Submitted:
29 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Viruses and Infection Procedures
2.3. Animals
2.4. Viral Burden Measurements
2.5. Leukocyte Isolation and Flow Cytometry
2.6. Flow Cytometry Antibodies and Tetramers
2.7. RNA Isolation and qRT-PCR
2.8. RNA Sequencing
2.9. Multiplexed Error-Robust In Situ Hybridization (MERFISH): Sample Preparation and Imaging
2.10. MERFISH: Post-Imaging Data Processing and Analysis
2.11. Gene Module Score Calculation
2.12. Generation of Lgals3bp-/- C57BL6/J Mice
2.13. Lgals3bp-/- Mice Genotyping
2.14. Statistical Analysis
3. Results
3.1. Microglia Activated by WNV Share Transcriptional Signatures with Aged/Senescent Microglia
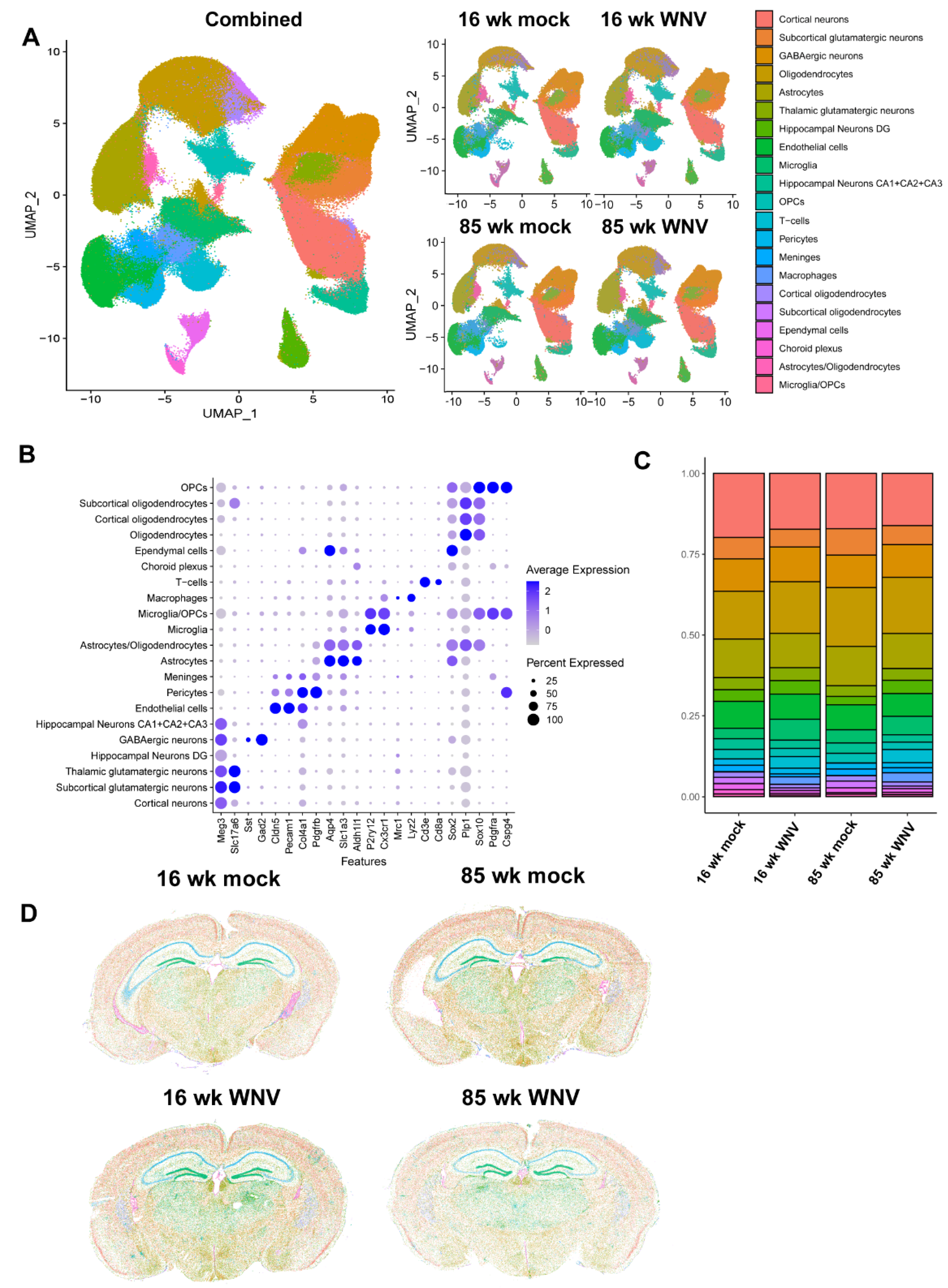
3.2. Spatial Imaging Reveals Infection and Aging-Dependent Transcriptomic Changes in Mouse Brain
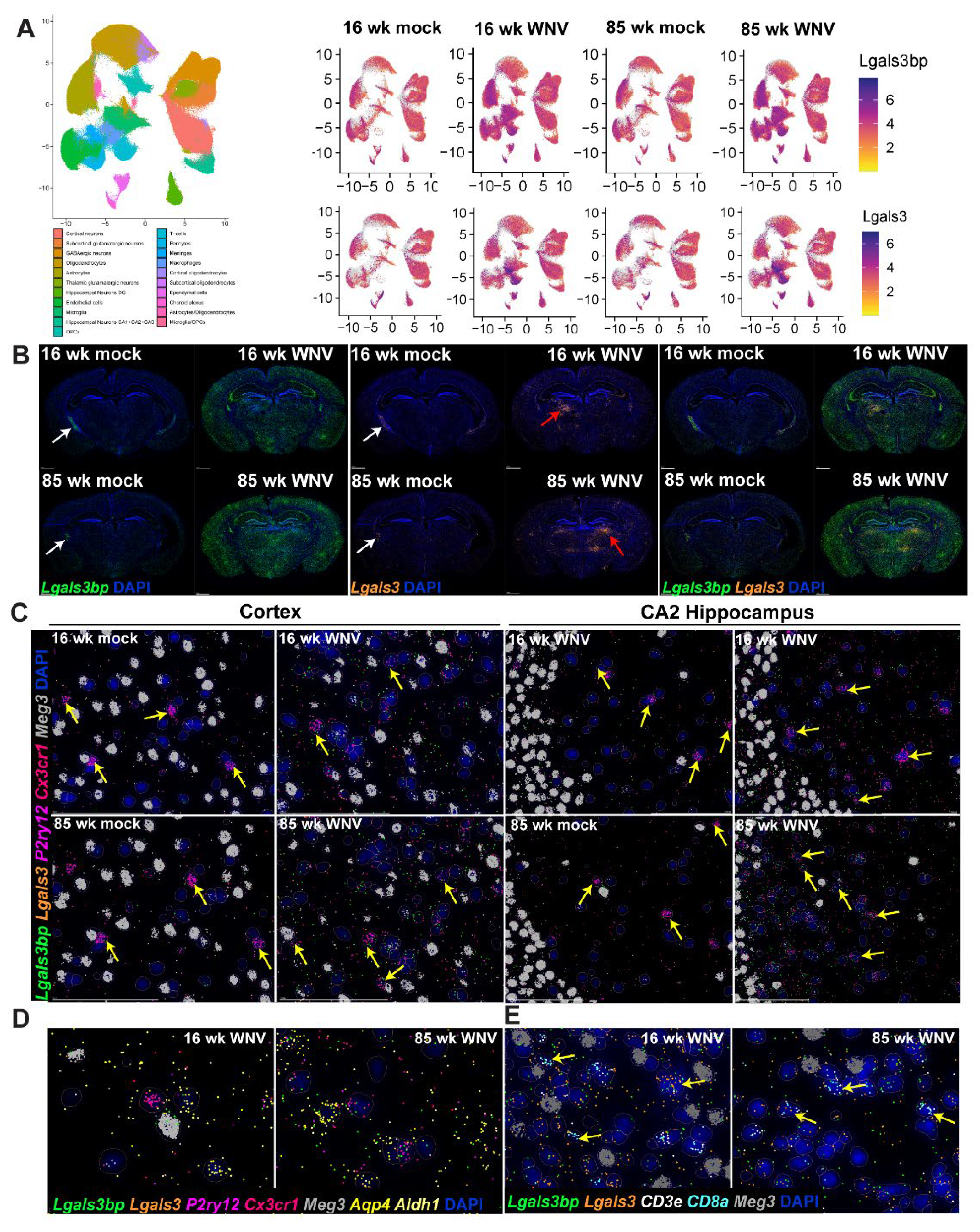
3.3. Lgals3bp mRNA Is Broadly Expressed in the CNS by Microglia, Astrocytes, Neurons and, Most Prominently, by Ependymal Cells and the Choroid Plexus
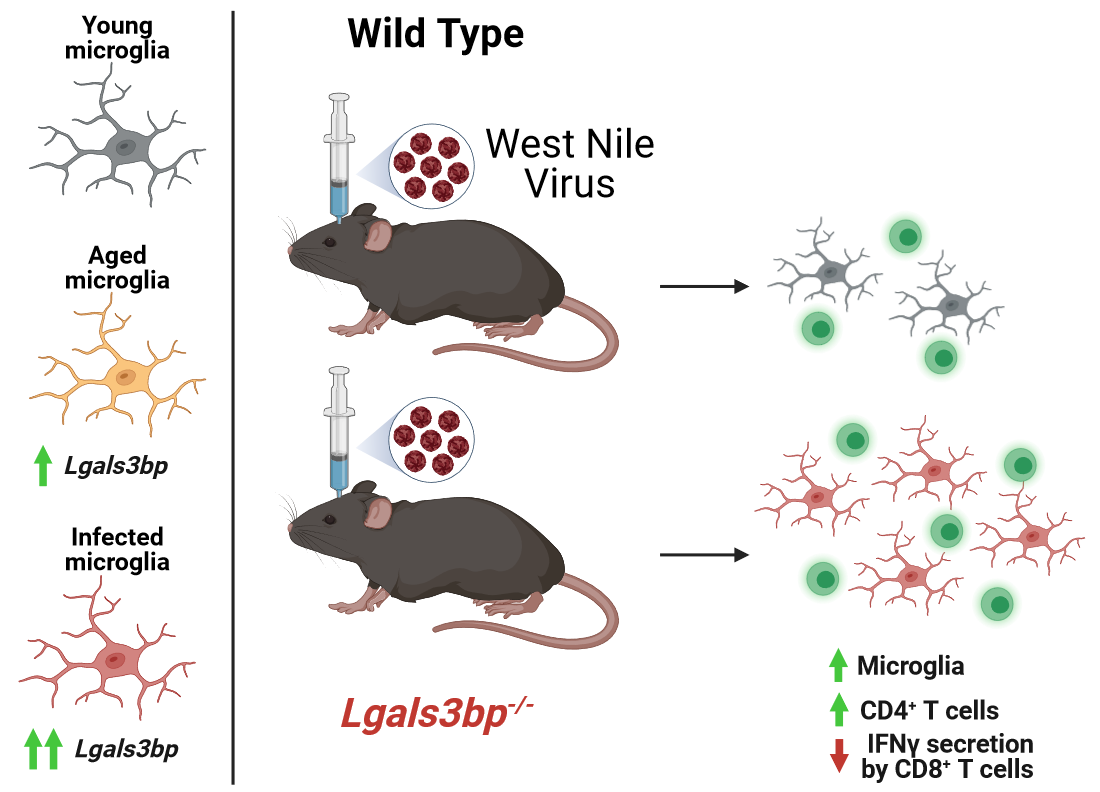
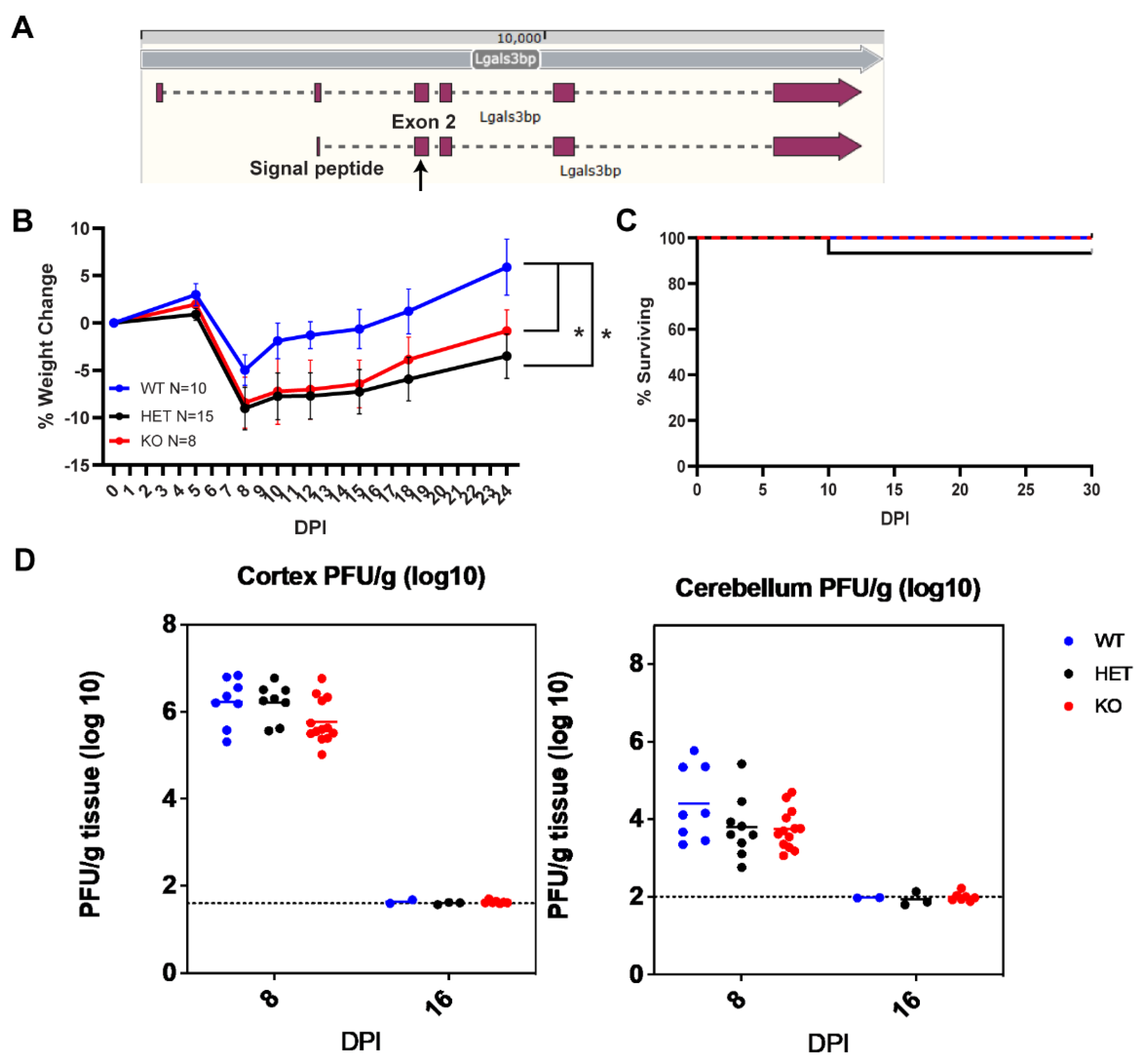
3.4. WNV Infection of Lgals3bp-/- C57BL6/J Mice Exhibit Increased Severity of Infection without Differences in Virologic Control or Survival
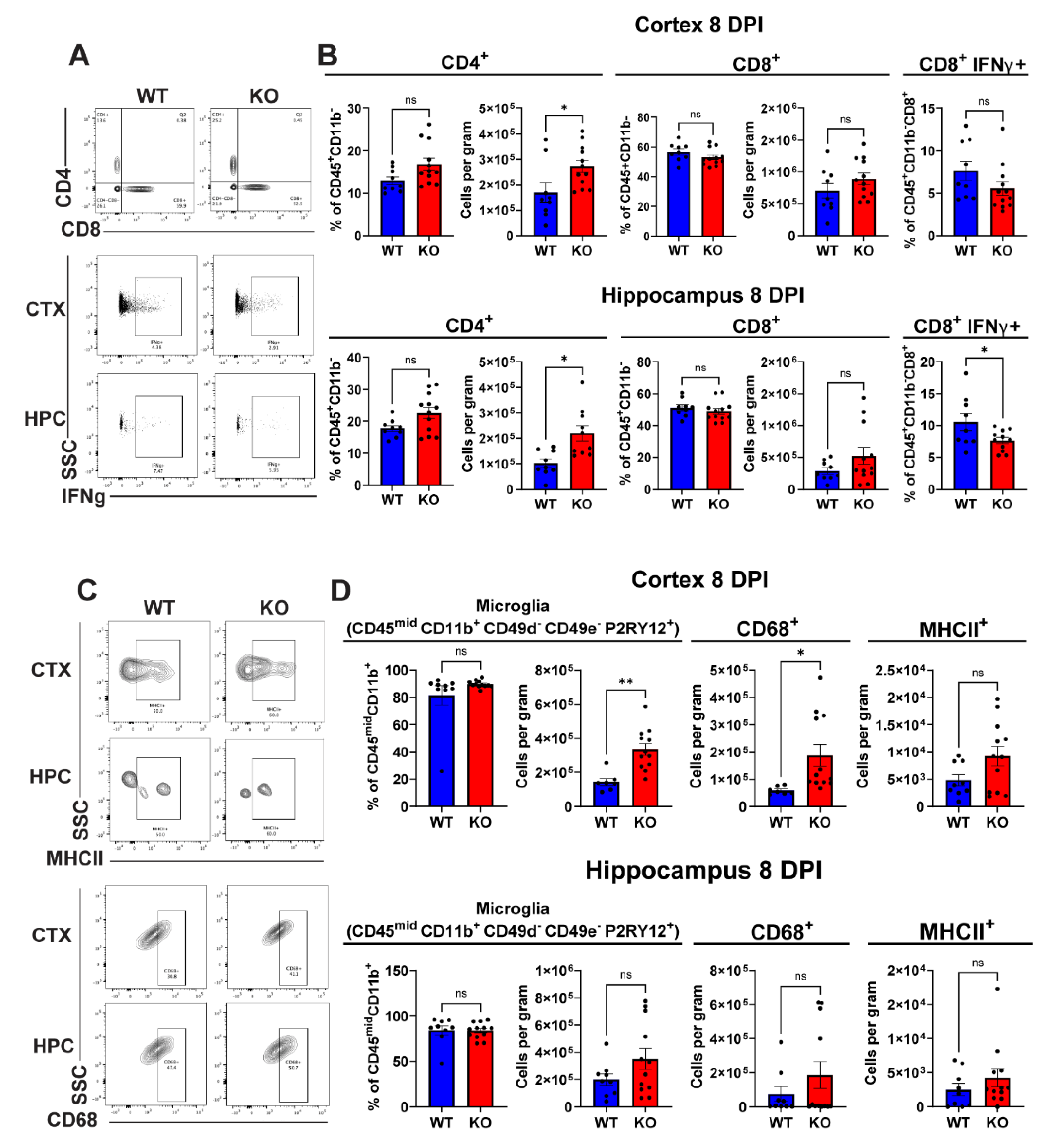
3.5. Lgals3bp-/- C57BL6/J Mice Exhibit More Homeostatic Microglia and Reduced Neuroinflammation during WNV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Li, X.; Li, Y.; Jin, Y.; Zhang, Y.; Wu, J.; Xu, Z.; Huang, Y.; Cai, L.; Gao, S.; Liu, T.; et al. Transcriptional and Epigenetic Decoding of the Microglial Aging Process. Nat Aging 2023, 3, 1288–1311. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Tillman, G.; Kraut, M.A.; Chiang, H.-S.; Strain, J.F.; Li, Y.; Agrawal, A.G.; Jester, P.; Gnann, J.W.; Whitley, R.J.; et al. West Nile Virus Neuroinvasive Disease: Neurological Manifestations and Prospective Longitudinal Outcomes. BMC Infectious Diseases 2014, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Sander, B.; Nelder, M.P. Long-Term Sequelae of West Nile Virus-Related Illness: A Systematic Review. Lancet Infect Dis 2015, 15, 951–959. [Google Scholar] [CrossRef]
- Szretter, K.J.; Daniels, B.P.; Cho, H.; Gainey, M.D.; Yokoyama, W.M.; Jr, M.G.; Virgin, H.W.; Klein, R.S.; Sen, G.C.; Diamond, M.S. 2′-O Methylation of the Viral mRNA Cap by West Nile Virus Evades Ifit1-Dependent and -Independent Mechanisms of Host Restriction In Vivo. PLOS Pathogens 2012, 8, e1002698. [Google Scholar] [CrossRef] [PubMed]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Perez-Torres, C.; Frouin, A.; Wilton, D.K.; et al. A Complement–Microglial Axis Drives Synapse Loss during Virus-Induced Memory Impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.; Vasek, M.J.; Vollmer, L.L.; Sun, T.; Jiang, X.; Klein, R.S. Astrocytes Decrease Adult Neurogenesis during Virus-Induced Memory Dysfunction via Interleukin-1. Nat Immunol 2018, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.F.; Soung, A.L.; Yang, W.; Ai, S.; Kanmogne, M.; Davé, V.A.; Artyomov, M.; Magee, J.A.; Klein, R.S. Single-Cell