Submitted:
03 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Results
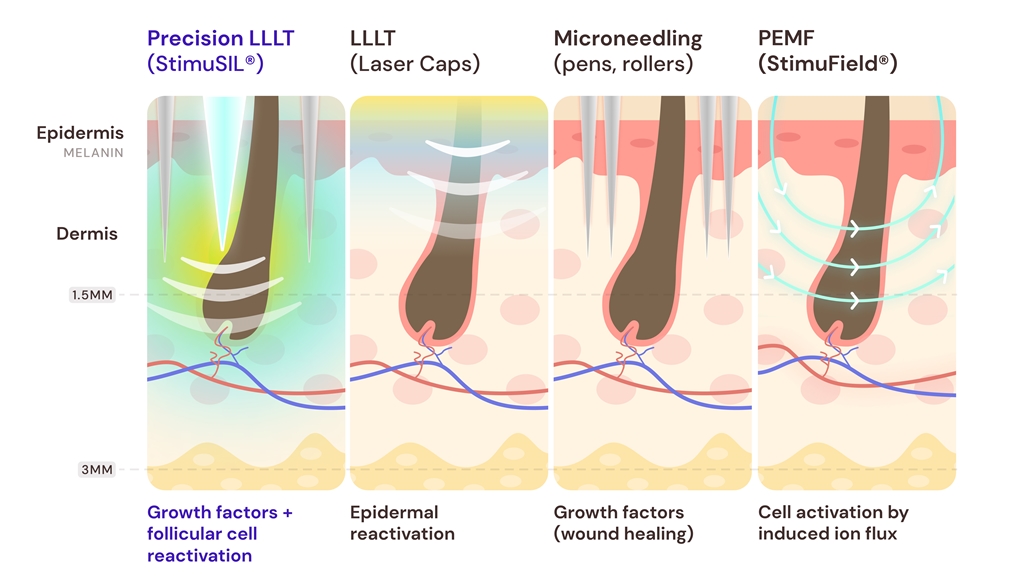
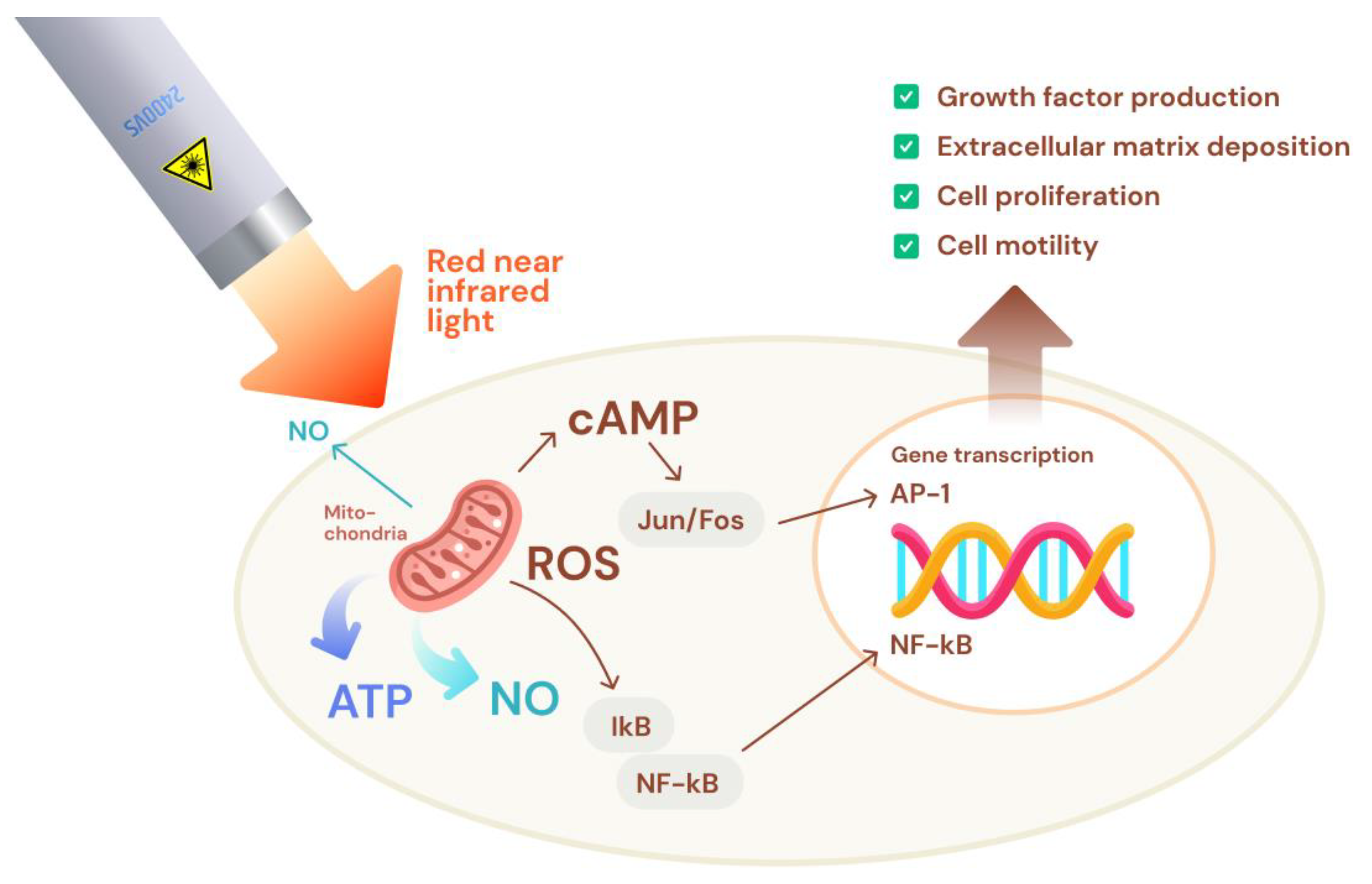
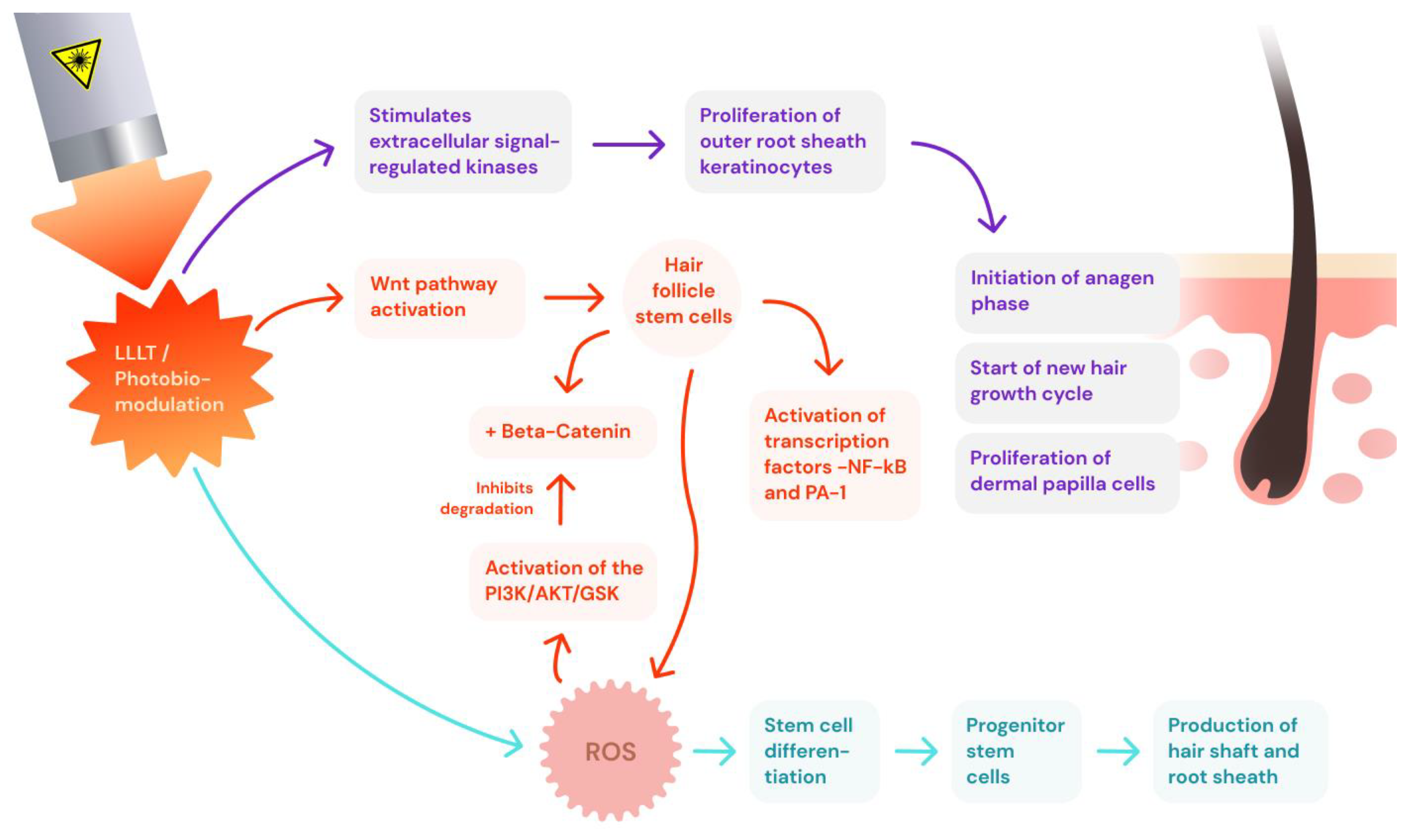
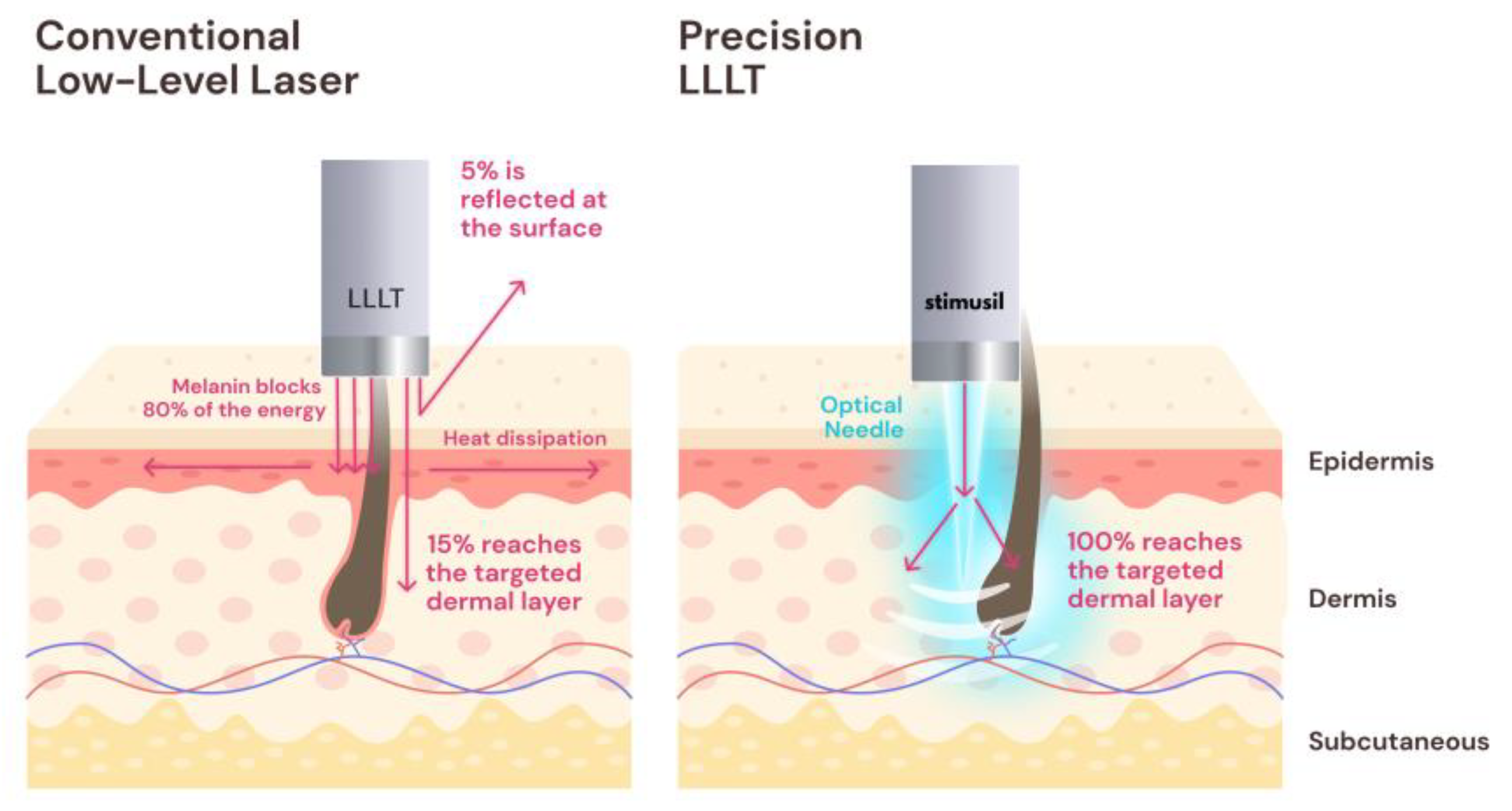
3.1. Low-Level Laser Therapy (LLLT)
3.2. Alternative Forms of Laser Hair Therapy
3.2.1. Fractional Lasers
3.2.2. Nonablative Lasers
3.2.3. Blue and Yellow LLLT
3.3. Pulsed Electromagnetic Field Therapy (PEMF)
3.3.1. PEMF Treatments for AGA
3.3.2. Multimodal PEMF Treatments for AGA
3.4. Microneedling
3.4.1. Microneedling as an Adjunct Treatment
3.4.2. Delivering LLLT via Microneedling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, C.H.; Sood, T.; Zito, P.M. Androgenetic Alopecia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20(12), 3759–3781. [Google Scholar] [CrossRef]
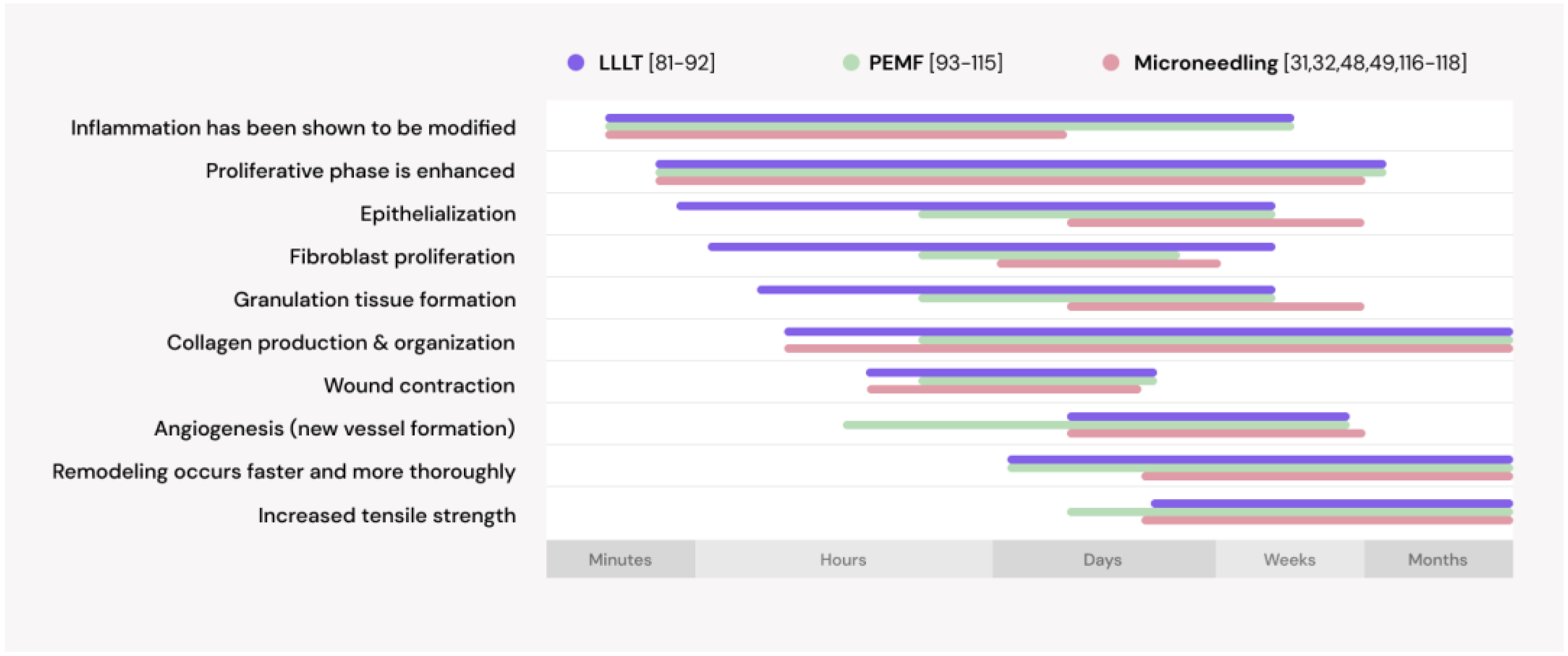
