Submitted:
31 May 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
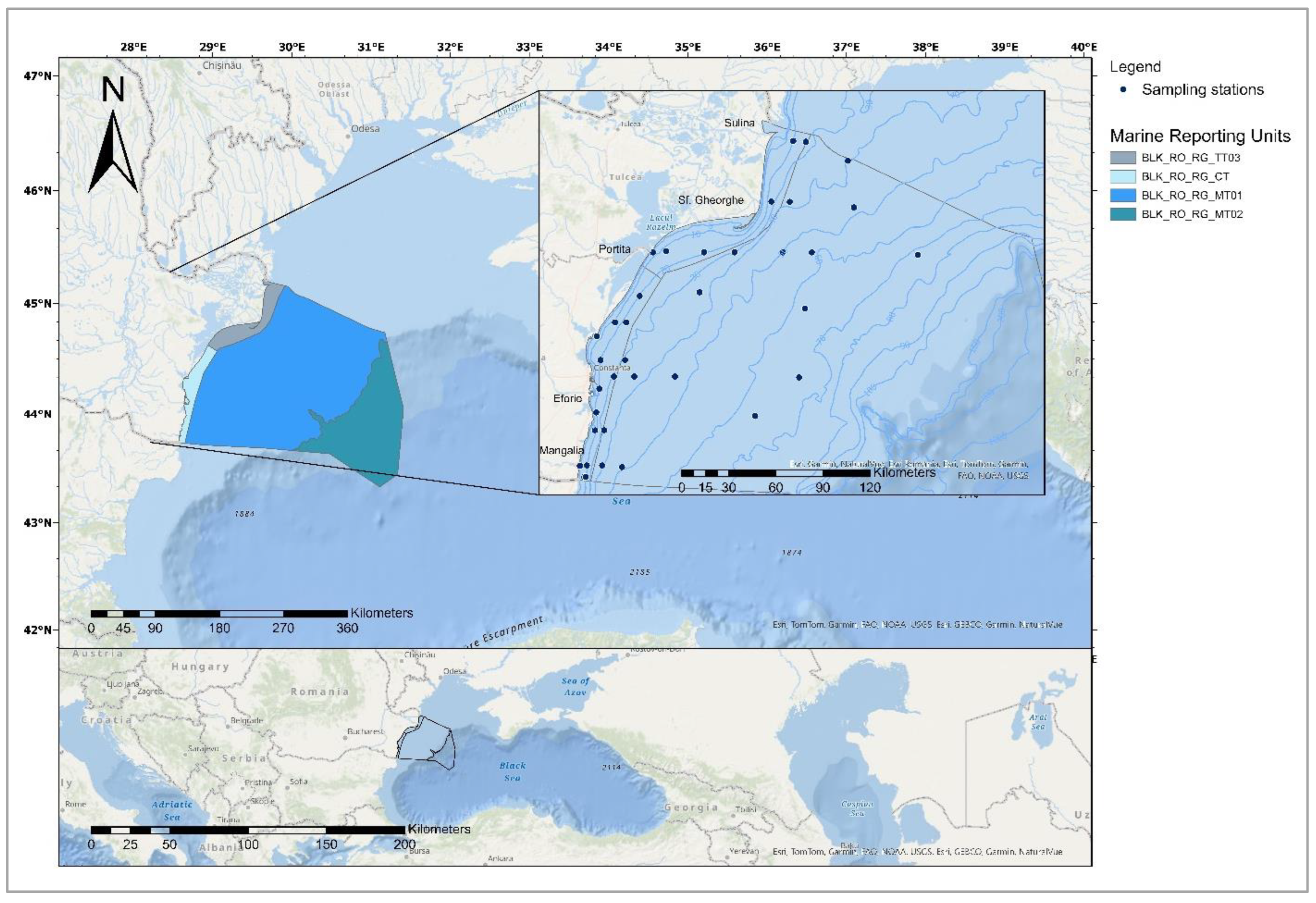
2.1. Sampling and Preliminary Preparation Methods
2.2. Analytical Methods
2.3. Human Health Risk Assessment
Estimated Daily Intake (EDI) and Estimated Weekly Intake (EWI)
Non-Carcinogenic Hazard (the Target Hazard Quotient (THQ)) and Hazardous Risk (Total Hazard Quotient (TTHQ))
Carcinogenic Risk Index (CRI)
4. Results
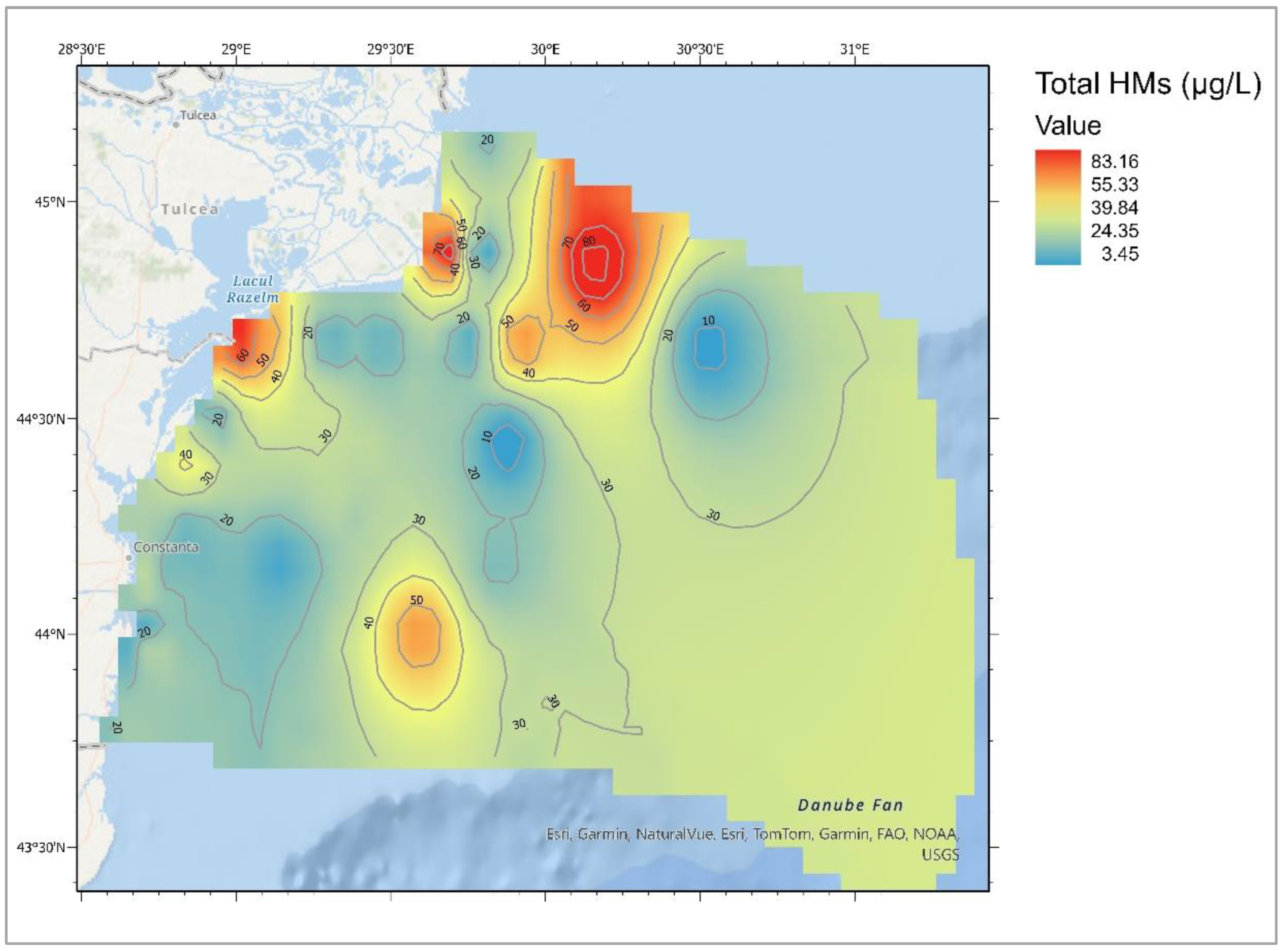
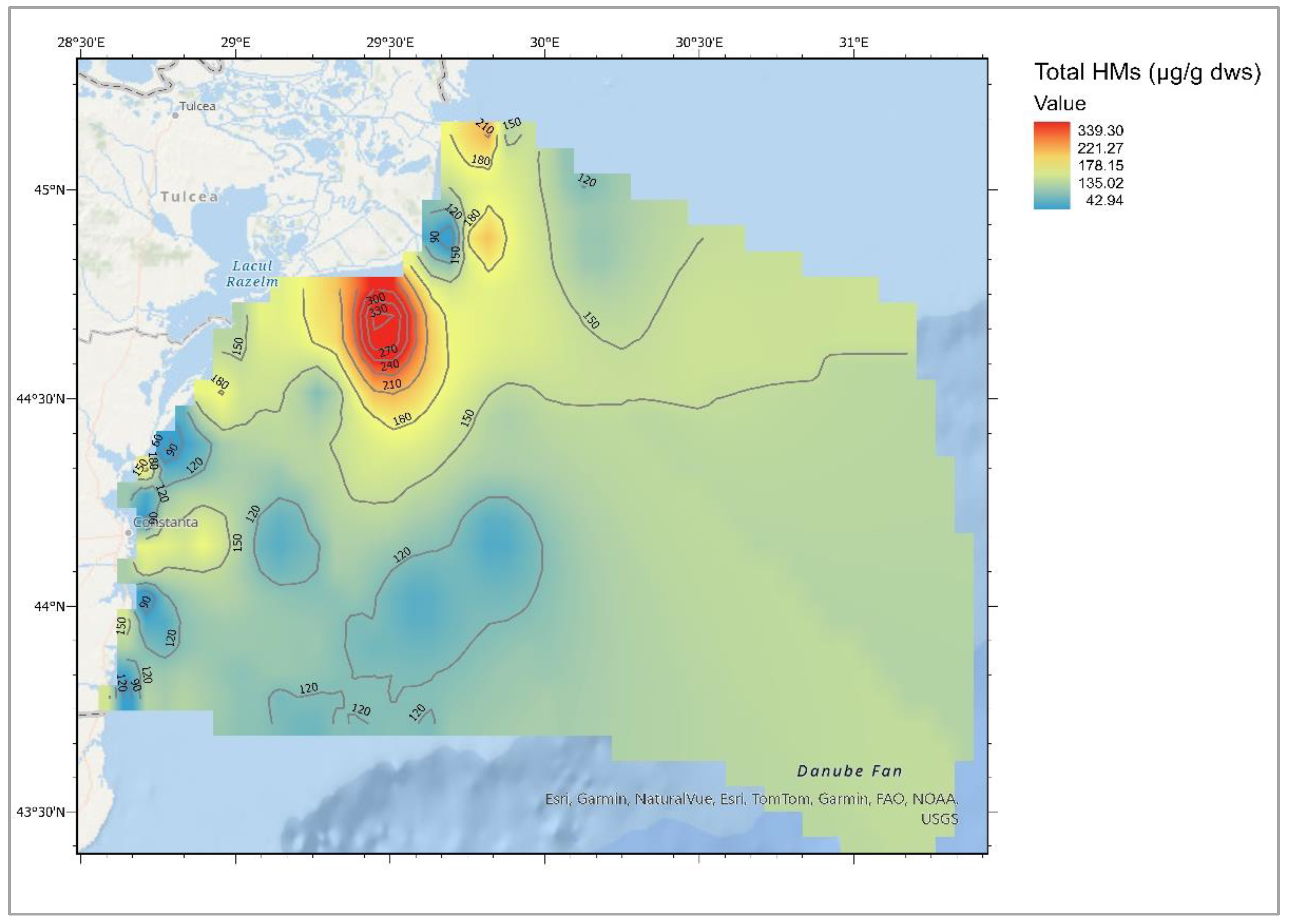
Heavy Metals
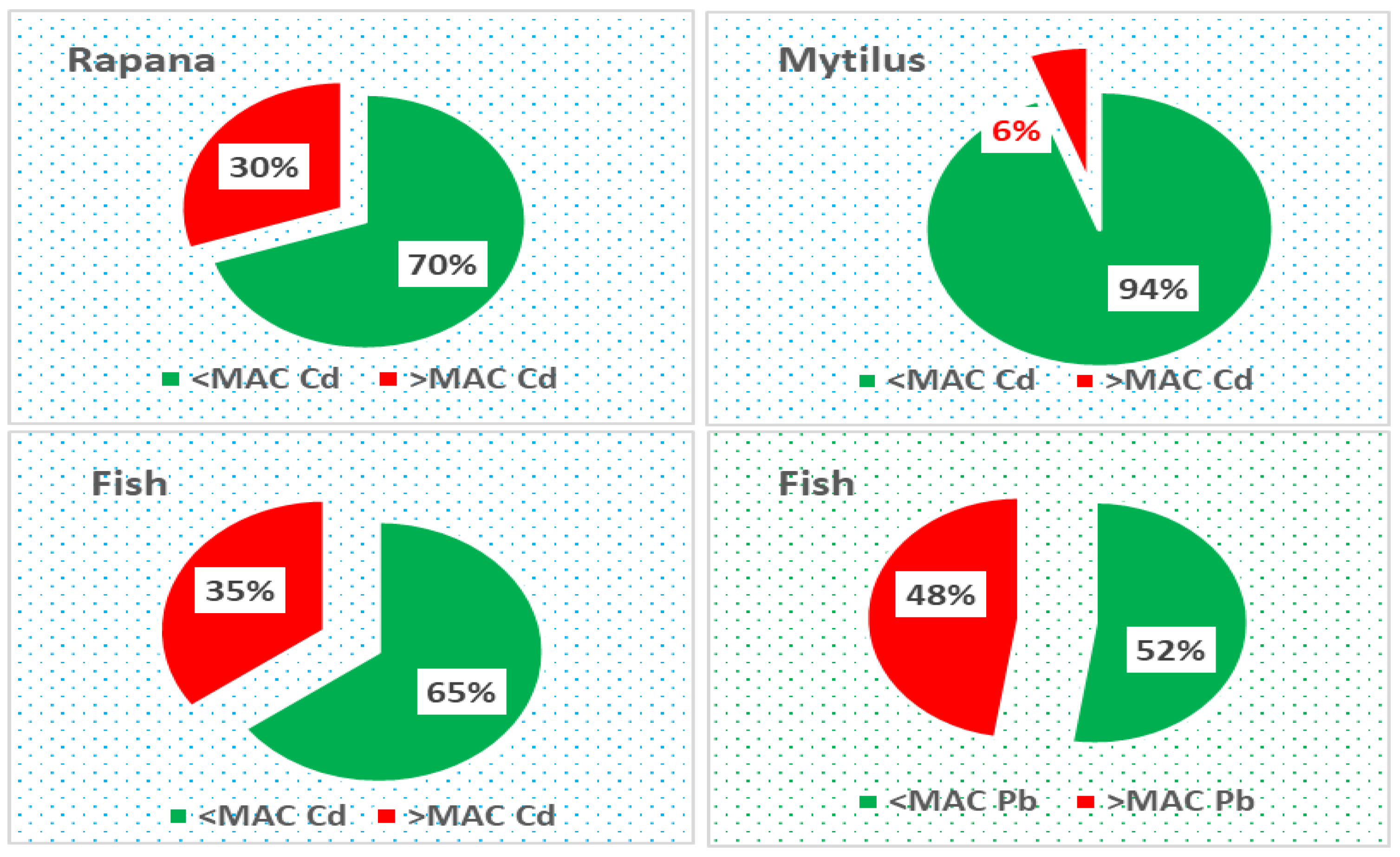
Human Health Risk Assessment
- TTHQ > 1: Indicates potential chronic danger, suggesting that the combined effects of metals exceed safe levels.
