1. Introduction
Fragile X syndrome (FXS) is a neurodevelopmental disorder caused by the full mutation (FM) in the Fragile X Messenger Ribonucleoprotein 1 (
FMR1) gene on the Xq27.3 chromosome region [
1]. The FM of the
FMR1 gene is caused by an expansion of the CGG trinucleotide repeats (more than 200 CGGs) in the 5’ untranslated region [
2]. The
FMR1 gene codes FMR1 protein (FMRP) which is an RNA binding protein important for translation of certain mRNAs involved primarily in brain development, neuronal synapse formation and synaptic plasticity [
3]. FMRP is known as a translation repressor which limits various protein synthesis in the brain.
FMR1 transcription is suppressed by methylation of the
FMR1 gene, occurring in the FM. In the absence of FMRP, neuronal circuit formation and higher cognitive functions can be impaired [
4]. FMRP has a crucial role in synaptic function due to regulation of the translation of the metabotropic Glutamate Receptor 5 (mGluR5) in astrocytes and myelin production in oligodendrocytes [
5,
6,
7]. On the other hand, FMRP can regulate transcription and RNA synthesis by interaction with transcription factors and chromatin modifying enzymes [
4]. Finally, FMRP can interact with some proteins such as ion channels and regulate their function [
4]. FMRP through the interaction with the large conductance Ca
2+-activated K+ (BK) channels, modulates action potential duration and consequently regulates neurotransmitter release and short-term plasticity in CA3 hippocampal pyramidal neurons [
3]. All these factors together contribute to the clinical presentation of FXS.
FXS is the most common monogenic cause of Autism Spectrum Disorder (ASD) and inherited intellectual disability (ID) [
8]. IQ levels in FXS patients can be correlated with gender, methylation status and FMRP abundance [
9,
10]. Approximately 85% of males and 25-30% of females with FXS have ID [
6]. On the other side, more than half (50–60%) of males and 20% of females with FXS are diagnosed with ASD (reviewed in: Protic et al., 2022 [
11]). Generally, females with FXS have less severe symptomatology due to compensatory activation of the unaffected X [
11,
12].
Besides ASD, patients with FXS exhibit ID, sleep problems, changes in circadian rhythm (CR), delay in the development of motor functions, of speech and of language in early childhood, and often attention deficit hyperactivity disorder (ADHD) [
13]. FXS can be also characterized by other comorbidities such as neurological seizures, endocrinological problems like obesity, cardiac abnormalities (mitral valve prolapse), and macroorchidism in puberty [
14,
15]. Recurrent otitis media infections in childhood and strabismus are also present in patients with FXS. Physical appearance can also be characterized by an elongated face, prominent ears, mandibular prognathism, and joint hypermobility [
14]. As mentioned above, sleep problems are often present in individuals with FXS and manifest mostly as hardly falling asleep, impaired sleep quality and waking up many times during the night. Sleep problems tend to affect quality of patient’s life and have a negative impact on the whole family [
11,
16].
FXS preclinical research on animals is limited to a few models, such as the
Fmr1 knock-out (KO) mice, the
Fmr1 KO zebrafish and the
Drosophila melanogaster model of FXS (
Fmr1, FBgn0028734 [
17]; herein named
dFMR1) such as
dFMR1B55 mutants [
18,
19,
20]. The
dFMR1B55 allele is one example of the
Drosophila melanogaster model of FXS. In
dFMR1B55, the 2.5 kb deletion including exons 2, 3, and 4 of the
dFMR1 gene was generated by an imprecise excision of the EP(3)3422 P-element [
21]. The
Drosophila melanogaster models of FXS, such as
dFMR1B55, show impairment in CR, climbing abilities, social interaction, olfactory learning and memory etc. [
22,
23]. Tracking of locomotor activity enables sleep and CR analysis in the
Drosophila melanogaster models of FXS. Until now, CRs have been studied in
Drosophila mostly with infrared-based beam-crossing methods [
21,
23,
24,
25,
26,
27]. These methods may lead to under-estimation of the total activity of flies throughout the day, which may, in turn, affect estimation of critical CR and sleep statistics [
28,
29].
The aim of this study is to analyze sleep pattern and CR in dFMR1B55 mutants as an animal model for further research in the field of FXS, using a new platform based on continuous high resolution video monitoring in integration with customized version of the open-source app VANESSA.
2. Results
2.1. CR Rhythm Analysis in the w1118, per01 and dFMR1B55 Groups
The CR of white1118 (w1118), period01 (per01) and dFMR1B55 flies was analyzed using video monitoring and VANESSA application.