- Badri, T.; Nessel, T.A.; Kumar, D.D. Minoxidil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Choi, M.C.; Cheung, K.K.; Li, X.; Cheing, G.L.Y. Pulsed electromagnetic field (PEMF) promotes collagen fibre deposition associated with increased myofibroblast population in the early healing phase of diabetic wound. Arch. Dermatol. Res. 2016, 308(1), 21–29. [Google Scholar] [CrossRef]
- Goren, A.; McCoy, J.; Situm, M.; et al. Controversies in the treatment of androgenetic alopecia: The history of finasteride. Dermatol. Ther. 2019, 32(2), e12647. [Google Scholar]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil Use in Dermatology, Side Effects and Recent Patents. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6(2), 130–136. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: a review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Birch, M.P.; Messenger, J.F.; Messenger, A.G. Hair density, hair diameter and the prevalence of female pattern hair loss. Br. J. Dermatol. 2001, 144(2), 297–304. [Google Scholar] [CrossRef]
- Olsen, E.A.; Messenger, A.G.; Shapiro, J.; Bergfeld, W.F.; Hordinsky, M.K.; Roberts, J.L.; Stough, D.; Washenik, K.; Whiting, D.A. Evaluation and treatment of male and female pattern hair loss. J. Am. Acad. Dermatol. 2005, 52(2), 301–311. [Google Scholar] [CrossRef]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. 2021, 84(5), 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32(1), 41–52. [Google Scholar]
- Glass, G.E. Photobiomodulation: A review of the molecular evidence for low level light therapy. J. Plast. Reconstr. Aesthet. Surg. 2021b, 74(5), 1050–1060. [Google Scholar] [CrossRef]
- Glass, G.E. Photobiomodulation: The Clinical Applications of Low-Level Light Therapy. Aesthet. Surg. J. 2021a, 41(6), 723-738.
