Submitted:
25 June 2024
Posted:
27 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction

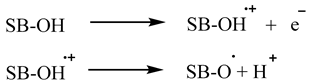
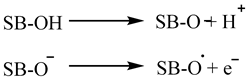
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Schiff Based Isatin-Thiosemicarbazones Synthesis
2.2.2. Preparations of Biodiesel-Diesel Blends
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Thermogravimetric Analysis (TGA)
2.2.5. Fourier Transform Infrared Spectroscopy (FT- IR)
2.2.6. DPPH. Method for Antioxidant Activity
2.2.7. Kinetics of Isatin-Thiosemicarbazone Derivatives
2.2.8. Analytical Statistics
3. Results and Discussion
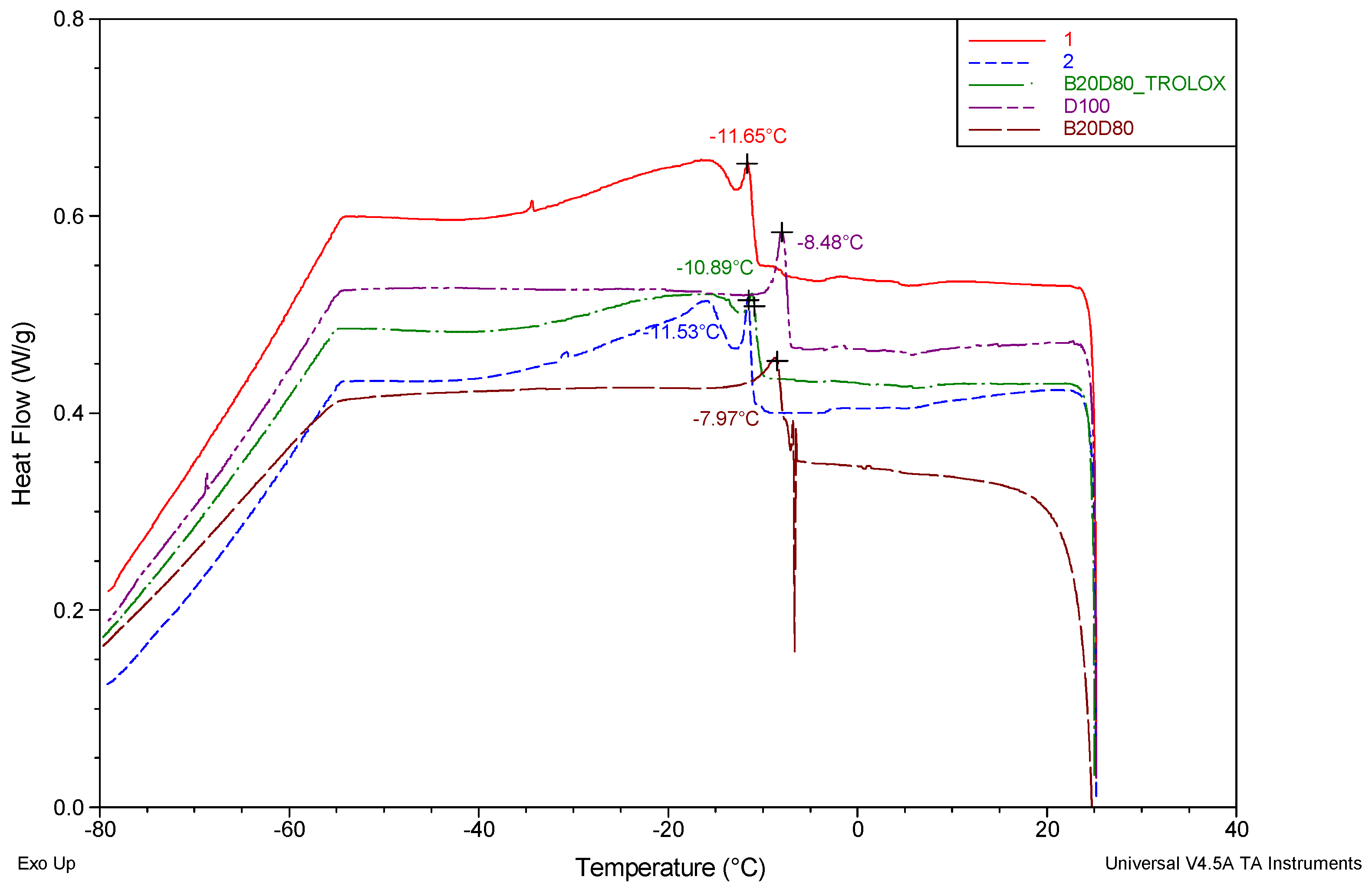
3.1. Differential Scanning Calorimetry (DSC)
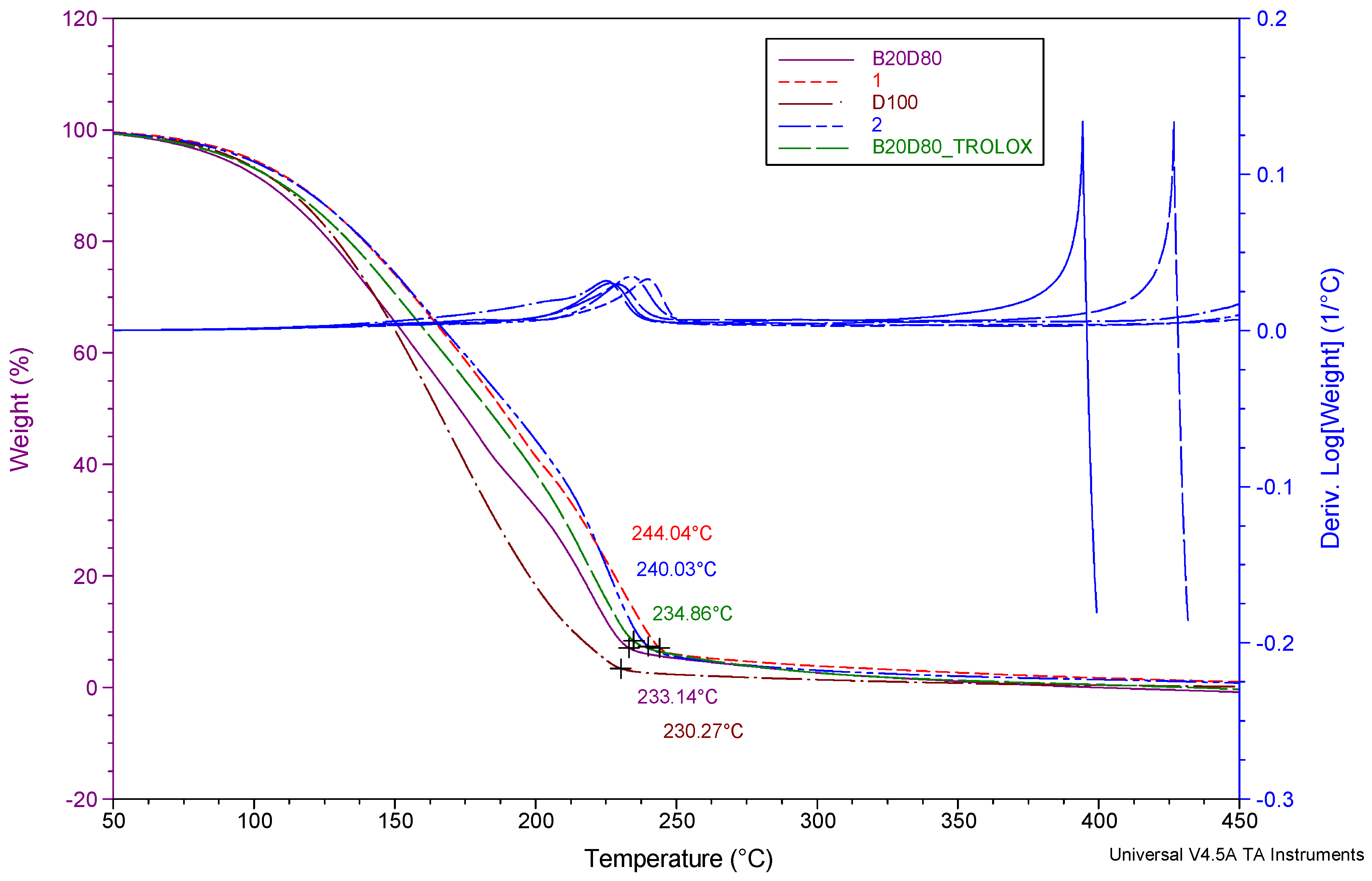
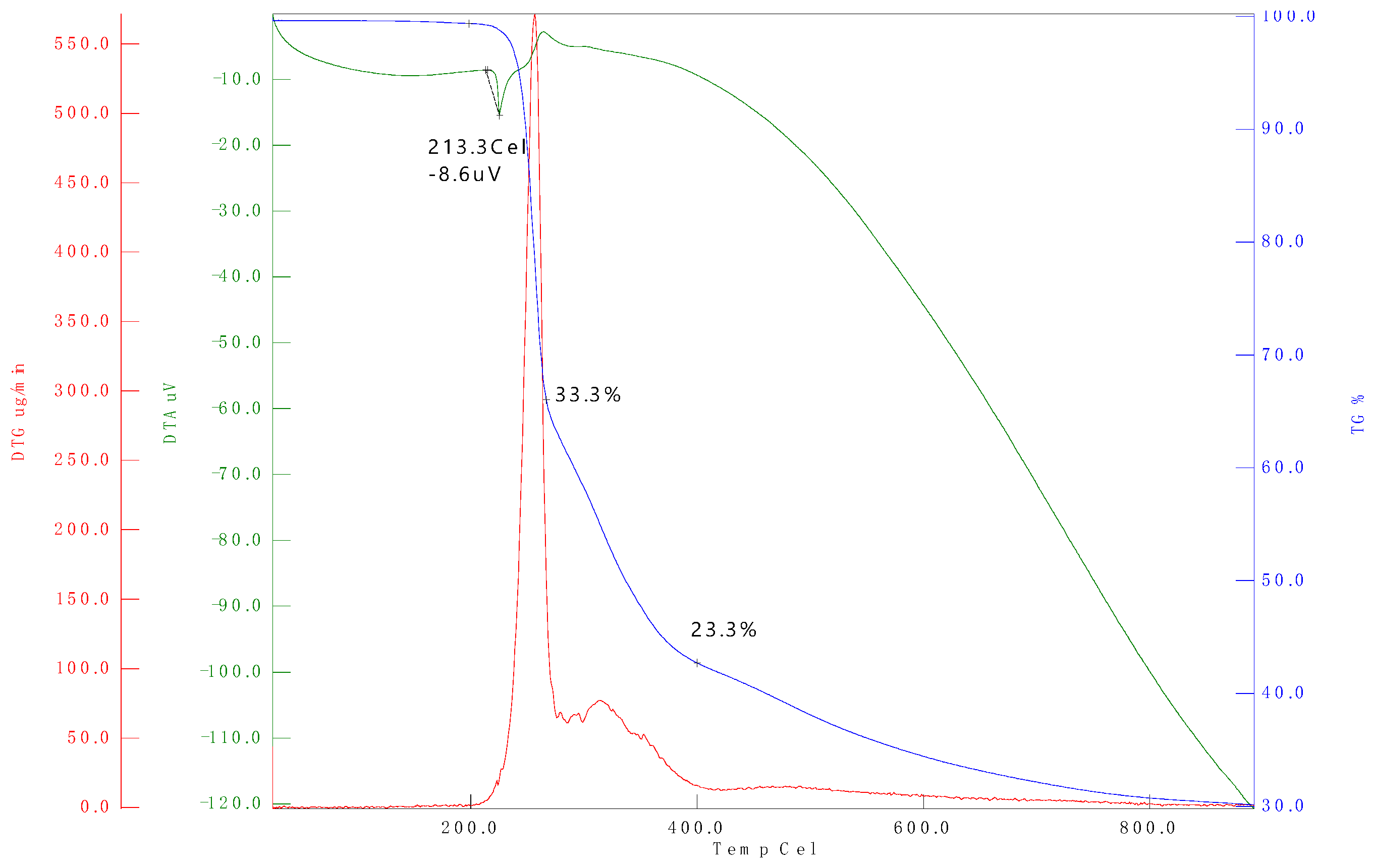
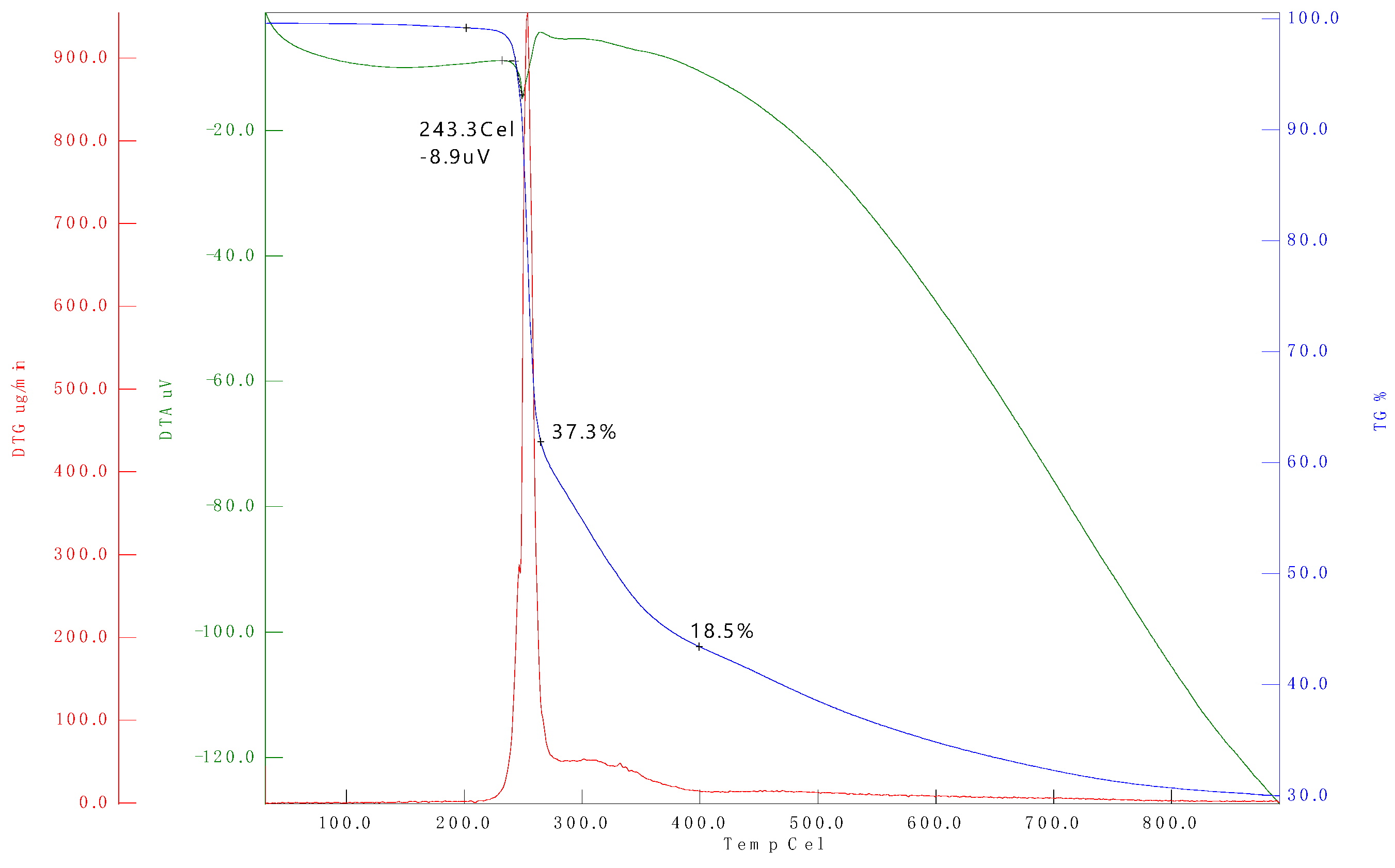
3.2. Thermogravimetric Analysis (TGA)
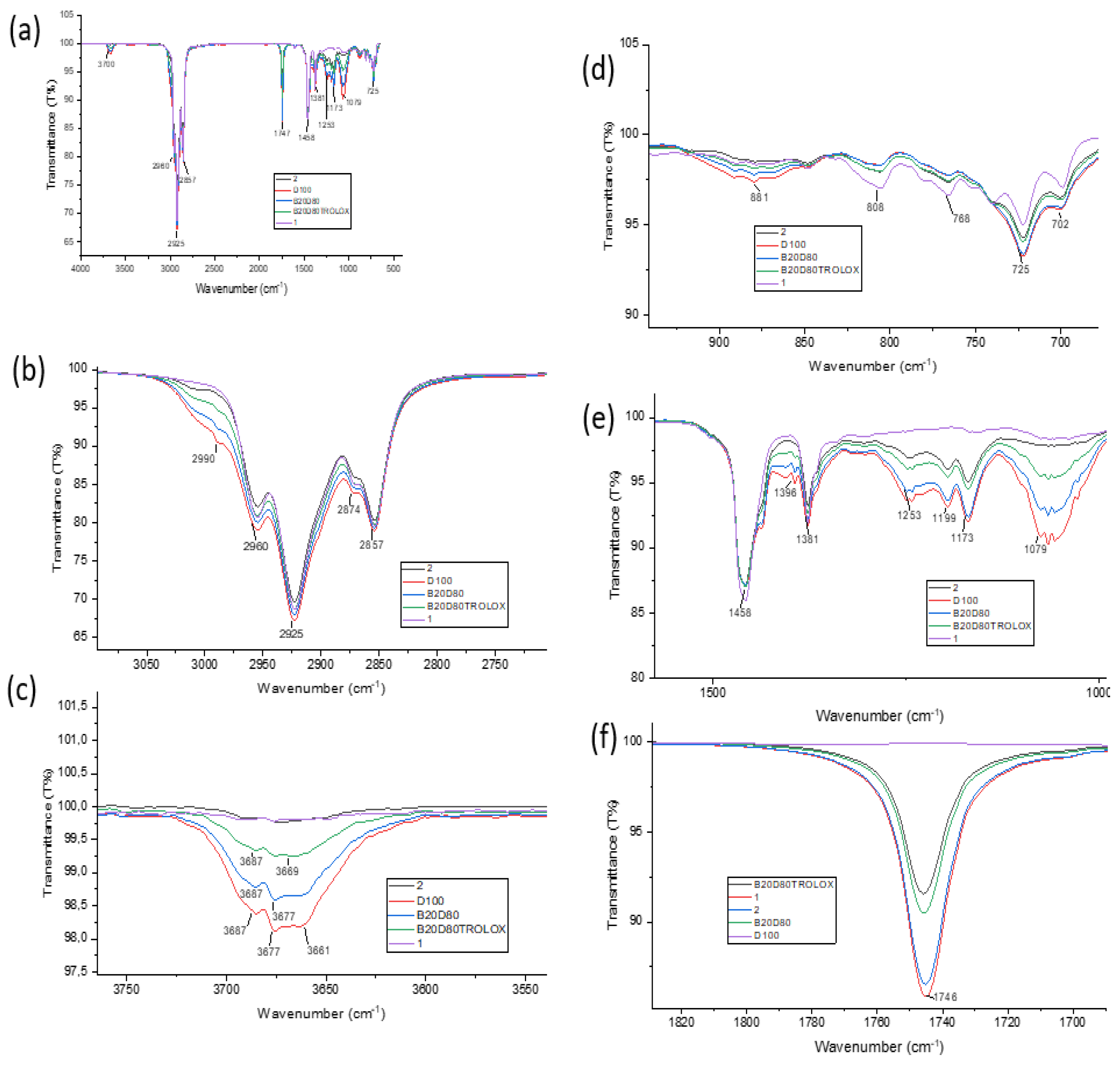
3.3. Fourier Transform Infrared Spectroscopy (FT- IR)
