Submitted:
30 May 2024
Posted:
03 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Data Source
Sample Selection
Statistical Analysis
Results
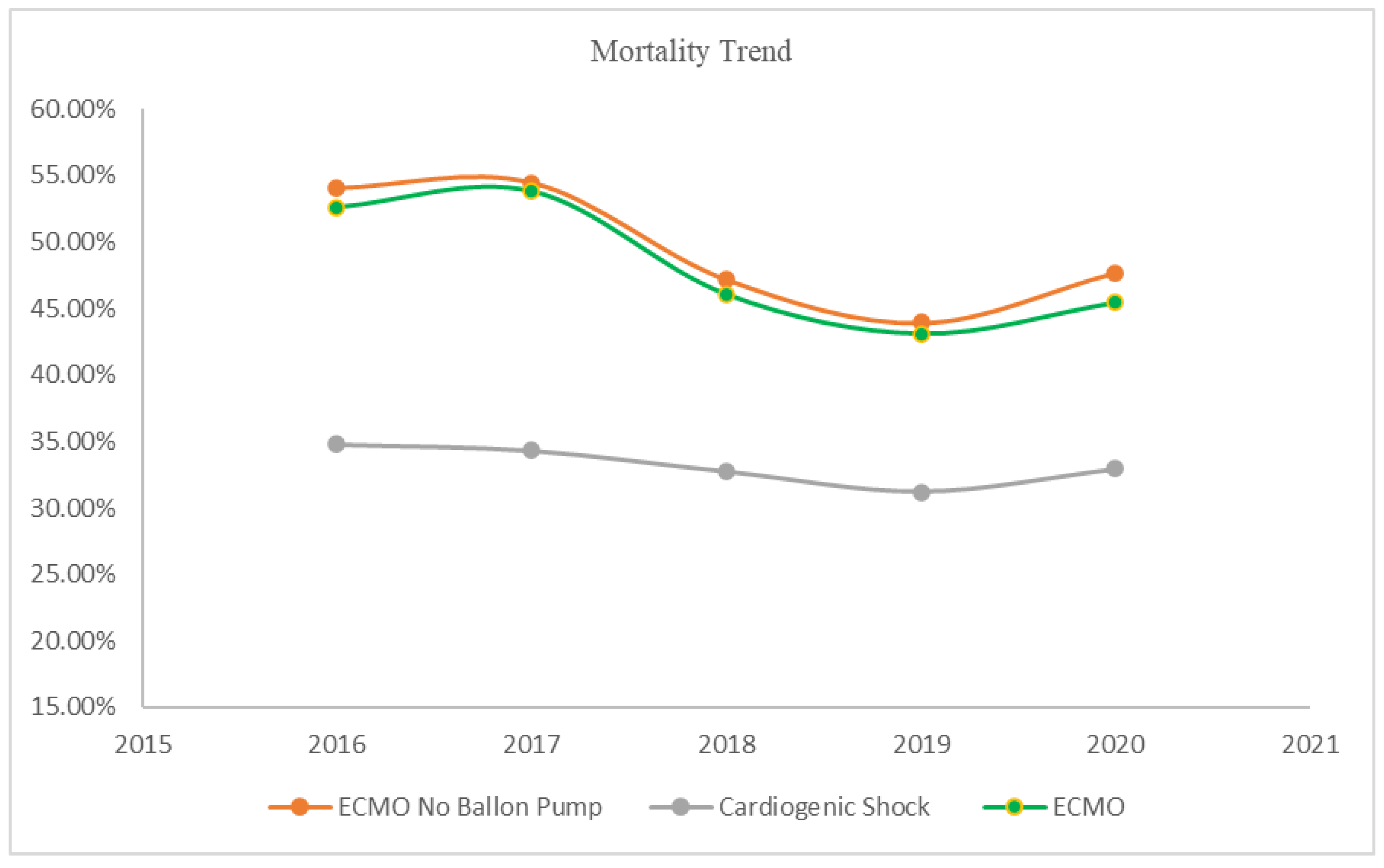
Mortality
Multivariate and Subgroup Analysis
Complications
Discussion
Conclusion
Limitations
Conflict of Interest:
Funding
References
- Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340(15):1162-8.
- Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119(9):1211-9.
- Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc. 2019;8(8):e011991.
- Telukuntla KS, Estep JD. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist Debakey Cardiovasc J. 2020;16(1):27-35.
- Malik A, Basu T, VanAken G, Aggarwal V, Lee R, Abdul-Aziz A, et al. National Trends for Temporary Mechanical Circulatory Support Utilization in Patients With Cardiogenic Shock From Decompensated Chronic Heart Failure: Incidence, Predictors, Outcomes, and Cost. Journal of the Society for Cardiovascular Angiography & Interventions. 2023;2(6, Part B):101177.
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287-96.
- Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M, et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation. 2019;139(3):395-403. [CrossRef]
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165.
- Tehrani BN, Truesdell AG, Psotka MA, Rosner C, Singh R, Sinha SS, et al. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020;8(11):879-91.
- Yuan S, He J, Cai Z, Zhang R, Song C, Qiao Z, et al. Intra-aortic balloon pump in cardiogenic shock: A propensity score matching analysis. Catheter Cardiovasc Interv. 2022;99 Suppl 1:1456-64. [CrossRef]
- Zein R, Patel C, Mercado-Alamo A, Schreiber T, Kaki A. A Review of the Impella Devices. Interv Cardiol. 2022;17:e05.
- Zhang Q, Han Y, Sun S, Zhang C, Liu H, Wang B, et al. Mortality in cardiogenic shock patients receiving mechanical circulatory support: a network meta-analysis. BMC Cardiovasc Disord. 2022;22(1):48.
- Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ Heart Fail. 2018;11(9):e004905.
- Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of Mechanical Circulatory Support. Journal of the American College of Cardiology. 2015;66(23):2663-74. [CrossRef]
- Tsangaris A, Alexy T, Kalra R, Kosmopoulos M, Elliott A, Bartos JA, et al. Overview of Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support for the Management of Cardiogenic Shock. Front Cardiovasc Med. 2021;8:686558.
- Piechura LM, Coppolino A, Mody GN, Rinewalt DE, Keshk M, Ogawa M, et al. Left ventricle unloading strategies in ECMO: A single-center experience. J Card Surg. 2020;35(7):1514-24.
- Shen J, Tse JR, Chan F, Fleischmann D. CT Angiography of Venoarterial Extracorporeal Membrane Oxygenation. Radiographics. 2022;42(1):23-37.
- Keebler ME, Haddad EV, Choi CW, McGrane S, Zalawadiya S, Schlendorf KH, et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC: Heart Failure. 2018;6(6):503-16.
- Richardson AC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO Journal. 2021;67(3):221-8.
- Zeymer U, Freund A, Hochadel M, Ostadal P, Belohlavek J, Rokyta R, et al. Venoarterial extracorporeal membrane oxygenation in patients with infarct-related cardiogenic shock: an individual patient data meta-analysis of randomised trials. Lancet. 2023;402(10410):1338-46. [CrossRef]
- Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287-303.
- El Sibai R, Bachir R, El Sayed M. Outcomes in Cardiogenic Shock Patients with Extracorporeal Membrane Oxygenation Use: A Matched Cohort Study in Hospitals across the United States. BioMed Research International. 2018;2018:2428648.
- García-Gigorro R, Renes-Carreño E, Pérez-Vela JL, Marín-Mateos H, Gutiérrez J, Corrés-Peiretti MA, et al. Mechanical support with venoarterial extracorporeal membrane oxygenation (ECMO-VA): Short-term and long-term prognosis after a successful weaning. Med Intensiva. 2017;41(9):513-22.
- Koerner MM, Harper MD, Gordon CK, Horstmanshof D, Long JW, Sasevich MJ, et al. Adult cardiac veno-arterial extracorporeal life support (VA-ECMO): prevention and management of acute complications. Ann Cardiothorac Surg. 2019;8(1):66-75.
- Krasivskyi I, Ivanov B, Vehrenberg J, Eghbalzadeh K, Gerfer S, Gaisendrees C, et al. Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program. Life (Basel). 2022;12(11).
