1. Introduction
Spinal meningiomas (SM) are benign, intradural, juxtamedullary tumors situated within the spinal canal that may cause neurological deficits and pain contingent upon their localization. The primary objective of the treatment is to achieve gross total resection (GTR) to allow neurological recovery and mitigating the risk of tumor recurrence [
1,
2,
3,
4]. Due to the benign nature of these tumors the neurological deficits manifest slowly. A subset of patients may exhibit incidental radiological findings indicating the presence of SM with no or minor neurological deficits.
There is a growing focus on quality of life (QoL) and quality of the delivered care [
5]. Quality indicators (QI), predominantly proposed and implemented by healthcare policymakers, such as readmission and reoperation rates evaluating treatment and care, are continually evolving and have been utilized for reimbursement purposes [
6,
7].Beyond that, for the patients, QoL is a critical factor, as it significantly impact their ability to resume social and employment activities.
In the past several publications showed relatively good outcome and QoL after resection of intraspinal, intradural tumors including meningiomas. However, most of these publications reported bilateral laminectomy as the main approach for tumor resection [
4,
8,
9,
10]. Recently we demonstrated the resection of spinal meningiomas is feasible via a less invasive unilateral approach, showing a similar rate of gross total resection (GTR) in comparison to a bilateral laminectomy. Furthermore, we demonstrated that patients undergoing unilateral hemilaminectomy, with less muscle detachment and bone resection had significantly less blood loss (EBL) during the surgical procedure and faster recovery with significantly shorter length of hospital stay (LOS) [
11]. Minimal invasive spine surgery (MISS) can further improve surgical outcomes though reduced surgical impact, decreased pain, quicker recovery [
12,
13,
14].
The aim of this study is to question the optimal timing of resection spinal meningiomas and its influence on QoL and QI. For these reasons we compared patients who were operated in an early stage of the disease with mild symptoms (McCormick scale 1-2) and patients operated with more severe symptoms (McCormick scale >2). The primary outcome was favorable neurological recovery (McCormick scale 1), and whether performing surgery on patients with mild neurological symptoms is recommended. The secondary outcomes are QI, including length of hospital stay, nosocomial infections, 90 days unplanned readmission and re-surgery. In addition, we evaluated QoL and postoperative disability according to short form 36 (SF36), Oswestry disability index (ODI), and neck disability index (NDI) questionnaires.
2. Materials and Methods
Study Design
We included all patients who underwent a resection of spinal meningioma between 2011 and 2021 at our neurosurgical department in this retrospective study. The following data of the hospital electronic records were analyzed: age, sex, tumor volume, spinal canal occupancy ratio, neurological symptoms, localization of the tumor within the spinal canal and its relation to the spinal cord, the presence of cord edema, surgical approach, the extent of resection (EOR) according to the Simpson classification [
15], use of intraoperative neuromonitoring (IOM), estimated blood loss (EBL, ml), duration of surgery (minutes), length of hospital stay (LOS; days), 90-day nosocomial infections, 90-day surgical site infection, unplanned 90-day readmission and 90-day re-operation, 90-day mortality and tumor progress or recurrence.
Furthermore, we contacted the patients and sent them questionnaires including SF-36 questionnaire to evaluate QoL. To evaluate their functional outcome we used the Oswestry disability index (ODI) and Neck-Disability index (NDI) depending on the localization of the tumor within the spinal canal [
16,
17]. Back, neck or extremity pain was determined according to visual analogue scale (VAS) from 0 to 10. Prior to sending these questionnaires patients were contacted by telephone and informed about the study. Informed consent was obtained from each patient participating in this part of the study.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Münster, Germany (reference number 2021-714-f-S, February 15, 2022).
Surgical Intervention
Surgical resection was performed via dorsal or dorso-lateral approach using hemilaminectomy according to the tumor´s location in the spinal cord. During surgery, intra-operative neurophysiological monitoring (IOM, inomed Medizintechnik GmbH, Emmendingen, Germany) was conducted, including motor-evoked potentials (MEP), sensory-evoked potentials (SEP) of upper and lower extremities. After the exposure of the dura, intraoperative sonography was performed to detect the tumor and the dura was opened. After the durotomy the nerve roots were identified and in ventrally located tumors the dentate ligaments were also identified and cut. Subsequently, the tumor poles were visualized. In order to be able to remove the tumor through the laminotomy it was debulked using an ultrasonic aspirator (CUSA®, Integra lifesciences, Princeton, NJ, USA). Finally, the meningioma was removed, and the dura attachments were coagulated to achieve resection grade 2 according to the Simpson classification whenever feasible [
15]. The dura was closed using a 6-0 monofil continuous suture. The first postoperative MRI was performed three months after surgery.
Outcome and Assessment of QoL and functionality
The neurological status of the patients was assessed three months after surgery using the modified McCormick Scale [
18], ranging from 1 (no symptoms or minimal dysesthesia) to 5 (paraplegic/quadriplegic). The status was evaluated both before and after surgery independently by two of the authors (MS and WS). For further dichotomic calculations, the McCormick scales 1 and 2 were considered as ’mild symptoms’, and 3 to 5 as ‘severe symptoms’. Postoperatively, McCormick scale of I was considered as ‘favorable recovery’.
To assess QoL and functional disabilities patients were subsequently contacted and requested to complete standardized questionnaires. QoL was assessed by the SF-36 questionnaire. It contains questions evaluating general health, physical functioning, limitations due to physical health, limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain and health change. We compared the result of patients’ population with the general population from data published previously [
19].
