Submitted:
14 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Isolation, Identification and Characterization of Strains
2.2. Tested Prebiotics and Cultivation Media Composition
2.3. Testing of Bacterial Growth
2.4. Enzymatic Assay
2.5. Statistical Analyses
3. Results
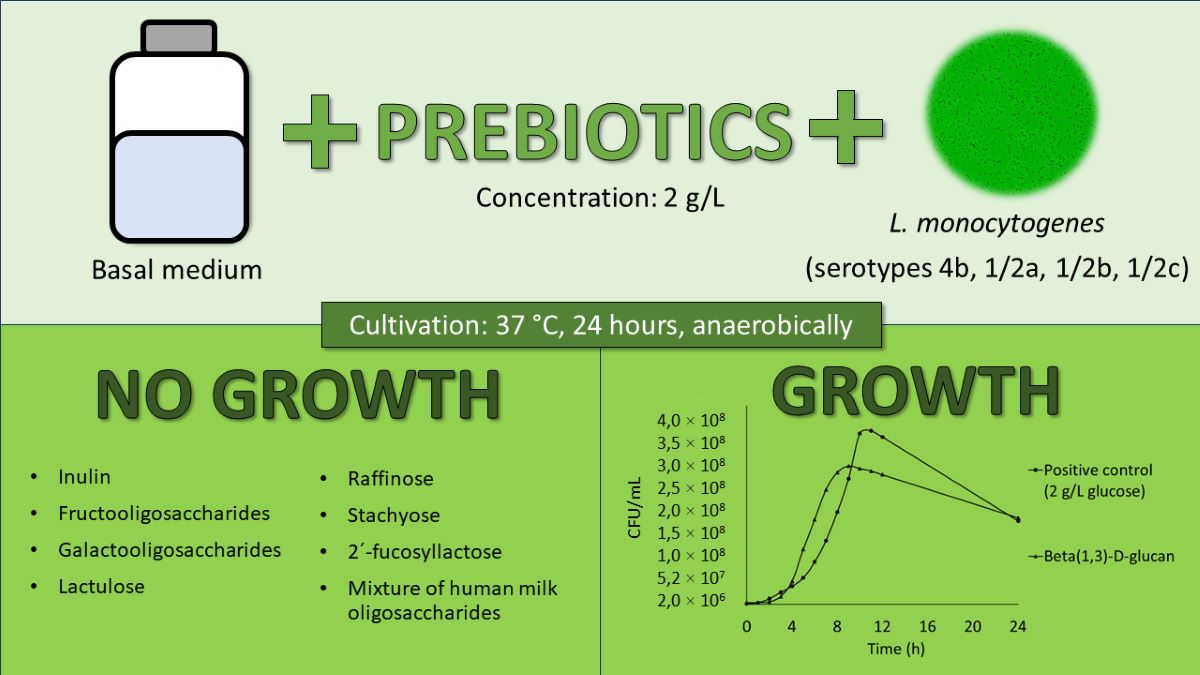
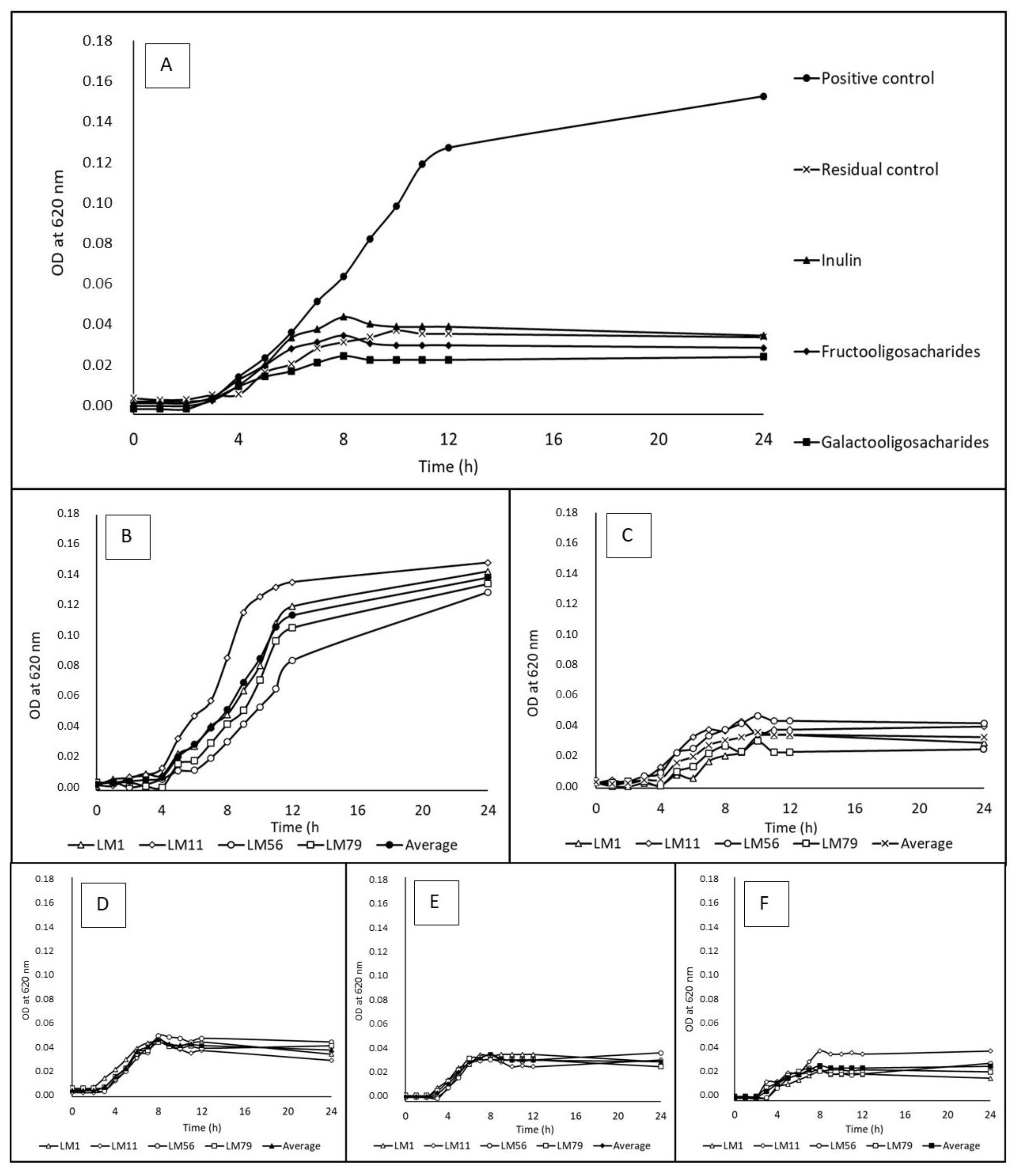
3.1. Utilization of Water-Soluble Prebiotics