- Pang S, Miao G, Zhao X. Effects and safety of extracorporeal membrane oxygenation in the treatment of patients with ST-segment elevation myocardial infarction and cardiogenic shock: A systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:963002.
- Senoner T, Treml B, Breitkopf R, Oezpeker UC, Innerhofer N, Eckhardt C, et al. ECMO in Myocardial Infarction-Associated Cardiogenic Shock: Blood Biomarkers as Predictors of Mortality. Diagnostics. 2023;13(24):3683.
- Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation. 2023;147(6):454-64.
- Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N Engl J Med. 2023;389(14):1286-97.
- Paddock S, Meng J, Johnson N, Chattopadhyay R, Tsampasian V, Vassiliou V. The impact of extracorporeal membrane oxygenation on mortality in patients with cardiogenic shock post-acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J Open. 2024;4(1):oeae003.
- Burgos LM, Seoane L, Diez M, Baro Vila RC, Furmento JF, Vrancic M, et al. Multiparameters associated to successful weaning from VA ECMO in adult patients with cardiogenic shock or cardiac arrest: Systematic review and meta-analysis. Ann Card Anaesth. 2023;26(1):4-11.
- Henry TD, Yannopoulos D, van Diepen S. Extracorporeal Membrane Oxygenation for Cardiogenic Shock: When to Open the Parachute? Circulation. 2023;147(6):465-8.
- Lo Coco V, Lorusso R, Raffa GM, Malvindi PG, Pilato M, Martucci G, et al. Clinical complications during veno-arterial extracorporeal membrane oxigenation in post-cardiotomy and non post-cardiotomy shock: still the achille's heel. J Thorac Dis. 2018;10(12):6993-7004.
- Geller BJ, Sinha SS, Kapur NK, Bakitas M, Balsam LB, Chikwe J, et al. Escalating and De-escalating Temporary Mechanical Circulatory Support in Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2022;146(6):e50-e68.
- Møller JE, Engstrøm T, Jensen LO, Eiskjær H, Mangner N, Polzin A, et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. New England Journal of Medicine. 2024;390(15):1382-93.
- Hajjar LA, Teboul JL. Mechanical Circulatory Support Devices for Cardiogenic Shock: State of the Art. Crit Care. 2019;23(1):76.
- Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922-34.
- Jeong JH, Kook H, Lee SH, Joo HJ, Park JH, Hong SJ, et al. Prediction of In-Hospital Mortality for Ischemic Cardiogenic Shock Requiring Venoarterial Extracorporeal Membrane Oxygenation. J Am Heart Assoc. 2024;13(4):e032701.
| Cardiogenic Shock | No ECMO | ECMO | ECMO No Balloon Pump | p-value | |
|---|---|---|---|---|---|
| Age N(Mean±SD) | 796,585(66.57±14.40) | 779,225(66.83±14.28) | 17,360(54.87±15.40) | 13,160(53.72±15.43) | <0.001 |
| Mortality | 33.07% | 32.74% | 47.91% | 49.05% | <0.001 |
| Gender % | <0.001 | ||||
| Male | 62.00% | 61.91% | 66.16% | 64.29% | |
| Female | 38.00% | 38.09% | 33.84% | 35.71% | |
| Race % | <0.001 | ||||
| White | 67.40% | 67.46% | 64.27% | 63.34% | |
| Black | 16.40% | 16.39% | 17.01% | 17.80% | |