Postoperative disability was measured with ODI or NDI questionnaires. Patients having meningiomas in the thoracic and lumbar spine answered the ODI questionnaire and those with tumors in the cervical spine the NDI questionnaire. In addition, all patients were asked to evaluate back, neck or extremity pain using VAS score 0 to 10.
Evaluation of Images
Tumor volume was semiautomatically measured using Brainlab elements® software (Brainlab AG, Munich, Germany) and expressed in milliliters (ml). Furthermore, we calculated the occupancy ratio of the meningioma in comparison to the spinal canal, by measuring the area of the meningioma on the slide with the largest extension and dividing it by the area of the spinal canal, the ratio is shown in percentage [
20]. In addition, we evaluated whether the meningioma was localized ventrally of the dentate ligament of posterior to it, and whether a spinal cord edema was present or not.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 29.0 (IBM Corp., Armonk, NY, USA). Categorical variables are shown as absolute and relative frequencies. Parametric values are presented in mean and standard deviation (SD). Non-parametric values are presented as the median and interquartile range (IQR, 25% quartile and 75% quartile). Fisher’s exact test was performed to compare groups of binary categorical variables. A two-tailed Student t-test was used as a parametric and a two-sided Mann–Whitney U-test (MWU) as a non-parametric test. A probability value less than p < .05 was considered statistically significant.
3. Results
3.1. Patients’ Characteristics
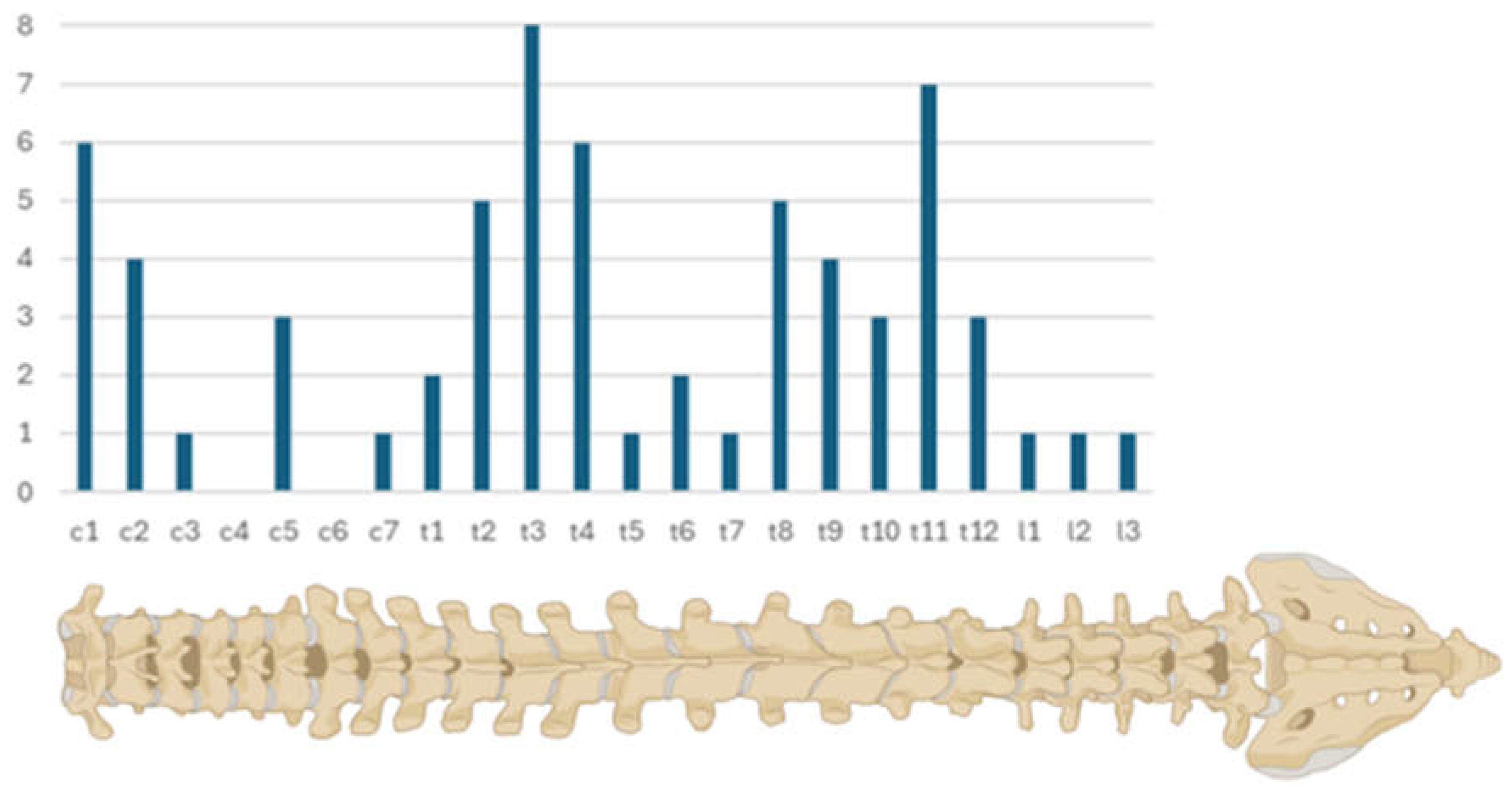
In the period of ten years between 2011 and 2021 65 cases with spinal meningiomas were operated at our department. With 59 cases (90.77%), female patients accounted the vast majority. Two of the patients underwent surgery due to tumor recurrence (N = 2, 3.08%) and one due to progression after partial resection (N = 1, 1.54%). Patients’ ages at time of surgery ranged between 25 and 86 years, with a mean age of 58.4 (±14.10) years. In most of the cases the meningiomas were located in the thoracic spine (N = 45, 69.23%), followed by 17 (26.15%) cases in the cervical spine and only rarely in the lumbar spine (N = 3, 4.62%). We noticed more cases at junction areas C1, T1 to 4 and T 8 to 12 (
Figure 1). Mean tumor volume was 1.22 ml (±0.85), and mean occupancy ratio of the spinal canal was 51.88% (±19.85%). Cord compression was visible in 91% of the cases (N = 59) and 20% of the cases cord edema was present (N = 13). Interesting was the finding that in seven cases (10.77%) patients had a history of cranial meningioma surgery, too.