- TTHQ < 1: Implies no potential health risk, as the cumulative impact remains below critical thresholds.
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, M.; Whitfield, A. Challenging paradigms in estuarine ecology and management. Estuarine, Coast. Shelf Sci. 2011, 94, 306–314, . [CrossRef]
- Damir, N.-A.; Coatu, V.; Pantea, E.D.; Galațchi, M.; Botez, E.; Birghilă, S. Assessment of Polycyclic Aromatic Hydrocarbons Content in Marine Organisms of Commercial Interest from the Romanian Black Sea Coast. Polycycl. Aromat. Compd. 2022, 42, 7595–7606, . [CrossRef]
- Valentina Coatu Influente Ale Poluarii Chimice a Apei Marii Negre Asupra Unor Procese Biochimice La Moluste; Constanta, 2017; Vol. 502;.
- Rodríguez-Hernández, .; Camacho, M.; Henríquez-Hernández, L.A.; Boada, L.D.; Ruiz-Suárez, N.; Valerón, P.F.; González, M.A.; Zaccaroni, A.; Zumbado, M.; Luzardo, O.P. Assessment of human health hazards associated with the dietary exposure to organic and inorganic contaminants through the consumption of fishery products in Spain. Sci. Total. Environ. 2016, 557-558, 808–818, . [CrossRef]
- EEA Hazardous Substances in Marine Organisms. Https://Www.Eea.Europa.Eu/Data-and-Maps/Indicators/Hazardous-Substances-in-Marine-Organisms-3/Assessment. Accesed on 27 May 2024.
- Yunus, K.; Zuraidah, M.; John, A. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofeminism Clim. Chang. 2020, 1, 21–35, . [CrossRef]
- Chiarelli, R.; Roccheri, M.C. Marine Invertebrates as Bioindicators of Heavy Metal Pollution. Open J. Met. 2014, 04, 93–106, . [CrossRef]
- Sun, Y.; Wu, S.; Gong, G. Trends of research on polycyclic aromatic hydrocarbons in food: A 20-year perspective from 1997 to 2017. Trends Food Sci. Technol. 2019, 83, 86–98, . [CrossRef]
- Venkateswarlu, V.; Venkatrayulu, C. Bioaccumulation of heavy metals in edible marine fish from coastal areas of Nellore, Andhra Pradesh, India. GSC Biol. Pharm. Sci. 2020, 10, 018–024, . [CrossRef]
- Bat, L.; Gökkurt, O.; Sezgin, M.; Üstün, F.; Sahin, F. Evaluation of the Black Sea Land Based Sources of Pollution the Coastal Region of Turkey. Open Mar. Biol. J. 2009, 3, 112–124, . [CrossRef]
- Ismail, T. H. T., Adnan, N. A. F., & Samah, M. A. A. (2017). Study on Accumulation of Fe, Pb, Zn, Ni and Cd in Nerita Lineata and Thais Bitubercularis from Tanjung Harapan and Teluk Kemang, Malaysia. . Journal Cleanwas 2017, 1, 6–16. doi.org/10.1007/978-981-4560-70-2_72.
- Morankar, N.H.; Kurhe, A.R. An overview of the impact of heavy metal accumulation on marine molluscs. Int. J. Multidiscip. Res. Growth Evaluation 2023, 4, . [CrossRef]
- Bmw, A.-T.; K, I.A. Evaluation of Polycyclic Aromatic Hydrocarbons and Some Heavy Metals in Roasted Food Snacks in Amassoma, Niger Delta, Nigeria. 2013, 7, 961–966. [CrossRef]
- Bouwman, H.; Kylin, H.; Yive, N.S.C.K.; Tatayah, V.; Løken, K.; Skaare, J.U.; Polder, A. First report of chlorinated and brominated hydrocarbon pollutants in marine bird eggs from an oceanic Indian Ocean island. Environ. Res. 2012, 118, 53–64, . [CrossRef]
- Bouwman H. POPs in Southern Africa. The Handbook of Environmental Chemistry 2003, 297–320. doi.org/10.1007/10751132_11.
- Wikoff, D., F.L., & B.L. Persistent Organic Pollutants: An Overview. . Dioxins and health: including other persistent organic pollutants and endocrine disruptors 2012, 1–35. doi.org/10.1002/9781118184141.ch1.
- Lallas, P.L. The Stockholm Convention on Persistent Organic Pollutants. Am. J. Int. Law 2001, 95, 692–708, . [CrossRef]
- Danilov, D.; Dediu, L.; Damir, N.A.; Coatu, V.; Lazar, L. Screening for Organic Pollutants in the Black Sea Turbot (Scophthalmus maeoticus). Fishes 2023, 8, 265, . [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Heal. 2020, 17, 1363, . [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123, . [CrossRef]
- Directive, E.C.; 60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy I Establishing a Framework for Community Action in the Field of Water Policy; 2000;
- Pratt, M.M.; John, K.; MacLean, A.B.; Afework, S.; Phillips, D.H.; Poirier, M.C. Polycyclic Aromatic Hydrocarbon (PAH) Exposure and DNA Adduct Semi-Quantitation in Archived Human Tissues. Int. J. Environ. Res. Public Heal. 2011, 8, 2675–2691, . [CrossRef]
- Tongo, I.; Ogbeide, O.; Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017, 4, 55–61, . [CrossRef]
- Perra, G., Renzi, M., Guerranti, C., & E Focardi, S. Polycyclic Aromatic Hydrocarbons Pollution in Sediments: Distribution and Sources in a Lagoon System (Orbetello, Central Italy). Transit Water Bull 2009, 3(1), 45-58. 10.1285/i1825229Xv3n1p45.
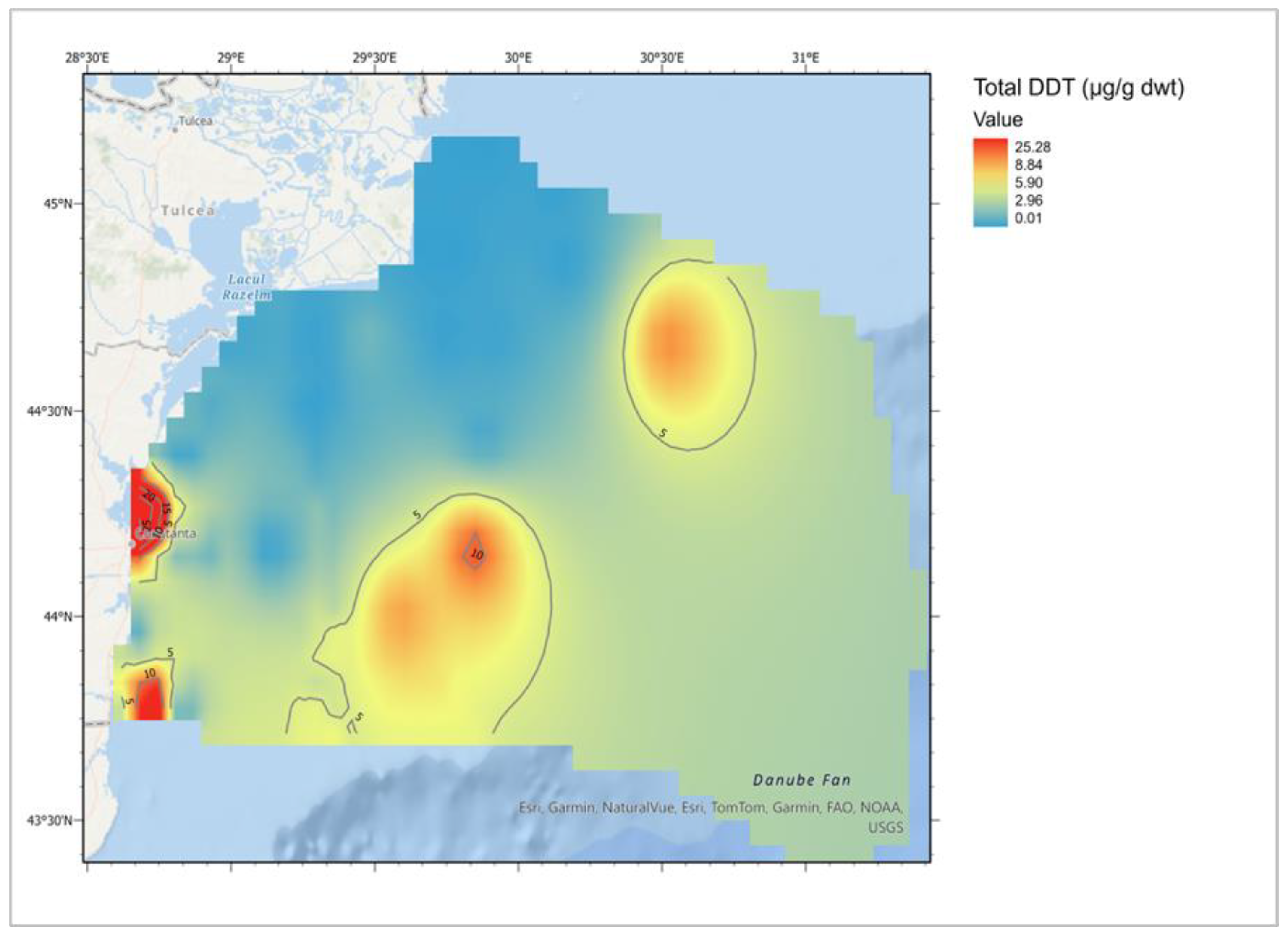
- Damir, N.; Danilov, D.; Oros, A.; Lazăr, L.; Coatu, V. Chemical status evaluation of the Romanian Black Sea marine environment based on benthic organisms’ contamination. 2022, 52, 52–77, . [CrossRef]
- COMMISSION REGULATION (EU) 2023/915 Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 2023.