3.4. DPPH. Free Radical Scavenger Effect for Isatin-Thiosemicarbazones
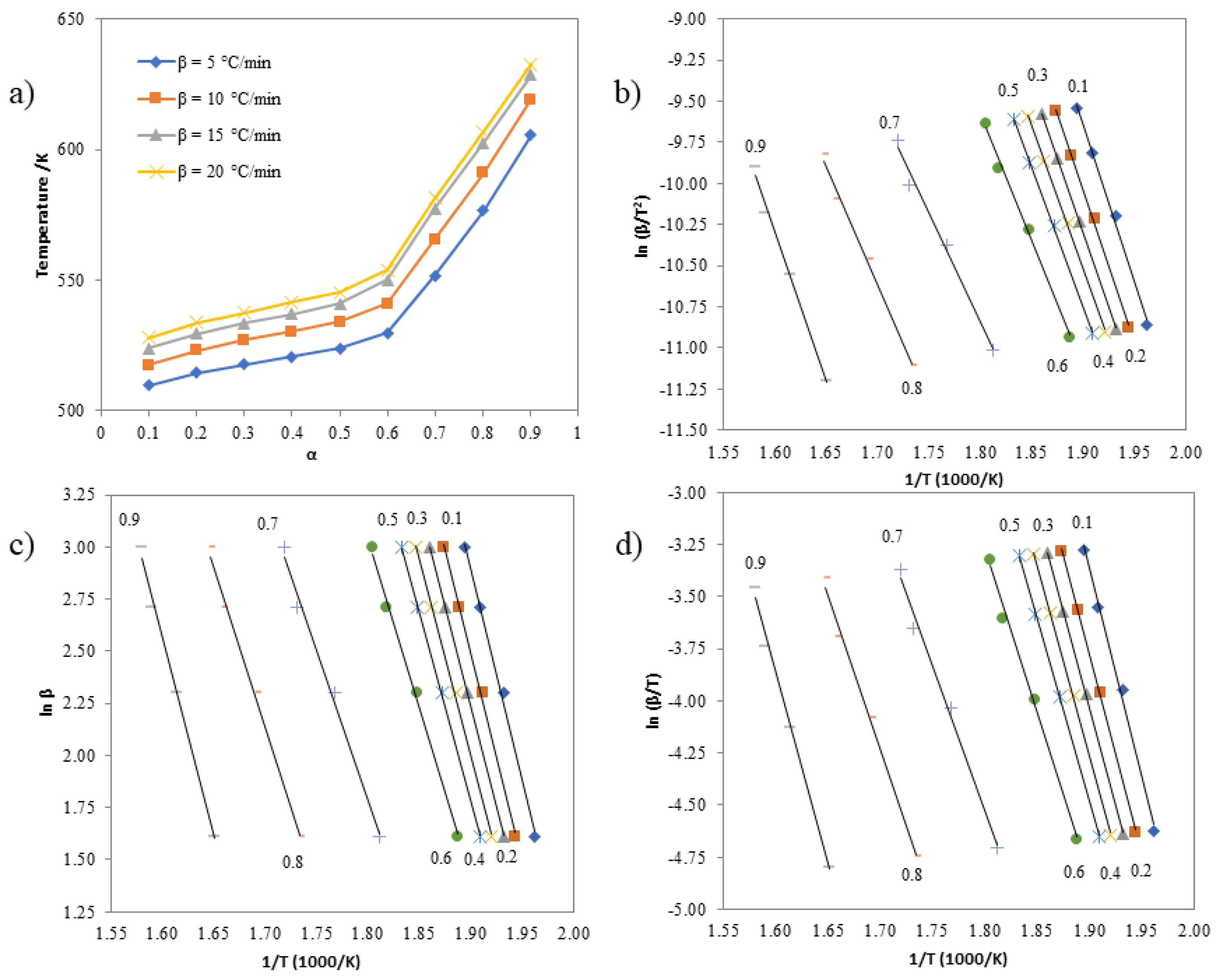
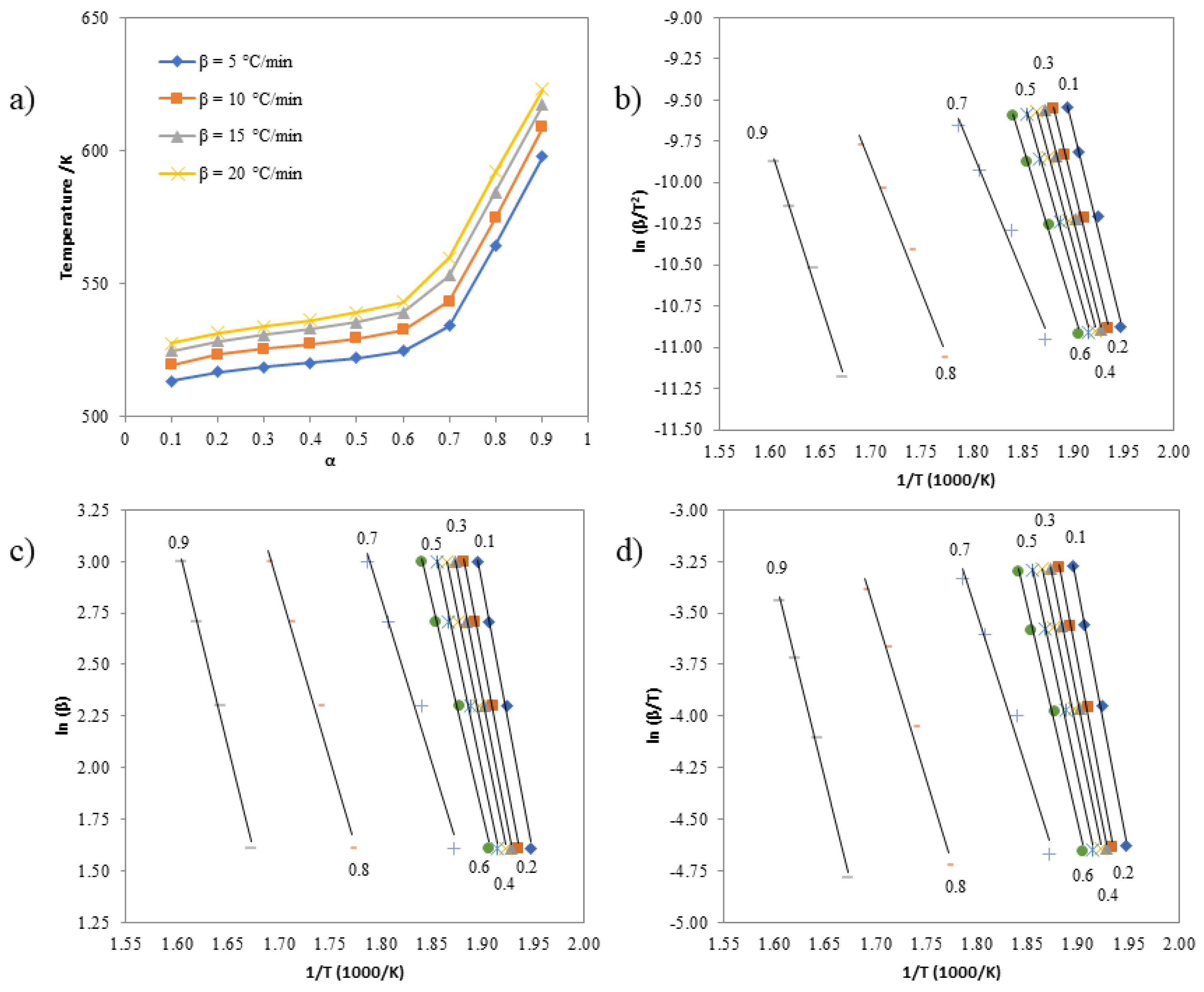
3.6. Investigating the Kinetics of Isatin-Thiosemicarbazones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, H.L.; McCormick, R.L. Spectroscopic Study of Biodiesel Degradation Pathways. SAE Technical Papers 2006, 776–790. [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. International Journal of Food Properties 2017, 20, 1689–1699. [CrossRef]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Masum, B.M.; Imtenan, S.; Ashraful, A.M. Effect of Antioxidants on Oxidation Stability of Biodiesel Derived from Vegetable and Animal Based Feedstocks. Renewable and Sustainable Energy Reviews 2014, 30, 356–370. [CrossRef]
- Pullen, J.; Saeed, K. An Overview of Biodiesel Oxidation Stability. Renewable and Sustainable Energy Reviews 2012, 16, 5924–5950. [CrossRef]