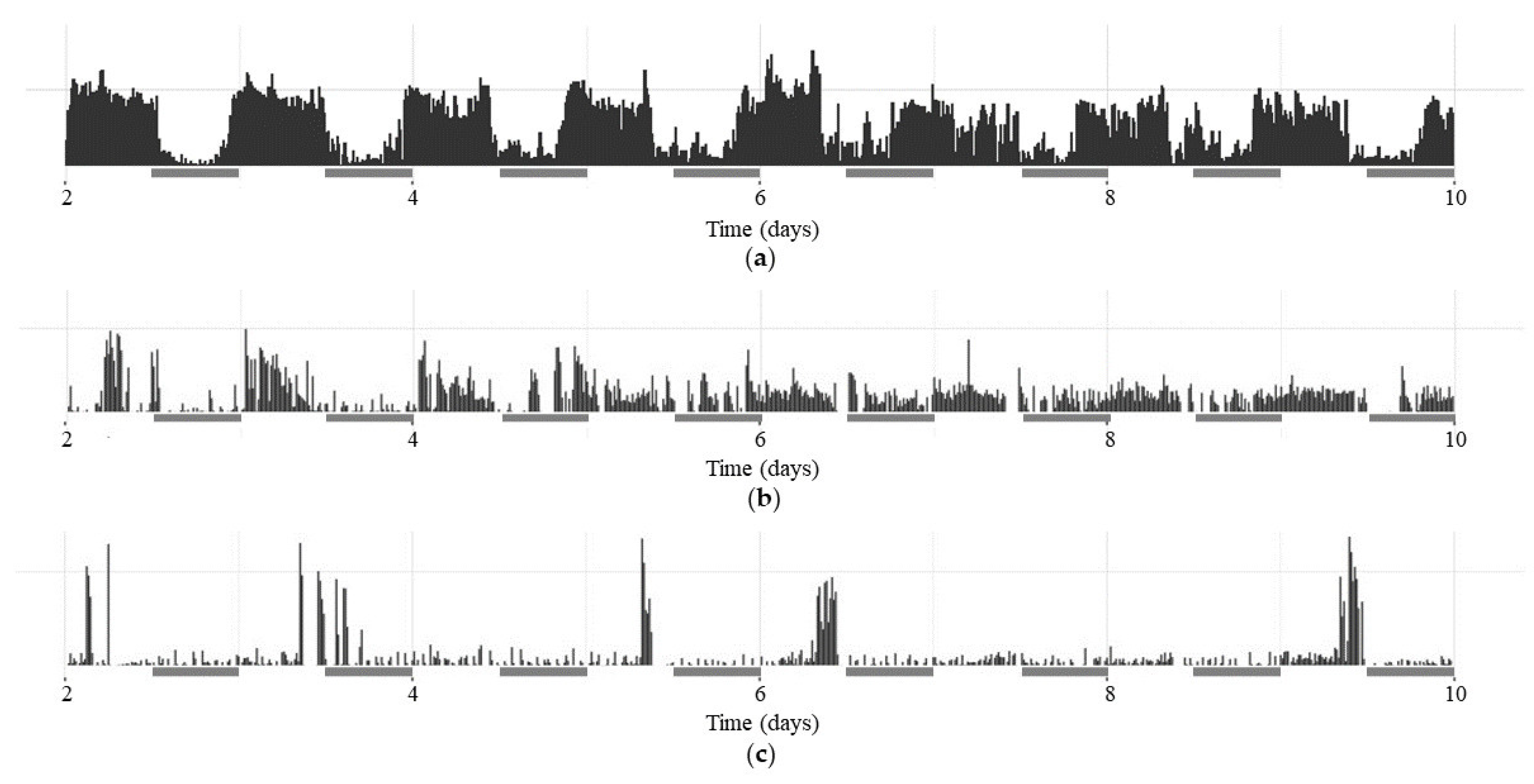
A graphical representation of the activity patterns (CRs) of selected flies of the
white1118 (
w1118),
period01 (
per01) and
dFMR1B55 groups in the dark-dark (DD) period (from 3
rd to 10
th experimental day) and their synchronization with environmental cues (light) is presented in actograms within
Figure 1.
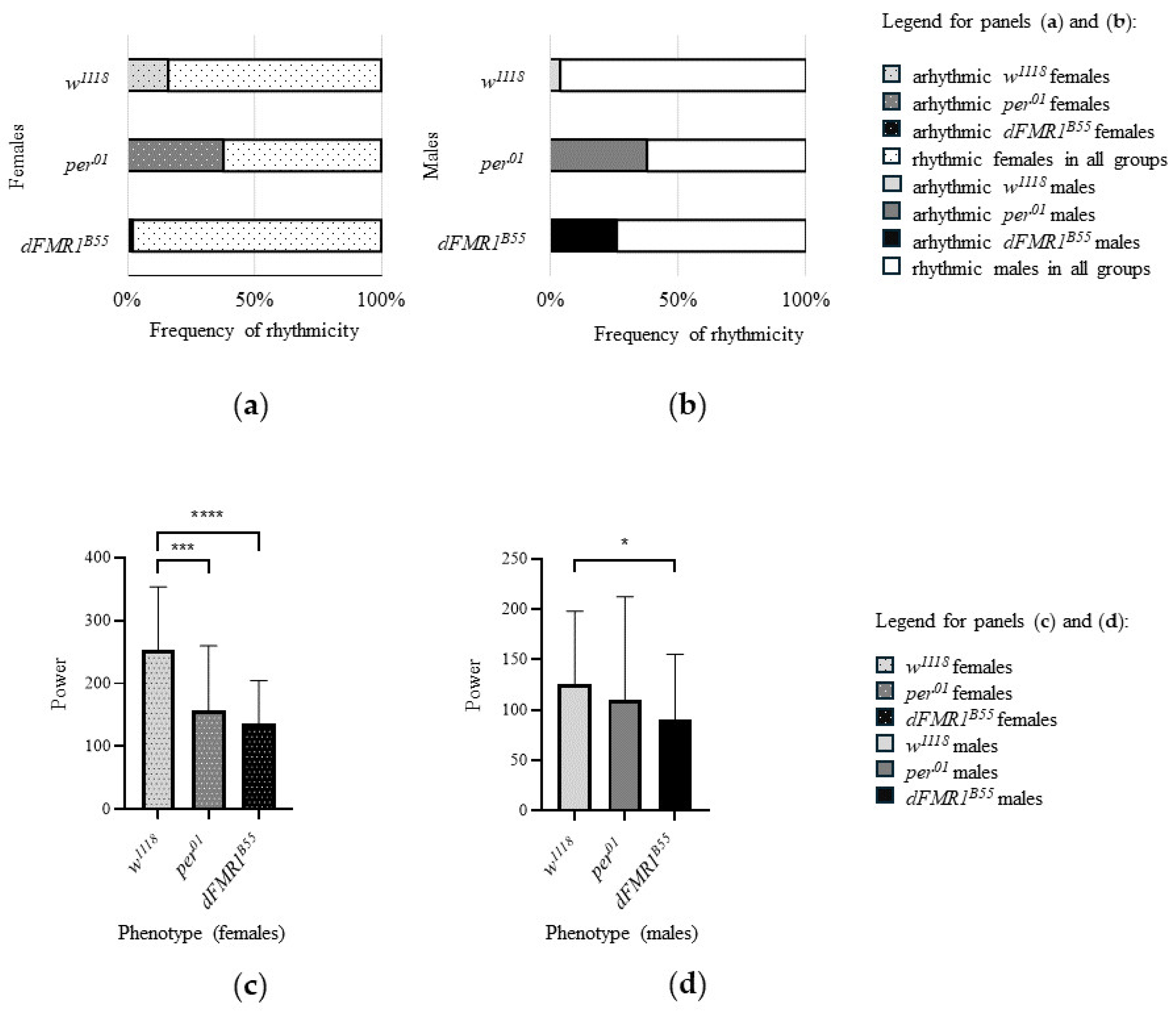
In the control w1118 groups (N = 50 for males and N = 50 for females), the rhythmicity was observed in 84% (42/50) of females and in 96% (48/50) of male flies. Comparison between sexes showed a significantly higher frequency of rhythmic flies in males (p = 0.04). The mean period for rhythmic individuals was 23.70 ± 0.17 h for females (N = 42) and 23.78 ± 0.27 h for males (N = 48) and there was no statistically significant difference in period values (p = 0.14) between sexes. In addition, w1118 rhythmic females had significantly higher (p < 0.0001, both) median and mean values of rhythm power (median = 263.90, with range: 68.85 - 423.60; mean ± SD: 252.60 ± 100.90) in comparison with w1118 rhythmic males (median = 110.90, with range: 21.17 - 385.00, mean ± SD: 125.60 ± 72.49).
In the per01 groups, the rhythmicity was observed in 62% (31/50) of females and in 62% (31/50) of male flies. Comparison between sexes showed the same frequency of rhythmic flies (p > 0.99). The mean period for rhythmic individuals