- Guo, Y.; Qu, Q.; Chen, J.; Miao, Y.; Hu, Z. Proposed mechanisms of low-level light therapy in the treatment of androgenetic alopecia. Lasers Med. Sci. 2021, 36(4), 703–713. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation for the management of alopecia: Mechanisms of action, patient selection and perspectives. Clin. Cosmet. Investig. Dermatol. 2019, 12, 669–678. [Google Scholar] [CrossRef]
- Liu, K.-H.; Liu, D.; Chen, Y.-T.; Chin, S.-Y. Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: A system review and meta-analysis of randomized controlled trials. Lasers Med. Sci. 2019, 34(6), 1063–1069. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; Shibli, J.A.; Angelova Volponi, A.; Kempisty, B.; Dyszkiewicz-Konwińska, M. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9(6), Article 6.
- Gentile, P.; Garcovich, S. The Effectiveness of Low-Level Light/Laser Therapy on Hair Loss. Facial Plast. Surg. Aesthet. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Gavazzoni, M.F.; Trüeb, R.M. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int. J. Trichology 2014, 6(2), 45–49. [Google Scholar]
- Egger, A.; Resnik, S.R.; Aickara, D.; Maranda, E.; Kaiser, M.; Wikramanayake, T.C.; Jimenez, J.J. Examining the safety and efficacy of low-level laser therapy for male and female pattern hair loss: A review of the literature. Skin Appendage Disord. 2020, 6(5), 259–267. [Google Scholar] [CrossRef] [PubMed]
- Galadari, H.; Shivakumar, S.; Lotti, T.; Wollina, U.; Goren, A.; Rokni, G.R.; Grabbe, S.; Goldust, M. Low-level laser therapy and narrative review of other treatment modalities in androgenetic alopecia. Lasers Med. Sci. 2020, 35(6), 1239–1244. [Google Scholar] [CrossRef]
- Leavitt, M.; Charles, G.; Heyman, E.; Michaels, D. HairMaxLaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin. Drug Investig. 2009, 29, 283–292. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.W.; Kim, J.Y.; Shin, J.W.; Lee, S.J.; Huh, C.H. Low-level light therapy for androgenetic alopecia: A 24-week, randomized double-blind, sham device-controlled multicenter trial. Dermatol. Surg. 2013, 39, 1177–1183. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Wikramanayake, T.C.; Bergfeld, W.; Hordinsky, M.; Hickman, J.G.; Hamblin, M.R.; et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: A multicenter, randomized, sham device-controlled, double-blind study. Am. J. Clin. Dermatol. 2014, 15, 115–127. [Google Scholar] [CrossRef]
- Mai-Yi Fan, S.; Cheng, Y.-P.; Lee, M.-Y.; Lin, S.-J.; Chiu, H.-Y. Efficacy and safety of a low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, self-comparison, sham device-controlled trial. Dermatol. Surg. 2018, 44(11), 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Park, M.W.; Lee, C.J. Phototherapy of Androgenetic Alopecia with Low Level Narrow Band 655-nm Red Light and 780-nm Infrared Light. J. Am. Acad. Dermatol. 2007, 56, AB112. [Google Scholar]
- Satino, J.L.; Markou, M. Hair regrowth and increased hair tensile strength using the HairMaxLaserComb for low-level laser therapy. Int. J. Cos. Surg. Aest. Dermatol. 2003, 5, 113–117. [Google Scholar] [CrossRef]
- Lanzafame, R.; Blanche, R.; Bodian, A.; Chiacchierini, R.; Fernandez-Obregon, A.; Kazmirek, E.; et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg. Med. 2013, 45(S25), 12. [Google Scholar] [CrossRef]
- Avram, M.R.; Rogers, N.E. The use of low-level light for hair growth: Part I. J. Cosmet. Laser Ther. 2009, 11, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Esmat, S.M.; Hegazy, R.A.; Gawdat, H.I.; Abdel Hay, R.M.; Allam, R.S.; El Naggar, R.; et al. Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: A randomized controlled study. Lasers Surg. Med. 2017, 49, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.K.; Inamadar, A.C.; Palit, A. A randomized controlled, single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J. Cutan. Aesthet. Surg. 2018, 11(4), 211–216. [Google Scholar] [PubMed]
- Dhurat, R.; Sukesh, M.; Avhad, G.; Dandale, A.; Pal, A.; Pund, P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: A pilot study. Int. J. Trichol. 2013, 5(1), 6–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Park, B.C. The efficacy and safety of the combination of photobiomodulation therapy and pulsed electromagnetic field therapy on androgenetic alopecia. J. Cosmet. Dermatol. 2022. [Google Scholar] [CrossRef]
- Bureau, J.P.; Guilbaud, J.; Roux, F.M.E. Essential Oils and Low-Intensity Electromagnetic Pulses in the Treatment of Andro-gen-Dependent Alopecia. Adv. Ther. 2003, 20, 220–229. [Google Scholar] [CrossRef]
- Maddin, W.S.; Bell, P.W.; James, J.H.M. The Biological Effects of a Pulsed Electrostatic Field with Specific Reference to Hair Electrotrichogenesis. Int. J. Dermatol. 1990, 29(6), 446–450. [Google Scholar] [CrossRef] [PubMed]
- Maddin, W.S.; Amara, I.; Sollecito, W.A. Electrotrichogenesis: Further evidence of efficacy and safety on extended use. Int. J. Dermatol. 1992, 31(12), 878–880. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Sannino, M.; Cannarozzo, G.; Giudice, A.; Del Duca, E.; Tamburi, F.; Bennardo, L.; Nisticò, S.P. Blue light-emitting diodes in hair regrowth: the first prospective study. Lasers Med. Sci. 2021, 36(8), 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Lee, G. Y.; Lee, S. J.; Kim, W. S. The effect of a 1550 nm fractional erbium–glass laser in female pattern hair loss. J. Eur. Acad. Dermatol. Venereol. 2011, 25(12), 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Warning Letter to Transdermal Cap Inc., April 24, 2015. Archived by WebCite® at https://web.archive.org/web/20190424174411/https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm458008.htm (accessed on April 12, 2024).
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Jedlowski PM, Anthony M. Use of fractionated laser therapy for the treatment of androgenetic alopecia: a systematic review and meta-analysis. Lasers Med Sci. 2023 Dec 13; 39(1):4.
- Dabek, R.J.; Austen, W.G.; Bojovic, B. Laser-assisted Hair Regrowth: Fractional Laser Modalities for the Treatment of Androgenic Alopecia. Plast. Reconstr. Surg. Glob. Open 2019, 7(4), e2157. [Google Scholar] [CrossRef]
- Balazic, E.; Muskat, A.; Kost, Y.; Cohen, J.L.; Kobets, K. The role of laser and energy-assisted drug delivery in the treatment of alopecia. Lasers Med. Sci. 2024, 39(1), 73. [Google Scholar] [CrossRef]
- Bernabe, R.M.; Choe, D.; Calero, T.; Lin, J.; Pham, C.; Dang, J.; Madrigal, P.; Yenikomshian, H.A.; Gillenwater, T.J. Laser-Assisted Drug Delivery in the Treatment of Hypertrophic Scars and Keloids: A Systematic Review. J. Burn Care Res. 2024: irae023.