- Bat, L., Yardım, Ö., Arıcı, E., Hasançavuşoğlu, Z., & Öztekin, A. Heavy Metals Risk Assesement for Consumption of Wild Mediterranean Mussels Mytilus Galloprovincialis LAMARCK, 1819 along Samsun Coasts of the Black Sea. Pakistan Journal of Marine Sciences 2023, 32.
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. (Eds.) Methods of Seawater Analysis; Willey-VCH: Weinheim, Germany, 1999.
- IAEA-MEL Training Manual on the Measurement of Heavy Metals in Environmental Samples; Monaco, 1999;
- IAEA-MEL Marine Environmental Studies Laboratory Training Manual on the Measurement of Organochlorine and Petroleum Hydrocarbons in Environmental Samples; 1995;
- Bat, L., Kaya, Y., & Oztekin, H. C. Heavy Metal Levels in the Black Sea Anchovy (Engraulis encrasicolus) as Biomonitor and Potential Risk of Human Health. Turkish Journal of Fisheries and Aquatic Sciences 2014, 14: 845-851. 10.4194/1303-2712-v14_4_01.
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Mendelsohn, R.; Deziel, N.C. Health risks of heavy metals in food and their economic burden in Armenia. Environ. Int. 2023, 172, 107794, . [CrossRef]
- Https://Www.Fao.Org/Faostat/ .Accesed on 27 May 2024.
- Bat, L.; Yardım, .; Öztekin, A.; Arıcı, E. Assessment of heavy metal concentrations in Scophthalmus maximus (Linnaeus, 1758) from the Black Sea coast: Implications for food safety and human health. J. Hazard. Mater. Adv. 2023, 12, . [CrossRef]
- RAIS Https://Rais.Ornl.Gov/Cgi-Bin/Tools/TOX_search. Accesed on 27 May 2024.
- USEPA Https://Www.Epa.Gov/Risk/Regional-Screening-Levels-Rsls-Generic-Tables. Accesed on 27 May 2024.
- Alexander, J., Benford, D., Boobis, A., Ceccatelli, S., Cravedi, J. P., Di Domenico, A. & van Leeuwen, R. Scientific opinion on lead in food EFSA panel on contaminants in the food chain (CONTAM). EFSA Journal 2010, 8, 1570.
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, 6268, doi:10.2903/j.efsa.2020.6268.
- Alexander, J., Benford, D., Boobis, A., Ceccatelli, S., Cravedi, J. P., Di Domenico, A.,& van Leeuwen, R. Statement on Tolerable Weekly Intake for Cadmium. EFSA Journal 2011, 9, 1975.
- Order 147/2004 for the Approval of Veterinary Sanitary Rules and Food Safety Regarding Pesticide Residues in Products of Animal and Non-Animal Origin and Residues of Veterinary Drugs in Products of Animal.;
- Https://Www.Info-Delta.Ro/Agricultura-Delta-Dunarii/. Accesed on 27 May 2024.
- Bat, L.; Öztekin, A.; Arici, E.; Şahin, F. Human Health Risk Assessment of Heavy Metals in the Black Sea: Evaluating Mussels. Current World Environment 2018, 13, 15–31. [CrossRef]
- BAT, L. HEAVY METALS IN EDIBLE TISSUES OF BENTHIC ORGANISMS FROM SAMSUN COASTS, SOUTH BLACK SEA, TURKEY AND THEIR POTENTIAL RISK TO HUMAN HEALTH. J. Food Heal. Sci. 2016, 57–66, . [CrossRef]
- Bakan, G.; Özkoç, H.B. An ecological risk assessment of the impact of heavy metals in surface sediments on biota from the mid-Black Sea coast of Turkey. Int. J. Environ. Stud. 2007, 64, 45–57, . [CrossRef]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol. 2014, 32, 369–378, . [CrossRef]
- Levent Bat, Öztekin, H. C. Heavy Metals in Mytilus Galloprovincialis, Rapana Venosa and Eriphia Verrucosa from The Black Sea Coasts of Turkey As Bioindicators of Pollution. Walailak J Sci & Tech 2015, 13.
- Zhelyazkov, G.; Yankovska-Stefanova, T.; Mineva, E.; Stratev, D.; Vashin, I.; Dospatliev, L.; Valkova, E.; Popova, T. Risk assessment of some heavy metals in mussels (Mytilus galloprovincialis) and veined rapa whelks (Rapana venosa) for human health. Mar. Pollut. Bull. 2018, 128, 197–201, . [CrossRef]
- Copat, C.; Bella, F.; Castaing, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy Metals Concentrations in Fish from Sicily (Mediterranean Sea) and Evaluation of Possible Health Risks to Consumers. Bull. Environ. Contam. Toxicol. 2011, 88, 78–83, . [CrossRef]
- Younis, E.M.; Abdel-Warith, A.-W.A.; Al-Asgah, N.A.; Elthebite, S.A.; Rahman, M. Nutritional value and bioaccumulation of heavy metals in muscle tissues of five commercially important marine fish species from the Red Sea. Saudi J. Biol. Sci. 2020, 28, 1860–1866, . [CrossRef]
- Fazio, F.; D’iglio, C.; Capillo, G.; Saoca, C.; Peycheva, K.; Piccione, G.; Makedonski, L. Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy). Animals 2020, 10, 1739, . [CrossRef]
- Görür, F.K.; Keser, R.; Akçay, N.; Dizman, S. Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere 2012, 87, 356–361, . [CrossRef]
- Galaţchi, M.; Oros, A.; Coatu, V.; Costache, M.; Coprean, D.; Galaţchi, L.-D. Pollutant bioaccumulation in anchovy (Engraulis encrasicolus) tissue, fish species of commercial interest at the Romanian Black Sea coast. Ovidius Univ. Ann. Chem. 2017, 28, 11–17, . [CrossRef]
- Makedonski, L.; Peycheva, K.; Stancheva, M. Determination of heavy metals in selected black sea fish species. Food Control. 2017, 72, 313–318, . [CrossRef]
- Simionov, I.-A.; Cristea, V.; Petrea, S.-M.; Mogodan, A.; Nicoara, M.; Baltag, E.S.; Strungaru, S.-A.; Faggio, C. Bioconcentration of Essential and Nonessential Elements in Black Sea Turbot (Psetta Maxima Maeotica Linnaeus, 1758) in Relation to Fish Gender. J. Mar. Sci. Eng. 2019, 7, 466, . [CrossRef]
- Azizi, G., Akodad, M., Baghour, M., Layachi, M., & Moumen, A. The Use of Mytilus Spp. Mussels as Bioindicators of Heavy Metal Pollution in the Coastal Environment. A Review. . Journal of Materials and Environmental Science 2018, 9(4), 1170-1181.10.26872/jmes.2018.9.4.129.
- Barhoumi, B.; El Megdiche, Y.; Clérandeau, C.; Ben Ameur, W.; Mekni, S.; Bouabdallah, S.; Derouiche, A.; Touil, S.; Cachot, J.; Driss, M.R. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in mussel (Mytilus galloprovincialis) and eel (Anguilla anguilla) from Bizerte lagoon, Tunisia, and associated human health risk assessment. Cont. Shelf Res. 2016, 124, 104–116, . [CrossRef]
- Balcıoğlu, E.B. Assessment of polycyclic aromatic hydrocarbons (PAHs) in mussels ( Mytilus galloprovincialis ) of Prince Islands, Marmara Sea. Mar. Pollut. Bull. 2016, 109, 640–642, . [CrossRef]
- Kucuksezgin, F.; Pazi, I.; Yucel-Gier, G.; Akcali, B.; Galgani, F. Monitoring of heavy metal and organic compound levels along the Eastern Aegean coast with transplanted mussels. Chemosphere 2013, 93, 1511–1518, . [CrossRef]
- León, V.M.; Martínez-Gómez, C.; García, I.; Campillo, J.A.; Benedicto, J. Spatial distribution and temporal trends of polycyclic aromatic hydrocarbons in Mytilus galloprovincialis from the Iberian Mediterranean coast. Environ. Monit. Assess. 2013, 185, 1055–1070, . [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O.; M. M. STORELLI and G. O. MARCOTRIGIANO*Pharmacal-Biologic Department, Chemistry and Biochemistry Section, Medicine Veterinary Faculty, University of Bari, Strada prov. le per Casamassima, km 3, 70010 Valenzano (BA), Italy Polycyclic Aromatic Hydrocarbons in Mussels (Mytilus galloprovincialis) from the Ionian Sea, Italy. J. Food Prot. 2001, 64, 405–409, . [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Triantafillaki, S.; Dassenakis, M.; Androutsos, F.; Scoullos, M. Polycyclic aromatic hydrocarbons in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Saronikos Gulf (Greece). Estuarine, Coast. Shelf Sci. 2008, 79, 733–739, . [CrossRef]
- Oros, A., Coatu, V., Tolun, L. G., Atabay, H., Denga, Y., Damir, N., Kolosov, V. ANEMONE Deliverable 3.1."Hazardous Substances Assessment in Black Sea Biota”; 2021;Ed. CD Press.