- Rashedul, H.K.; Masjuki, H.H.; Kalam, M.A.; Teoh, Y.H.; How, H.G.; Rizwanul Fattah, I.M. Effect of Antioxidant on the Oxidation Stability and Combustion-Performance-Emission Characteristics of a Diesel Engine Fueled with Diesel-Biodiesel Blend. Energy Conversion and Management 2015, 106, 849–858. [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A Comprehensive Review on Biodiesel as an Alternative Energy Resource and Its Characteristics. Renewable and Sustainable Energy Reviews 2012, 16, 2070–2093. [CrossRef]
- Srivastava, A.; Prasad, R. Triglycerides-Based Diesel Fuels. Renewable & sustainable energy reviews 2000, 4, 111–133. [CrossRef]
- Hosseinzadeh-bandbafha, H.; Kumar, D.; Singh, B.; Shahbeig, H. Biodiesel Antioxidants and Their Impact on the Behavior of Diesel Engines : A Comprehensive Review. Fuel Processing Technology 2022, 232, 107264. [CrossRef]
- Ravi Krishna, E.; Muralidhar Reddy, P.; Sarangapani, M.; Hanmanthu, G.; Geeta, B.; Shoba Rani, K.; Ravinder, V. Synthesis of N4 Donor Macrocyclic Schiff Base Ligands and Their Ru (II), Pd (II), Pt (II) Metal Complexes for Biological Studies and Catalytic Oxidation of Didanosine in Pharmaceuticals. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2012, 97, 189–196. [CrossRef]
- Kumar, R.; Kumar, V.; Sham, R. Stability of Biodiesel – A Review. Renewable and Sustainable Energy Reviews 2016, 62, 866–881. [CrossRef]
- Uddin, M.N.; Ahmed, S.S.; Alam, S.M.R. REVIEW: Biomedical Applications of Schiff Base Metal Complexes. Journal of Coordination Chemistry 2020, 73, 3109–3149. [CrossRef]
- Koteswara Rao, N.S.R.R.M.M.; Ram Reddy, M.G. Studies on the Synthesis, Characterisation and Antimicrobial Activity of New Co(II), Ni(II) and Zn(II) Complexes of Schiff Base Derived from Ninhydrin and Glycine. Biology of Metals 1990, 3, 19–23. [CrossRef]
- Kalem, E.; Ağar, E. Schiff Bazlarinin Biyolojik Aktivitesi Biological Activity of Schiff Bases Öz : Abstract : 2021, 8, 57–76.