was 23.60 ± 0.31 h for females (N = 31) and 23.86 ± 0.27 h for males (N = 31). Furthermore, for rhythmic females, the median value of the power of the rhythm was 145.00 (range: 17.99 - 395.50) and the mean value was 157.30 ± 102.50. In addition, the median and mean values for rhythmic males were 68.49 (range: 15.10 - 373.80 and 109.60 ± 102.70, respectively. Interestingly, male per01 rhythmic individuals had a significantly longer period (p = 0.0002), while per01 rhythmic females had a significantly higher power of the rhythm than the rhythmic per01 males (p = 0.0180).
Finally, in the experimental dFMR1B55 groups, rhythmicity was observed in 98% (49/50) of females and in 74% (37/50) of male flies indicating a significantly lower frequency of rhythmic flies in males (p = 0.0005). The mean period for rhythmic individuals was 23.66 ± 0.36 h for females (N = 49) and 23.69 ± 0.49 h for males (N = 37) without statistically significant difference in period values (p = 0.51) between sexes. The median values of the power of the rhythm were 128.90 (range: 14.18 - 314.80) for rhythmic females and 82.65 (range: 15.12 - 292.20) for rhythmic males; while the mean values of the same parameter were 135.60 ± 68.81 for females and 90.18 ± 65.16 for males. Like w118 and per01 females, dFMR1B55 rhythmic females had a significantly higher power of rhythm than the rhythmic dFMR1B55 males (p = 0.001).