- Modena, D.A.; Miranda, A.C.; Grecco, C.; Liebano, R.E.; Cordeiro, R.C.; Guidi, R.M. Efficacy, safety, and guidelines of application of the fractional ablative laser erbium YAG 2940 nm and non-ablative laser erbium glass in rejuvenation, skin spots, and acne in different skin phototypes: a systematic review. Lasers Med. Sci. 2020, 35(9), 1877–1888. [Google Scholar] [CrossRef]
- Nobari, N.N.; Tabavar, A.; Sadeghi, S.; Dehghani, A.; Kalantari, Y.; Ghassemi, M.; Atefi, N.; Goodarzi, A. A systematic review of the comparison between needling (RF-needling, meso-needling, and micro-needling) and ablative fractional lasers (CO2, erbium YAG) in the treatment of atrophic and hypertrophic scars. Lasers Med. Sci. 2023, 38(1), 67. [Google Scholar] [CrossRef]
- Cho, S.; Choi, M.J.; Zheng, Z.; Goo, B.; Kim, D.Y.; Cho, S.B. Clinical effects of non-ablative and ablative fractional lasers on various hair disorders: a case series of 17 patients. J. Cosmet. Laser Ther. 2013, 15(2), 74–79. [Google Scholar] [CrossRef]
- Abd ElKawy, F.A.E.W.; Aly, S.H.M.; Ibrahim, S.M.A. Fractional CO2 laser versus microneedling as a transepidermal drug delivery system for the treatment of alopecia areata: A clinical dermoscopic evaluation. Dermatol. Ther. 2022, 35(7), e15553. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.; Obaid, Z.M.; Sayedahmed, O.M.E. Comparative study between topical application of triamcinolone acetonide after fractional carbon dioxide laser versus microneedling in the treatment of resistant alopecia areata. Dermatol. Ther. 2022, 35(12), e15913. [Google Scholar] [CrossRef] [PubMed]
- Day, D.; McCarthy, M.; Talaber, I. Non-ablative Er: YAG laser is an effective tool in the treatment arsenal of androgenetic alopecia. J. Cosmet. Dermatol. 2022, 21(5), 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Azari, A.; Golmohammadi, M.G. Effects of 780-nm low-level laser therapy with a pulsed gallium aluminum arsenide laser on the healing of a surgically induced open skin wound of rat. Photomed. Laser Surg. 2010, 28, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Buscone, S.; Mardaryev, A.N.; Westgate, G.E.; Uzunbajakava, N.E.; Botchkareva, N.V. Cryptochrome 1 is modulated by blue light in human keratinocytes and exerts positive impact on human hair growth. Exp. Dermatol. 2021, 30(2), 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Sannino, M.; Cannarozzo, G.; Giudice, A.; Del Duca, E.; Tamburi, F.; Bennardo, L.; Nisticò, S.P. Blue light-emitting diodes in hair regrowth: the first prospective study. Lasers Med. Sci. 2021, 36(8), 1719–1723. [Google Scholar] [CrossRef]
- Kwon, H.H.; Lee, J.B.; Yoon, J.Y.; Park, S.Y.; Ryu, H.H.; Park, B.M.; Kim, Y.J.; Suh, D.H. The clinical and histological effect of home-use, combination blue–red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: A double-blind, randomized controlled trial. Br. J. Dermatol. 2013, 168(5), 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Ki, M.-S. Hair Growth Booster Effects of Micro-Needling with Low-Level Led Therapy and Growth Factors on Subjects Treated with Finasteride®. Appl. Sci. 2022, 12, 9164. [Google Scholar] [CrossRef]
- Hong, C.; Zhang, G.; Zhang, W.; Liu, J.; Zhang, J.; Chen, Y.; Peng, H.; Cheng, Y.; Ding, X.; Xin, H.; Wang, X. Hair grows hair: Dual-effective hair regrowth through a hair enhanced dissolvable microneedle patch cooperated with the pure yellow light irradiation. Appl. Mater. Today 2021, 25, 101188. [Google Scholar] [CrossRef]
- Moraveji, M.; Haghighipour, N.; Keshvari, H.; Abbariki, T.N.; Shokrgozar, M.A.; Amanzadeh, A. Effect of Extremely Low-Frequency Electromagnetic Field on MAP2 and Nestin Gene Expression of Hair Follicle Dermal Papilla Cells. Int. J. Artif. Organs 2016, 39(6), 294–299. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.M.; Park, H.J.; Nam, M.H.; Seo, Y.K. Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling. Biomedicines 2022, 10(4). [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Bai, L.; Zhao, P.; Zhang, M. Exposure to 50 Hz electromagnetic fields enhances hair follicle regrowth in C57BL/6 mice. Exp. Biol. Med. 2019, 244(5), 389–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, Q.; Zhang, N.; Wang, B.; Yang, Z.; Ryaby, J.T.; Waldorff, E.I.; Lee, W.Y.W.; Li, G. A Novel Pulsed Electromagnetic Field Promotes Distraction Osteogenesis Via Enhancing Osteogenesis and Angiogenesis in a Rat Model. J. Orthop. Transl. 2020, 25, 87–95. [Google Scholar] [CrossRef]
- Selvam, R.; Ganesan, K.; Raju, K.N.; Gangadharan, A.C.; Manohar, B.M.; Puvanakrishnan, R. Low frequency and low intensity pulsed electromagnetic field exerts its antiinflammatory effect through restoration of plasma membrane calcium ATPase activity. Life Sci. 2007, 80(26), 2403–2410. [Google Scholar] [CrossRef]
- Hadshiew, I.M.; Foitzik, K.; Arck, P.C.; Paus, R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J. Investig. Dermatol. 2004, 123(3), 455–457. [Google Scholar] [CrossRef] [PubMed]
- Prie, B.E.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative stress in androgenetic alopecia. J. Med. Life 2016, 9(1), 79. [Google Scholar] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: a review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Hochfeld, L.M.; Henne, S.K.; Nöthen, M.M. Hormonal regulation in male androgenetic alopecia—Sex hormones and beyond: Evidence from recent genetic studies. Exp. Dermatol. 2020, 29(9), 814–827. [Google Scholar] [CrossRef]