- Andersen, J.H.; Murray, C.; Larsen, M.M.; Green, N.; Høgåsen, T.; Dahlgren, E.; Garnaga-Budrė, G.; Gustavson, K.; Haarich, M.; Kallenbach, E.M.; et al. Development and testing of a prototype tool for integrated assessment of chemical status in marine environments. Environ. Monit. Assess. 2016, 188, . [CrossRef]
- Oros, A.; Coatu, V.; Tolun, L.G.; Atabay, H.; Denga, Y.; Damir, N.; Danilov, D.; Aslan, E.; Litvinova, M.; Oleinik, Y.; et al. Hazardous Substances Assessment in Black Sea Biota. 2021, 51, 27–48, . [CrossRef]
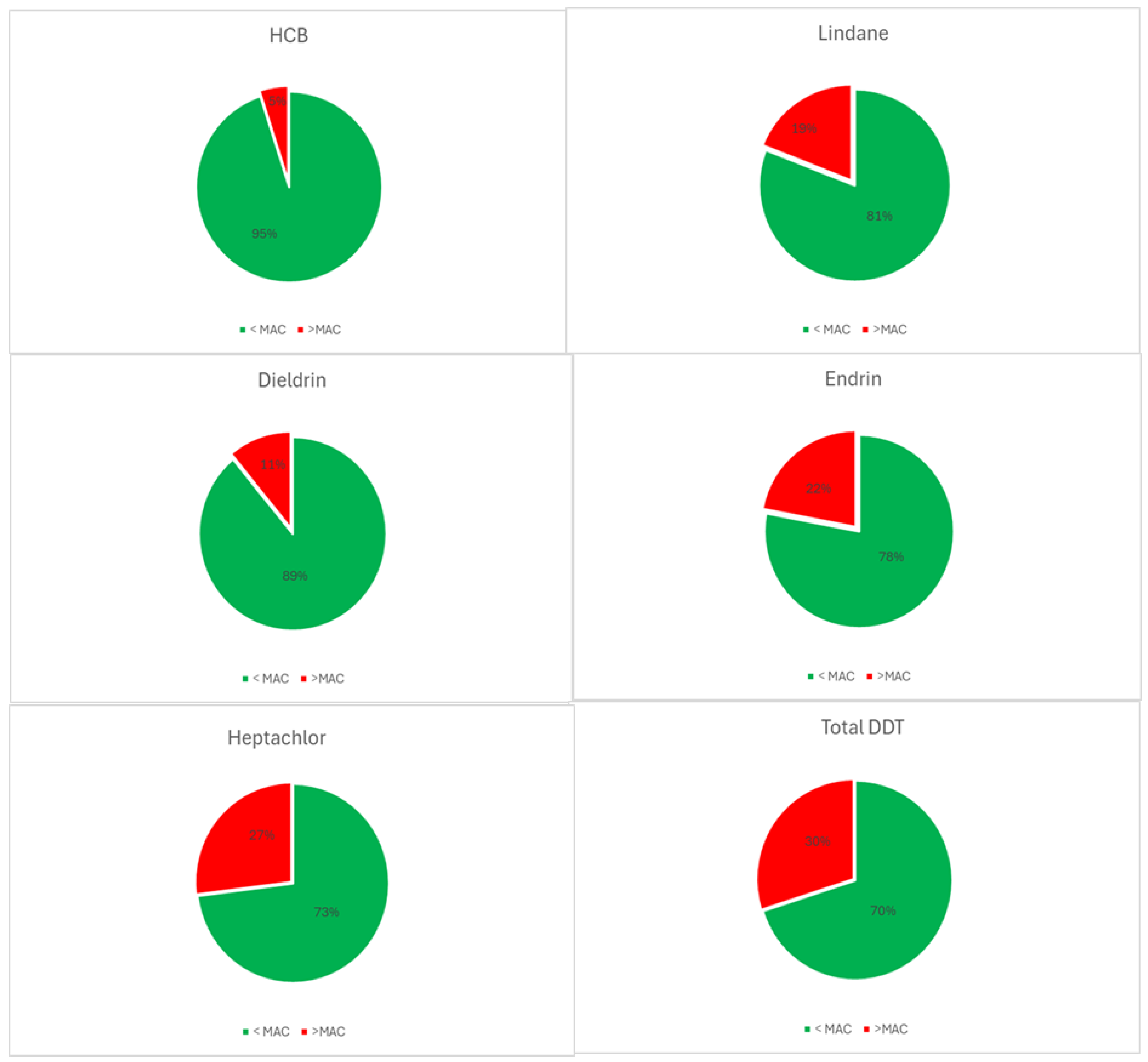
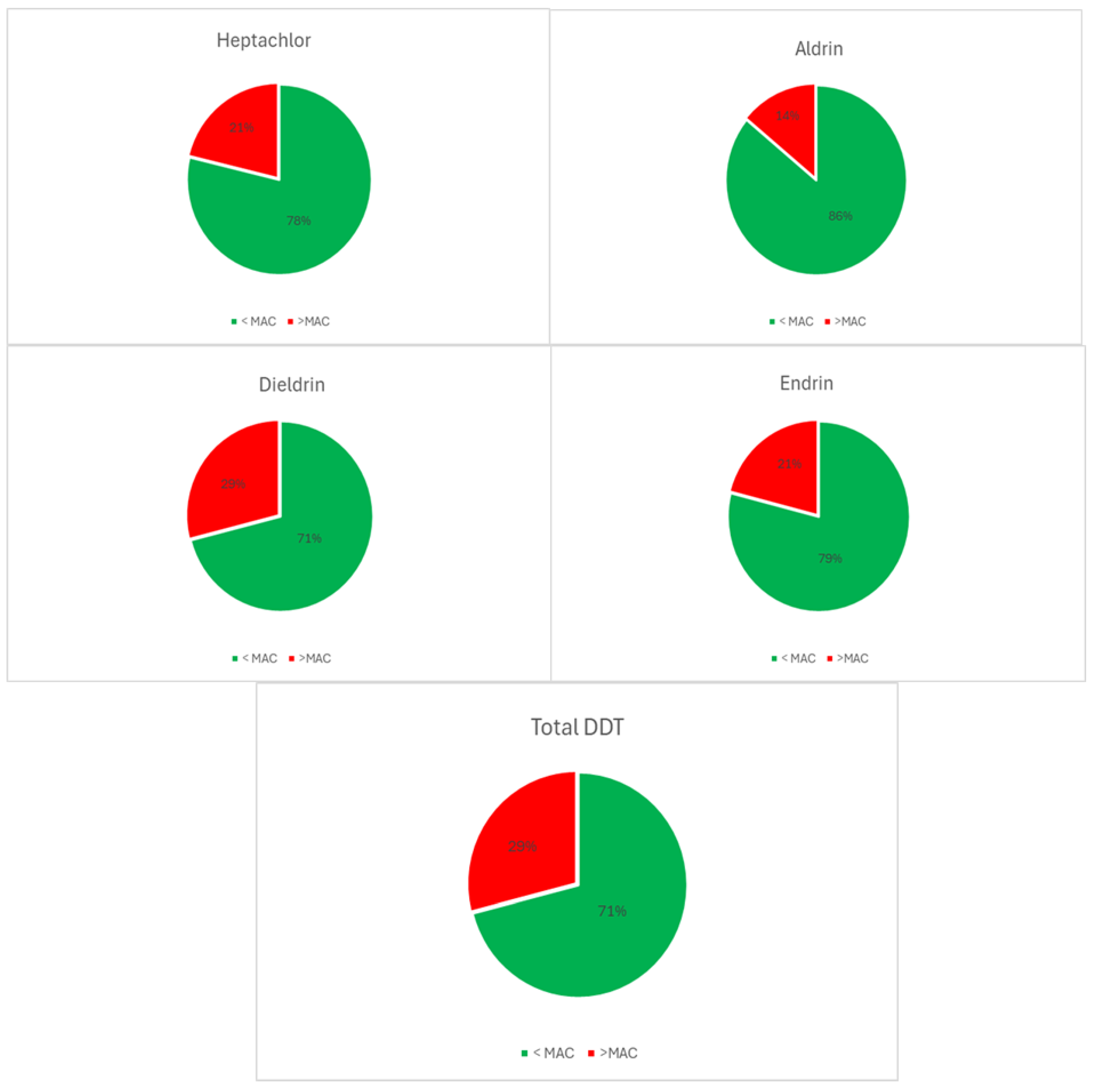
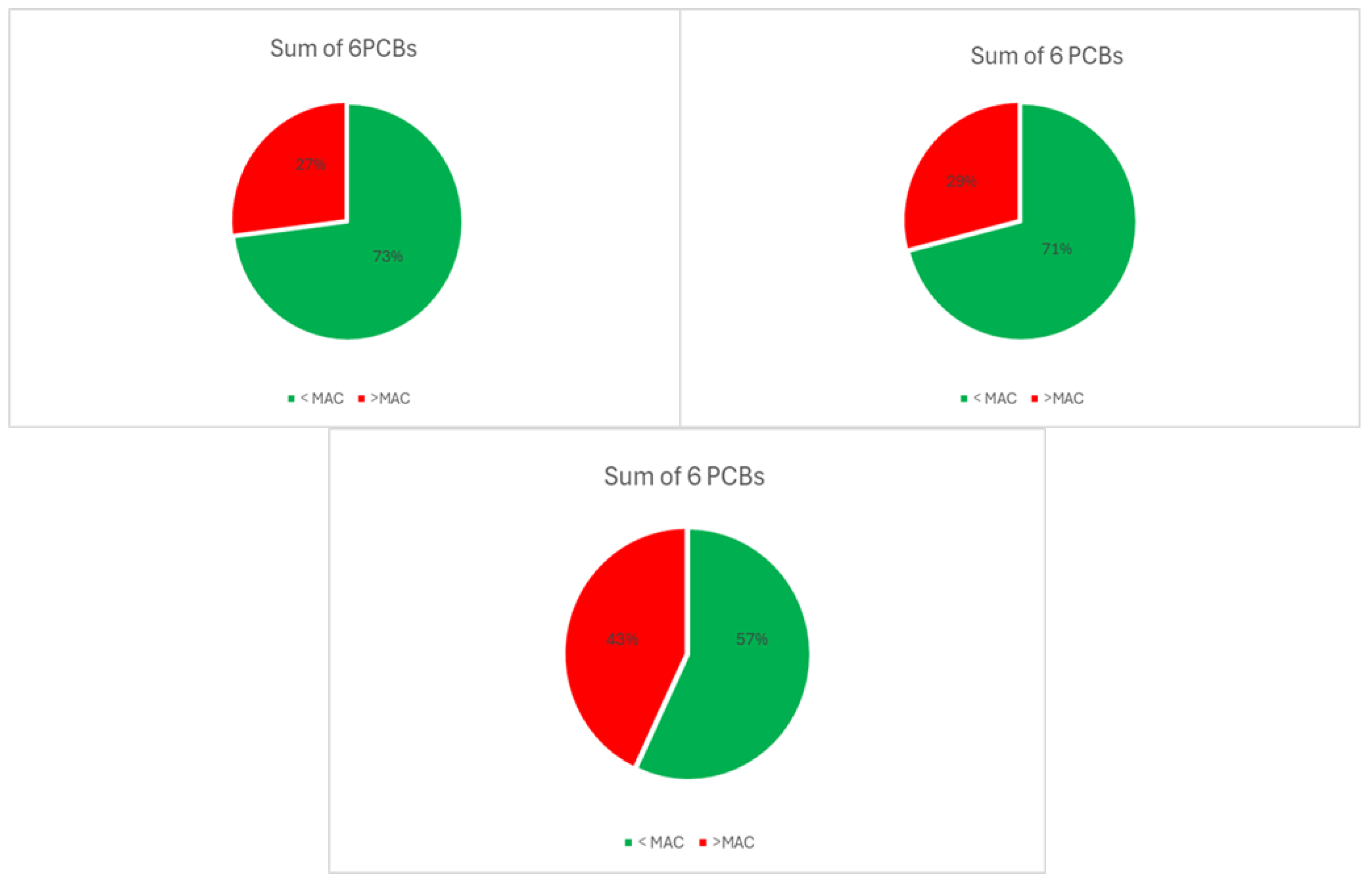
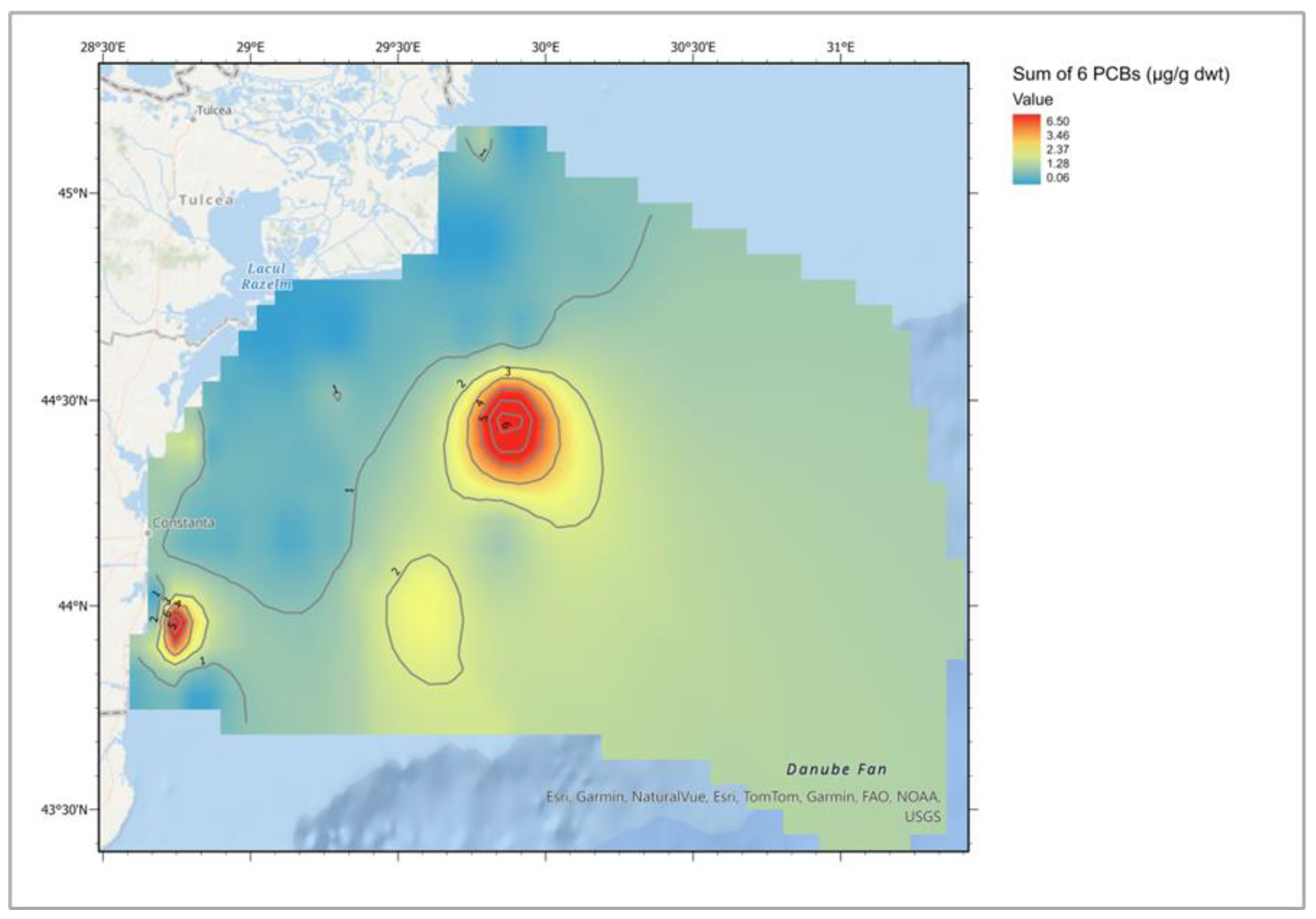
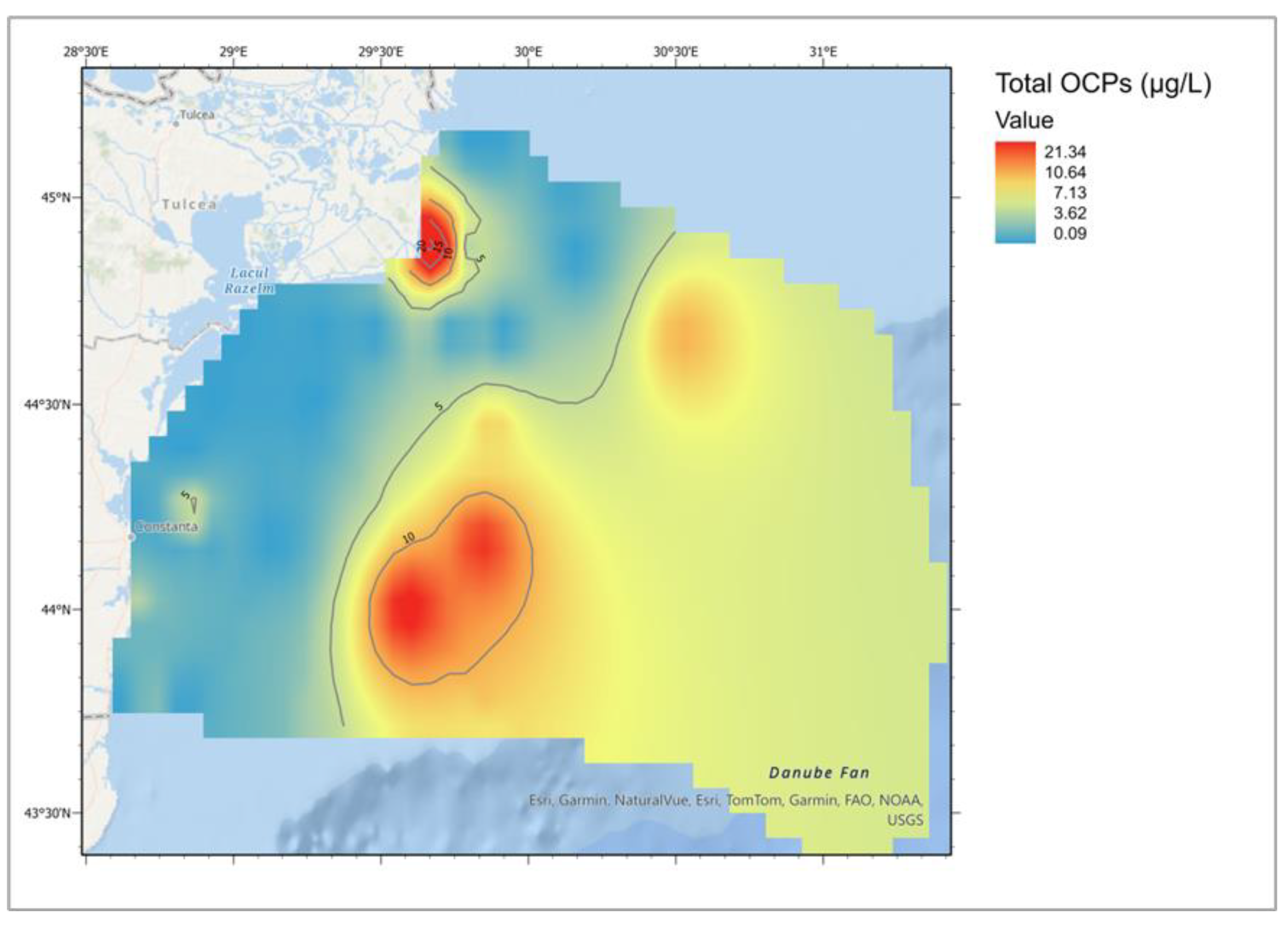
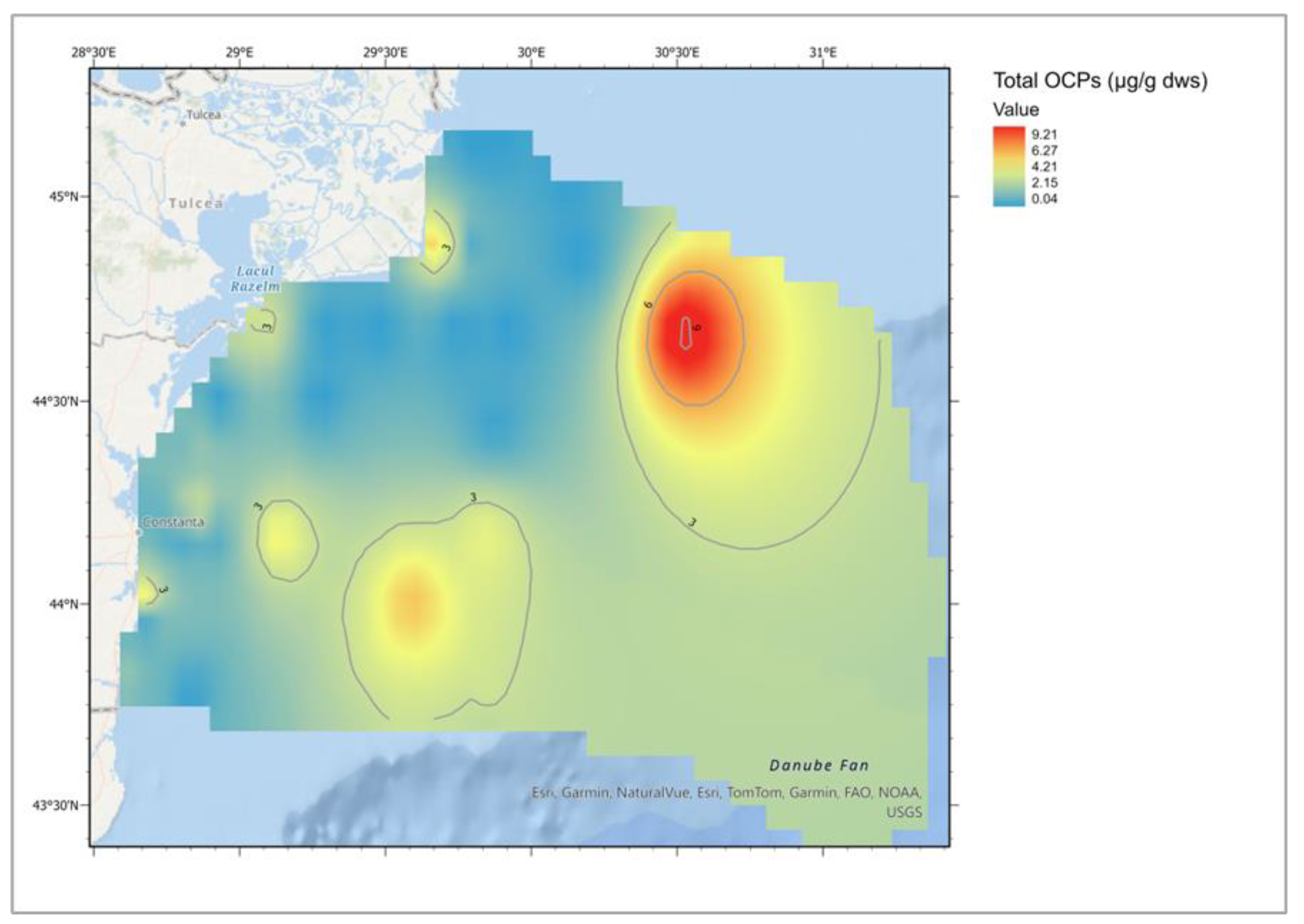
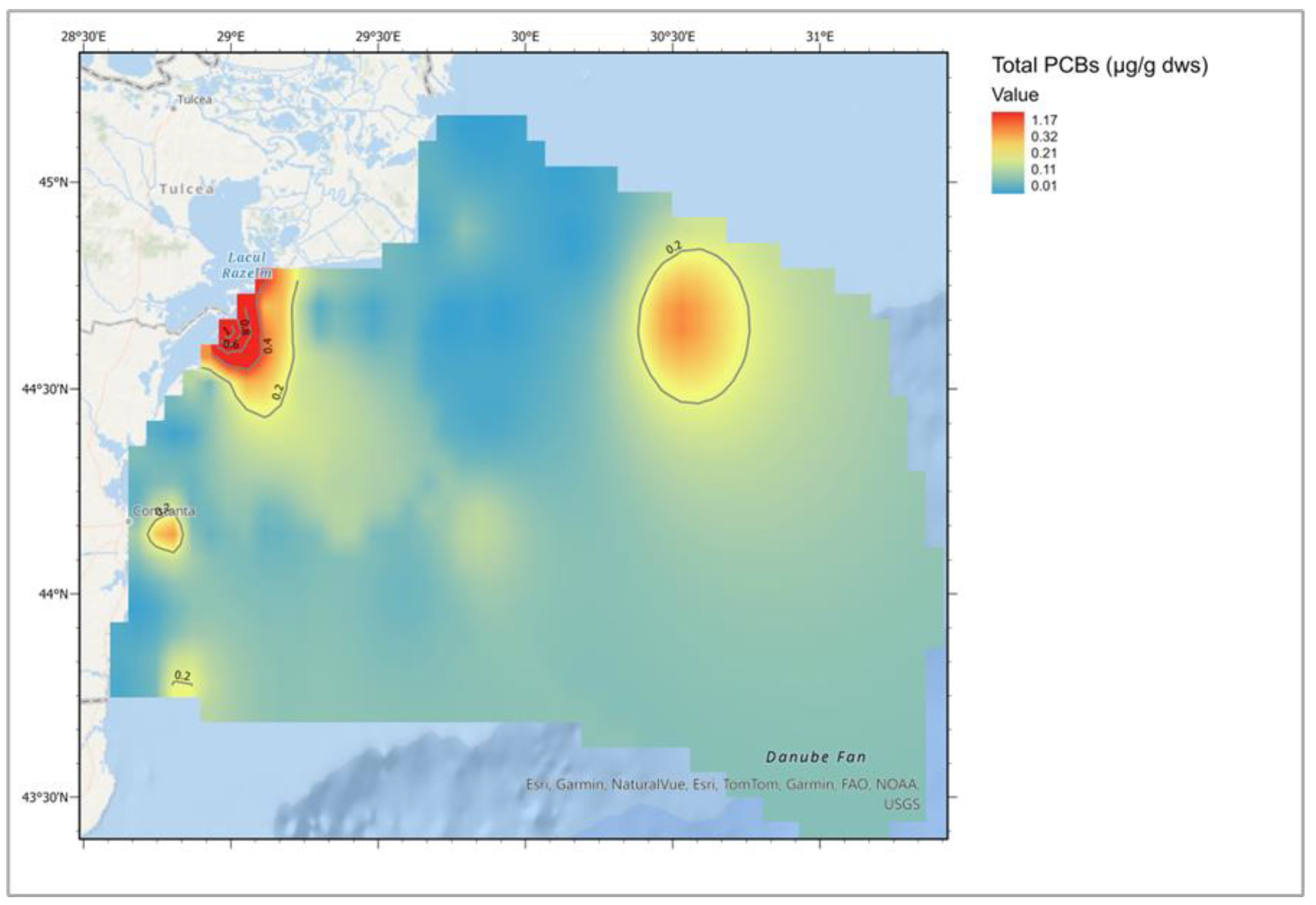
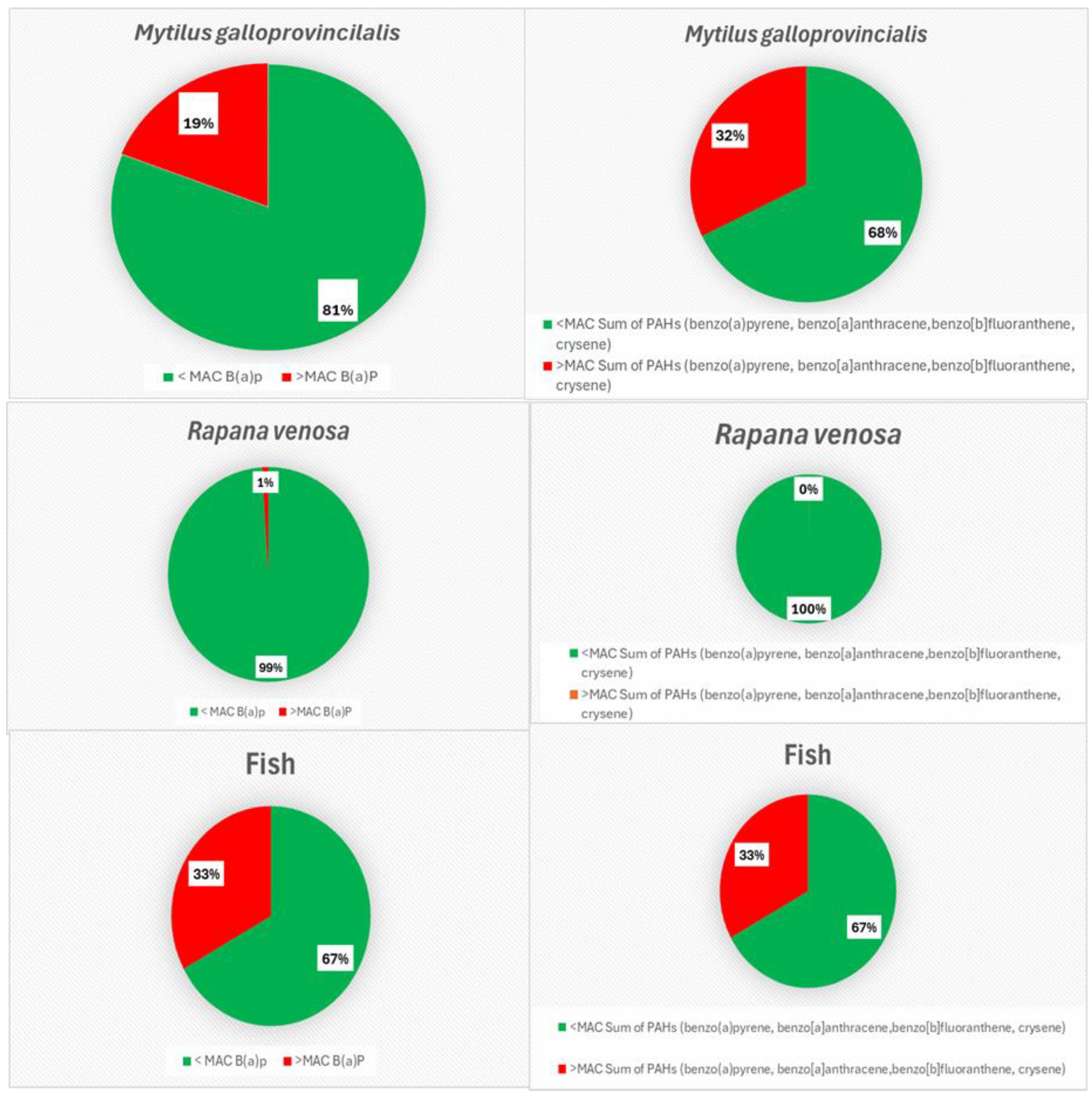
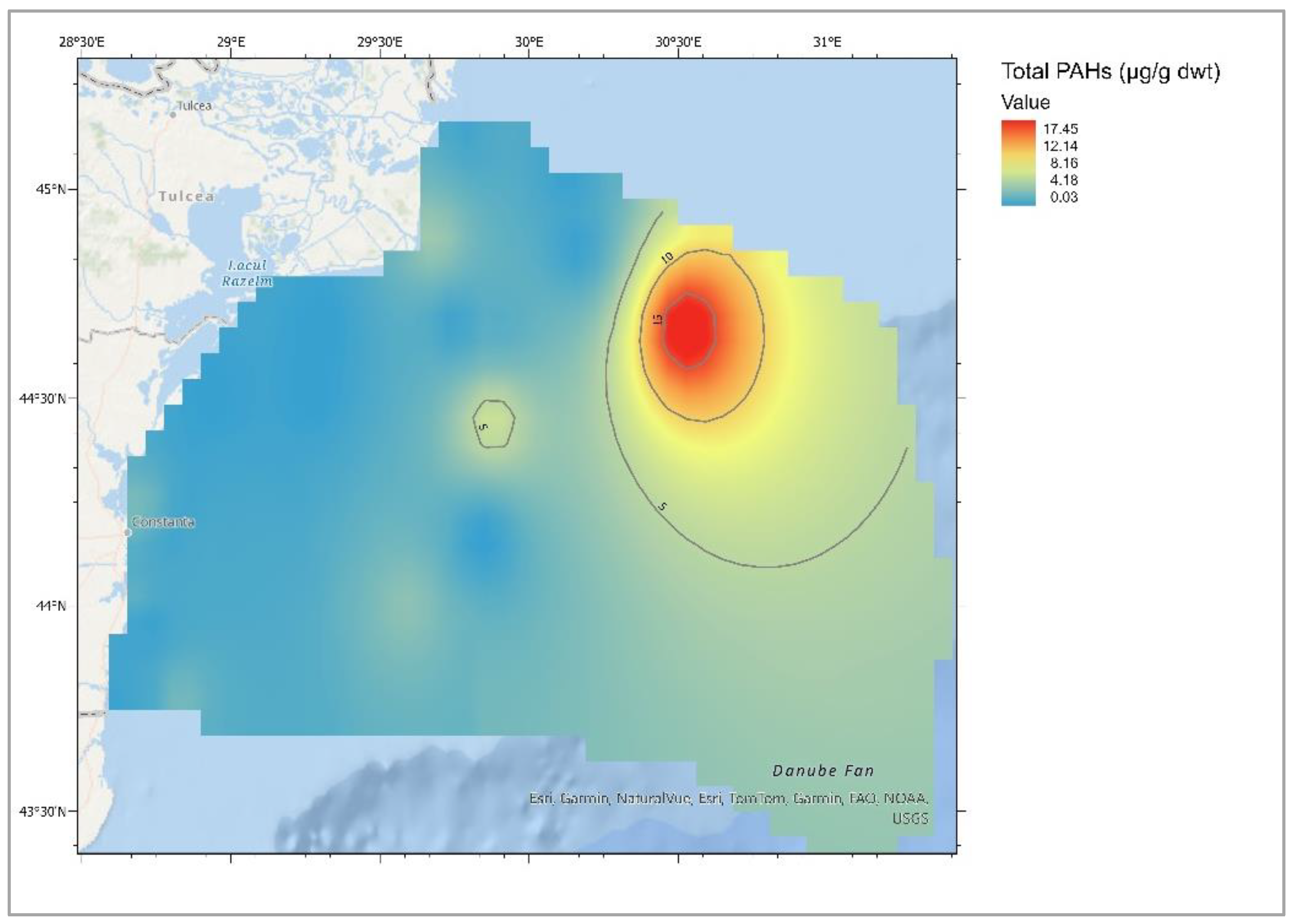
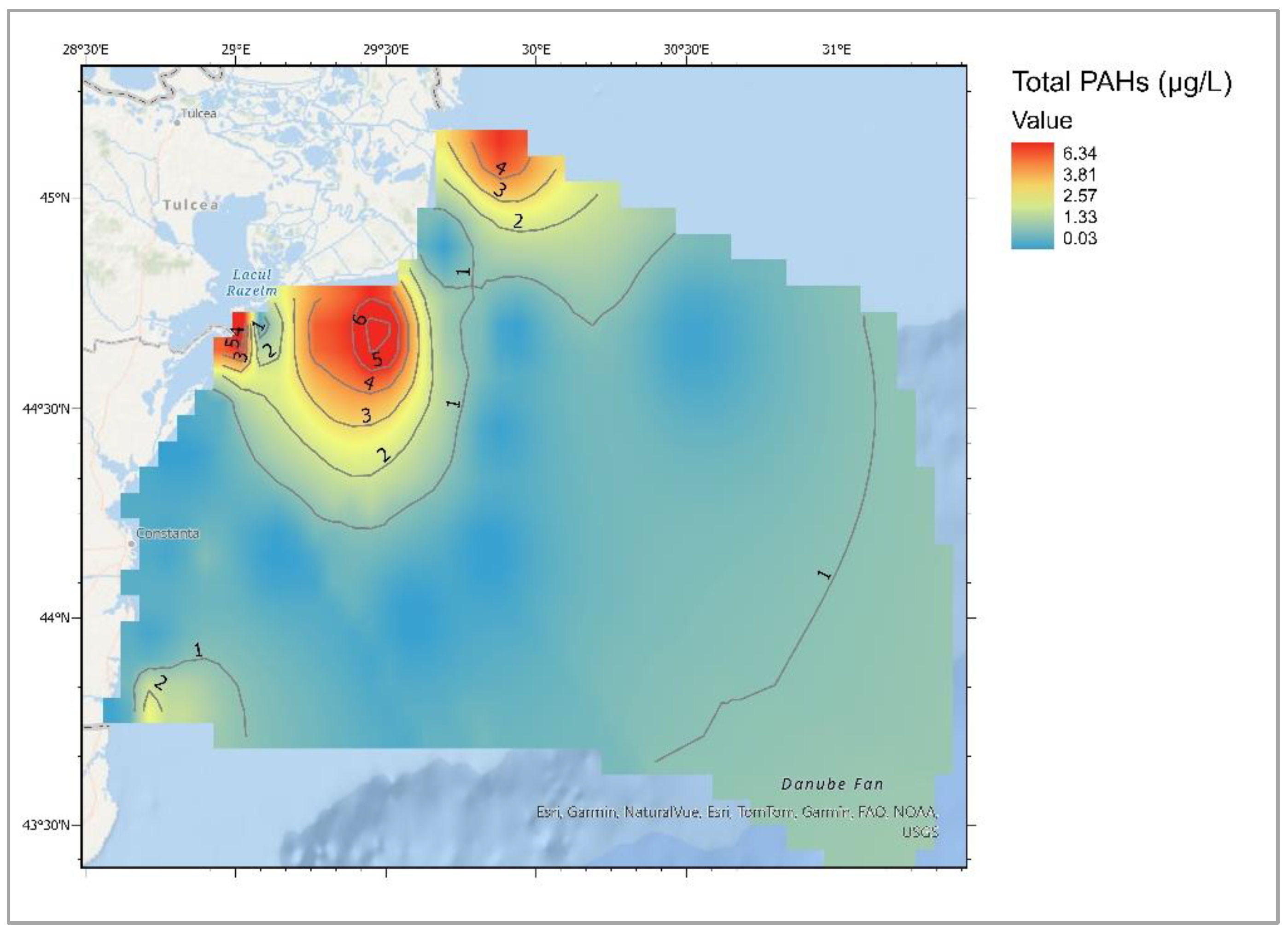
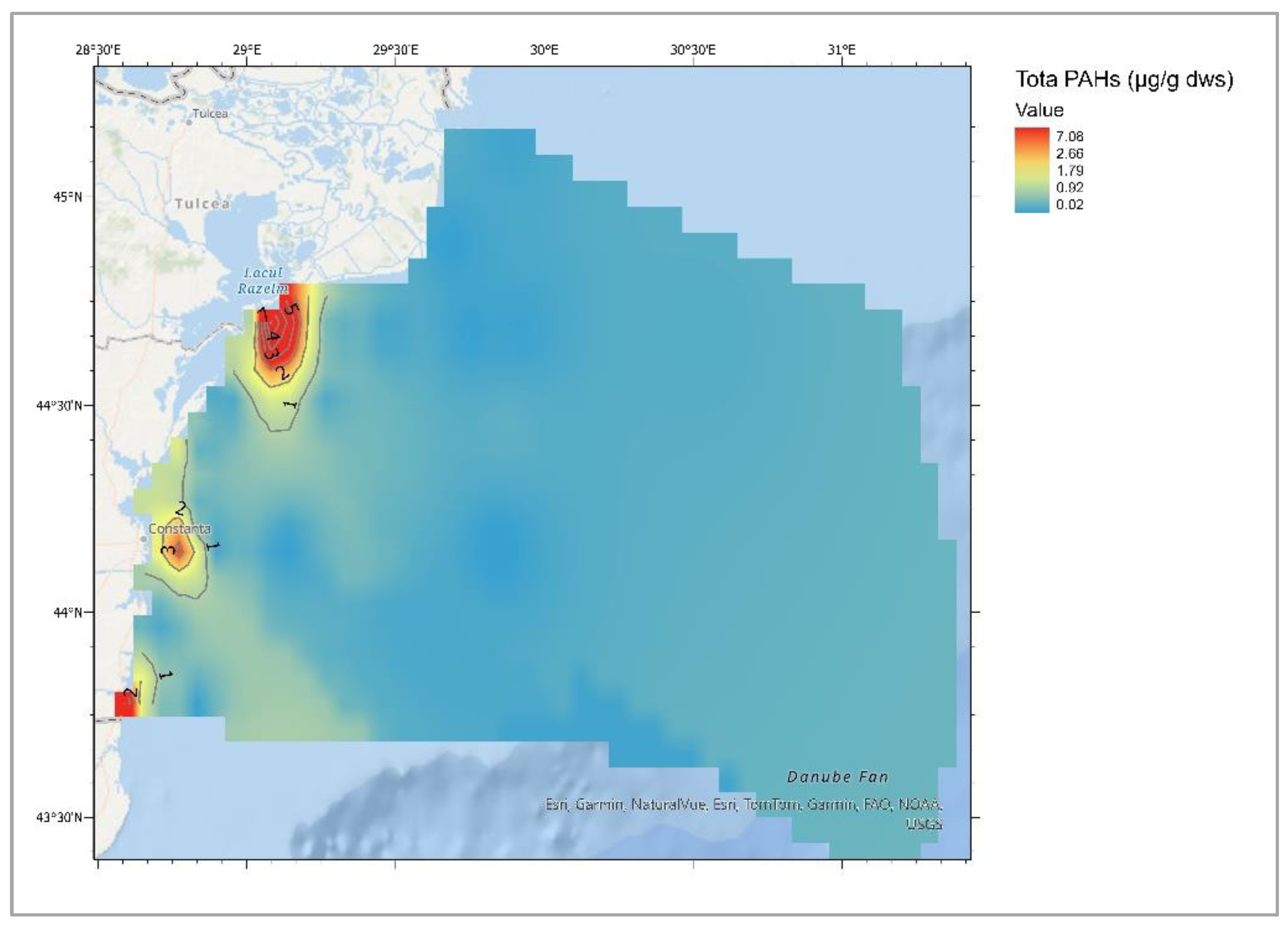
| Descriptive Statistics (Mytilus galloprovincialis, 2016-2023) | ||||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | Skewness | Kurtosis | |
| Cu (µg/g ww) |
45 | 2.112 | 2.154 | 0.460 | 5.110 | 1.038 | 2.756 | 56.536 | 0.540 | -0.311 |
| Cd (µg/g ww) |
45 | 0.520 | 0.377 | 0.143 | 2.018 | 0.276 | 0.630 | 71.461 | 2.169 | 5.969 |
| Pb (µg/g ww) |
45 | 0.149 | 0.034 | 0.002 | 1.587 | 0.0192 | 0.102 | 196.19 | 3.445 | 13.486 |
| Ni (µg/g ww) |
45 | 1.005 | 0.758 | 0.143 | 5.744 | 0.450 | 1.140 | 98.097 | 3.090 | 12.160 |
| Cr (µg/g ww) |
45 | 0.957 | 0.390 | 0.080 | 4.384 | 0.233 | 1.169 | 119.59 | 1.748 | 2.091 |
| Descriptive Statistics (Rapana venosa, 2016 - 2021) | ||||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | Skewness | Kurtosis | |
| Cu (µg/g ww) |
15 | 5.994 | 5.2180 | 0.93 | 12.867 | 4.640 | 7.672 | 55.47 | 0.643 | 0.450 |
| Cd (µg/g ww) |
15 | 0.814 | 0.578 | 0.111 | 2.915 | 0.192 | 1.199 | 100.657 | 1.480 | 1.827 |
| Pb (µg/g ww) |
15 | 0.137 | 0.023 | 0.001 | 1.310 | 0.010 | 0.065 | 245.915 | 3.443 | 12.292 |
| Ni (µg/g ww) |
15 | 0.432 | 0.386 | 0.010 | 1.208 | 0.040 | 0.744 | 85.49 | 0.493 | -0.612 |
| Cr (µg/g ww) |
15 | 0.843 | 0.5765 | 0.116 | 2.376 | 0.324 | 1.390 | 81.921 | 1.052 | 0.495 |
| Descriptive Statistics (Fish, 2016 - 2019) | ||||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | Skewness | Kurtosis | |
| Cu (µg/g ww) |
21 | 2.990 | 2.592 | 0.565 | 7.907 | 1.275 | 4.725 | 68.518 | 0.770 | -0.263 |
| Cd (µg/g ww) |
21 | 0.065 | 0.030 | 0.007 | 0.230 | 0.016 | 0.075 | 109.52 | 1.295 | 0.323 |
| Pb (µg/g ww) |
21 | 0.601 | 0.260 | 0.002 | 4.625 | 0.003 | 0.520 | 175.92 | 3.134 | 10.949 |
| Ni (µg/g ww) |
21 | 4.245 | 3.172 | 0.092 | 18.46 | 0.332 | 5.808 | 127.52 | 1.758 | 2.736 |
| Cr (µg/g ww) |