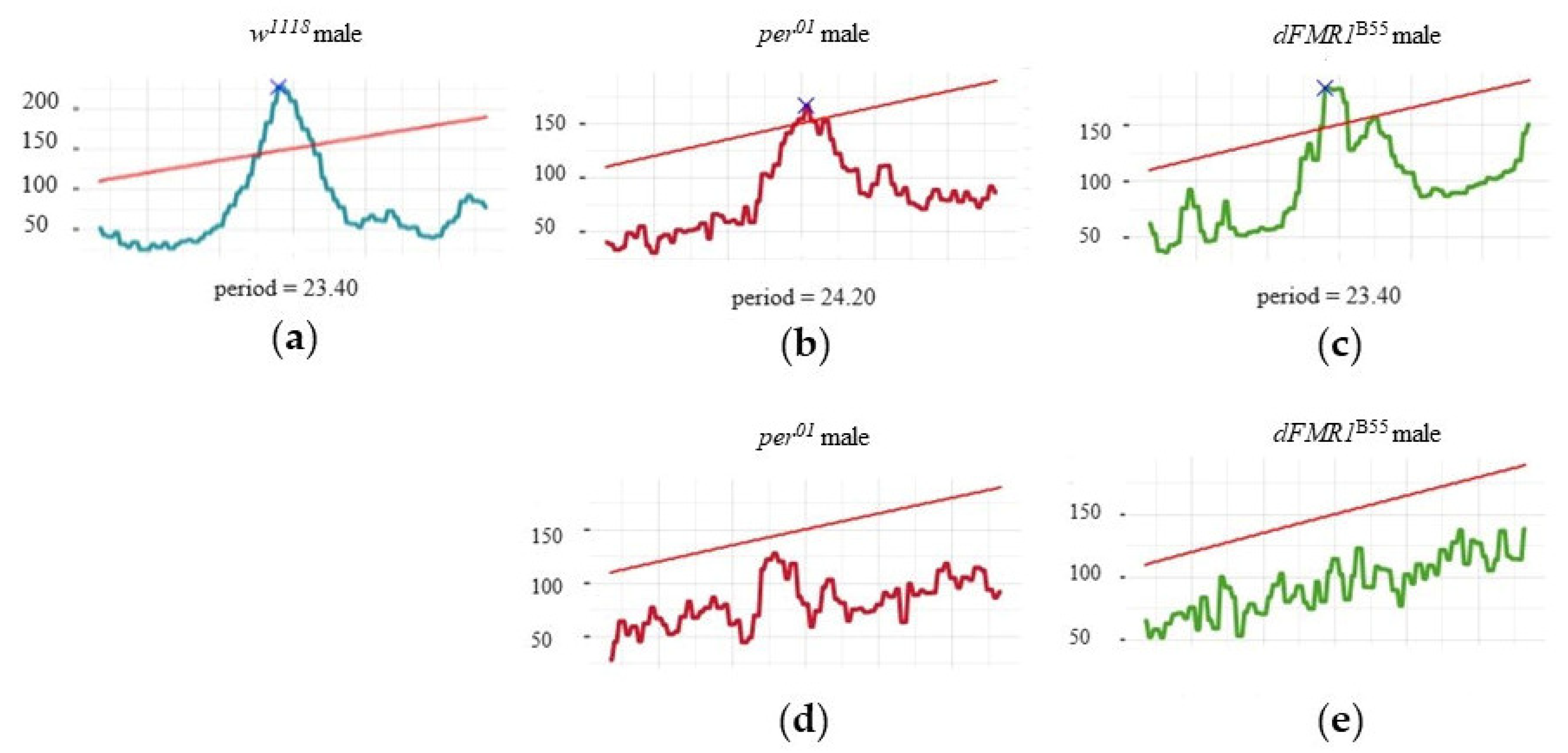
The described values of periods and power of the CRs, in individual rhythmic and arhythmic
w1118,
per01, and
dFMR1B55 males, are visualized within selected chi-square periodograms in
Figure 2.
Comparisons of examined variables related to CR (frequency of rhythmicity, period and power of rhythm) among included groups of flies revealed that the lowest frequencies of rhythmic flies of both sexes were noticed in per01 (61% in both, males and females). However, a significantly lower frequency of rhythmicity was shown for dFMR1B55 males compared to w1118 males (p = 0.002). Interestingly, dFMR1B55 females had a higher frequency of rhythmicity compared to w1118 of the same sex (p = 0.01). On the other hand, there were not statistically significant differences between dFMR1B55 and per01 males (p = 0.20) in term of frequency of rhythmic flies, while for females, dFMR1B55 showed a higher frequency of rhythmicity compared to per01 (p < 0.0001). Finally, for both sexes, w1118 had a higher frequency of rhythmicity compared to per01 (females: p = 0.01; males: p < 0.0001).