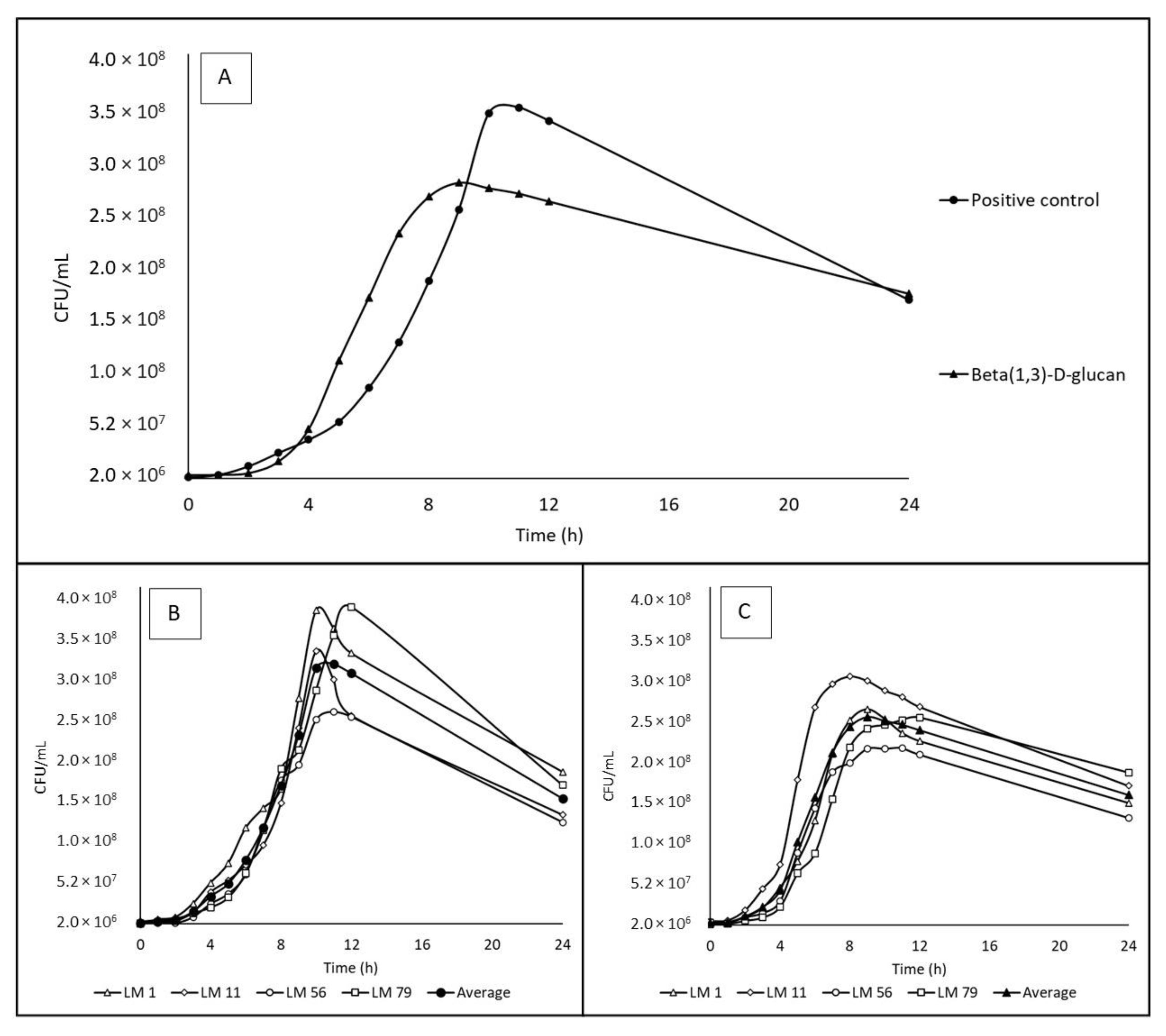
3.2. Utilization of Beta-(1,3)-D-glucan (Partially Water-Soluble Prebiotic)
3.3. Changes in pH Values in the Fermentation System
3.4. Growth Curves and Rates
3.5. Enzymatic Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhunia, A.K. Foodborne Microbial Pathogens, Mechanisms and Pathogenesis; 2018; ISBN 978-1-4939-7347-7.