RNA Transcriptome Analysis of CNS Immune Cells Reveals CXCL16/CXCR6 as Maintenance Factors for Tissue-Resident T Cells That Drive Synapse Elimination. Genome Medicine 2022, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.; Soung, A.; Vollmer, L.L.; Kanmogne, M.; Last, A.; Brown, J.; Klein, R.S. T Cells Promote Microglia-Mediated Synaptic Elimination and Cognitive Dysfunction during Recovery from Neuropathogenic Flaviviruses. Nat Neurosci 2019, 22, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Capone, E.; Iacobelli, S.; Sala, G. Role of Galectin 3 Binding Protein in Cancer Progression: A Potential Novel Therapeutic Target. J Transl Med 2021, 19, 405. [Google Scholar] [CrossRef]
- Dufrusine, B.; Capone, E.; Ponziani, S.; Lattanzio, R.; Lanuti, P.; Giansanti, F.; De Laurenzi, V.; Iacobelli, S.; Ippoliti, R.; Mangiola, A.; et al. Extracellular LGALS3BP: A Potential Disease Marker and Actionable Target for Antibody–Drug Conjugate Therapy in Glioblastoma. Mol Oncol 2023, 17, 1460–1473. [Google Scholar] [CrossRef]
- Xu, G.; Xia, Z.; Deng, F.; Liu, L.; Wang, Q.; Yu, Y.; Wang, F.; Zhu, C.; Liu, W.; Cheng, Z.; et al. Inducible LGALS3BP/90K Activates Antiviral Innate Immune Responses by Targeting TRAF6 and TRAF3 Complex. PLoS Pathog 2019, 15, e1008002. [Google Scholar] [CrossRef]
