Submitted:
31 May 2024
Posted:
31 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Cells
2.2. MTT Assay
2.3. Protein Purification
2.4. Immunoblotting
2.5. Rab25 Knockdown with siRNA Transfection
2.6. Confocal Microscopy
2.7. Statistical Analysis
3. Results
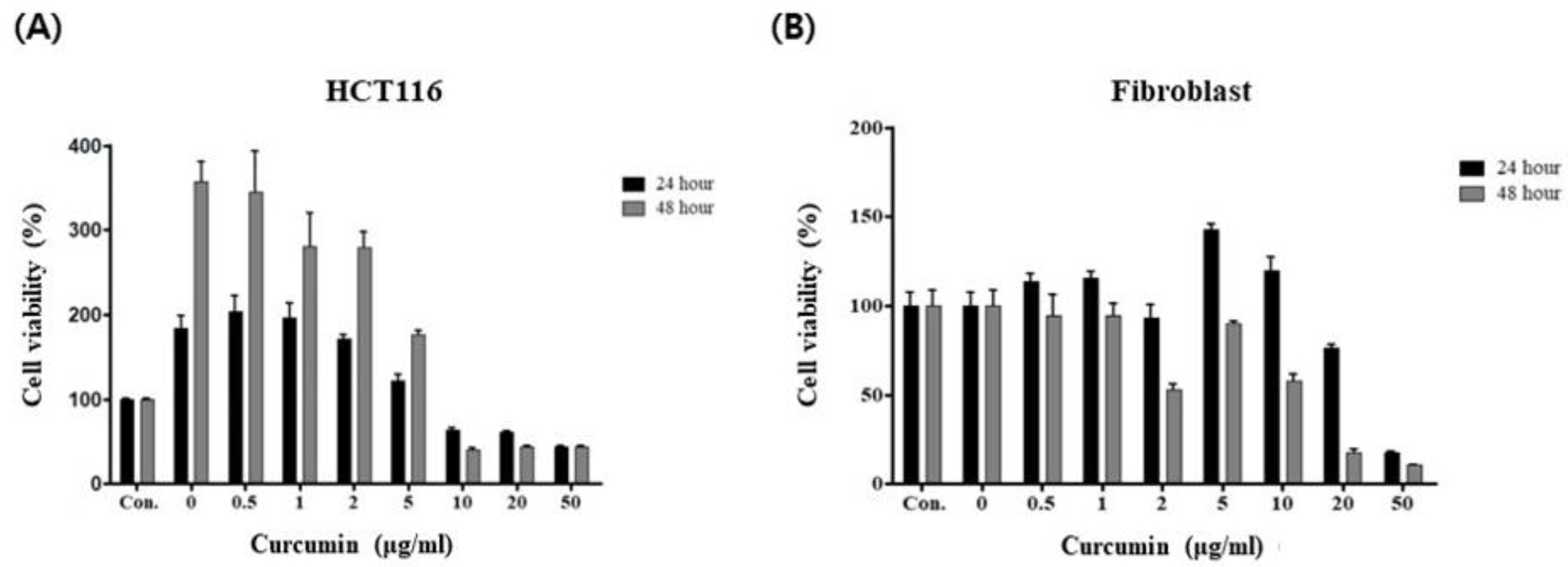
3.1. Curcumin Induces Cancer-Specific Cytotoxicity
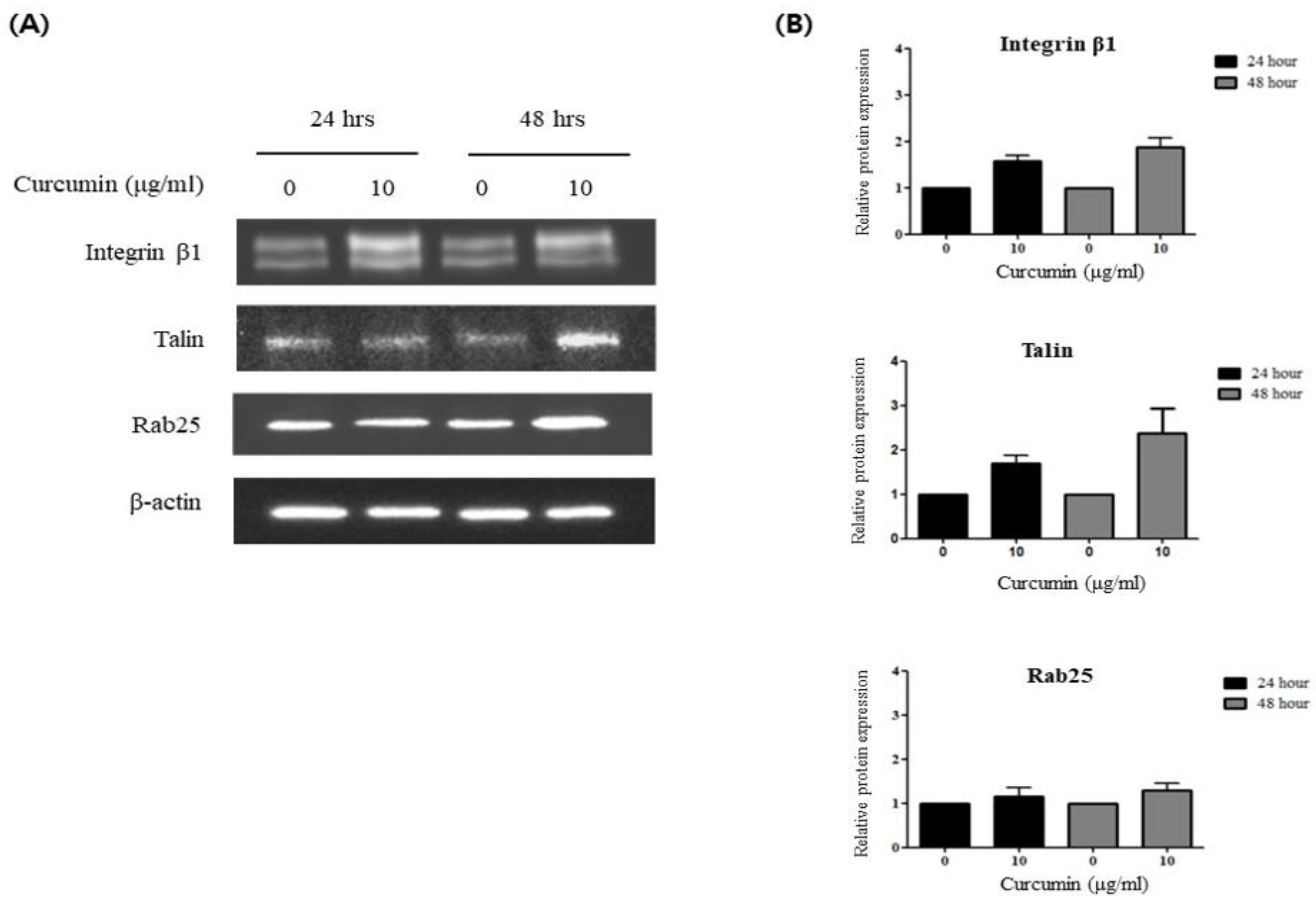
3.2. Curcumin Induces Integrin β1 Activation via a Talin-Dependent Pathway
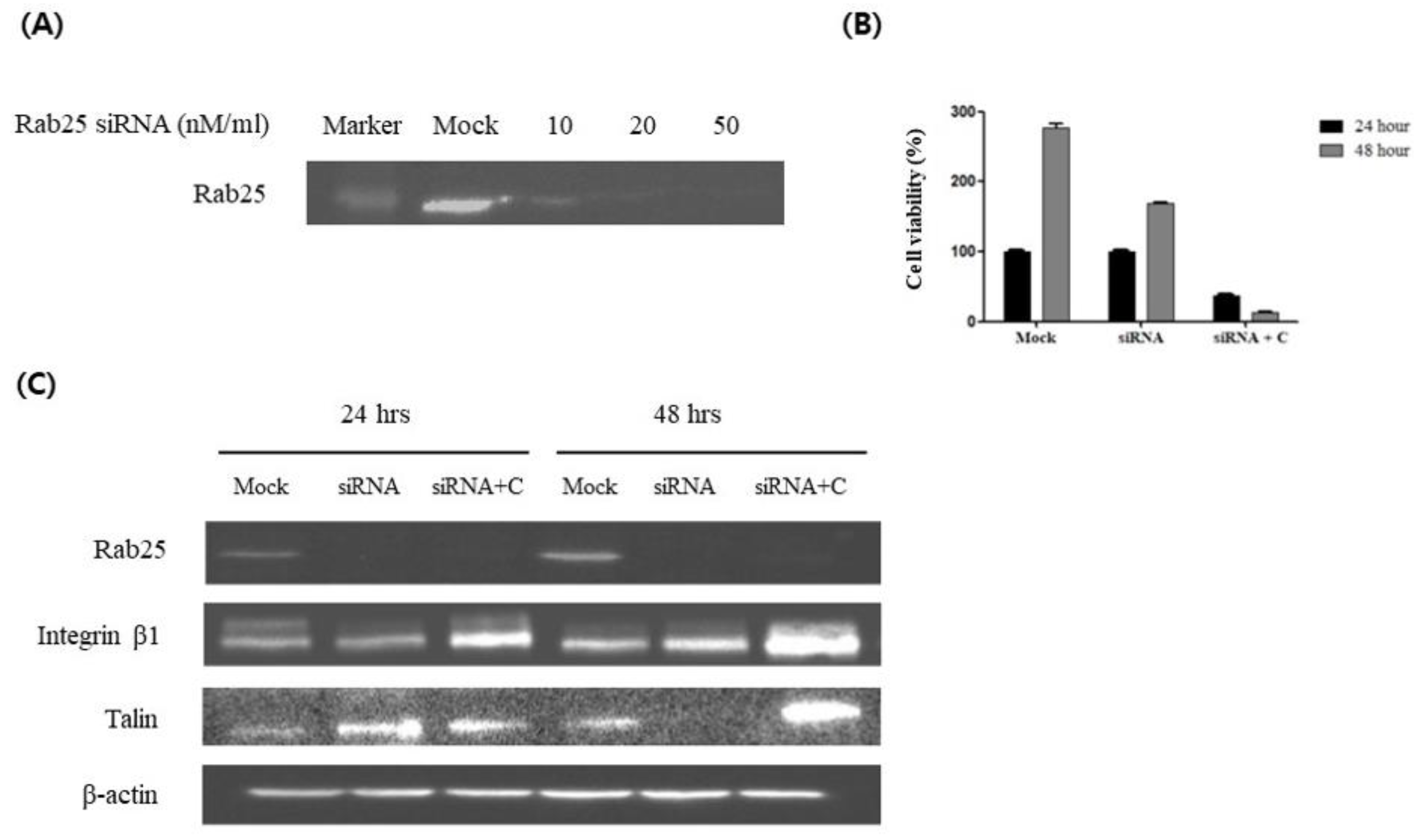
3.3. Curcumin Induces Integrin β1 Activation via a Rab25-Independent Pathway
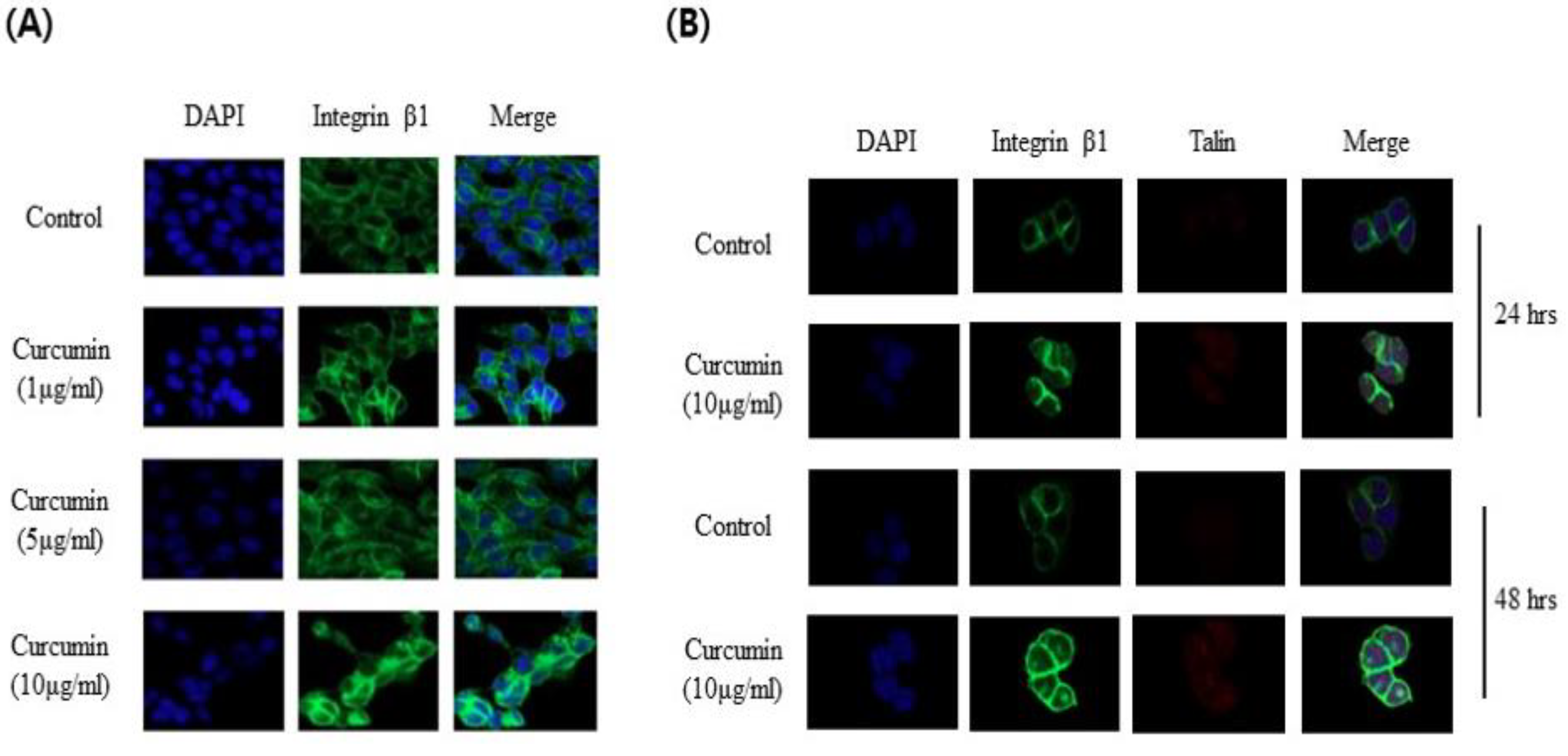
3.4. Visualization of the Expression of Integrin β1 and Talin in Response to Curcumin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Valadbeigi, S.; Sajjadi, S.E.; Shokoohinia, Y.; Azizian, H.; Taheripak, G. Grandivittin as a natural minor groove binder extracted from Ferulago macrocarpa to ct-DNA, experimental and in silico analysis. Chem Biol Interact 2016, 258, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Koroth, J.; Nirgude, S.; Tiwari, S.; Gopalakrishnan, V.; Mahadeva, R.; Kumar, S.; Karki, S.S.; Choudhary, B. Investigation of anti-cancer and migrastatic properties of novel curcumin derivatives on breast and ovarian