- Han, X.; Chang, L.; Qiu, Z.; Lin, M.; Wang, Y.; Liu, D.; Diao, Q.; Zhong, J.L.; Xu, W. Micro-Injury Induces Hair Regeneration and Vitiligo Repigmentation Through Wnt/β-Catenin Pathway. Stem Cells Dev. 2022, 31((5-6)), 111–118. [Google Scholar] [CrossRef]
- Huang, P.; Yan, R.; Zhang, X.; Wang, L.; Ke, X.; Qu, Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol. Ther. 2019, 196, 79–90. [Google Scholar] [CrossRef]
- Jha, A.K.; Vinay, K.; Zeeshan, M.; Roy, P.K.; Chaudhary, R.K.P.; Priya, A. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil. J. Cosmet. Dermatol. 2019, 18(5), 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, Y.; Huang, Y.; Wang, J.; Yang, K.; Zhang, Y.; Pu, W.; Liu, J.; Shi, X.; Ma, Y.; Ni, C.; Zhang, Y.; Zhu, Y.; Li, H.; Wang, J.; Lin, J.; Wu, W. Insights into male androgenetic alopecia using comparative transcriptome profiling: hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways. Br. J. Dermatol. 2022, 187(6), 936–947. [Google Scholar] [CrossRef] [PubMed]
- Premanand, A.; Reena Rajkumari, B. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch. Dermatol. Res. 2018, 310(5), 391–399. [Google Scholar] [CrossRef]
- Lu, G.Q.; Wu, Z.B.; Chu, X.Y.; Bi, Z.G.; Fan, W.X. An investigation of crosstalk between Wnt/β-catenin and transforming growth factor-β signaling in androgenetic alopecia. Medicine (Baltimore). 2016, 95(30), e4297. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Zaiac, M.N.; Canazza, A.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Alis, R.; Lucia, A.; Emanuele, E. Topical application of the Wnt/β-catenin activator methyl vanillate increases hair count and hair mass index in women with androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15(4), 469–474. [Google Scholar] [CrossRef]
- Bao, L.; Gong, L.; Guo, M.; Liu, T.; Shi, A.; Zong, H.; Xu, X.; Chen, H.; Gao, X.; Li, Y. Randomized trial of electrodynamic microneedle combined with 5% minoxidil topical solution for the treatment of Chinese male Androgenetic alopecia. J. Cosmet. Laser Ther. 2020, 22(1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesari, K.K.; Behari, J. The therapeutic effect of a pulsed electromagnetic field on the reproductive patterns of male Wistar rats exposed to a 2.45-GHz microwave field. Clinics 2011, 66(7), 1237–1245. [Google Scholar] [CrossRef]
- Keszler, A.; Lindemer, B.; Weihrauch, D.; Jones, D.; Hogg, N.; Lohr, N.L. Red/near infrared light stimulates release of an endothelium-dependent vasodilator and rescues vascular dysfunction in a diabetes model. Free Radic. Biol. Med. 2017, 113, 157-164. Erratum in: Free Radic. Biol. Med. 2019, 131, 443.
- Keszler, A.; Lindemer, B.; Broeckel, G.; Weihrauch, D.; Gao, Y.; Lohr, N.L. In Vivo Characterization of a Red Light-Activated Vasodilation: A Photobiomodulation Study. Front. Physiol. 2022, 13, 880158. [Google Scholar] [CrossRef]
- Weihrauch, D.; Keszler, A.; Broeckel, G.; Aranda, E.; Lindemer, B.; Lohr, N.L. Red light mediates the exocytosis of vasodilatory vesicles from cultured endothelial cells: a cellular, and ex vivo murine model. Photochem. Photobiol. Sci. 2024, 23(2), 355–364. [Google Scholar] [CrossRef]
- Weihrauch, D.; Keszler, A.; Lindemer, B.; Krolikowski, J.; Lohr, N.L. Red light stimulates vasodilation through extracellular vesicle trafficking. J. Photochem. Photobiol. B: Biol. 2021, 220, 112212. [Google Scholar] [CrossRef]
- Wunsch, A.; Matuschka, K. A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomed. Laser Surg. 2014, 32(2), 93–100. [Google Scholar] [CrossRef] [PubMed]
- "Assessing the Safety and Efficacy of a Novel Microneedling and Laser Device for Male Pattern Hair Loss (CS-SAGA-001)." ClinicalTrials.gov Identifier: NCT05970809. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05970809 (accessed on 1 April 2024).
- Jahangiri Noudeh Y, Shabani M, Vatankhah N, Hashemian SJ, Akbari K. A combination of 670 and 810nm diode lasers for wound healing acceleration in diabetic rats. Photomed. Laser Surg. 2010, 28, 621–7.
- Meirelles GC, Santos JN, Chagas PO, Moura AP, Pinheiro AL. A comparative study of the effects of laser photobiomodulation on the healing of third-degree burns: a histological study in rats. Photomed. Laser Surg. 2008, 26, 159–66.
- Pessoa ES, Melhado RM, Theodoro LH, Garcia VG. A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed. Laser Surg. 2004, 22, 199–204.
- Rodrigo SM, Cunha A, Pozza DH, Blaya DS, Moraes JF, Weber JB, de Oliveira MG. Analysis of the systemic effect of red and infrared laser therapy on wound repair. Photomed. Laser Surg. 2009, 27, 929–35.
- Treichel JL, Henry MM, Skumatz CM, Eells JT, Burke JM. Formate, the toxic metabolite of methanol, in cultured ocular cells. Neurotoxicology 2003, 24, 825–34.
- Byrnes KR, Barna L, Chenault VM, Waynant RW, Ilev IK, Longo L, Miracco C, Johnson B, Anders JJ. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed. Laser Surg. 2004, 22, 281–90.
- Gal P, Vidinsky B, Toporcer T, Mokry M, Mozes S, Longauer F, Sabo J. Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed. Laser Surg. 2006, 24, 480–8.
- Iordanou P, Lykoudis EG, Athanasiou A, Koniaris E, Papaevangelou M, Fatsea T, Bellou P. Effect of visible and infrared polarized light on the healing process of full-thickness skin wounds: an experimental study. Photomed. Laser Surg. 2009, 27, 261–7.
- Oliveira PC, Pinheiro AL, Reis Junior JA, de Castro IC, Gurgel C, Noia MP, Meireles GC, Cangussu MC, Ramalho LM. Polarized light (lambda400 –2000nm) on third-degree burns in diabetic rats: immunohistochemical study. Photomed. Laser Surg. 2010, 28, 613–9.