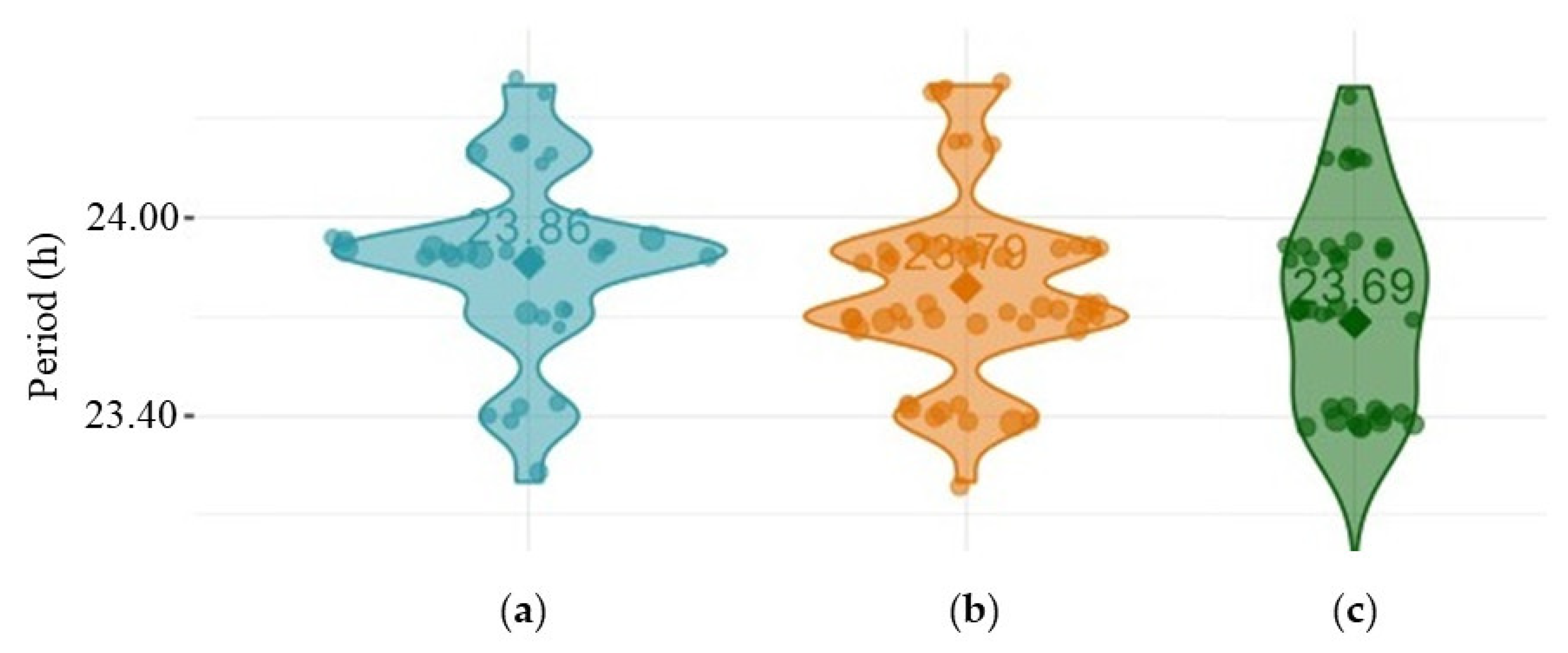
Periods for rhythmic individuals were not statistically different for
w1118 vs per01 vs dFMR1B55 (females: p = 0.25; males: p = 0.10). Multiple comparisons among included groups divided by sex did not show statistically significant differences in period values (p > 0.05, all). Violin plots of periods, presented in
Figure 3, show that there were approximately the same period values in rhythmic males of all examined groups
(w1118,
per01 and
dFMR1B55).
The power of the rhythm comparison among w1118, per01 and dFMR1B55 revealed statistically significant differences among them (females: p < 0.0001; males: p = 0.02). Specifically, for both sexes, dFMR1B55 had significantly lower power than w1118 (females: p < 0.0001; males: p = 0.03). There were no significant differences between dFMR1B55 and per01 in power values (both sexes: p > 0.9999).
Bar plots of frequency of rhythmicity and power of rhythm in
w1118,