| Hipanic | 8.99% | 9.00% | 8.74% | 8.80% | |
| Asian/Pac Isl | 3.32% | 3.31% | 3.70% | 3.58% | |
| Native-American | 0.67% | 0.67% | 0.91% | 1.07% | |
| Others | 3.22% | 3.17% | 5.37% | 5.41% | |
| Smoking | 23.11% | 23.30% | 14.92% | 15.05% | <0.001 |
| Peripheral Vascular Diseases | 5.27% | 5.31% | 3.08% | 2.96% | <0.001 |
| Cardiomyopathy | 41.98% | 42.17% | 33.41% | 29.86% | <0.001 |
| Diabetes | 39.48% | 39.77% | 26.56% | 25.87% | <0.001 |
| CKD | 39.78% | 40.15% | 22.96% | 21.39% | <0.001 |
| Systolic Heart Failure | 52.81% | 52.93% | 47.35% | 43.84% | <0.001 |
| PCI Three Vessel | 0.56% | 0.55% | 1.18% | 0.80% | <0.001 |
| Left main STEMI | 0.12% | 0.11% | 0.52% | 0.46% | 0.56 |
| STEMI | 20.46% | 20.42% | 22.09% | 18.73% | <0.001 |
| Non-STEMI | 18.35% | 18.52% | 10.92% | 9.19% | <0.001 |
| Anterior Wall STEMI | 5.89% | 5.82% | 9.27% | 7.56% | <0.001 |
| History of MI | 12.03% | 12.17% | 5.88% | 5.32% | <0.001 |
| Cachexia | 2.62% | 2.64% | 1.84% | 1.98% | 0.005 |
| Morbid Obesity | 8.29% | 8.27% | 8.99% | 9.80% | <0.001 |
| Obesity | 8.42% | 8.42% | 8.24% | 8.13% | <0.001 |
| Chronic Liver Disease | 19.18% | 18.74% | 38.88% | 39.06% | <0.001 |
| Atrial Fibrillation/Flutter | 43.54% | 43.76% | 33.87% | 32.60% | <0.001 |
| COPD | 23.46% | 23.73% | 11.18% | 11.06% | <0.001 |
| ALL Valvular Heart Disease | 22.73% | 22.88% | 20.54% | 18.35% | <0.001 |
| History of Stroke | 2.02% | 2.05% | 0.86% | 0.80% | 0.006 |
| Acute Lactic Acidosis | 36.93% | 36.68% | 48.30% | 48.44% | <0.001 |
| Cardiac Arrest | 9.57% | 9.49% | 13.10% | 13.34% | <0.001 |
| Mechanical Ventilation | 45.96% | 45.47% | 68.23% | 69.83% | <0.001 |
| Renal Replacement Therapy | 12.41% | 12.11% | 26.04% | 26.75% | <0.001 |
| Heart Failure | 70.37% | 70.44% | 67.25% | 64.67% | <0.001 |
| Presence of Coronary Angioplasty & Graft | 9.17% | 9.22% | 6.94% | 6.00% | <0.001 |
| Presence of Aortocoronary Bypass Graft | 7.83% | 7.91% | 4.35% | 4.48% | 0.1 |
| Presence of Cardiac Pacemaker | 3.14% | 3.18% | 1.24% | 1.03% | 0.04 |
| Prosthetic Heart Valve | 2.72% | 2.73% | 2.42% | 2.51% | <0.001 |
| Presence of Automatic (implantable) Cardiac Defibrillator | 8.27% | 8.37% | 4.03% | 3.76% | 0.002 |
| Coronary Angioplasty Status | 1.04% | 1.04% | 0.86% | 0.80% | 0.24 |
| Right Ventricular Infarction | 1.35% | 1.34% | 1.73% | 1.14% | <0.001 |
| Rotational Atherectomy | 0.10% | 0.10% | 0.14% | 0.11% | 0.09 |
| Mortality | ECMO No Balloon Pump | Risk ratio (CI) | p-value |
|---|---|---|---|
| Age<50 | 41.24% | 2.73 (2.40-3.11) | <0.001 |
| Age>=50 | 53.48% | 2.11 (2.00-2.22) | |
| Male | 48.23% | 2.12 (2.00-2.25) | <0.001 |
| Female | 50.53% | 1.81 (1.68-1.95) | |
| Diabetes | 52.72% | 2.07 (1.91-2.24) | 0.73 |
| No Diabetes | 47.77% | 2.03 (1.92-2.15) | |
| STEMI | 54.56% | 1.95 (1.79-2.12) | 0.009 |
| No STEMI | 47.78% | 2.24 (2.11-2.37) | |
| Non-STEMI | 50.83% | 2.20 (1.92-2.51) | 0.13 |
