T. Improved Scoring of Functional Groups from Gene Expression Data by Decorrelating GO Graph Structure. Bioinformatics 2006, 22, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS Fusions: Beta-glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher Plants. EMBO J 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Malamy, J.E.; Benfey, P.N. Down and out in Arabidopsis: The Formation of Lateral Roots. trends in plant sciences 1997, 2, 390–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