- Ribeiro MS, Silva DF, Maldonado EP, de Rossi W, Zezell DM. Effects of 1047-nm neodymium laser radiation on skin wound healing. J. Clin. Laser Med. Surg. 2002, 20, 37–40.
- Pinheiro AL, Pozza DH, Oliveira MG, Weissmann R, Ramalho LM. Polarized light (400–2000nm) and non-ablative laser (685 nm): a description of the wound healing process using immunohistochemical analysis. Photomed. Laser Surg. 2005, 23, 485–92.
- Vasilenko T, Slezak M, Kovac I, Bottkova Z, Jakubco J, Kostelnikova M, Tomori Z, Gal P. The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670nm on wound tensile strength in rats: a short report. Photomed. Laser Surg. 2010, 28, 281–3.
- Keskin, Y.; Taştekin, N.; Kanter, M.; Top, H.; Özdemir, F.; Erboğa, M.; Taşpınar, Ö.; Süt, N. The effect of magnetic field therapy and electric stimulation on experimental burn healing. Turkish J. Phys. Med. Rehabil. 2019, 65(4), 352. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Karkambounas, S.; Batistatou, A.; Lykoudis, E.; Katsaraki, A.; Kartsiouni, T.; Papalois, A.; Evangelou, A. The effect of pulsed electromagnetic fields on secondary skin wound healing: an experimental study. Bioelectromagnetics 2007, 28(5), 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Gengadharan, A.C.; Balachandran, C.; Manohar, B.M.; Puvanakrishnan, R. Low frequency pulsed electromagnetic field—a viable alternative therapy for arthritis. Indian J. Exp. Biol. 2009, 47(12), 939–948. [Google Scholar] [PubMed]
- Tenuta, M.; Tarsitano, M.G.; Mazzotta, P.; Lucchini, L.; Sesti, F.; Fattorini, G.; Pozza, C.; Olivieri, V.; Naro, F.; Gianfrilli, D.; Lenzi, A. Therapeutic use of pulsed electromagnetic field therapy reduces prostate volume and lower urinary tract symptoms in benign prostatic hyperplasia. Andrology 2020, 8(5), 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Monti, D.; Bersani, F.; Cantini, M.; Cadossi, R.; Sacchi, A.; Franceschi, C. Extremely low frequency pulsed electromagnetic fields increase cell proliferation in lymphocytes from young and aged subjects. Biochem. Biophys. Res. Commun. 1989, 160(2), 692–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kwon, U.H.; Kim, H.; Kim, H.J.; Kim, B.; Park, J.O.; Moon, E.S.; Moon, S.H. Pulsed electromagnetic field stimulates cellular proliferation in human intervertebral disc cells. Yonsei Med. J. 2010, 51(6), 954. [Google Scholar] [CrossRef] [PubMed]
- Pezzetti, F.; De Mattei, M.; Caruso, A.; Cadossi, R.; Zucchini, P.; Carinci, F.; Traina, G.C.; Sollazzo, V. Effects of pulsed electromagnetic fields on human chondrocytes: an in vitro study. Calcif. Tissue Int. 1999, 65, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kwan, L.C.R. A comprehensive assessment and management program for diabetic ulcer. Doctoral dissertation, Hong Kong Polytechnic University, 2012.
- Duran, V.; Zamurović, A.; Stojanović, S.; Poljacki, M.; Jovanović, M.; Durisić, S. Therapy of venous ulcers using pulsating electromagnetic fields—personal results. Medicinski Pregled 1991, 44(11-12), 485–488. [Google Scholar]
- Lee, E.W.C.; Maffulli, N.; Li, C.K.; Chan, K.M. Pulsed magnetic and electromagnetic fields in experimental achilles tendonitis in the rat: a prospective randomized study. Arch. Phys. Med. Rehabil. 1997, 78(4), 399–404. [Google Scholar] [CrossRef]
- Girgin, S.; Gedik, E.; Ozturk, H.; Akbulut, V.; Kale, E.; Buyukbayram, H.; Celik, S. Effects of combined pulse electromagnetic field stimulation plus glutamine on the healing of colonic anastomosis in rats. Digest. Dis. Sci. 2009, 54, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.E.D.; Anwar, H.W.; Elnakib, M.M.; Mosaad, A.E. Assessment of the effect of pulsed electromagnetic field therapy in the treatment of chronic wounds. Microbes Infect. Dis. 2023, 4(3). [Google Scholar]
- Amareswari, V.H. Evaluation of Effect of Pulsed electromagnetic field therapy in chronic non-healing Diabetic foot ulcers. Doctoral dissertation, Madras Medical College, Chennai, 2016.
- Ahmadian, S.; Zarchi, S.R.; Bolouri, B. Effects of Extremely-Low-Frequency Pulsed Electromagnetic Fields on Collagen Synthesis in Rat Skin. Biotechnol. Appl. Biochem. 2006, 43(2), 71–75. [Google Scholar] [CrossRef] [PubMed]
- El Rasheed, N.A.A.; Mahmoud, N.F.; Hamada, H.A.; El Khatib, A. Pulsed Electromagnetic Fields Versus Laser Therapy on Enhancing Recovery of Diabetic Foot Ulcer: A Single Blind Randomized Controlled Trial. Biomed. Res. 2017, 28(19). [Google Scholar]
- Cheing, G.L.Y.; Li, X.; Huang, L.; Kwan, R.L.C.; Cheung, K.K. Pulsed Electromagnetic Fields (PEMF) Promote Early Wound Healing and Myofibroblast Proliferation in Diabetic Rats. Bioelectromagnetics 2014, 35(3), 161–169. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Wong-Gibbons, D.; Maultsby, J. Microcirculatory Effects of Pulsed Electromagnetic Fields. J. Orthop. Res. 2004, 22(1), 80–84. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, C.; Wang, L.; Zhang, Q.; Liang, Z.; He, C.; Wei, Q. The Effect of Pulsed Electromagnetic Fields on Angiogenesis. Bioelectromagnetics 2021, 42(3), 250–258. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, C.; Liang, Z.; Zhang, Q.; Xiong, F.; Chen, L.; He, C.; Wei, Q. Pulsed Electromagnetic Fields Increase Angiogenesis and Improve Cardiac Function After Myocardial Ischemia in Mice. Circ. J. 2020, 84(2), 186–193. [Google Scholar] [CrossRef]