| No Non-STEMI | 48.87% | 1.97 (1.87-2.07) | |
| Left main STEMI | 66.67% | 2.94 (1.73-5.02) | 0.16 |
| No Left main STEMI | 48.97% | 2.01 (1.92-2.10) | |
| Anterior Wall STEMI | 51.76% | 2.04 (1.76-2.35) | 0.95 |
| No Anterior Wall STEMI | 48.83% | 2.03 (1.93-2.13) | |
| PCI Three Vessel | 47.62% | 1.92 (1.19-3.10) | 0.84 |
| No PCI Three Vessel | 49.06% | 2.01 (1.92-2.11) | |
| Cardiac Arrest | 56.13% | 1.15 (1.04-1.28) | <0.001 |
| No Cardiac Arrest | 47.96% | 2.17 (2.07-2.29) | |
| Acute Lactic Acidosis | 56.47% | 1.44 (1.36-1.53) | <0.001 |
| No Acute Lactic Acidosis | 42.08% | 2.37 (2.21-2.55) | |
| Peripheral Vascular Diseases | 57.69% | 1.95 (1.58-2.41) | 0.74 |
| No Peripheral Vascular Diseases | 48.79% | 2.03 (1.93-2.12) | |
| Obesity | 52.34% | 2.59 (2.22-3.01) | <0.001 |
| No Obesity | 48.76% | 1.96 (1.87-2.06) | |
| Smoking | 50.76% | 2.13 (1.91-2.38) | 0.24 |
| No Smoking | 48.75% | 1.99 (1.89-2.09) | |
| Hypertension | 50.51% | 2.12 (2.00-2.25) | <0.001 |
| No Hypertension | 47.22% | 1.80 (1.66-1.94) |
| Complications | Balloon Pump No ECMO | ECMO No Balloon Pump | p-value | Odds Ratio (C.I) |
|---|---|---|---|---|
| Pericardial Effusion | 3.72% | 7.79% | <0.001 | 2.19(1.84-2.60) |
| Cardiac Tamponade | 1.90% | 6.19% | <0.001 | 3.40(2.80-4.13) |
| Postprocedural Acute Kidney Failure | 0.42% | 0.34% | 0.59 | 0.81(0.39-1.71) |
| Acute Posthemorrhagic Anemia | 28.90% | 54.33% | <0.001 | 2.93(2.65-3.23) |
| Acquired Hemolytic Anemia | 0.07% | 0.49% | <0.001 | 6.92(3.08-15.56) |
| Postprocedural Hemorrhage | 0.96% | 5.09% | <0.001 | 5.55(4.42-6.97) |
| Acute Postprocedural Respiratory Failure | 4.49% | 5.47% | 0.02 | 1.23(1.03-1.48) |
| Disseminated Intravascular Coagulation | 1.58% | 8.89% | <0.001 | 6.07(5.13-7.19) |
| Cardiac Perforation (Accidental puncture and laceration of a circulatory system organ) | 0.87% | 2.20% | <0.001 | 2.58(1.94-3.44) |
| Procedural Bleeding | 0.10% | 1.18% | <0.001 | 11.63(6.60-20.49) |
| Intraoperative Cardiac Functional Disturbances | 0.33% | 1.33% | <0.001 | 4.04(2.66-6.14) |
| Postprocedural Cerebrovascular Infarction | 0.07% | 0.15% | 0.18 | 2.12(0.70-6.45) |
| Amputation of limb | 0.06% | 0.15% | 0.09 | 2.70(0.86-8.49) |
| Hemopericardium as current complication following acute myocardial infarction | 0.11% | 0.04% | 0.29 | 0.34(0.05-2.49) |
| Ventricular septal defect as current complication following acute myocardial infarction | 0.65% | 0.61% | 0.82 | 0.94(0.56-1.59) |
| Rupture of cardiac wall without hemopericardium as current complication following acute myocardial infarction | 0.15% | 0.11% | 0.66 | 0.77(0.23-2.52) |
| Rupture of chordae tendineae as current complication following acute myocardial infarction | 0.32% | 0.46% | 0.27 | 1.42(0.76-2.63) |
| Other current complications following acute myocardial infarction | 0.09% | 0.08% | 0.8 | 0.82(0.19-3.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