- Farber, J.M.; Peterkin, P.I. Listeria Monocytogenes, a Food-Borne Pathogen. Microbiol Rev 1991, 55, 476–511, doi:10.1128/MR.55.3.476-511.1991. [CrossRef]
- Shamloo, E.; Hosseini, H.; Moghadam, A.Z.; Larsen, H.M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria Monocytogenes in Food Safety: A Review of Its Prevalence, Detection, and Antibiotic Resistance. Iran J Vet Res 2019, 20, 241.
- Azari, S.; Johnson, L.J.; Webb, A.; Kozlowski, S.M.; Zhang, X.; Rood, K.; Amer, A.; Seveau, S. Hofbauer Cells Spread Listeria Monocytogenes among Placental Cells and Undergo Pro-Inflammatory Reprogramming While Retaining Production of Tolerogenic Factors. mBio 2021, 12, doi:10.1128/MBIO.01849-21/ASSET/AF596A8D-3D9F-4F02-BD95-CA0CC4DB8AFE/ASSETS/IMAGES/MEDIUM/MBIO.01849-21-T001.GIF. [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control The European Union One Health 2020 Zoonoses Report. EFSA Journal 2021, 19, doi:10.2903/J.EFSA.2021.6971. [CrossRef]
- Das, S.; Surendran, P.K.; Thampuran, N. Detection and Differentiation of Listeria Monocytogenes and Listeria Innocua by Multiplex PCR. Fishery Technology 2010, 47, 91–94.
- Nadon, C.A.; Woodward, D.L.; Young, C.; Rodgers, F.G.; Wiedmann, M. Correlations between Molecular Subtyping and Serotyping of Listeria Monocytogenes. J Clin Microbiol 2001, 39, 2704, doi:10.1128/JCM.39.7.2704-2707.2001. [CrossRef]
- Bortolussi, R. Public Health: Listeriosis: A Primer. CMAJ : Canadian Medical Association Journal 2008, 179, 795, doi:10.1503/CMAJ.081377. [CrossRef]
- Miceli, A.; Settanni, L. Influence of Agronomic Practices and Pre-Harvest Conditions on the Attachment and Development of Listeria Monocytogenes in Vegetables. Annals of Microbiology 2019 69:3 2019, 69, 185–199, doi:10.1007/S13213-019-1435-6. [CrossRef]
- Simonetti, T.; Peter, K.; Chen, Y.; Jin, Q.; Zhang, G.; LaBorde, L.F.; Macarisin, D. Prevalence and Distribution of Listeria Monocytogenes in Three Commercial Tree Fruit Packinghouses. Front Microbiol 2021, 12, 652708, doi:10.3389/FMICB.2021.652708/BIBTEX. [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo, J. Van; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr Res Rev 2004, 17, 259–275, doi:10.1079/NRR200479. [CrossRef]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal Bacteria: An Emerging Player in Defense Against Respiratory Pathogens. Front Immunol 2019, 10, 1203, doi:10.3389/FIMMU.2019.01203. [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br J Nutr 2010, 104 Suppl 2, doi:10.1017/S0007114510003363. [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat Rev Gastroenterol Hepatol 2017, 14, 491–502, doi:10.1038/NRGASTRO.2017.75. [CrossRef]
- Charalampopoulos, D.; Rastall, R.A. Prebiotics in Foods. Curr Opin Biotechnol 2012, 23, 187–191, doi:10.1016/J.COPBIO.2011.12.028. [CrossRef]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial Effects of Human Milk Oligosaccharides on Gut Microbiota. Benef Microbes 2014, 5, 273–283, doi:10.3920/BM2013.0080. [CrossRef]
- Bunešová, V.; Vlková, E.; Rada, V.; Kňazovická, V.; Ročková, Š.; Geigerová, M.; Božik, M. Growth of Infant Fecal Bacteria on Commercial Prebiotics. Folia Microbiol (Praha) 2012, 57, 273–275, doi:10.1007/S12223-012-0123-8. [CrossRef]
- Rada, V.; Nevoral, J.; Trojanová, I.; Tománková, E.; Šmehilová, M.; Killer, J. Growth of Infant Faecal Bifidobacteria and Clostridia on Prebiotic Oligosaccharides in in Vitro Conditions. Anaerobe 2008, 14, 205–208, doi:10.1016/J.ANAEROBE.2008.05.003. [CrossRef]
- Sauer, J.-D.; Herskovits, A.A.; O’Riordan, M.X.D. Metabolism of the Gram-Positive Bacterial Pathogen Listeria Monocytogenes. Microbiol Spectr 2019, 7, doi:10.1128/MICROBIOLSPEC.GPP3-0066-2019. [CrossRef]
- Friedman, M.E.; Roessler, W.G. GROWTH OF LISTERIA MONOCYTOGENES IN DEFINED MEDIA. J Bacteriol 1961, 82, 528, doi:10.1128/JB.82.4.528-533.1961. [CrossRef]