- Grassadonia, A.; Graziano, V.; Pagotto, S.; Veronese, A.; Giuliani, C.; Marchisio, M.; Lanuti, P.; De Tursi, M.; D’Egidio, M.; Di Marino, P.; et al. Tgf-Β1 Transcriptionally Promotes 90K Expression: Possible Implications for Cancer Progression. Cell Death Discov. 2021, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Pronto-Laborinho, A.; Pinto, S.; Gromicho, M.; Bonucci, S.; Tranfield, E.; Correia, C.; Alexandre, B.M.; de Carvalho, M. Investigating LGALS3BP/90 K Glycoprotein in the Cerebrospinal Fluid of Patients with Neurological Diseases. Sci Rep 2020, 10, 5649. [Google Scholar] [CrossRef]
- Kyrousi, C.; O’Neill, A.C.; Brazovskaja, A.; He, Z.; Kielkowski, P.; Coquand, L.; Di Giaimo, R.; D’ Andrea, P.; Belka, A.; Forero Echeverry, A.; et al. Extracellular LGALS3BP Regulates Neural Progenitor Position and Relates to Human Cortical Complexity. Nat Commun 2021, 12, 6298. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.-Y.; Tilgner, M.; Lo, M.K.; Kent, K.A.; Bernard, K.A. Infectious cDNA Clone of the Epidemic West Nile Virus from New York City. Journal of Virology 2002, 76, 5847–5856. [Google Scholar] [CrossRef]
- Zhou, Y.; Ray, D.; Zhao, Y.; Dong, H.; Ren, S.; Li, Z.; Guo, Y.; Bernard, K.A.; Shi, P.-Y.; Li, H. Structure and Function of Flavivirus NS5 Methyltransferase. Journal of Virology 2007, 81, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.D.; Lazear, H.M.; Diamond, M.S. Propagation, Quantification, Detection, and Storage of West Nile Virus. Curr Protoc Microbiol 2013, 31, 15D.3.1–15D.3.18. [Google Scholar] [CrossRef]