21 | 0.235 | 0.215 | 0.028 | 0.880 | 0.135 | 0.277 | 74.113 | 2.614 | 9.363 |
| Metal | EDIs | EWIs | THQs | CRIs | ||||
| Children | Adults | Children | Adults | Children | Adults | Children | Adults | |
| Copper (Cu) | 8.27E-05 | 3.54E-05 | 5.79E-04 | 2.48E-04 | 2.07E-03 | 8.86E-04 | ||
| Cadmium (Cd) | 1.89E-05 | 8.09E-06 | 1.32E-04 | 5.66E-05 | 1.89E-01 | 8.09E-02 | ||
| Chromium (Cr) | 3.88E-05 | 1.66E-05 | 2.72E-04 | 1.17E-04 | 1.29E-02 | 5.55E-03 | ||
| Nickel (Ni) | 4.12E-05 | 1.77E-05 | 2.88E-04 | 1.24E-04 | 2.06E-03 | 8.83E-04 | ||
| Lead (Pb) | 1.05E-05 | 4.52E-06 | 7.38E-05 | 3.16E-05 | 8.96E-08 | 3.84E-08 | ||
| TTHQ | 2.06E-01 | 8.81E-02 | ||||||
| Descriptive Statistics (M. galloprovincialis, 2016 - 2023) | ||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | |
| HCB (µg/g ww) |
46 | 0.0335 | 0.00021 | 0.00008 | 0.4505 | 0.00008 | 0.0176 | 0.0858 |
| Lindane (µg/g ww) | 46 | 0.3312 | 0.00324 | 0.00006 | 7.3458 | 0.00006 | 0.1041 | 1.2112 |
| Heptachlor (µg/g ww) | 46 | 0.4533 | 0.00005 | 0.00005 | 9.0988 | 0.00005 | 0.0415 | 1.5225 |
| Aldrin (µg/g ww) |
46 | 0.0050 | 0.00005 | 0.00005 | 0.0577 | 0.00005 | 0.0019 | 0.0121 |
| Dieldrin (µg/g ww) | 46 | 0.0827 | 0.00032 | 0.00005 | 1.4328 | 0.00003 | 0.0137 | 0.2893 |
| Endrin (µg/g ww) | 46 | 0.0857 | 0.00212 | 0.00006 | 0.7117 | 0.00006 | 0.0893 | 0.1721 |
| p,p' DDE (µg/g ww) | 46 | 0.0589 | 0.00041 | 0.00003 | 1.1550 | 0.00003 | 0.0103 | 0.2073 |
| p,p' DDD (µg/g ww) | 46 | 0.5189 | 0.00089 | 0.00003 | 15.1661 | 0.00003 | 0.0553 | 2.2678 |
| p,p' DDT (µg/g wwt | 46 | 0.1560 | 0.00003 | 0.00003 | 5.3864 | 0.00003 | 0.0025 | 0.8001 |
| Descriptive Statistics (M. galloprovincialis, 2016 - 2023) | ||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | |
| PCB28 (µg/g ww) | 46 | 0.0497 | 0.0001 | 0.00006 | 1.3773 | 0.00006 | 0.0011 | 0.2139 |
| PCB52 (µg/g ww) | 46 | 0.0555 | 0.0003 | 0.00005 | 0.9289 | 0.00005 | 0.0540 | 0.1515 |
| PCB101 (µg/g ww) | 46 | 0.0195 | 0.0004 | 0.00009 | 0.1863 | 0.00009 | 0.0091 | 0.0458 |
| PCB118 (µg/g ww) | 46 | 0.0188 | 0.0005 | 0.00006 | 0.1260 | 0.00006 | 0.0296 | 0.0310 |
| PCB153 (µg/g ww) | 46 | 0.0068 | 0.0001 | 0.00009 | 0.0688 | 0.00009 | 0.0010 | 0.0182 |
| PCB138 (µg/g ww) | 46 | 0.0856 | 0.0001 | 0.00011 | 2.1570 | 0.00011 | 0.0047 | 0.3439 |
| PCB180 (µg/g ww) | 46 | 0.0045 | 0.0002 | 0.00005 | 0.0547 | 0.00005 | 0.0048 | 0.0102 |
| Descriptive Statistics (Rapana venosa, 2016 - 2021) | ||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | |
| HCB (µg/g ww) |
15 | 0.0084 | 0.0037 | 0.0001 | 0.0323 | 0.0003 | 0.0161 | 0.0105 |
| Lindane (µg/g ww) | 15 | 0.0596 | 0.0044 | 0.0001 | 0.3320 | 0.0003 | 0.1252 | 0.1009 |
| Heptachlor (µg/g ww) | 15 | 0.2029 | 0.0062 | 0.0001 | 1.2954 | 0.0009 | 0.0977 | 0.4271 |
| Aldrin (µg/g ww) |