- Umiatin, U.; Hadisoebroto Dilogo, I.; Sari, P.; Kusuma Wijaya, S. Histological Analysis of Bone Callus in Delayed Union Model Fracture Healing Stimulated with Pulsed Electromagnetic Fields (PEMF). Scientifica 2021. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; He, F.; Li, S.; He, R.; Liu, Q.; Dong, Q.; Zhou, S.; Miao, H.; Lu, Q.; Li, F. Low Frequency Pulsed Electromagnetic Fields Exposure Alleviate the Abnormal Subchondral Bone Remodeling at the Early Stage of Temporomandibular Joint Osteoarthritis. BMC Musculoskelet. Disord. 2022, 23(1), 987. [Google Scholar] [CrossRef]
- Jing, D.; Zhai, M.; Tong, S.; Xu, F.; Cai, J.; Shen, G.; Wu, Y.; Li, X.; Xie, K.; Liu, J.; Xu, Q. Pulsed Electromagnetic Fields Promote Osteogenesis and Osseointegration of Porous Titanium Implants in Bone Defect Repair Through a Wnt/β-Catenin Signaling-Associated Mechanism. Sci. Rep. 2016, 6(1), 32045. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.H.; van der Jagt, O.P.; Punt, B.J.; Verhaar, J.A.; van Leeuwen, J.P.; Weinans, H.; Jahr, H. Stimulation of Osteogenic Differentiation in Human Osteoprogenitor Cells by Pulsed Electromagnetic Fields: An In Vitro Study. BMC Musculoskelet. Disord. 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Barakat, M.; Awad, S.; Medhat, W.; El-Fakahany, H.; Farag, H. Multiple Microneedling Sessions for Minimally Invasive Facial Rejuvenation: An Objective Assessment. Int. J. Dermatol. 2015, 54(12), 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Mehta-Ambalal, S.R. Neocollagenesis and Neoelastinogenesis: From the Laboratory to the Clinic. J. Cutan. Aesthet. Surg. 2016, 9(3), 145–151. [Google Scholar] [CrossRef]
- Zduńska, K.; Kołodziejczak, A.; Rotsztejn, H. Is Skin Microneedling a Good Alternative Method of Various Skin Defects Removal. Dermatol. Ther. 2018, 31(6), e12714. [Google Scholar]
| Study | AGA diagnosis |
Treatment regimen | Treatment | Subject demographics and numbers | Duration (weeks) | Improvement in hair density (total hairs /cm2) |
|---|---|---|---|---|---|---|
| Kumar et al., 2018 [31] | 2x daily treatment | Minoxidil 5% | 34 M | 12 | 1.89 ± 8.94 | |
| NH III-IV |
2x daily treatment, as well as 8 sessions: four 1x per week, then four fortnightly sessions |
Minoxidil 5% Microneedling |
34 M | 12 | 12.82 ± 6.82 | |
|
Dhurat et al., 2013 [32] |
2x daily treatment | Minoxidil 5% | 44 M | 12 | 22 | |
| NH III-IV |
2x daily treatment, as well as 1x weekly microneedling (with no minoxidil on day of microneedling) | Minoxidil 5% Microneedling |
50 M | 12 | 91.4 |
|
|
Choi and Park 2022 [33] |
NH II+ L I+ |
15 min LLLT and 10 min PEMF 17 treatments: 1x per week for 12 weeks, then 1x every 2 weeks for 8 weeks, then 1x in 4 weeks |
PEMF & LLLT | 31 M 9 F |
23 | 24.8 ± 2.65 |
| Sham | 28 M 12 F |
23 | 6.5 ± 1.85 | |||
| Bureau et al., 2003 [34] | NH I-VII L I-II |
30 min 3x per week |
PEMF & Nutraceutical | 31 M 9 F |
26 | 34 |
| Nutraceutical | 21 M 8 F |
26 | 9 | |||
|
Maddin et al., 1990 [35] |
12 minutes 20 1x per week 4 2x per week |
PEMF using Electrotricho-genesis | 30 M | 12 | 3.67 ± 6.72* | |
| PEMF using Electrotricho-genesis | 30 M | 24 | 7.08 ± 8.59* | |||
| NH III-IV |
PEMF using Electrotricho-genesis | 30 M | 36 | 11.87 ± 10.61* | ||
| Sham | 26 M | 12 | -1.97 ± 8.79* | |||
| Sham | 26 M | 24 | 1.93 ± 8.3* | |||
| Sham | 26 M | 36 | 5.61 ± 10.25* | |||
| Maddin et al., 1992 [36] |
NH III-IV |
12 minutes 20 1x per week 4 2x per week |
PEMF using Electrotricho-genesis | 14 M | 24 | 11.66 ± 1.38* |
| 12 minutes 26 1x per week 4 2x per week |
PEMF using Electrotricho-genesis | 14 M | 30 | 17.59 ± 1.98* | ||
| Leavitt et al., 2009 [22] | NH IIa-V FS I-IV |
15 minutes 3x per week |
LLLT using the HairMax LaserComb |
71 M | 26 | 17.3 ± 11.9 |
| Sham | 39 M | 26 | -8.9 ± 11.7 | |||
| Kim et al., 2013 [23] | NH III-VII L I-III |
18 minutes daily | LLLT using the Oaze 3R Helmet | 15 M & F | 24 | 17.2 ± 12.1 |
| Sham | 14 M & F | 24 | -2.1 ± 18.3 | |||
| Mai-Yi Fan et al., 2018 [25] | NH IIa-V LI-4, II-1, II-2 FS I-IV |
30 minutes 3x per week |
LLLT using the Restore ID-520 | 61 M 13 F 61 M |
24 24 |
6 ± 12.5 |
| Sham | 13 F | -2 ± 12.6 | ||||
| 11 minutes 3x per week |
LLLT using HairMax LaserComb | 42 F | 26 | 20.2 ± 11.2 | ||
| Sham | 21 F | 26 | 2.8 ± 16.5 | |||
| 18 minutes 3x per week |
LLLT using HairMax LaserComb | 39 F | 26 | 20.2 ± 11.6 | ||
| Jimenez et al., 2014 [24] | NH IIa-V | Sham | 18 F | 26 | 3.0 ± 9.3 | |
| 15 minutes 3x per week |
LLLT using HairMax LaserComb | 24 M | 26 | 18.4 ± 13.7 | ||
| Sham | 14 M | 26 | 3.0 ± 9.3 | |||
| 8 minutes 3x per week |
LLLT using HairMax LaserComb | 19 M 42 F |
26 | 25.7 ± 17.1 | ||
| Sham | 21 M | 26 | 9.4 ± 12.9 | |||
| Lodi et al., 2021 [37] | NH III-VI | 24 minutes 2x per week |
Blue LLLT | 20 M | 10 | 11 ± 3 |
| Lee et al., 2011 [38] | L I-II | 10 treatments (every fortnight) | 1550 fractional Er:Glass Laser | 27 F | 20 | 57 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