- Funk, K.E.; Klein, R.S. CSF1R Antagonism Limits Local Restimulation of Antiviral CD8+ T Cells during Viral Encephalitis. J Neuroinflammation 2019, 16, 22. [Google Scholar] [CrossRef]
- Sentmanat, M.F.; White, J.M.; Kouranova, E.; Cui, X. Highly Reliable Creation of Floxed Alleles by Electroporating Single-Cell Embryos. BMC Biol 2022, 20, 31. [Google Scholar] [CrossRef]
- Advanced Protocols for Animal Transgenesis: An ISTT Manual; Pease, S., Saunders, T.L., Eds.; Springer Protocols Handbooks; Springer: Berlin, Heidelberg, 2011. ISBN 978-3-642-20791-4.
- Manipulating the Mouse Embryo : A Laboratory Manual - NLM Catalog - NCBI Available online:. Available online: https://www.ncbi.nlm.nih.gov/nlmcatalog/101622901 (accessed on 22 May 2024).
- Keane, L.; Antignano, I.; Riechers, S.-P.; Zollinger, R.; Dumas, A.A.; Offermann, N.; Bernis, M.E.; Russ, J.; Graelmann, F.; McCormick, P.N.; et al. mTOR-Dependent Translation Amplifies Microglia Priming in Aging Mice. J Clin Invest 2021, 131, 132727. [Google Scholar] [CrossRef]
- Arutyunov, A.; Klein, R.S. Microglia at the Scene of the Crime: What Their Transcriptomics Reveal about Brain Health. Curr Opin Neurol 2023, 36, 207–213. [Google Scholar] [CrossRef]
- Gómez Morillas, A.; Besson, V.C.; Lerouet, D. Microglia and Neuroinflammation: What Place for P2RY12? Int J Mol Sci 2021, 22, 1636. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A New Gene Set Identifies Senescent Cells and Predicts Senescence-Associated Pathways across Tissues. Nat Commun 2022, 13, 4827. [Google Scholar] [CrossRef] [PubMed]