- Balay, D.R.; Gänzle, M.G.; McMullen, L.M. The Effect of Carbohydrates and Bacteriocins on the Growth Kinetics and Resistance of Listeria Monocytogenes. Front Microbiol 2018, 9, 347, doi:10.3389/FMICB.2018.00347/BIBTEX. [CrossRef]
- Gopal, S.; Berg, D.; Hagen, N.; Schriefer, E.M.; Stoll, R.; Goebel, W.; Kreft, J. Maltose and Maltodextrin Utilization by Listeria Monocytogenes Depend on an Inducible ABC Transporter Which Is Repressed by Glucose. PLoS One 2010, 5, doi:10.1371/JOURNAL.PONE.0010349. [CrossRef]
- Pine, L.; Malcolm, G.B.; Brooks, J.B.; Daneshvar, M.I. Physiological Studies on the Growth and Utilization of Sugars by Listeria Species. Can J Microbiol 1989, 35, 245–254, doi:10.1139/M89-037. [CrossRef]
- Schardt, J.; Jones, G.; Müller-Herbst, S.; Schauer, K.; D’Orazio, S.E.F.; Fuchs, T.M. Comparison between Listeria Sensu Stricto and Listeria Sensu Lato Strains Identifies Novel Determinants Involved in Infection. Sci Rep 2017, 7, doi:10.1038/S41598-017-17570-0. [CrossRef]
- Bae, D.; Seo, K.S.; Zhang, T.; Wang, C. Characterization of a Potential Listeria Monocytogenes Virulence Factor Associated with Attachment to Fresh Produce. Appl Environ Microbiol 2013, 79, 6855, doi:10.1128/AEM.01006-13. [CrossRef]
- Paspaliari, D.K.; Loose, J.S.M.; Larsen, M.H.; Vaaje-Kolstad, G. Listeria Monocytogenes Has a Functional Chitinolytic System and an Active Lytic Polysaccharide Monooxygenase. FEBS J 2015, 282, 921–936, doi:10.1111/FEBS.13191. [CrossRef]
- Salmonová, H.; Killer, J.; Bunešová, V.; Geigerová, M.; Vlková, E. Cultivable Bacteria from Pectinatella Magnifica and the Surrounding Water in South Bohemia Indicate Potential New Gammaproteobacterial, Betaproteobacterial and Firmicutes Taxa. FEMS Microbiol Lett 2018, 365, doi:10.1093/FEMSLE/FNY118. [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J Bacteriol 1991, 173, 697–703, doi:10.1128/JB.173.2.697-703.1991. [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An Important Software for Molecular Biology. GERF Bull Biosci 2011, 2, 60–61.
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res 1997, 25, 4876–4882, doi:10.1093/NAR/25.24.4876. [CrossRef]
- McGinnis, S.; Madden, T.L. BLAST: At the Core of a Powerful and Diverse Set of Sequence Analysis Tools. Nucleic Acids Res 2004, 32, W20–W25, doi:10.1093/NAR/GKH435. [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int J Syst Evol Microbiol 2017, 67, 1613–1617, doi:10.1099/IJSEM.0.001755/CITE/REFWORKS. [CrossRef]
- Liu, D.; Ainsworth, A.J.; Austin, F.W.; Lawrence, M.L. Characterization of Virulent and Avirulent Listeria Monocytogenes Strains by PCR Amplification of Putative Transcriptional Regulator and Internalin Genes. J Med Microbiol 2003, 52, 1065–1070, doi:10.1099/JMM.0.05358-0. [CrossRef]
- Hungate, R.E. Chapter IV A Roll Tube Method for Cultivation of Strict Anaerobes. Methods in Microbiology 1969, 3, 117–132, doi:10.1016/S0580-9517(08)70503-8. [CrossRef]
- Rockova, S.; Nevoral, J.; Rada, V.; Marsik, P.; Sklenar, J.; Hinkova, A.; Vlkova, E.; Marounek, M. Factors Affecting the Growth of Bifidobacteria in Human Milk. Int Dairy J 2011, 21, 504–508, doi:10.1016/J.IDAIRYJ.2011.02.005. [CrossRef]
- Bai, Y.P.; Zhou, H.M.; Zhu, K.R.; Li, Q. Effect of Thermal Processing on the Molecular, Structural, and Antioxidant Characteristics of Highland Barley β-Glucan. Carbohydr Polym 2021, 271, doi:10.1016/J.CARBPOL.2021.118416. [CrossRef]
- Zhao, Y.; Zhou, H.M.; Huang, Z.H.; Zhao, R.Y. Different Aggregation States of Barley β-Glucan Molecules Affects Their Solution Behavior: A Comparative Analysis. Food Hydrocoll 2020, 101, doi:10.1016/J.FOODHYD.2019.105543. [CrossRef]