- Chen, Y.; Mi, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis of Schiff Bases Modified Inulin Derivatives for Potential Antifungal and Antioxidant Applications. International Journal of Biological Macromolecules 2020, 143, 714–723. [CrossRef]
- Ren, S.; Wang, R.; Komatsu, K.; Bonaz-Krause, P.; Zyrianov, Y.; McKenna, C.E.; Csipke, C.; Tokes, Z.A.; Lien, E.J. Synthesis, Biological Evaluation, and Quantitative Structure -Activity Relationship Analysis of New Schiff Bases of Hydroxysemicarbazide as Potential Antitumor Agents. Journal of Medicinal Chemistry 2002, 45, 410–419. [CrossRef]
- Duff, B.; Reddy Thangella, V.; Creaven, B.S.; Walsh, M.; Egan, D.A. Anti-Cancer Activity and Mutagenic Potential of Novel Copper(II) Quinolinone Schiff Base Complexes in Hepatocarcinoma Cells. European Journal of Pharmacology 2012, 689, 45–55. [CrossRef]
- Jarrahpour, A.; Sheikh, J.; Mounsi, I. El; Juneja, H.; Hadda, T. Ben Computational Evaluation and Experimental in Vitro Antibacterial, Antifungal and Antiviral Activity of Bis-Schiff Bases of Isatin and Its Derivatives. Medicinal Chemistry Research 2013, 22, 1203–1211. [CrossRef]
- Singh, K.; Raparia, S.; Surain, P. Co(II), Ni(II), Cu(II) and Zn(II) Complexes of 4-(4-Cyanobenzylideneamino)-3-Mercapto-5-Oxo-1,2,4-Triazine: Synthesis, Characterization and Biological Studies. Medicinal Chemistry Research 2015, 24, 2336–2346. [CrossRef]
- Tople, M.S.; Patel, N.B.; Patel, P.P.; Purohit, A.C.; Ahmad, I.; Patel, H. An in Silico-in Vitro Antimalarial and Antimicrobial Investigation of Newer 7-Chloroquinoline Based Schiff-Bases. Journal of Molecular Structure 2023, 1271, 134016. [CrossRef]
- Petrović, Z.D.; Orović, J.; Simijonović, D.; Petrović, V.P.; Marković, Z. Experimental and Theoretical Study of Antioxidative Properties of Some Salicylaldehyde and Vanillic Schiff Bases. RSC Advances 2015, 5, 24094–24100. [CrossRef]
- Marković, Z.; Đorović, J.; Petrović, Z.D.; Petrović, V.P.; Simijonović, D. Investigation of the Antioxidant and Radical Scavenging Activities of Some Phenolic Schiff Bases with Different Free Radicals. Journal of Molecular Modeling 2015, 21. [CrossRef]
- Amić, A.; Marković, Z.; Marković, J.M.D.; Jeremić, S.; Lučić, B.; Amić, D. Free Radical Scavenging and COX-2 Inhibition by Simple Colon Metabolites of Polyphenols: A Theoretical Approach. Computational Biology and Chemistry 2016, 65, 45–53. [CrossRef]
- Marković, Z. Study of the Mechanisms of Antioxidative Action of Different Antioxidants. Journal of the Serbian Society for Computational Mechanics 2016, 10, 135–150. [CrossRef]
- Agarwal, S.; Singhal, S.; Singh, M.; Arora, S.; Tanwer, M. Role of Antioxidants in Enhancing Oxidation Stability of Biodiesels. 2018. [CrossRef]
- Rice-Evans, C. Plant Polyphenols: Free Radical Scavengers or Chain-Breaking Antioxidants? Biochemical Society symposium 1995, 61, 103–116. [CrossRef]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, Anti-Bacterial and Anti-Fungal Activities of Some Novel Schiff Bases Containing 2,4-Disubstituted Thiazole Ring. European Journal of Medicinal Chemistry 2010, 45, 651–660. [CrossRef]
- Bal, T.R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and Evaluation of Anti-HIV Activity of Isatin β-Thiosemicarbazone Derivatives. Bioorganic and Medicinal Chemistry Letters 2005, 15, 4451–4455. [CrossRef]
- Cvijetić, I.N.; Herlah, B.; Marinković, A.; Perdih, A.; Bjelogrlić, S.K. Phenotypic Discovery of Thiocarbohydrazone with Anticancer Properties and Catalytic Inhibition of Human DNA Topoisomerase IIα. Pharmaceuticals 2023, 16, 341. [CrossRef]
- de Oliveira, R.B.; de Souza-Fagundes, E.M.; Soares, R.P.P.; Andrade, A.A.; Krettli, A.U.; Zani, C.L. Synthesis and Antimalarial Activity of Semicarbazone and Thiosemicarbazone Derivatives. European Journal of Medicinal Chemistry 2008, 43, 1983–1988. [CrossRef]
- Fayed, E.A.; Ragab, A.; Ezz Eldin, R.R.; Bayoumi, A.H.; Ammar, Y.A. In Vivo Screening and Toxicity Studies of Indolinone Incorporated Thiosemicarbazone, Thiazole and Piperidinosulfonyl Moieties as Anticonvulsant Agents. Bioorganic Chemistry 2021, 116, 105300. [CrossRef]
- Jacob, Í.T.T.; Gomes, F.O.S.; de Miranda, M.D.S.; de Almeida, S.M.V.; da Cruz-Filho, I.J.; Peixoto, C.A.; da Silva, T.G.; Moreira, D.R.M.; de Melo, C.M.L.; de Oliveira, J.F.; et al. Anti-Inflammatory Activity of Novel Thiosemicarbazone Compounds Indole-Based as COX Inhibitors. Pharmacological Reports 2021, 73, 907–925. [CrossRef]
- Yakan, H. Preparation, Structure Elucidation, and Antioxidant Activity of New Bis(Thiosemicarbazone) Derivatives. Turkish Journal of Chemistry 2020, 44, 1085–1099. [CrossRef]
- Pelosi, G.; Bisceglie, F.; Bignami, F.; Ronzi, P.; Schiavone, P.; Re, M.C.; Casoli, C.; Pilotti, E. Antiretroviral Activity of Thiosemicarbazone Metal Complexes. Journal of Medicinal Chemistry 2010, 53, 8765–8769. [CrossRef]
- Yakan, H.; Muğlu, H.; Türkeş, C.; Demir, Y.; Erdoğan, M.; Çavuş, M.S.; Beydemir, Ş. A Novel Series of Thiosemicarbazone Hybrid Scaffolds: Design, Synthesis, DFT Studies, Metabolic Enzyme Inhibition Properties, and Molecular Docking Calculations. Journal of Molecular Structure 2023, 1280. [CrossRef]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel Thiosemicarbazone Chitosan Derivatives: Preparation, Characterization, and Antifungal Activity. Carbohydrate Polymers 2012, 87, 2664–2670. [CrossRef]