15 | 0.1592 | 0.0020 | 0.0001 | 1.2146 | 0.0001 | 0.0925 | 0.3744 |
| Dieldrin (µg/g ww) | 15 | 0.3487 | 0.0238 | 0.0001 | 1.9202 | 0.0001 | 0.3265 | 0.6307 |
| Endrin (µg/g ww) | 15 | 0.2421 | 0.0168 | 0.0001 | 1.3636 | 0.0077 | 0.2814 | 0.4306 |
| p,p' DDE (µg/g ww) | 15 | 0.0029 | 0.0001 | 0.0001 | 0.0185 | 0.0001 | 0.0026 | 0.0057 |
| p,p' DDD (µg/g ww) | 15 | 1.2885 | 0.0020 | 0.0001 | 10.6067 | 0.0001 | 0.6253 | 2.9083 |
| p,p' DDT (µg/g ww) | 15 | 0.4965 | 0.0289 | 0.0001 | 3.7823 | 0.0001 | 0.0983 | 1.1062 |
| Descriptive Statistics (Rapana venosa, 2016 - 2021) | ||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | |
| PCB28 (µg/g ww) | 15 | 0.0043 | 0.0005 | 0.0001 | 0.0290 | 0.0001 | 0.0016 | 0.0100 |
| PCB52 (µg/g ww) | 15 | 0.0204 | 0.0063 | 0.0001 | 0.1169 | 0.0012 | 0.0417 | 0.0330 |
| PCB101 (µg/g ww) | 15 | 0.0055 | 0.0005 | 0.0002 | 0.0383 | 0.0002 | 0.0061 | 0.0111 |
| PCB118 (µg/g ww) | 15 | 0.0044 | 0.0001 | 0.0001 | 0.0300 | 0.0001 | 0.0023 | 0.0099 |
| PCB153 (µg/g ww) | 15 | 0.0074 | 0.0002 | 0.0002 | 0.0549 | 0.0002 | 0.0017 | 0.0164 |
| PCB138 (µg/g ww) | 15 | 0.0084 | 0.0002 | 0.0002 | 0.0545 | 0.0002 | 0.0044 | 0.0177 |
| PCB180 (µg/g ww) | 15 | 0.0090 | 0.0002 | 0.0001 | 0.0692 | 0.0001 | 0.0069 | 0.0189 |
| Descriptive Statistics (Fish, 2016 - 2019) | ||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | |
| HCB (µg/g ww) |
21 | 0.0315 | 0.0197 | 0.0001 | 0.2223 | 0.0001 | 0.0285 | 0.0537 |
| Lindane (µg/g ww) | 21 | 0.0100 | 0.0001 | 0.0001 | 0.0875 | 0.0001 | 0.0049 | 0.0210 |
| Heptachlor (µg/g ww) | 21 | 0.0251 | 0.0020 | 0.0001 | 0.1974 | 0.0001 | 0.0271 | 0.0483 |
| Aldrin (µg/g ww) |
21 | 0.0089 | 0.0001 | 0.0001 | 0.0844 | 0.0001 | 0.0020 | 0.0205 |
| Dieldrin (µg/g ww) | 21 | 0.0536 | 0.0101 | 0.0001 | 0.5735 | 0.0001 | 0.0283 | 0.1333 |
| Endrin (µg/g ww) | 21 | 0.0326 | 0.0040 | 0.0001 | 0.2291 | 0.0001 | 0.0107 | 0.0663 |
| p,p' DDE (µg/g ww) | 21 | 0.0155 | 0.0033 | 0.0001 | 0.1288 | 0.0019 | 0.0096 | 0.0312 |
| p,p' DDD (µg/g ww) | 21 | 0.0688 | 0.0053 | 0.0001 | 0.4481 | 0.0031 | 0.0406 | 0.1272 |
| p,p' DDT (µg/g wwt | 21 | 0.0309 | 0.0028 | 0.0001 | 0.2195 | 0.0008 | 0.0081 | 0.0659 |
| Descriptive Statistics (Fish, 2016 - 2019) | ||||||||
| N | Mean | Median | Min | Max | 25th percentile | 75th percentile | Coef. Var. | |
| PCB28 (µg/g ww) | 21 | 0.0108 | 0.0008 | 0.0001 | 0.0516 | 0.0001 | 0.0164 | 0.0163 |
| PCB52 (µg/g ww) | 21 | 0.0147 | 0.0014 | 0.0001 | 0.1636 | 0.0001 | 0.0074 | 0.0370 |
| PCB101 (µg/g ww) | 21 | 0.0260 | 0.0006 | 0.0002 | 0.2390 | 0.0004 | 0.0138 | 0.0649 |
| PCB118 (µg/g ww) | 21 | 0.0342 | 0.0008 | 0.0001 | 0.1939 | 0.0001 | 0.0822 | 0.0579 |
| PCB153 (µg/g ww) | 21 | 0.0265 | 0.0003 | 0.0002 | 0.2220 | 0.0002 | 0.0052 | 0.0558 |
| PCB138 (µg/g ww) | 21 | 0.0205 | 0.0002 | 0.0002 | 0.1126 | 0.0002 | 0.0485 | 0.0335 |