- Jones, G.S.; D’Orazio, S.E.F. Listeria Monocytogenes: Cultivation and Laboratory. Curr Protoc Microbiol 2013, 31, 9B.2.1, doi:10.1002/9780471729259.MC09B02S31. [CrossRef]
- Humble, M.W.; King, A.; Phillips, I. API ZYM: A Simple Rapid System for the Detection of Bacterial Enzymes. J Clin Pathol 1977, 30, 275, doi:10.1136/JCP.30.3.275. [CrossRef]
- Durica-Mitic*, S.; Göpel*, Y.; Görke, B. Carbohydrate Utilization in Bacteria: Making the Most Out of Sugars with the Help of Small Regulatory RNAs. Microbiol Spectr 2018, 6, doi:10.1128/MICROBIOLSPEC.RWR-0013-2017/ASSET/6C5BDC76-9F0F-46C8-8AC6-96325512130C/ASSETS/GRAPHIC/RWR-0013-2017-FIG5.GIF. [CrossRef]
- Gahan, C.G.M.; Hill, C. Listeria Monocytogenes: Survival and Adaptation in the Gastrointestinal Tract. Front Cell Infect Microbiol 2014, 5, 1–7, doi:10.3389/fcimb.2014.00009. [CrossRef]
- Liu, S.; Graham, J.E.; Bigelow, L.; Morse, P.D.; Wilkinson, B.J. Identification of Listeria Monocytogenes Genes Expressed in Response to Growth at Low Temperature. Appl Environ Microbiol 2002, 68, 1697–1705, doi:10.1128/AEM.68.4.1697-1705.2002/ASSET/C7E94927-C352-4AF0-91D5-0DE52FFB4AAA/ASSETS/GRAPHIC/AM0421319003.JPEG. [CrossRef]
- Lockyer, S.; Stanner, S. Prebiotics – an Added Benefit of Some Fibre Types. Nutr Bull 2019, 44, 74–91, doi:10.1111/NBU.12366. [CrossRef]
- Ibrahim, O.O. Functional Oligosaccharides: Chemicals Structure, Manufacturing, Health Benefits, Applications and Regulations. Journal of Food Chemistry and Nanotechnology 2018, 4, 65–76, doi:10.17756/JFCN.2018-060. [CrossRef]
- Kim, K.S.; Yun, H.S. Production of Soluble β-Glucan from the Cell Wall of Saccharomyces Cerevisiae. Enzyme Microb Technol 2006, 39, 496–500, doi:10.1016/J.ENZMICTEC.2005.12.020. [CrossRef]
- Mudgil, D. The Interaction Between Insoluble and Soluble Fiber. Dietary Fiber for the Prevention of Cardiovascular Disease: Fiber’s Interaction between Gut Micoflora, Sugar Metabolism, Weight Control and Cardiovascular Health 2017, 35–59, doi:10.1016/B978-0-12-805130-6.00003-3. [CrossRef]
- Stone, B.A. Chemistry of β-Glucans. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides 2009, 5–46, doi:10.1016/B978-0-12-373971-1.00002-9. [CrossRef]
- del Corral, F.; Buchanan, R.L. Evaluation of the API-ZYM System for Identification OfListeria. Food Microbiol 1990, 7, 99–106, doi:10.1016/0740-0020(90)90015-A. [CrossRef]
- Singhania, R.R.; Patel, A.K.; Sukumaran, R.K.; Larroche, C.; Pandey, A. Role and Significance of Beta-Glucosidases in the Hydrolysis of Cellulose for Bioethanol Production. Bioresour Technol 2013, 127, 500–507, doi:10.1016/J.BIORTECH.2012.09.012. [CrossRef]
- Ouyang, B.; Wang, G.; Zhang, N.; Zuo, J.; Huang, Y.; Zhao, X. Recent Advances in β-Glucosidase Sequence and Structure Engineering: A Brief Review. Molecules 2023, 28, doi:10.3390/MOLECULES28134990. [CrossRef]
- Synytsya, A.; Novak, M. Structural Analysis of Glucans. Ann Transl Med 2014, 2, 17, doi:10.3978/J.ISSN.2305-5839.2014.02.07. [CrossRef]
- Kumar, K.; Correia, M.A.S.; Pires, V.M.R.; Dhillon, A.; Sharma, K.; Rajulapati, V.; Fontes, C.M.G.A.; Carvalho, A.L.; Goyal, A. Novel Insights into the Degradation of β-1,3-Glucans by the Cellulosome of Clostridium Thermocellum Revealed by Structure and Function Studies of a Family 81 Glycoside Hydrolase. Int J Biol Macromol 2018, 117, 890–901, doi:10.1016/J.IJBIOMAC.2018.06.003. [CrossRef]
- Ramos, O.S.; Malcata, F.X. Food-Grade Enzymes. Comprehensive Biotechnology, Second Edition 2011, 3, 555–569, doi:10.1016/B978-0-08-088504-9.00213-0. [CrossRef]
- Romick, T.L.; Fleming, H.P.; Mcfeeters, R.F. Aerobic and Anaerobic Metabolism of Listeria Monocytogenes in Defined Glucose Medium. Appl Environ Microbiol 1996, 62, 304–307. [CrossRef]
- Kunová, G.; Rada, V.; Lisová, I.; Ročková, Š.; Vlková, E. In Vitro Fermentability of Prebiotic Oligosaccharides by Lactobacilli. Czech J. Food Sci 2011, 29, 49–54. [CrossRef]