- Khan, S.A.; Kumar, P.; Joshi, R.; Iqbal, P.F.; Saleem, K. Synthesis and in Vitro Antibacterial Activity of New Steroidal Thiosemicarbazone Derivatives. European Journal of Medicinal Chemistry 2008, 43, 2029–2034. [CrossRef]
- Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Cremonini, M.A.; Placucci, G.; et al. New Isatin Derivatives with Antioxidant Activity. European Journal of Medicinal Chemistry 2010, 45, 1374–1378. [CrossRef]
- Elsaman, T.; Mohamed, M.S.; Eltayib, E.M.; Abdel-aziz, H.A.; Abdalla, A.E.; Munir, M.U.; Mohamed, M.A. Isatin Derivatives as Broad-Spectrum Antiviral Agents: The Current Landscape. Medicinal Chemistry Research 2022, 31, 244–273. [CrossRef]
- Chohan, Z.H.; Pervez, H.; Rauf, A.; Khan, K.M.; Supuran, C.T. Isatin-Derived Antibacterial and Antifungal Compounds and Their Transition Metal Complexes. Journal of Enzyme Inhibition and Medicinal Chemistry 2004, 19, 417–423. [CrossRef]
- Guo, H. Isatin Derivatives and Their Anti-Bacterial Activities. European Journal of Medicinal Chemistry 2019, 164, 678–688. [CrossRef]
- Tangadanchu, V.K.R.; Sui, Y.F.; Zhou, C.H. Isatin-Derived Azoles as New Potential Antimicrobial Agents: Design, Synthesis and Biological Evaluation. Bioorganic and Medicinal Chemistry Letters 2021, 41, 128030. [CrossRef]
- Jiang, D.; Wang, G.Q.; Liu, X.; Zhang, Z.; Feng, L.S.; Liu, M.L. Isatin Derivatives with Potential Antitubercular Activities. Journal of Heterocyclic Chemistry 2018, 55, 1263–1279. [CrossRef]
- Nain, S. Recent Advancement in Synthesis of Isatin as Anticonvulsant Agents: A Review. Medicinal Chemistry 2014, 4. [CrossRef]
- Van Den Berg, R.; Haenen, G.R.M.M.; Van Den Berg, H.; Bast, A. Applicability of an Improved Trolox Equivalent Antioxidant Capacity (TEAC) Assay for Evaluation of Antioxidant Capacity Measurements of Mixtures. Food Chemistry 1999, 66, 511–517. [CrossRef]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Ferreira, H.; Lima, J.L.F.C.; Reis, S. Antioxidant Activity of Vitamin E and Trolox: Understanding of the Factors That Govern Lipid Peroxidation Studies in Vitro. Food Biophysics 2009, 4, 312–320. [CrossRef]
- Volkan, Y.M. Investigation of The Evaluation of Diasporic Bauxite from Islahiye Region in Alumina Production, Sakarya University, 2022.
- Božić, A.R.; Filipović, N.R.; Novaković, I.T. Synthesis , Antioxidant and Antimicrobial Activity of Carbohydrazones. 2017, 82, 495–508.
- Gangarapu, K.; Manda, S.; Jallapally, A.; Thota, S.; Karki, S.S.; Balzarini, J.; De Clercq, E.; Tokuda, H. Synthesis of Thiocarbohydrazide and Carbohydrazide Derivatives as Possible Biologically Active Agents. Medicinal Chemistry Research 2014, 23, 1046–1056. [CrossRef]
- Muğlu, H. Synthesis, Characterization, and Antioxidant Activity of Some New N 4-Arylsubstituted-5-Methoxyisatin-β-Thiosemicarbazone Derivatives. Research on Chemical Intermediates 2020, 46, 2083–2098. [CrossRef]
- Wang, X.; Dai, M.; Xie, Y.; Han, J.; Ma, Y.; Chen, C. Experimental Investigation of Evaporation Characteristics of Biodiesel-Diesel Blend Droplets with Carbon Nanotubes and Nanoceria as Nanoadditives. Applied Surface Science 2020, 505, 144186. [CrossRef]
- Dunn, R.O. Effect of Antioxidants on the Oxidative Stability of Methyl Soyate ( Biodiesel ) B. 2005, 86, 1071–1085. [CrossRef]
- Maru, M.M.; Lucchese, M.M.; Legnani, C.; Quirino, W.G.; Balbo, A.; Aranha, I.B.; Costa, L.T.; Vilani, C.; de Sena, L.Á.; Damasceno, J.C.; et al. Biodiesel Compatibility with Carbon Steel and HDPE Parts. Fuel Processing Technology 2009, 90, 1175–1182. [CrossRef]
- Soykan, C. Synthesis , Characterization , and Biological Activity of N - ( 4- Acetylphenyl ) Maleimide and Its Oxime , Carbazone , Thiosemicarbazone Derivatives and Their Polymers. 2003, 1942–1951.
- Aniza, R.; Chen, W.H.; Kwon, E.E.; Bach, Q.V.; Hoang, A.T. Lignocellulosic Biofuel Properties and Reactivity Analyzed by Thermogravimetric Analysis (TGA) toward Zero Carbon Scheme: A Critical Review. Energy Conversion and Management: X 2024, 22, 100538. [CrossRef]
- Kutuk, H.; Turkoz, N. Microwave-Assisted Synthesis of Disulfides. Phosphorus, Sulfur and Silicon and the Related Elements 2011, 186, 1515–1522. [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30. [CrossRef]
- Yakan, H.; Cakmak, S.; Kutuk, H.; Yenigun, S.; Ozen, T. Synthesis, Characterization, Antioxidant, and Antibacterial Activities of New 2,3-Dimethoxy and 3-Acetoxy-2-Methyl Benzamides. Research on Chemical Intermediates 2020, 46, 2767–2787. [CrossRef]
- Vlase, T.; Doca, N.; Vlase, G.; Bolcu, C.; Borcan, F. Kinetics of Non-Isothermal Decomposition of Three IRGANOX-Type Antioxidants. Journal of Thermal Analysis and Calorimetry 2008, 92, 15–18. [CrossRef]
- Haji, I., Yildız, K. Decomposition Kinetics of Diasporitic Bauxite from Gaziantep Region. In Proceedings of the 9th International Scientific Research Congress, Ankara.; 2021; pp. 439–447.
- 60. Angelopoulos, P, Samouhos, M, Taxiarchou, M. Thermal Decomposition Kinetics of Greek Diasporic Bauxite. Proceedings of OPMR 2016- Opportunities in Processing of Metal Resources in South East Europe 2016, 191–200.