- Antignano, I.; Liu, Y.; Offermann, N.; Capasso, M. Aging Microglia. Cell Mol Life Sci 2023, 80, 126. [Google Scholar] [CrossRef] [PubMed]
- Sams, E.C. Oligodendrocytes in the Aging Brain. Neuronal Signal 2021, 5, NS20210008. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat Rev Endocrinol 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.C.; Sehl, M.E.; Shih, R.; Langfelder, P.; Wang, R.; Horvath, S.; Bream, J.H.; Duggal, P.; Martinson, J.; Wolinsky, S.M.; et al. Accelerated Aging with HIV Occurs at the Time of Initial HIV Infection. iScience 2022, 25. [Google Scholar] [CrossRef] [PubMed]
- Durso, D.F.; Silveira-Nunes, G.; Coelho, M.M.; Camatta, G.C.; Ventura, L.H.; Nascimento, L.S.; Caixeta, F.; Cunha, E.H.M.; Castelo-Branco, A.; Fonseca, D.M.; et al. Living in Endemic Area for Infectious Diseases Accelerates Epigenetic Age. Mechanisms of Ageing and Development 2022, 207, 111713. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated Biological Aging in COVID-19 Patients. Nat Commun 2022, 13, 2135. [Google Scholar] [CrossRef]
- Jarrard, L.E. On the Role of the Hippocampus in Learning and Memory in the Rat. Behav Neural Biol 1993, 60, 9–26. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Kwon, M.-S. Aged Microglia in Neurodegenerative Diseases: Microglia Lifespan and Culture Methods. Front. Aging Neurosci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Malvaso, A.; Gatti, A.; Negro, G.; Calatozzolo, C.; Medici, V.; Poloni, T.E. Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells 2023, 12, 2824. [Google Scholar] [CrossRef] [PubMed]