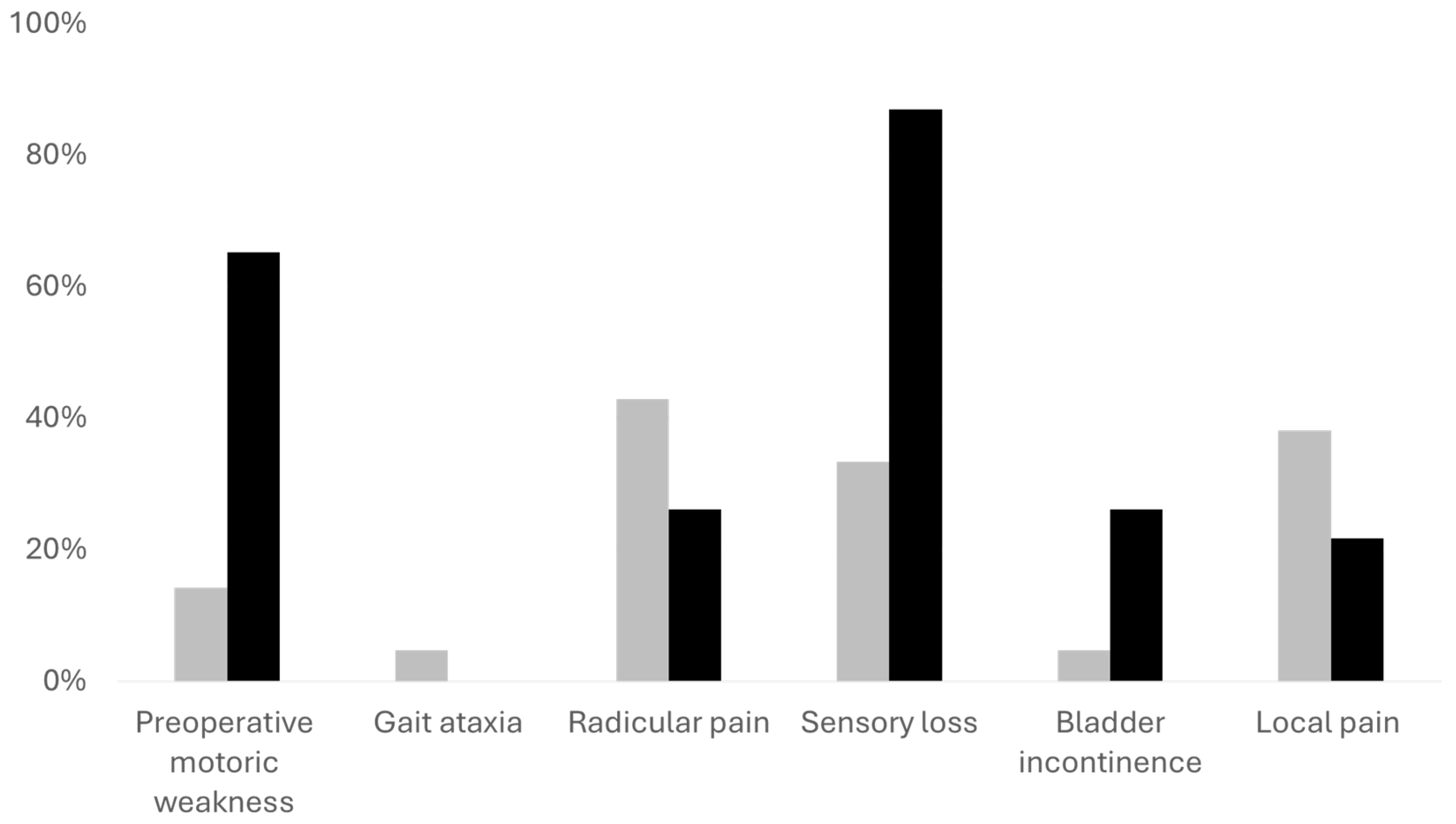
For further analysis we divided the patients into two cohorts. The first consisted of patients with mild symptoms (McCormick scale 1 and 2) and the second patients with more severe symptoms (McCormick 3 and higher). In this analysis we found out that patients with mild symptoms were younger (p = .015). Preoperatively, they had better Karnofsky performance scale, less gait ataxia, less motor weakness, and less sensory deficits (all p < .001) as compared to patients with more severe symptoms. In addition, patients with mild symptoms had a higher percentage of radicular and local pain (N = 18, 42.86% and N = 16, 38.10% respectively in comparison to N = 6, 26.09%) and N = 5, 21.74%), however, not reaching statical significance (both p > .05).
Although the tumor volume was comparable in both cohorts, the spinal canal occupancy ratio - measured at the level of the tumor’s largest diameter - was higher in the cohort with more severe neurological symptoms (p = .016). In addition, patients with severe symptoms had more frequently meningiomas in the thoracic spine (p = .026) and spinal cord edema (p = .049). See
Table 1 for further information.
Figure 2.