per01 and
dFMR1B55 are presented in
Figure 4.
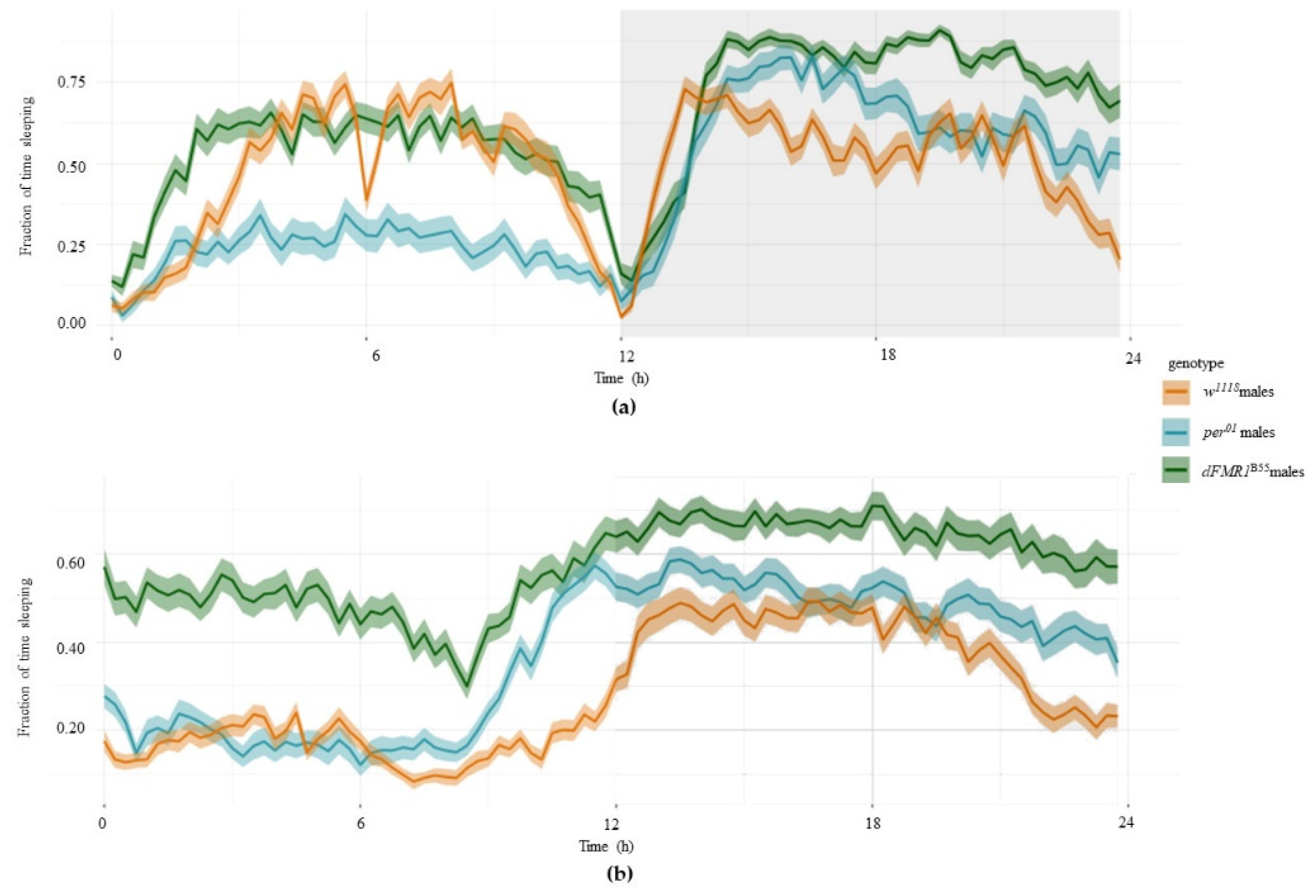
2.2. Sleep Analysis in the w1118, per01 and dFMR1B55 Groups
The sleep patterns of white1118 (w1118), period01 (per01) and dFMR1B55 flies was analyzed using video monitoring and VANESSA application.
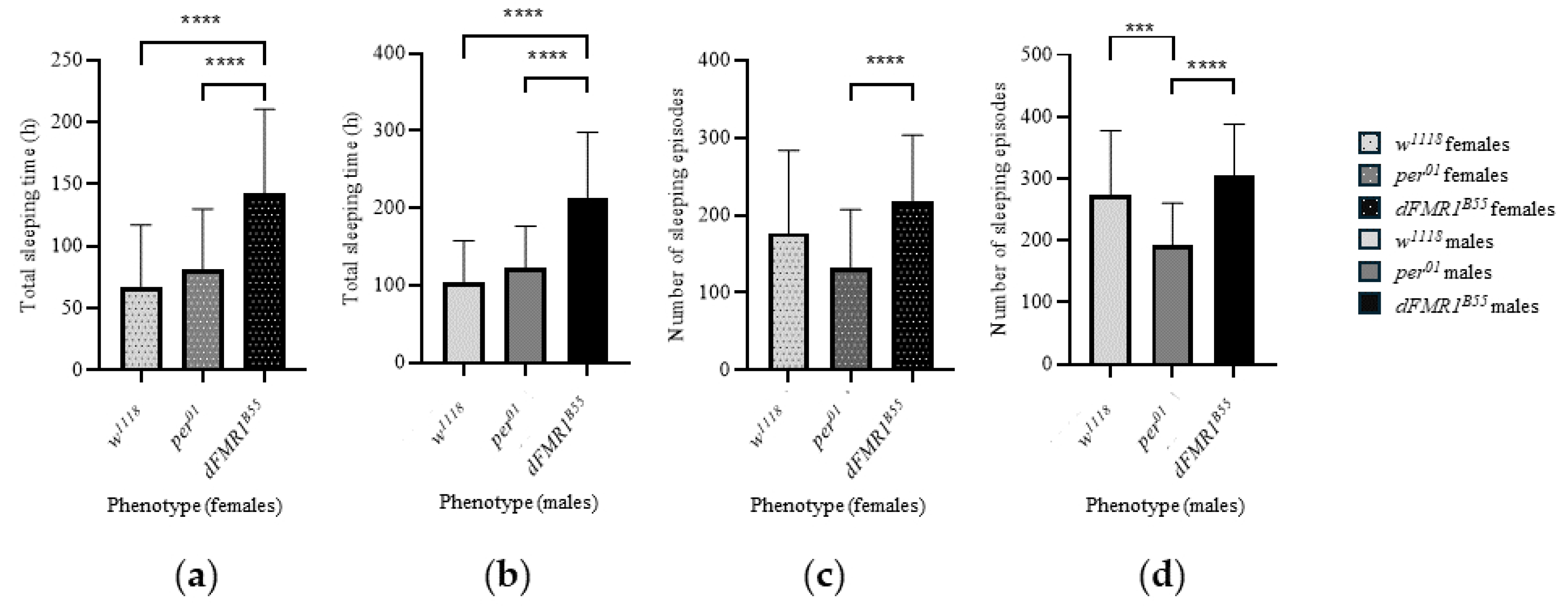
In the control w1118 groups, the total sleeping time during DD (3rd to 10th day) was 66.95 ± 50.12 h (median: 61.03 h with range: 3.52 - 172.30 h) for females and 104.70 ± 53.05 h (median: 100.40 h with range: 17.27 - 228.30 h) for males. Females had a total of 177.30 ± 106.60 sleeping episodes (median: 174.50 with a range 17 - 443) during the whole DD period, while males had 272.60 ± 104.90 (median: 267.50 with a range 63.00 - 493.00). Comparison between sexes showed that w1118 males had a significantly longer total sleeping time in DD (p = 0.0005) with a larger number of sleeping episodes compared to w1118 females (p < 0.0001).