- Zhao, J.; Cheung, P.C.K. Fermentation of β-Glucans Derived from Different Sources by Bifidobacteria: Evaluation of Their Bifidogenic Effect. J Agric Food Chem 2011, 59, 5986–5992, doi:10.1021/JF200621Y. [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A Critical Review on the Impacts of β-Glucans on Gut Microbiota and Human Health. J Nutr Biochem 2018, 61, 101–110, doi:10.1016/J.JNUTBIO.2018.06.010. [CrossRef]
- Shokri, H.; Asadi, F.; Khosravi, A.R.; Shokriy, H.; Khosraviy, A.R. Isolation of β -Glucan from the Cell Wall of Saccharomyces Cerevisiae. http://dx.doi.org/10.1080/14786410701591622 2009, 22, 414–421, doi:10.1080/14786410701591622. [CrossRef]
- Kumar, A.; Naraian, R. Differential Expression of the Microbial β-1,4-Xylanase, and β-1,4-Endoglucanase Genes. New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Genes Biochemistry and Applications 2019, 95–111, doi:10.1016/B978-0-444-63503-7.00006-1. [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, doi:10.3390/FOODS8030092. [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 1–28, doi:10.3390/BIOM11030440. [CrossRef]
- Wang, S.; Xiao, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Rational Use of Prebiotics for Gut Microbiota Alterations: Specific Bacterial Phylotypes and Related Mechanisms. J Funct Foods 2020, 66, 103838, doi:10.1016/J.JFF.2020.103838. [CrossRef]
- Buddington, K.K.; Donahoo, J.B.; Buddington, R.K. Dietary Oligofructose and Inulin Protect Mice from Enteric and Systemic Pathogens and Tumor Inducers. J Nutr 2002, 132, 472–477, doi:10.1093/JN/132.3.472. [CrossRef]
- Chen, P.; Reiter, T.; Huang, B.; Kong, N.; Weimer, B.C. Prebiotic Oligosaccharides Potentiate Host Protective Responses against L. Monocytogenes Infection. Pathogens 2017, 6, doi:10.3390/PATHOGENS6040068. [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front Nutr 2021, 8, 672925, doi:10.3389/FNUT.2021.672925/BIBTEX. [CrossRef]
- Sangwan, V.; Tomar, S.K.; Ali, B.; Singh, R.R.B.; Singh, A.K. Galactooligosaccharides Reduce Infection Caused by Listeria Monocytogenes and Modulate IgG and IgA Levels in Mice. Int Dairy J 2015, 41, 58–63, doi:10.1016/J.IDAIRYJ.2014.09.010. [CrossRef]
- Kupfahl, C.; Geginat, G.; Hof, H. Lentinan Has a Stimulatory Effect on Innate and Adaptive Immunity against Murine Listeria Monocytogenes Infection. Int Immunopharmacol 2006, 6, 686–696, doi:10.1016/J.INTIMP.2005.10.008. [CrossRef]
- Li, W.; Yajima, T.; Saito, K.; Nishimura, H.; Fushimi, T.; Ohshima, Y.; Tsukamoto, Y.; Yoshikai, Y. Immunostimulating Properties of Intragastrically Administered Acetobacter-Derived Soluble Branched (1,4)-β-D-Glucans Decrease Murine Susceptibility to Listeria Monocytogenes. Infect Immun 2004, 72, 7005–7011, doi:10.1128/IAI.72.12.7005-7011.2004/ASSET/FAA091FE-69E9-48F9-9FCB-A2C1C19F9160/ASSETS/GRAPHIC/ZII0120444520007.JPEG. [CrossRef]
- Torello, C.O.; De Souza Queiroz, J.; Oliveira, S.C.; Queiroz, M.L.S. Immunohematopoietic Modulation by Oral β-1,3-Glucan in Mice Infected with Listeria Monocytogenes. Int Immunopharmacol 2010, 10, 1573–1579, doi:10.1016/J.INTIMP.2010.09.009. [CrossRef]
- Alonso, V.P.P.; Harada, A.M.M.; Kabuki, D.Y. Competitive and/or Cooperative Interactions of Listeria Monocytogenes With Bacillus Cereus in Dual-Species Biofilm Formation. Front Microbiol 2020, 11, 177, doi:10.3389/FMICB.2020.00177/BIBTEX. [CrossRef]
- Amézquita, A.; Brashears, M.M. Competitive Inhibition of Listeria Monocytogenes in Ready-to-Eat Meat Products by Lactic Acid Bacteria. J Food Prot 2002, 65, 316–325, doi:10.4315/0362-028X-65.2.316. [CrossRef]
- Corr, S.C.; Gahan, C.G.M.; Hill, C. Impact of Selected Lactobacillus and Bifidobacterium Species on Listeria Monocytogenes Infection and the Mucosal Immune Response. FEMS Immunol Med Microbiol 2007, 50, 380–388, doi:10.1111/J.1574-695X.2007.00264.X. [CrossRef]