Overview of the preoperative neurological symptoms. The gray bars represent patients with mild preoperative neurological symptoms, black bars represent patients with more severe neurological symptoms. The Y axis demonstrates the percentage of patients with each symptom in each cohort. Motor weakness, sensory deficits and bladder dysfunction were significantly more prevalent in the cohort with severe symptoms (all p < .001). Neurological outcome and quality indicators
Figure 2.
Overview of the preoperative neurological symptoms. The gray bars represent patients with mild preoperative neurological symptoms, black bars represent patients with more severe neurological symptoms. The Y axis demonstrates the percentage of patients with each symptom in each cohort. Motor weakness, sensory deficits and bladder dysfunction were significantly more prevalent in the cohort with severe symptoms (all p < .001). Neurological outcome and quality indicators
3.2. Neurological Outcome and Quality Indicators
We noticed an improvement of at least one of the neurological symptoms and pain in most of the cases (N = 64, 98.46%). However, patients with mild symptoms had higher odds for favorable outcome (postoperative McCormick scale 1; 14.7778 (95%CI 3.9175-55.746, p < .001). The least improvement was noticed in bladder function in both cohorts. Furthermore, the recovery of patients with mild symptoms preoperatively was faster and their LOS was significantly lower, with mean LOS of 7.07 days (±2.4) in comparison to 10.04 days (±5.36, p = .003). While the Karnofsky performance scale improved in both cohorts, it was still significantly better in the cohort of the patient with mild symptoms (p = .004). Although improved in both cohorts, sensory loss was more frequent in the cohort with severe symptoms (p = .006). All other symptoms and deficits were comparable.
Adverse events were very rare in both cohorts, and we noticed only one nosocomial infection, one unplanned readmission, and one re-surgery due to CSF leakage within 90 days, see
Table 2 for further information.
Table 2.
Surgical data and postoperative outcome (SD: standard deviation).
Table 2.
Surgical data and postoperative outcome (SD: standard deviation).
| Variable |
Mild symptoms (N = 42) |
Severe symptoms (N = 23) |
P Value |
| Duration of surgery (min, mean, SD) |
238.34 (±111.77) |
231.13 (±68.51) |
.785 |
| Uni-lateral approach (N, %) |
37 (88.1%) |
22 (95.65%) |
.411 |
| Bilateral approach (N, %) |
5 (11.9%) |
1 (4.35%) |
.411 |
| Extent of resection |
|
|
|
| Simpson grade 2 (N, %) |
40 (95.24%) |
22 (95.65%) |
>.99 |
| Simpson grade 3 (N, %) |
2 (4.76%) |
1 (4.35%) |
|
| Estimated blood loss (ml, mean, SD) |
236.72 (±315.70) |
356.13 (±384) |
.191 |
| Length of hospital stay (days, mean, SD) |
7.07 (±2.4) |
10.04 (±5.36) |
.003 |
| Adverse events (N, %) |
2 (4.76%) |
2 (8.7%) |
>.99 |
| CSF leak (N, %) |
1 (2.38%) |
0 |
>.99 |
| Pulmonary embolism (N, %) |
1 (2.38%) |
0 |
>.99 |
| Cardial decompensation (N, %) |
0 |
1 (4.35%) |
.354 |
| Urinary tract infection |
0 |
1 (4.35%) |
.354 |
| Histology WHO 1 (N, %) |
42 (100%) |
23 (100%) |
>.99 |
| Karnofsky performance scale postoperative (Median, IQR) |
90 (90-100) |
80 (70-90) |
.004 |
| Postoperative McCormick scale (Median, IQR) |
1 (1-1) |
2 (1-2) |
.001 |
| Postoperative McCormick scale 1 (N, %) |
38 (90.48%) |
9 (39.13%) |
<.001 |
| Postoperative McCormick scale 2 (N, %) |
4 (9.52%) |
10 (43.48%) |
.003 |
| Postoperative McCormick scale 3 (N, %) |
0 |
3 (13.04%) |
.037 |
| Postoperative McCormick scale 4 (N, %) |
0 |
1 (4.35%) |
.354 |
| Postoperative McCormick scale 5 (N, %) |
0 |
0 |
>.99 |
| Postoperative motoric weakness (N, %) |
0 |
2 (8.70%) |
.122 |
| Gait ataxia (N, %) |
1 (2.38%) |
1 (4.35%) |
>.99 |
| Radicular pain (N, %) |
4 (9.52%) |
1 (4.35%) |
.649 |
| Sensory loss (N, %) |
1 (2.38%) |
6 (26.09%) |
.006 |
| Bladder incontinence (N, %) |
2 (4.76%) |
4 (17.39%) |
.174 |
| Local pain (N, %) |
5 (11.90%) |
0 |
.152 |
| Readmission in 90 days (N, %) |
1 (2.38%) |
0 |
>.99 |
| Re-Surgery in 90 days (N, %) |
1 (2.38%) |
0 |
>.99 |
| Tumor recurrence (N, %) |
3 (7.14%) |
0 |
.547 |
| Progression free survival (years, mean, SD) |
7.45 (±3.95) |
6 (±3.40) |
.121 |
Figure 3.