| PCB180 (µg/g ww) | 21 | 0.0082 | 0.0005 | 0.0001 | 0.1262 | 0.0001 | 0.0025 | 0.0274 |
| Variable | Descriptive Statistics (M. galloprovincialis, 2016-2023) | |||||
| Mean | Median | Min. | Max. | 75th percentile | Coef. Var. | |
| Naphthalene (µg/g ww) | 3.23E-4 | 1.3E-5 | nd | 3.088E-3 | 2.23E-3 | 246.2413 |
| Acenaphtylene (µg/g ww) | 1.00E-5 | 0.000 | nd | 2.65E-4 | 0.000 | 501.5951 |
| Acenaphtene (µg/g ww) | 1.00E-5 | 0.000 | nd | 2.82E-4 | 0.000 | 495.4992 |
| Fluorene (µg/g ww) | 9.10E-4 | 0.000 | nd | 9.169E-3 | 2.5E-5 | 297.9722 |
| Phenanthrene (µg/g ww) | 1.385E-3 | 2.0E-6 | nd | 1.1100E-2 | 1.332E-3 | 214.7091 |
| Anthracene (µg/g ww) | 4.80E-4 | 0.000 | nd | 1.0778E-2 | 1.61E-4 | 408.3381 |
| Fluoranthene (µg/g ww) | 1.59E-4 | 0.000 | nd | 1.214E-3 | 1.0E-5 | 235.3721 |
| Pyrene (µg/g ww) | 4.57E-4 | 0.000 | nd | 7.539E-3 | 1.6E-5 | 321.3973 |
| Benzo[a]anthracene (µg/g ww) | 2.32E-4 | 0.000 | nd | 6.865E-3 | 0.000 | 540.3274 |
| Crysene (µg/g ww) | 1.4E-5 | 0.000 | nd | 3.05E-4 | 0.000 | 393.7274 |
| Benzo[b]fluoranthene (µg/g ww) | 1.12E-4 | 0.000 | nd | 1.463E-3 | 0.000 | 267.7644 |
| Benzo[k]fluoranthene (µg/g ww) | 3.3E-5 | 0.000 | nd | 5.89E-4 | 0.000 | 384.8164 |
| Benzo[a]pyrene (µg/g ww) | 7.7E-5 | 0.000 | nd | 8.87E-4 | 4.0E-6 | 292.5008 |
| Benzo(g,h,i) perylene (µg/g ww) | 1.8E-5 | 0.000 | nd | 2.31E-4 | 0.000 | 326.2723 |
| Dibenzo(a,h) anthracene (µg/g ww) | 1.5E-5 | 0.000 | nd | 2.28E-4 | 0.000 | 372.3739 |
| Indeno (1,2,3-c, d) pyrene (µg/g ww) | 1.8E-5 | 0.000 | nd | 2.58E-4 | 0.000 | 369.8438 |
| Variable | Descriptive Statistics (Rapana venosa, 2016-2021) | ||||||
| Mean | Median | Min. | Max. | 25th percentile | 75th percentile | Coef. Var. | |
| Naphthalene (µg/g ww) | 2.772E-3 | 1.5E-6 | nd | 12.087E-2 | 1.5E-6 | 2.928E-3 | 152.4665 |
| Acenaphtylene (µg/g ww) | 1.5E-6 | 1.5E-6 | nd | 1.5E-6 | 1.5E-6 | 1.5E-6 | 0.000 |
| Acenaphtene (µg/g ww) | 1.94E-4 | 1.5E-6 | nd | 1.622E-3 | 1.5E-6 | 1.5E-6 | 276.7241 |
| Fluorene (µg/g ww) | 1.602E-3 | 1.5E-6 | nd | 9.835E-3 | 1.5E-6 | 1.5E-6 | 213.6379 |
| Phenanthrene (µg/g ww) | 3.4381E-2 | 1.800E-3 | nd | 0.135450 | 1.5E-6 | 0.049054 | 143.8718 |
| Anthracene (µg/g ww) | 0.001062 | 1.5E-6 | nd | 8.889E-3 | 1.5E-6 | 1.5E-6 | 276.8563 |
| Fluoranthene (µg/g ww) | 1.909E-3 | 1.5E-6 | nd | 0.011486 | 1.5E-6 | 2.597E-3 | 198.5881 |
| Pyrene (µg/g ww) | 2.751E-3 | 1.5E-6 | nd | 0.017988 | 1.5E-6 | 0.002387 | 214.8892 |
| Benzo[a]anthracene (µg/g ww) | 2.29E-4 | 1.5E-6 | nd | 1.937E-3 | 1.5E-6 | 1.5E-6 | 280.2920 |
| Crysene (µg/g ww) | 5.3E-5 | 1.5E-6 | nd | 3.54E-4 | 1.5E-6 | 1.5E-6 | 214.5570 |
| Benzo[b]fluoranthene (µg/g ww) | 1.499E-3 | 1.5E-6 | nd | 0.011766 | 1.5E-6 | 1.5E-6 | 259.3468 |