- da Silva Sabo, S.; Converti, A.; Todorov, S.D.; Domínguez, J.M.; de Souza Oliveira, R.P. Effect of Inulin on Growth and Bacteriocin Production by Lactobacillus Plantarum in Stationary and Shaken Cultures. Int J Food Sci Technol 2015, 50, 864–870, doi:10.1111/IJFS.12711. [CrossRef]
- García, M.J.; Ruíz, F.; Asurmendi, P.; Pascual, L.; Barberis, L. Searching Potential Candidates for Development of Protective Cultures: Evaluation of Two Lactobacillus Strains to Reduce Listeria Monocytogenes in Artificially Contaminated Milk. J Food Saf 2020, 40, e12723, doi:10.1111/JFS.12723. [CrossRef]
- Hascoët, A.S.; Ripolles-avila, C.; Cervantes-huamán, B.R.H.; Rodríguez-jerez, J.J. In Vitro Preformed Biofilms of Bacillus Safensis Inhibit the Adhesion and Subsequent Development of Listeria Monocytogenes on Stainless-Steel Surfaces. Biomolecules 2021, 11, 1–16, doi:10.3390/BIOM11030475. [CrossRef]
- Shao, X.; Fang, K.; Medina, D.; Wan, J.; Lee, J.L.; Hong, S.H. The Probiotic, Leuconostoc Mesenteroides, Inhibits Listeria Monocytogenes Biofilm Formation. J Food Saf 2020, 40, e12750, doi:10.1111/JFS.12750. [CrossRef]
- Tran, T.D.; Cid, C. Del; Hnasko, R.; Gorski, L.; McGarvey, J.A. Bacillus Amyloliquefaciens ALB65 Inhibits the Growth of Listeria Monocytogenes on Cantaloupe Melons. Appl Environ Microbiol 2020, 87, 1–10, doi:10.1128/AEM.01926-20. [CrossRef]
- Aké, F.M.D.; Joyet, P.; Deutscher, J.; Milohanic, E. Mutational Analysis of Glucose Transport Regulation and Glucose-Mediated Virulence Gene Repression in Listeria Monocytogenes. Mol Microbiol 2011, 81, 274–293, doi:10.1111/J.1365-2958.2011.07692.X. [CrossRef]
- Crespo Tapia, N.; Dorey, A.L.; Gahan, C.G.M.; den Besten, H.M.W.; O’Byrne, C.P.; Abee, T. Different Carbon Sources Result in Differential Activation of Sigma B and Stress Resistance in Listeria Monocytogenes. Int J Food Microbiol 2020, 320, 108504, doi:10.1016/J.IJFOODMICRO.2019.108504. [CrossRef]
- Jaradat, Z.W.; Bhunia, A.K. Glucose and Nutrient Concentrations Affect the Expression of a 104-Kilodalton Listeria Adhesion Protein in Listeria Monocytogenes. Appl Environ Microbiol 2002, 68, 4876–4883, doi:10.1128/AEM.68.10.4876-4883.2002. [CrossRef]
- Park, S.F.; Kroll, R.G. Expression of Listeriolysin and Phosphatidylinositol-Specific Phospholipase C Is Repressed by the Plant-Derived Molecule Cellobiose in Listeria Monocytogenes. Mol Microbiol 1993, 8, 653–661, doi:10.1111/J.1365-2958.1993.TB01609.X. [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J Nutr 1995, 125, 1401–1412, doi:10.1093/JN/125.6.1401. [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why Definitions Matter. Curr Opin Biotechnol 2016, 37, 1–7, doi:10.1016/J.COPBIO.2015.09.001. [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a More Comprehensive Concept for Prebiotics. Nat Rev Gastroenterol Hepatol 2015, 12, 303–310, doi:10.1038/NRGASTRO.2015.47. [CrossRef]
| Strain code | LM1 | LM11 | LM56 | LM79 | Average ± SD |
| Serotype | 4b | 1/2b | 1/2a | 1/2c | - |
| Positive control (2 g/L glucose) | 0.156 ± 0.036Aa | 0.140 ± 0.027Aa | 0.127 ± 0.032Aa | 0.130 ± 0.030Aa | 0.138 ± 0.032A |
| Residual control (0.1 g/L glucose) | 0.028 ± 0.002Ba | 0.029 ± 0.001BCa | 0.028 ± 0.001BCa | 0.026 ± 0.001BCa | 0.028 ± 0.002B |
| Negative control (no saccharides) | 0.016 ± 0.015Ba | -0.006 ± 0.026Cb | 0.008 ± 0.008BCab | 0.005 ± 0.005Cab | 0.006 ± 0.017C |
| Inulin | 0.027 ± 0.006Ba | 0.028 ± 0.004BCa | 0.047 ± 0.013Ba | 0.043 ± 0.005Ba | 0.036 ± 0.012B |
| Fructooligosaccharides | 0.026 ± 0.031Ba | 0.029 ± 0.008BCa | 0.034 ± 0.011BCa | 0.016 ± 0.005BCa | 0.027 ± 0.016B |
| Galactooligosaccharides | 0.008 ± 0.018Bb | 0.046 ± 0.006Ba | 0.018 ± 0.002BCb | 0.011 ± 0.007BCb | 0.021 ± 0.018BC |