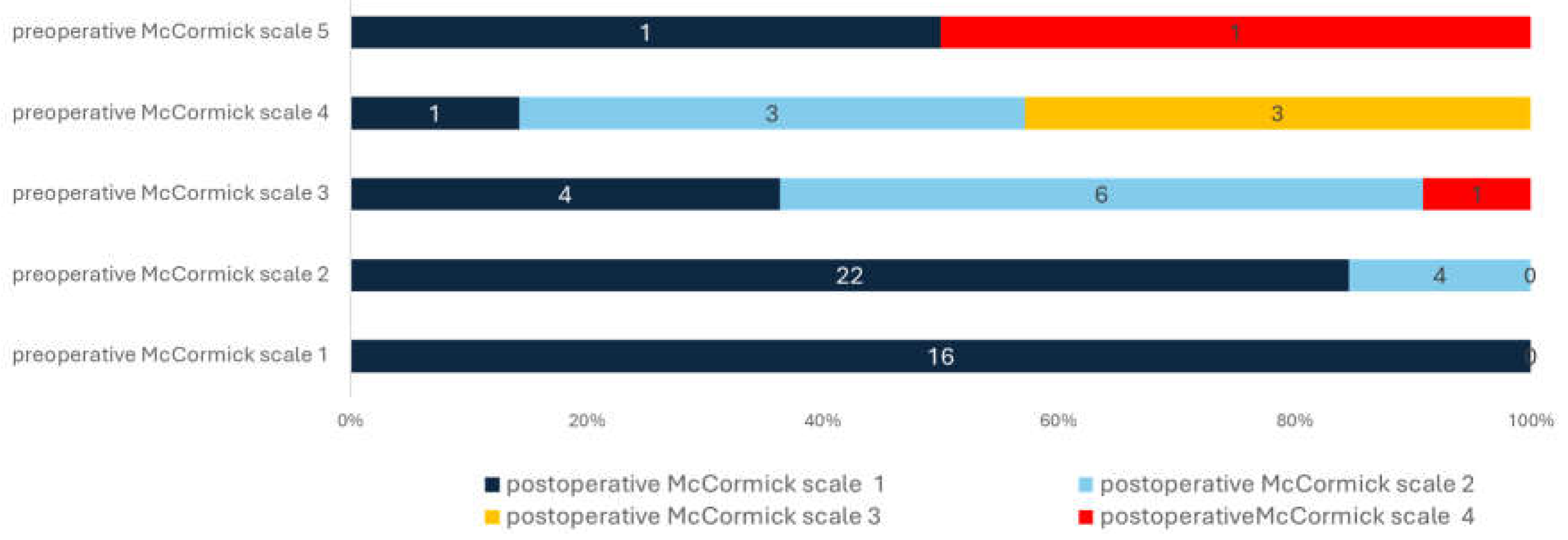
Relationship between preoperative and postoperative McCormick scale. All 16 patients with preoperative McCormick scale 1 had the same scale after surgery. Patients with preoperative McCormick scale 2 had also favorable outcome with McCormick scale 1 in most cases (N = 22, 84.62%). On the other hand, patients with McCormick scale 3 to 5, had much less odds for favorable outcome after surgery (p < .001). See text for further information.
Figure 3.
Relationship between preoperative and postoperative McCormick scale. All 16 patients with preoperative McCormick scale 1 had the same scale after surgery. Patients with preoperative McCormick scale 2 had also favorable outcome with McCormick scale 1 in most cases (N = 22, 84.62%). On the other hand, patients with McCormick scale 3 to 5, had much less odds for favorable outcome after surgery (p < .001). See text for further information.
Figure 4.
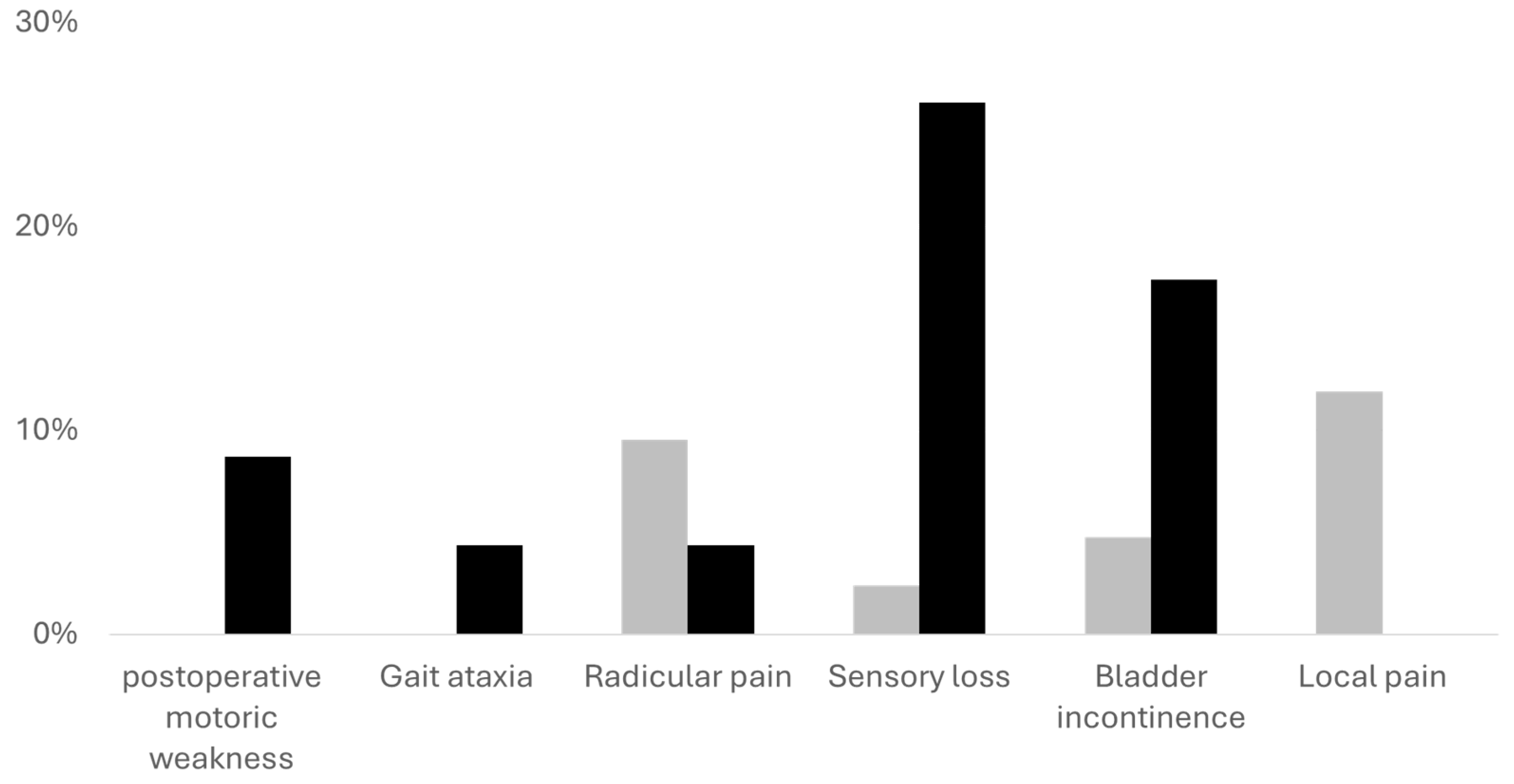
Remaining neurological symptoms after tumor resection. The gray bars represent patients with mild preoperative neurological symptoms, black bars represent patients with more severe neurological symptoms. The Y axis demonstrates the percentage of patients with each symptom in each cohort. Sensory deficits were significantly higher in the cohort with severe symptoms (p = .006). All other symptoms show no statistical significance.