- Soung, A.L.; Davé, V.A.; Garber, C.; Tycksen, E.D.; Vollmer, L.L.; Klein, R.S. IL-1 Reprogramming of Adult Neural Stem Cells Limits Neurocognitive Recovery after Viral Encephalitis by Maintaining a Proinflammatory State. Brain Behav Immun 2022, 99, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.E.; Blosser, T.R.; Sullivan, Z.A.; Dulac, C.; Zhuang, X. Molecular and Spatial Signatures of Mouse Brain Aging at Single-Cell Resolution. Cell 2023, 186, 194–208.e18. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-S.; Park, M.-R.; Sun, E.-G.; Choi, W.; Hwang, J.-E.; Bae, W.-K.; Rhee, J.H.; Cho, S.-H.; Chung, I.-J. Gal-3BP Negatively Regulates NF-κB Signaling by Inhibiting the Activation of TAK1. Front Immunol 2019, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Shim, H.-J.; Park, M.-R.; Choi, J.-N.; Akanda, M.R.; Hwang, J.-E.; Bae, W.-K.; Lee, K.-H.; Sun, E.-G.; Chung, I.-J. Lgals3bp Suppresses Colon Inflammation and Tumorigenesis through the Downregulation of TAK1-NF-κB Signaling. Cell Death Discov. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Funk, K.E.; Arutyunov, A.D.; Desai, P.; White, J.P.; Soung, A.L.; Rosen, S.F.; Diamond, M.S.; Klein, R.S. Decreased Antiviral Immune Response within the Central Nervous System of Aged Mice Is Associated with Increased Lethality of West Nile Virus Encephalitis. Aging Cell 2021, 20, e13412. [Google Scholar] [CrossRef]
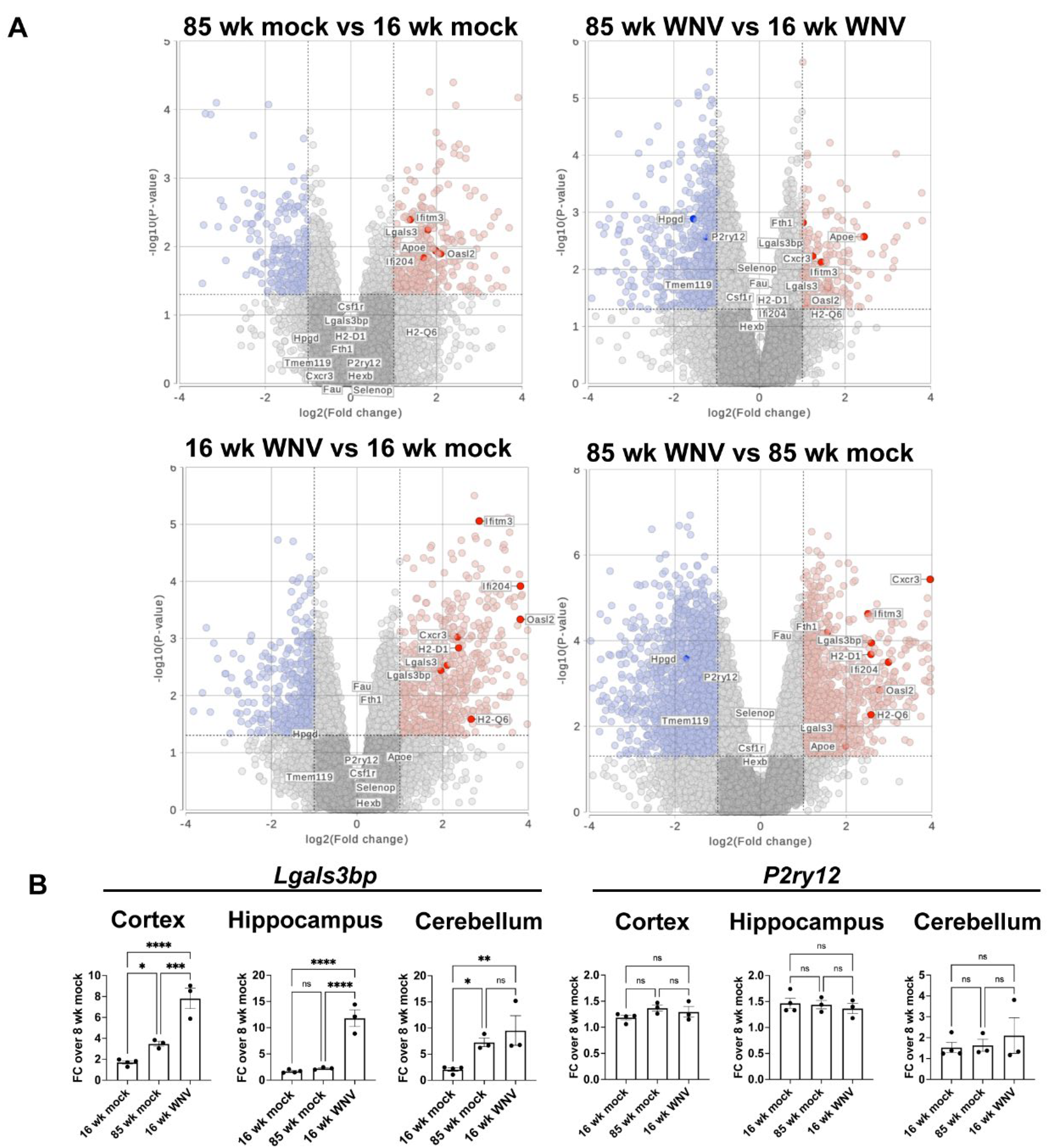