cancer cell lines. BMC Complement Altern Med 2019, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Current Science 2004, 87, 44–53. [Google Scholar]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: a short review. Life Sci 2006, 78, 2081-7. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res 2004, 75, 742–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted miR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother Radiopharm 2019, 34, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, S.; Lin, J.; Zhang, Q.J.; Chen, A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARgamma in rat activated hepatic stellate cell in vitro. Lab Invest 2007, 87, 488–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xu, J.; Johnson, A.C. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–87. [Google Scholar] [CrossRef]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.H.; Kong, A.N. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther 2008, 7, 2609–20. [Google Scholar] [CrossRef]
- Chaudhary, L.R.; Hruska, K.A. Inhibition of cell survival signal protein kinase B/Akt by curcumin in human prostate cancer cells. J Cell Biochem 2003, 89, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Squires, M.S.; Hudson, E.A.; Howells, L.; Sale, S.; Houghton, C.E.; Jones, J.L.; Fox, L.H.; Dickens, M.; Prigent, S.A.; Manson, M.M. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol 2003, 65, 361–76. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.T.; Soung, Y.H.; Surh, Y.J.; Cardelli, J.A.; Chung, J. Curcumin Prevents Palmitoylation of Integrin β4 in Breast Cancer Cells. PLoS One 2015, 10, e0125399. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Chung, J. Curcumin inhibition of the functional interaction between integrin α6β4 and the epidermal growth factor receptor. Mol Cancer Ther 2011, 10, 883–91. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Zhiani, M.; Mousavi, M.A.; Fathi, M. Crosstalk between Epidermal Growth Factor Receptors (EGFR) and integrins in resistance to EGFR tyrosine kinase inhibitors (TKIs) in solid tumors. Eur J Cell Biol 2020, 99, 151083. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Mohrenz, I.V.; Mirisola, V.; Schleicher, E.; Romeo, F.; Höhneke, C.; Jochum, M.; Nerlich, A.G.; Pfeffer, U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis 2008, 29, 779–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Margadant, C.; Kreft, M.; de Groot, D.J.; Norman, J.C.; Sonnenberg, A. Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking. Curr Biol 2012, 22, 1554–63. [Google Scholar] [CrossRef]