Figure 4.
Remaining neurological symptoms after tumor resection. The gray bars represent patients with mild preoperative neurological symptoms, black bars represent patients with more severe neurological symptoms. The Y axis demonstrates the percentage of patients with each symptom in each cohort. Sensory deficits were significantly higher in the cohort with severe symptoms (p = .006). All other symptoms show no statistical significance.
3.3. Functionality and Quality of Life
Out of the patients we were able to contact, we received 38 (60% of all patients) completed questionnaires. Six of the contacted patients did not return the questionnaire. Two sent inadequately filled forms, one patient had passed away and 16 patients could not be reached. Two of these patients who replied had undergone secondary surgery due to tumor recurrence. The mean time between surgery and contact was 6 years (±3.69). Overall, patients reported good functionality (ODI/NDI 0-20%) in 28 (73.68%) cases. 6 patients (15.79%) had moderate disabilities (ODI/NDI 21 to 40%) while 4 (10.53%) patients had more severe disabilities (ODI/NDI 41 to 62%). The four patients with severe disabilities were relatively old with a mean age of 76 (range 68 to 83). Remarkably, the three younger patients (age range 68-78) had severe preoperative neurological deficits, all classified as McCormick grade 4. This suggests that a poor neurological status in combination with advanced age played a major role for their functional disabilities.
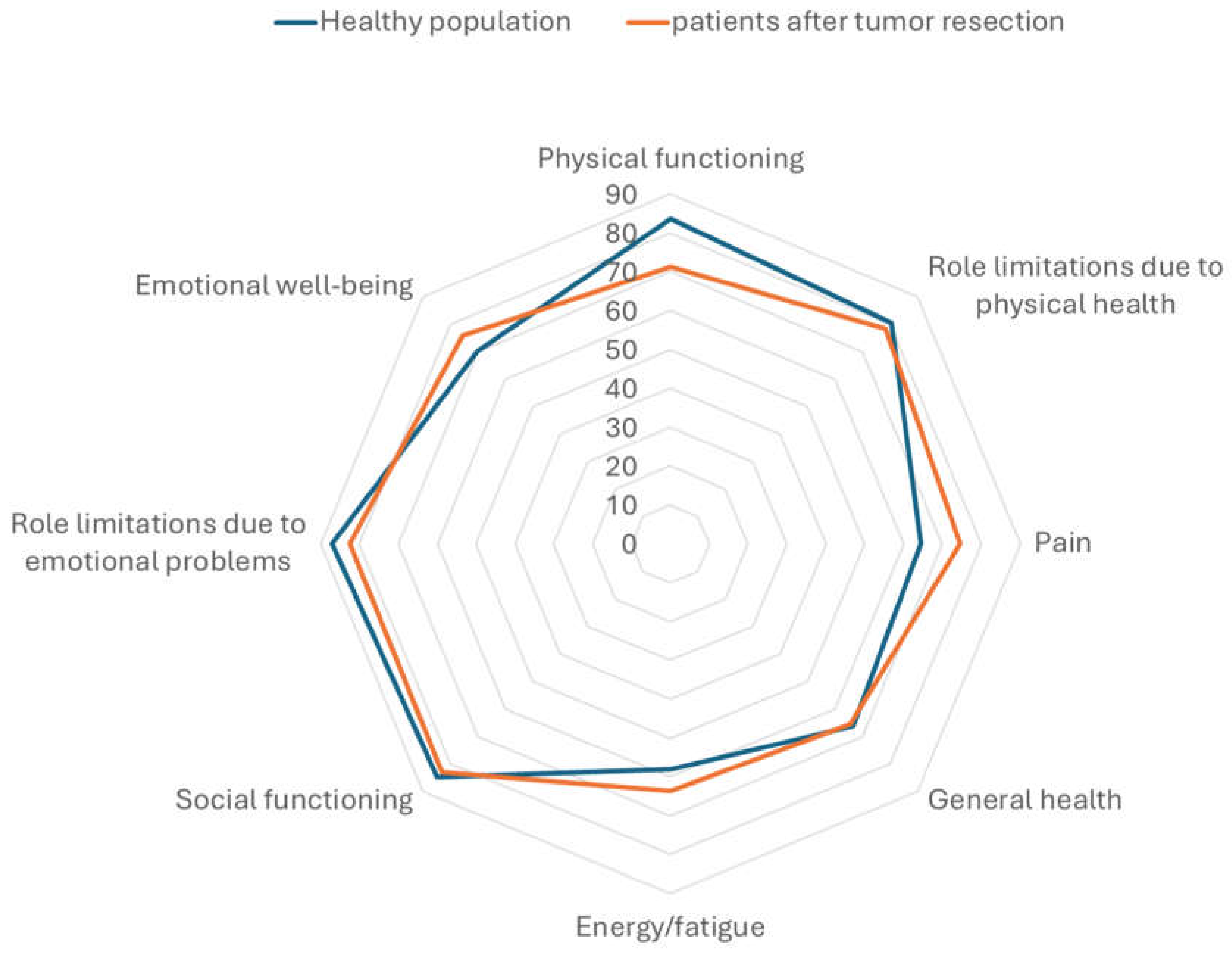
The results of the SF 36 questionnaire revealed that the QoL of patients after resection of spinal meningiomas is quite similar to the general population, when compared to data published previously [
19]. One exception is the slightly reduced physical function. Patients included in this study reported a mean of 71.32 in this part of the questionnaire in comparison to 83.7 in the general population. On the other hand, patients after resection of spinal meningiomas had slightly less complaints regarding pain, see
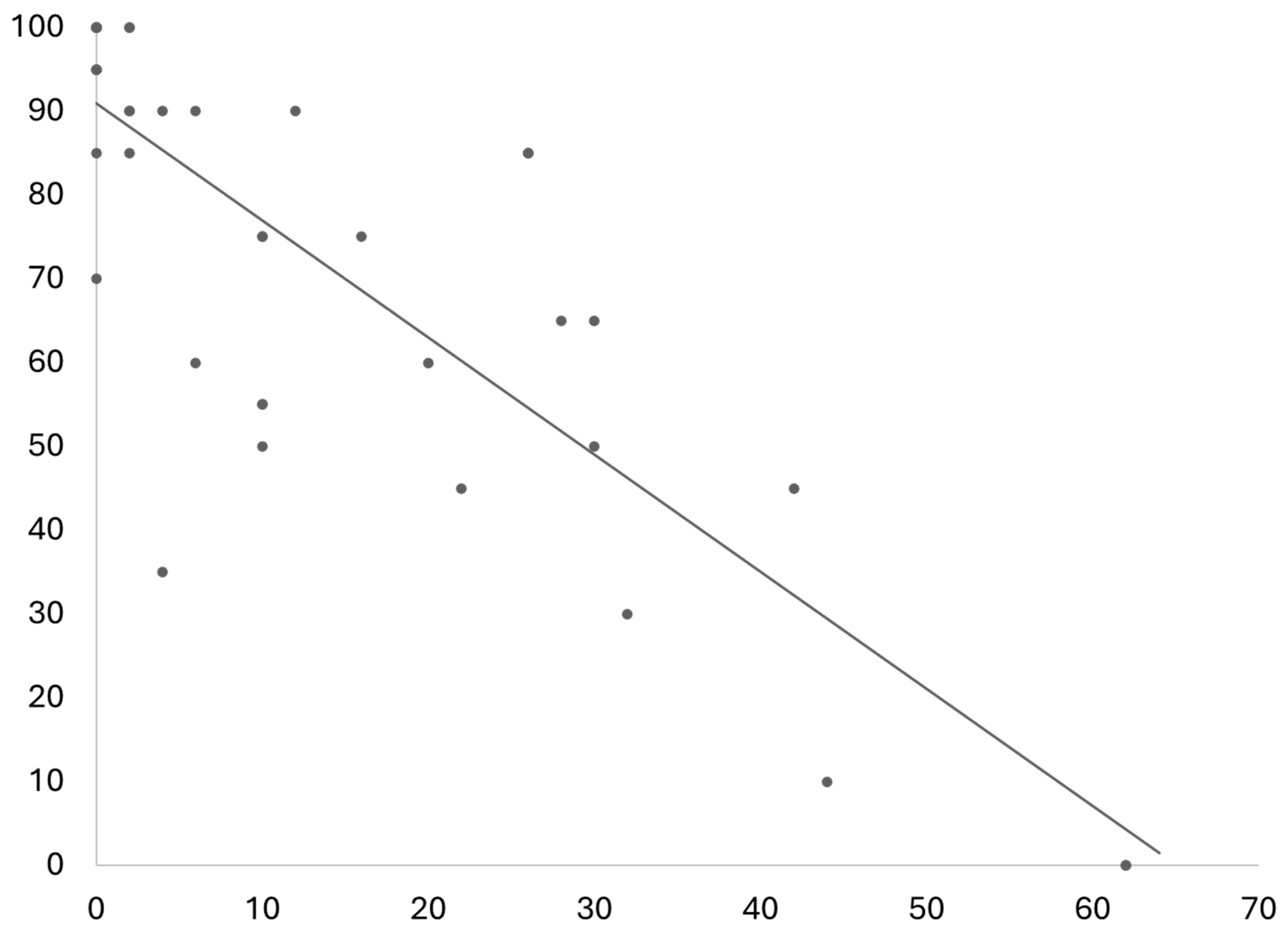
Figure 5. One important additional information is that the study on QoL in the general population the mean age of was notably lower than the mean age in this study. The physical function score had a strong negative correlation to the ODI/NDI values (r(36) = -.83, p < .001), see
Figure 6.