- Küçük, F.; Yildiz, K. The Decomposition Kinetics of Mechanically Activated Alunite Ore in Air Atmosphere by Thermogravimetry. Thermochimica Acta 2006, 448, 107–110. [CrossRef]
- Borugadda, V.B.; Goud, V. V Thermochimica Acta Thermal , Oxidative and Low Temperature Properties of Methyl Esters Prepared from Oils of Different Fatty Acids Composition : A Comparative Study. Thermochimica Acta 2014, 577, 33–40. [CrossRef]
- Çavuş, M.S.; Yakan, H.; Muğlu, H.; Bakır, T. Novel Carbohydrazones Including 5-Substituted Isatin: Synthesis, Characterization, and Quantum-Chemical Studies on the Relationship between Electronic and Antioxidant Properties. Journal of Physics and Chemistry of Solids 2020, 140. [CrossRef]
- Pitucha, M.; Ramos, P.; Wojtunik-Kulesza, K.; Głogowska, A.; Stefańska, J.; Kowalczuk, D.; Monika, D.; Augustynowicz-Kopeć, E. Thermal Analysis, Antimicrobial and Antioxidant Studies of Thiosemicarbazone Derivatives. Journal of Thermal Analysis and Calorimetry 2023, 148, 4223–4234. [CrossRef]
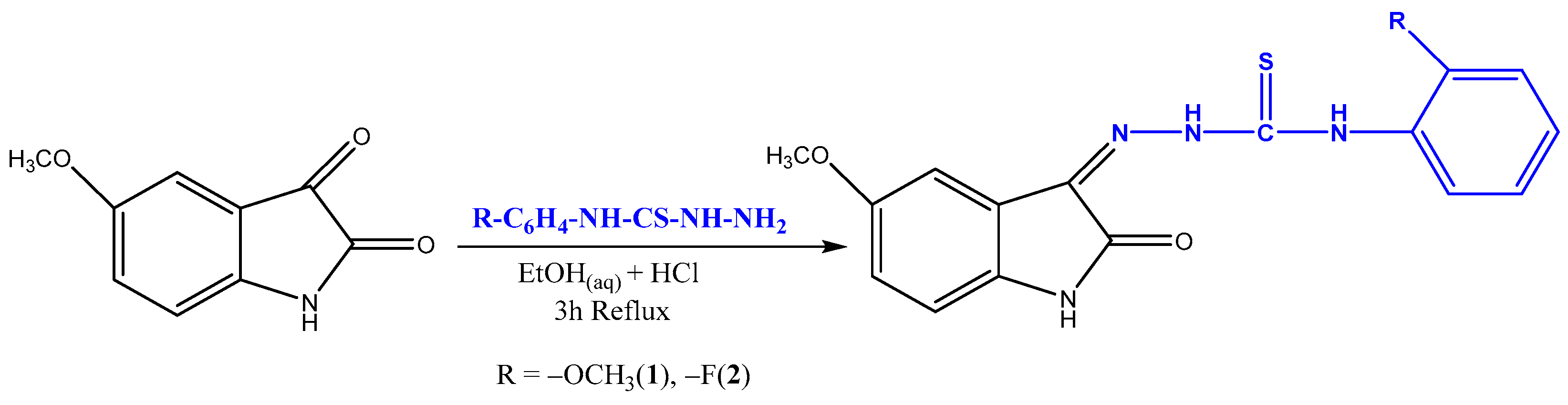
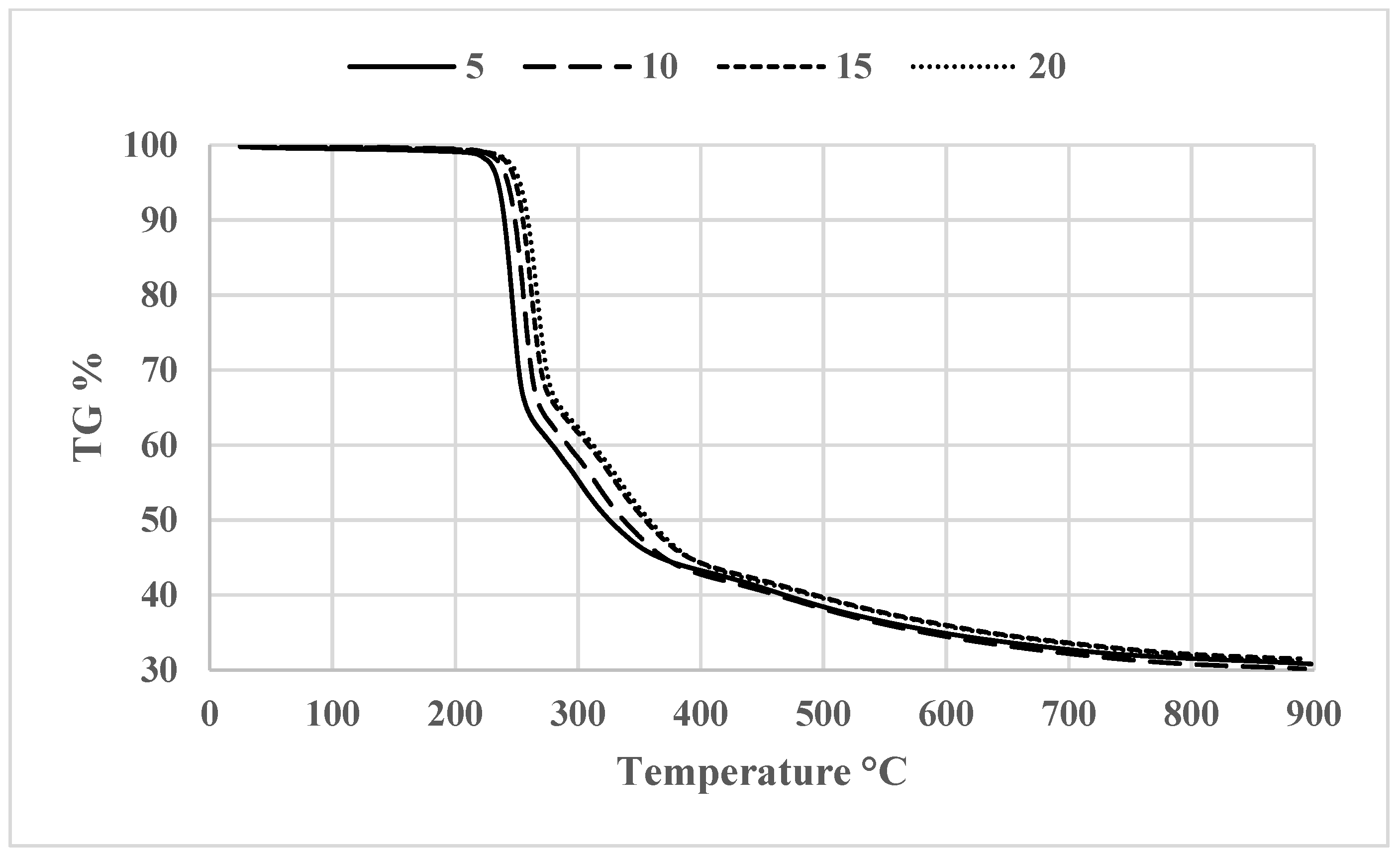
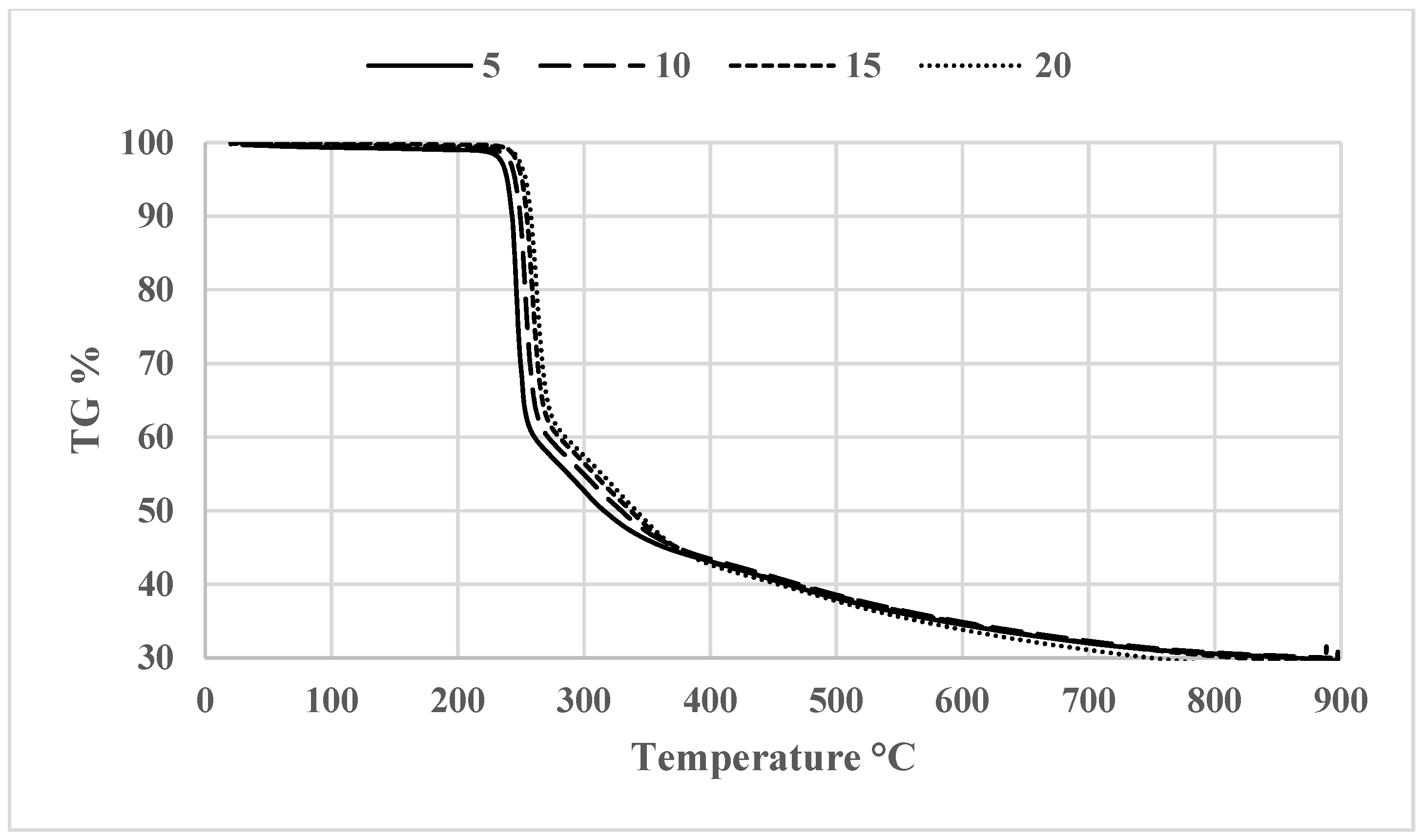
| Compound code | R | Molecular formula | Molecular weight | Melting point (°C) | Yield % |
|---|---|---|---|---|---|
| 1 | 2-OCH3 | C17H16N4O3S | 356.41 | 218–220 | 87 |
| 2 | 2-F | C16H13FN4O2S | 344.37 | 234–235 | 82 |
| Sample | Biodiesel (%) | Diesel (%) |
|---|---|---|
| D100 | - | 100 |
| B20D80 | 20 | 80 |
| B20D80TROLOX | 20 | 80 |
| B20D80_2 | 20 | 80 |
| B20D80_1 | 20 | 80 |
| Sample | (℃) | |
| D100 | 7.97 | 4.55 |
| B20D80 | 8.48 | 2.64 |
| B20D80TROLOX | 10.89 | 0.62 |
| B20D80_2 | 11.53 | 2.03 |
| B20D80_1 | 11.65 | 2.39 |
| Sample name | Temperature range (°C) | (°C) (Tonset) | (%) | ||
| D100 | 25-250 | 99.36 | 0.06 | 11.52 | 5.29 |
| B20D80 | 25-250 | 99.12 | 1.10-4 | 7.72 | 2.25 |
| B20D80TROLOX | 25-250 | 99.27 | 0.03 | 7.71 | 2.28 |