In the per01 groups, the total sleeping time during DD (3rd to 10th day) was 81.48 ± 48.48 h (median: 72.90 h with range: 12.42 - 244.50 h) for females and 123.30 ± 53.25 h (median: 124.60 h with range: 19.47 - 285.70 h) for males. Females had a total of 132.20 ± 74.96 sleeping episodes (median: 106.50 with range 31.00 - 289.00) during whole DD while males had 191.90 ± 68.29 (median: 182.00 with range 39.00 - 344.00). Comparison between sexes showed that per01 males had a significantly longer total sleeping time in DD (p < 0.0001) with a larger number of sleeping episodes (p < 0.0001).
Finally, in the experimental dFMR1B55 groups, the total sleeping time during DD (3rd to 10th day) was 142.70 ± 67.53 h (median: 146.50 h with range: 16.32 - 279.50 h) for females and 212.50 ± 85.32 h (median: 243.50 h with range: 40.38 - 318.80 h) for males. Females had a total of 218.50 ± 84.95 sleeping episodes (median: 215.50 with range 56.00 - 402.00) during whole DD, while males had 304.90 ± 83.44 (median: 314.00 with range 130.00 - 496.00). Comparison between sexes showed that dFMR1B55 males had a significantly longer total sleeping time in DD (p < 0.0001) with a larger number of sleeping episodes (p < 0.0001).
Comparison of examined variables related to sleep (total sleeping time during DD and number of sleeping episodes during DD) revealed statistically significant differences between those three groups, both in males and females (p < 0.0001, all). Longest total sleeping time during DD is noticed in dFMR1B55 in both sexes and average values and medians were twice larger than in w1118 and per01 flies (p < 0.0001, all). On the other hand, there were not statistically significant differences between w1118 and per01 in the total sleeping time during DD (females: p = 0.63; males: p = 0.40). Compared to per01, both dFMR1B55 males and females showed a statistically significant larger number of sleeping episodes in DD (p < 0.0001).
Bar plots and graphical presentation of sleep analyses in
w1118,
per01 and
dFMR1B55 are presented in
Figure 5 and
Figure 6, respectively.
3. Discussion
The current study is the first study that used high-resolution continuous video monitoring for a detailed analysis of circadian rhythm and sleep patterns in Drosophila Fmr1 mutants based on their locomotor activity. This study revealed a lower percentage of rhythmic males in the dFMR1B55 group in comparison to the control wild-type male flies. This observation may be based on the duration of their sleep, which revealed that dFMR1B55 generally sleeps more compared to the w1118 and per01 phenotypes. Although there is a high frequency of rhythmic flies in the dFMR1B55 group in both sexes, it is important to emphasize that they mostly had a significantly lower power of rhythm than w1118. On the other hand, their power of rhythm is similar to rhythmic per01 flies. All investigated groups of flies had a similar period of CR, which was around 24 hours.
CR changes through the measurement of locomotor activity have been previously tested in a few
Drosophila FXS models. All these studies showed
dFMR1 mutants as heterogeneous populations consisting of both rhythmic and arrhythmic individuals [
21,
23,
25,
26]. In accordance with our study, previous studies that investigated CR in
dFMR1B55 mutants also found differences in frequency of rhythmicity between
dFMR1B55 and wild-type of flies. All previously described types [
21,
26]. However, their results were independent of sexes, while groups of flies were divided by sexes in the current study. Although the frequency of rhythmicity of the
dFMR1B55 mutants found in previous studies [
21,
26] was much lower than in the current study, these results cannot be compared due to different experimental designs. Specifically, in the current study, the experimental design was based on video tracking of flies in subgroups divided by sex. However, similarly to our results, Inoue et al. (2002) calculated the period for the
dFMR1B55 mutants as 23.80 ± 0.50 h [
21].