| Gene | Activated microglia cluster [7] | Fold Change (Old/Young) [22] | P value |
|---|---|---|---|
| Apoe | A1 and A2 | 3.56 | 5.8E-18 |
| H2-D1 | A1 | 3.29 | 1.8E-20 |
| Fth1 | A1 and A2 | 2.74 | 3.0E-18 |
| Lgals3bp | A1 | 2.24 | 5.7E-23 |
| H2-Q6 | A1 | 2.23 | 1.1E-10 |
| Oasl2 | A1 | 1.89 | 1.9E-05 |
| Ifi204 | A1 | 1.79 | 1.0E-04 |
| Fcgr4 | A1 | 1.75 | 2.6E-04 |
| C1qa | A2 | 1.75 | 4.5E-06 |
| Ifitm3 | A1 | 1.72 | 4.0E-13 |
| ltm2b | A1 and A2 | 1.63 | 6.4E-05 |
| Ftl1 | A2 | 1.51 | 0.022 |
| Tyrobp | A2 | 1.50 | 0.002 |
| Ctsb | A1 and A2 | 1.47 | 1.8E-04 |
| Rps29 | A2 | 1.39 | 0.212 |
| C1qb | A2 | 1.37 | 0.026 |
| Fau | A2 | 1.36 | 0.213 |
| Fcer1g | A2 | 1.35 | 0.010 |
| H2-Ab1 | A1 and A2 | 1.34 | 0.002 |
| Ctss | A1 and A2 | 1.32 | 0.002 |
| Ly6e | A1 | 1.26 | 0.005 |
| H2-Eb1 | A1 and A2 | 1.25 | 0.014 |
| H2-Aa | A1 and A2 | 1.15 | 0.326 |
| CD74 | A1 and A2 | 1.14 | 0.404 |
| Cxcl9 | A2 | 1.12 | 0.722 |
| Psap | A2 | 0.93 | 0.846 |
| Selplg | Homeostatic | 1.11 | 0.652 |
| Fscn1 | Homeostatic | 1.04 | 0.917 |
| Hexb | Homeostatic | 1.00 | 0.986 |
| Cst3 | Homeostatic | 0.99 | 0.964 |
| Hpgd | Homeostatic | 0.88 | 0.326 |
| Pmp22 | Homeostatic | 0.86 | 0.149 |
| Selenop | Homeostatic | 0.85 | 0.242 |
| Csf1r | Homeostatic | 0.84 | 0.241 |
| Sparc | Homeostatic | 0.82 | 0.237 |
| Rnase4 | Homeostatic | 0.80 | 0.007 |
| Golm1 | Homeostatic | 0.74 | 0.091 |
| Siglech | Homeostatic | 0.73 | 0.002 |
| Cx3cr1 | Homeostatic | 0.73 | 0.157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