| Lactulose | 0.014 ± 0.040Ba | 0.010 ± 0.014BCa | -0.001 ± 0.005BCa | -0.005 ± 0.001Ca | -0.001 ± 0.010C |
| Raffinose | 0.004 ± 0.006Ba | 0.006 ± 0,002BCa | 0.004 ± 0.006BCa | -0.003 ± 0.007Ca | 0.003 ± 0.006C |
| Stachyose | 0.032 ± 0.005Ba | 0.011 ± 0.009BCb | 0.015 ± 0.005BCb | 0.003 ± 0.005BCb | 0.015 ± 0.012BC |
| 2´-fucosyllactose | 0.019 ± 0.002Ba | 0.013 ± 0.003BCa | 0.015 ± 0.004BCa | 0.011 ± 0.006BCa | 0.014 ± 0.005BC |
| Mixture of HMOs | 0.028 ± 0.014Ba | 0.010 ± 0.007BCab | 0.016 ± 0.007BCab | -0.006 ± 0.010Cb | 0.012 ± 0.015BC |
| Strain code | LM1 | LM11 | LM56 | LM79 | Average ± SD |
| Serotype | 4b | 1/2b | 1/2a | 1/2c | - |
| Positive control (2 g/L glucose) | 1.56 ± 0.72A | 1.2 ± 0.04A | 0.95 ± 0.01A | 1.24 ± 0.15A | 1.24 ± 0.39A |
| Negative control (no saccharides) | 0.33 ± 0.06B | 0.49 ± 0.12B | 0.16 ± 0.02B | 0.24 ± 0.11B | 0.31 ± 0.15B |
| Beta-(1-3)-D-glucan | 1.05 ± 0.09A | 1.43 ± 0.67A | 0.96 ± 0.07A | 1.48 ± 0.72A | 1.23 ± 0.48A |
| Strain code | LM1 | LM11 | LM56 | LM79 | Average ± SD |
| Serotype | 4b | 1/2b | 1/2a | 1/2c | - |
| Positive control (2 g/L glucose) | 5.70± 0.02Aab | 5.68 ± 0.01Aa | 5.73 ± 0.02Ac | 5.71 ± 0.01Abc | 5.70 ± 0.03A |
| Residual control (0.1 g/L glucose) | 6.63 ± 0.01Cab | 6.62 ± 0.00Ca | 6.63 ± 0.01Dab | 6.64 ± 0.00Cb | 6.63 ± 0.01C |
| Negative control (no saccharides) | 6.91 ± 0.01Db | 6.90 ± 0.00Da | 6.91 ± 0.01Eab | 6.90± 0.01Da | 6.90 ± 0.01D |
| Beta-(1,3)-D-glucan | 5.73± 0.01Ab | 5.67 ± 0.02Aa | 5.73 ± 0.01Ab | 5.69 ± 0.02Aab | 5.71 ± 0.03A |
| Inulin | 6.34 ± 0.02Bb | 6.31 ± 0.01Ba | 6.29 ± 0.01Ba | 6.30 ± 0.00Ba | 6.31 ± 0.03B |
| Fructooligosaccharides | 6.63 ± 0.02Cb | 6.59 ± 0.02Ca | 6.60 ± 0.02Cab | 6.63 ± 0.01Cab | 6.61 ± 0.02C |
| Galactooligosaccharides | 6.65 ± 0.04Ca | 6.60± 0.00Ca | 6.62 ± 0.02CDa | 6.62 ± 0.03Ca | 6.63 ± 0.03C |
| Lactulose | 6.90 ± 0.00Da | 6.89 ± 0.02Da | 6.91 ± 0.01Eab | 6.92 ± 0.01Eb | 6.91 ± 0.02D |
| Raffinose | 6.90 ± 0.00Da | 6.91 ± 0,01Dc | 6.90 ± 0.01Eb | 6.90 ± 0.00Da | 6.90 ± 0.01D |
| Stachyose | 6.89 ± 0.01Da | 6.90 ± 0.01Da | 6.90 ± 0.00Ea | 6.90 ± 0.00Da | 6.90 ± 0.01D |
| 2´-fucosyllactose | 6.90 ± 0.00Da | 6.90 ± 0.00Da | 6.90 ± 0.01Ea | 6.91 ± 0.01DEa | 6.90 ± 0.01D |
| Mixture of HMOs | 6.89 ± 0.01Da | 6.90 ± 0.01Dab | 6.90 ± 0.00Eb | 6.90 ± 0.01Dab | 6.90 ± 0.01D |
| Strain code | LM1 | LM11 | LM56 | LM79 | Average ± SD |
| Serotype | 4b | 1/2b | 1/2a | 1/2c | - |
| Positive control (2 g/L glucose) | 0.42 ± 0.21Aa | 0.63 ± 0.04Aa | 0.49 ± 0.02BCa | 0.64 ± 0.10Aa | 0.55 ± 0.14A |
| Residual control (0.1 g/L glucose) | 0.48 ± 0.07Aa | 0.32 ± 0.03Ba | 0.40 ± 0.08Ca | 0.55 ± 0.20ABa | 0.44 ± 0.14A |
| Inulin | 0.35 ± 0.02Ab | 0.63 ± 0.12Aa | 0.68 ± 0.03ABa | 0.51 ± 0.04ABab | 0.54 ± 0.11A |
| Fructooligosaccharides | 0.38 ± 0.10Ac | 0.67 ± 0.12Aab | 0.70 ± 0.09Aa | 0.47 ± 0.09ABbc | 0.55 ± 0.20A |
| Galactooligosaccharides | 0.19 ± 0.05Ab | 0.66 ± 0.03Aa | 0.73 ± 0.18ABa | 0.28 ± 0.13Bb | 0.46 ± 0.19A |
| Strain code | LM1 | LM11 | LM56 | LM79 | Average ± SD |
| Serotype | 4b | 1/2b | 1/2a | 1/2c | - |
| Positive control (2 g/L glucose) | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 | 0.47 ± 0.16 | 0.51 ± 0.07 |
| Beta-(1,3)-D-glucan | 0.53 ± 0.01 | 0.54 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 | 0.53 ± 0.01 |
| Strain code | Serotype | Enzyme* | ||||||
| Esterase (C4) | Esterase lipase (C8) | Leucine aminopeptidase |
Acid phosphatase |
Naphtol- AS-BI- -phosphohydrolase |
Alpha- glucosidase |
Beta- glucosidase |
||
| LM1 | 4b | 5 | 1 | 2 | 3 | 3 | 1 | 5 |
| LM11 | 1/2b | 4 | 1 | 2 | 3 | 2 | 0 | 4 |
| LM56 | 1/2a | 5 | 0 | 0 | 4 | 4 | 1 | 5 |
| LM79 | 1/2c | 4 | 0 | 0 | 4 | 4 | 0 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