Per is a key gene in CR regulation [
30] and
per01 was included as a control group in this study based on results in previously published articles that reported the majority of
dFMR1B55 males as arrhythmic [
21]. Interestingly, according to the results obtained in the current study using high-resolution videography and VANESSA software, the majority of these flies were rhythmic with a low power of rhythm. Clock
per01 mutants have a non-functional PER protein and cannot sustain rhythmicity properly [
28]. However, there are studies where weak CR components were detected in
per01 [
31,
32]. Klarsfeld et al. (2003) classified more than a third of
per01 individuals as rhythmic [
28], with weak power of rhythm, which is in concordance with the results obtained in the present study. In the current study, the frequency and power of
dFMR1B55 males` rhythmicity did not differ from
per01 males, suggesting that regarding CR,
dFMR1B55 males are more similar to the
per01 line, where weak rhythm was detected, and completely different than
w1118 same sex. Thus, the main research question is could scientists consider that
dFMR1B55 males are rhythmic with weak power of rhythmicity rather than they are being completely arhythmic? Based on our findings, it is evident that most
dFMR1B55 males exhibit weak rhythmicity compared to the control group (
w1118). This distinction in rhythmicity is supported by the methodology employed in our research. However, the same conclusion cannot be drawn for
dFMR1B55 females, as our results indicate that almost all of them displayed rhythmicity. Also,
w1118 females could not be a proper control group for comparison since, unexpectedly, the frequency of rhythmicity is lower in
w1118 females than in the
dFMR1B55 of the same sex.
Other studies investigating CR rhythm in the
Drosophila FXS model used different strains (
dFXRΔ113 and
dFMR3), and also showed differences in frequency of rhythmicity between FXS flies models and wild-type flies [
23,
25]. The power of rhythm as a CR parameter of
dFMR3 was only reported in the study conducted by Dockendorff et al (2002) using Fast Fourier Transform (FFT) analysis. Similar to our results, they showed much lower power of rhythmicity in
dFMR1 male mutants compared to wild-type [
23]. In addition, previous sleep study showed longer total sleep duration and a higher number of sleeping episodes in the
Drosophila FXS model compared to the wild-type, which is consistent with our results [
24]. In all these studies, no measures were made separately on females and males as done in the present study.
All previously described CR studies of
Drosophila FXS models used the infrared-based beam crossing method by
Drosophila Activity Monitoring (DAM) system [
21,
23,
24,
25,
26]. The previously used data analysis software was different in different studies. High-resolution continuous video monitoring with the Zantiks MWP Unit used in the current study is different from the most widely used infrared-based beam crossing method. While the infrared-based method considers the number of crossing beams events during time and is limited to movements near beams, the results of videography are presented as crossed distances. Micromovements of
Drosophila happening anywhere in the well can also be detected using this tracking system. Some scientists reported their concerns about the under- or over-estimation of infrared detection of some fly movements. These limitations are linked to fly positions in the system [
33]. In the current study, frequent short grooming bouts followed by short, crossed distances are being noticed in
dFMR1B55 mutants. In other words, the video monitoring used in the current study can detect those micromovements anywhere in the system. These micromovements could be probably the main reason of higher frequency of rhythmic flies in
per01 groups and general less total sleep values. Observed difference in methodology between DAN system and video-based monitoring may be also the main reason for variable results on CR in
dFMR1 mutants. Video monitoring as a more sensitive method could be useful especially in mutants with reduced overall motility [
34]. Bolduc et al. (2010) reported lower locomotor activity in
dFMR1B55 compared to
w1118 [
22], which is similar to the current study and could be related to significantly higher total sleeping in
dFMR1B55 mutants. In addition, video recording always shows less sleep than DAM systems [
35]. Micromovements less than 3 mm, i. e. activity records less than 1 body length of flies, will be considered for exclusion in the next iteration of our work. However, based on current and previous results, video monitoring used in our study could present a recommended methodology for sleep research and CR analyses in
dFMR1 mutants.
Zantiks MWP Unit is a fully automated system with no need for additional interventions during the whole experimental period [
36]. The system includes software, is controlled via a web browser and has full customer support. On the other hand, challenges are associated with software for CR and sleep analyses because almost all available software is adapted to the DAM system. However, VANESSA is an open-source, user-friendly application for CR and sleep data acquisition, analysis, and visualization [
37,
38], and it was adapted for analyses of fruit fly video tracking recordings in our research. The VANESSA apps have Graphical User Interfaces (GUIs), are also hosted on a server and can be directly used from a browser (available on
https://cryptodice.shinyapps.io/vanessa-dam-cra/ and
https://cryptodice.shinyapps.io/vanessadam-sa/) [
38]. Neither Zantiks MWP Unit nor VANESSA apps require high additional engineering or programming knowledge, which is an important advantage for research laboratories. Finally, the combination of Zantiks MWP and VANESSA presents modern, useful technology that provides a more sensitive methodology in the field of chronobiology.
Author Contributions
Conceptualization, D.P.; methodology, S.M., A.G. and M.S.; software, S.M. and A.G.; validation, D.P, and S.M.; formal analysis, S.M.; investigation, S.M, V.M. and M.S.; resources, D.P; data curation, S.M., V.M. and M.S.; writing—original draft preparation, S.M. and V.M.; writing—review and editing, D.P, M.C, and D.B.; visualization, S.M, and D.P.; supervision, D.P and D.B.; project administration, D.P.; funding acquisition, D.P. All authors have read and agreed to the published version of the manuscript.