| B20D80_2 | 25-250 | 99.24 | 0.02 | 8.94 | 0.08 |
| B20D80_1 | 25-250 | 98.56 | 0.30 | 10.20 | 0.86 |
| Compound | ν(O−H) | ν(C−H) Aromatic |
ν(C−H) Aliphatic |
ν(C=O) | ν(N−H) | ν(C−N) | ν(C−O) |
|---|---|---|---|---|---|---|---|
| B20D80_2 | – | 2925 | 2874 | 1746 | 1458 | 1199 | 1079 |
| B20D80_1 | – | 2925 | 2874 | 1746 | 1458 | 1173 | 1079 |
| B20D80 TROLOX | 3687 | 2925 | 2857 | 1746 | – | – | 1079 |
| B20D80 | 3687 | 2925 | 2857 | 1746 | – | – | 1079 |
| D100 | 3687 | 2925 | 2857 | 1746 | – | – | 1079 |
| Compound | IC50 values μM |
|---|---|
| 1 | 66.178±0.11b |
| 2 | 79.927±0.13c |
| Trolox | 8.757±0.07a |
| α | Activation Energies (kJ mol-1) | The standard deviation (s) for the Kissinger, Ozawa, and Boswell methods | ||
|---|---|---|---|---|
| Kissinger | Ozawa | Boswell | ||
| 0.1 | 163.05 | 171.68 | 167.37 | 4.31 |
| 0.2 | 155.18 | 163.89 | 159.53 | 4.35 |
| 0.3 | 152.00 | 160.77 | 156.38 | 4.38 |
| 0.4 | 146.72 | 155.54 | 151.13 | 4.41 |
| 0.5 | 143.70 | 152.59 | 148.15 | 4.44 |
| 0.6 | 130.64 | 139.65 | 135.14 | 4.50 |
| 0.7 | 114.96 | 124.38 | 119.67 | 4.71 |
| 0.8 | 123.40 | 133.24 | 128.32 | 4.92 |
| 0.9 | 153.22 | 163.51 | 158.36 | 5.14 |
| Standart Deviation (s) for Separate for each method | 16.09 | 15.88 | 15.98 | |
| α | Activation Energies (kJ mol-1) | The standard deviation (s) for the Kissinger, Ozawa, and Boswell methods | ||
|---|---|---|---|---|
| Kissinger | Ozawa | Boswell | ||
| 0.1 | 211.74 | 220.40 | 216.07 | 4.33 |
| 0.2 | 206.48 | 215.19 | 210.83 | 4.35 |
| 0.3 | 199.63 | 208.38 | 204.01 | 4.37 |
| 0.4 | 190.60 | 199.38 | 194.99 | 4.39 |
| 0.5 | 182.53 | 191.35 | 186.94 | 4.41 |
| 0.6 | 169.41 | 178.29 | 173.85 | 4.44 |
| 0.7 | 125.89 | 134.98 | 130.44 | 4.54 |
| 0.8 | 129.56 | 139.17 | 134.36 | 4.80 |
| 0.9 | 160.42 | 170.57 | 165.49 | 5.07 |
| Standart Deviation (s) for Separate for each method | 31.56 | 31.24 | 31.39 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