| Benzo[k]fluoranthene (µg/g ww) | 2.00E-4 | 1.5E-6 | nd | 1.681E-3 | 1.5E-6 | 1.5E-6 | 277.4948 |
| Benzo[a]pyrene (µg/g ww) | 4.659E-3 | 8.3E-5 | nd | 3.7211E-2 | 1.5E-6 | 2.173E-3 | 262.7476 |
| Benzo (g,h, i) perylene (µg/g ww) | 2.821E-3 | 1.5E-6 | nd | 0.023204 | 1.5E-6 | 2.98E-4 | 271.7314 |
| Dibenzo(a,h) anthracene (µg/g ww) | 1.609E-3 | 1.5E-6 | nd | 0.014360 | 1.5E-6 | 1.5E-6 | 297.2002 |
| Indeno (1,2,3-c, d) pyrene (µg/g ww) | 2.246E-3 | 1.5E-6 | nd | 0.020095 | 1.5E-6 | 1.5E-6 | 297.9945 |
| Variable | Descriptive Statistics (Fish, 2016-2019) | ||||||
| Mean | Median | Min. | Max. | 25th percentile | 75th percentile | Coef. Var. | |
| Naphthalene (µg/g ww) | 1.6683E-2 | 3.501E-3 | 2.5E-5 | 7.7349E-2 | 1.25E-5 | 0.015594 | 181.4329 |
| Acenaphtylene (µg/g ww) | 3.065E-3 | 5.26E-4 | 2.5E-5 | 0.011294 | 9.4E-5 | 5.924E-3 | 151.0249 |
| Acenaphtene (µg/g ww) | 3.523E-3 | 6.10E-4 | 2.5E-5 | 0.012942 | 1.44E-4 | 6.804E-3 | 150.4601 |
| Fluorene (µg/g ww) | 3.670E-3 | 6.63E-4 | 2.5E-5 | 0.013670 | 2.00E-4 | 6.799E-3 | 150.9471 |
| Phenanthrene (µg/g ww) | 0.019458 | 0,008764 | 2.38E-3 | 0.071160 | 3.188E-3 | 0.024633 | 138.1410 |
| Anthracene (µg/g ww) | 0.015274 | 2.609E-3 | 1.0E-5 | 0.078800 | 1.00E-4 | 7.528E-3 | 204.7746 |
| Fluoranthene (µg/g ww) | 3.425E-3 | 1.89E-4 | 2.5E-5 | 0.013209 | 6.3E-5 | 6.879E-3 | 160.6858 |
| Pyrene (µg/g ww) | 3.558E-3 | 6.55E-4 | 2.5E-5 | 0.013076 | 1.00E-4 | 6.837E-3 | 150.2645 |
| Benzo[a]anthracene (µg/g ww) | 3.614E-3 | 6.60E-4 | 2.5E-5 | 0.013280 | 1.68E-4 | 6.891E-3 | 149.9080 |
| Crysene (µg/g ww) | 3.585E-3 | 6.53E-4 | 2.5E-5 | 0.013139 | 1.54E-4 | 6.887E-3 | 149.7954 |
| Benzo[b]fluoranthene (µg/g ww) | 2.369E-3 | 4.27E-4 | 2.5E-5 | 8.647E-3 | 8.1E-5 | 4.605E-3 | 149.6779 |
| Benzo[k]fluoranthene (µg/g ww) | 2.488E-3 | 4.58E-4 | 2.5E-5 | 9.068E-3 | 1.46E-4 | 4.773E-3 | 148.7542 |
| Benzo[a]pyrene (µg/g ww) | 2.585E-3 | 4.58E-4 | 2.5E-5 | 9.466E-3 | 1.25E-4 | 4.976E-3 | 149.7488 |
| Benzo (g,h, i) perylene (µg/g ww) | 1.059E-4 | 1.60E-4 | 1.0E-5 | 5.192E-4 | 2.5E-5 | 8.19E-4 | 193.1788 |
| Dibenzo(a,h) anthracene (µg/g ww) | 1.127E-3 | 1.76E-4 | 1.0E-5 | 5.523E-3 | 2.5E-5 | 864E-4 | 193.0662 |
| Indeno (1,2,3-c,d)pyrene (µg/g ww) | 2.740E-3 | 4.98E-4 | 2.5E-5 | 1.0115E-2 | 2.5E-5 | 5.282E-3 | 151.2229 |
| Location | Concentration Range | References |
|---|---|---|
|
Prince Islands (Marmara,Turkey) Eastern Aegean Coast (Turkey) Iberian Mediterranean Coastal areas (Spain) Bizerte lagoon (north Tunisia) Ionian Sea (Italy) Saronikos Gulf (Greece) Black Sea coast (Romania) |
664-9083 29.4-64.2 75-390 107.4-430.7 14.8-645.3 1480-2400 1.0E-5- 0.0073926 |
Balcıoğlu[57] Küçüksezgin et al.[58] Leon et al.[59] Barhoumi et al.[56] Storelli et al.[60] Valavanidis et al.[61] Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