- Pernier, J.; Cardoso Dos Santos, M.; Souissi, M.; Joly, A.; Narassimprakash, H.; Rossier, O.; Giannone, G.; Helfer, E.; Sengupta, K.; Le Clainche, C. Talin and kindlin cooperate to control the density of integrin clusters. J Cell Sci 2023, 136. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Nam, K.T. Rab25 as a tumour suppressor in colon carcinogenesis. Br J Cancer 2011, 104, 33–6. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett 2005, 223, 181–90. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Naqvi, S.T.; Muhammad, S.A. Curcumin: a Polyphenol with Molecular Targets for Cancer Control. Asian Pac J Cancer Prev 2016, 17, 2735–9. [Google Scholar]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8. [Google Scholar] [CrossRef]
- Sullivan, D.E.; Ferris, M.; Nguyen, H.; Abboud, E.; Brody, A.R. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med 2009, 13, 1866–76. [Google Scholar] [CrossRef] [PubMed]
- Sa, G.; Das, T. Anti cancer effects of curcumin: cycle of life and death. Cell Div 2008, 3, 14. [Google Scholar] [CrossRef]
- Ray, S.; Chattopadhyay, N.; Mitra, A.; Siddiqi, M.; Chatterjee, A. Curcumin exhibits antimetastatic properties by modulating integrin receptors, collagenase activity, and expression of Nm23 and E-cadherin. J Environ Pathol Toxicol Oncol 2003, 22, 49–58. [Google Scholar] [PubMed]
- Shakibaei, M.; Schulze-Tanzil, G.; John, T.; Mobasheri, A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat 2005, 187, 487–97. [Google Scholar] [CrossRef]
- Mani, J.; Fleger, J.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.; Relja, B.; Juengel, E.; et al. Curcumin combined with exposure to visible light blocks bladder cancer cell adhesion and migration by an integrin dependent mechanism. Eur Rev Med Pharmacol Sci 2019, 23, 10564–10574. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett 2008, 267, 133–64. [Google Scholar] [CrossRef]
- Boon, H.; Wong, J. Botanical medicine and cancer: a review of the safety and efficacy. Expert Opin Pharmacother 2004, 5, 2485–501. [Google Scholar] [CrossRef]
- Wargovich, M.J. Nutrition and cancer: the herbal revolution. Curr Opin Gastroenterol 1999, 15, 177–80. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol Res 2020, 153, 104635. [Google Scholar] [CrossRef] [PubMed]
- Keyvani-Ghamsari, S.; Khorsandi, K.; Gul, A. Curcumin effect on cancer cells' multidrug resistance: An update. Phytother Res 2020, 34, 2534–2556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