To compare between functionality and QoL and neurological outcome we allocated between patients with favorable neurological outcome and those with incomplete neurological outcome. This analysis revealed a significantly better disability index in patients with favorable neurological outcome. The patients with favorable neurological outcome had a mean ODI/NDI score of 9.26% (±11.60%) in comparison to 23.27% (±24.10, p = .020) in the cohort of patients with incomplete neurological outcome. This was in concordance with the SF 36 subcategories physical function and role limitations due to physical health (both p < .05). All other subcategories showed no significant differences.
As described above, the main risk factor for incomplete neurological outcome was the preoperative neurological status. Patients with preoperative McCormick scale 1 and 2 had significantly more favorable outcomes than those with a preoperative McCormick scale 3 and 4 (p < .001). In addition, the neurological outcome was associated to LOS, with a mean of 6.85 days (±1.2) the LOS was significantly lower in patients with favorable neurological outcome in comparison to 9 days (±4.2) in cases with incomplete neurological outcome (p = .022). See
Table 3,
Table 4 and
Table 5 for further information.
3.4. Tumor Recurrence and Progression
Three patients (4.6%) had a tumor recurrence after a mean follow up period of 7.12 (±3.95) years. One of these patients had a resection grade 3 according to the Simpson classification, showing some tendency, however, without statistical significance (p = .196). On the other hand, two out the three patients with a resection grade 3 without progression had postoperative stereotactic irradiation.
4. Discussion
The neurological and functional outcomes following resection of spinal meningiomas are excellent in most cases. However, patients with mild preoperative symptoms experience more favorable recoveries compared to those with severe preoperative symptoms. A favorable neurological outcome is associated with better disability scores and improved quality of life (QoL). The findings of this study suggest that performing tumor resection in patients with mild neurological symptoms is justified to achieve optimal neurological outcomes, functionality, and quality of life.
Neurological Outcome after Minimal-Invasive Resection
Our results indicate that the postoperative status following resection of spinal meningiomas depends mainly on the preoperative neurological status of the patients, in addition, the age of the patients seems to play a role [
2,
8]. Patients with no or mild symptoms, who were classified preoperatively as McCormick scale 1 or 2 experienced favorable neurological outcome in most cases. In contrary, patients with more severe neurological symptoms had experienced improvement of their deficits in most cases, however, significantly less patients had favorable neurological outcome reaching McCormick scale 1 after surgery in comparison to patients with mild symptoms preoperatively. Nonetheless, in comparison to intramedullary lesions such as hemangioblastoma, the recovery rate was much better [
21]. These results are in line with previously published studies investigating the outcome after other intradural tumor resections [
2,
20,
22] and highlight that severe neuronal damage would not fully recover after surgery.
Patients with mild symptoms in this study had more frequent pain as primary symptom, in comparison to more severe neurological deficits such as gait ataxia, sensory deficits or motor weakness in the other cohort. Furthermore, patients in the cohort with severe symptoms were older, had more frequent tumors in the thoracic spine, with higher spinal canal occupancy ratio and spinal cord edema. The impact of the spinal canal ratio on neurological deficits was also demonstrated in previously published studies [
2,
20,
23,
24,
25,
26]. These all seem to be risk factors for developing more severe deficits or a delay in diagnostics [
20]. We suppose the localization of tumors within the cervical and lumbar spine cause typical radicular symptoms and accelerate diagnostics and treatment [
2,
26]. The smaller spinal canal occupancy ratio, the younger age of the patients, and the less frequent spinal canal edema all indicate that these tumors were identified in an earlier stage of the disease. Moreover, imaging is much more frequently performed on the cervical and lumbar spine due to the higher rate of degenerative conditions in these localizations, than imaging of the thoracic spine. We therefore encourage to perform more imaging of the thoracic spine in patients with refractory back pain, or when neurological findings are not correlated to findings in the more frequent images on the cervical and lumbar spine.
The results of this study indicate that resection of spinal meningiomas causing cord compression, even when causing only minor symptoms should be treated in early stage of the disease. Surgery in advanced stages may relief symptoms in most cases, but the recovery would not be as good as in patients with mild symptoms. A treatment strategy of wait and see, which is often propagated, seems to be less recommended like in other intradural tumors [
21,
27,
28,
29].
Effect of Neurological Outcome on Functionality and Quality of Life
The overall QoL of patients after resection of spinal meningiomas was relatively equivalent to the general population [
19], with minor deficits on the subcategory of physical functionality. However, in this study there were more elderly patients included, which also has influence on physical functionality [
19]. As expected, we observed an association between QoL and postoperative neurological outcome. Patients who showed a favorable neurological outcome after tumor resection graded their life quality significantly higher than those patients who had incomplete recovery after surgery. This was significantly different in the subcategories “physical functionality” and “role limitations due to physical health”. Furthermore, we found a very strong negative correlation between the subcategory physical functionality and disability according to the NDI and ODI questionnaires, which are validated for disabled due to spinal diseases [
9]. This indicates the influence of spinal diseases on the physical function subcategory of QoL [
4].
Similar results on the influence of permanent disabilities due to spinal tumors were published before [
2,
10,
20,
30,
31]. Other previously published studies highlight that supportive care of oncological patients who suffer from neurological symptoms reduces the rate of psychological disorders, pain, and anxiety [
32,
33,
34].
Quality Indicators and Adverse Events
The role of QI is getting more important in recent years [
7]. Therefore, it is important to report the typical measured QI in different pathologies and medical procedures for future evaluations. With four adverse events cases (6.15%), two of them in one patient, the rate is similar to previously published systematic review [
22]. In this study we found one patient who had a nosocomial urinary tract infection. This patient was 75 old when admitted with paraplegia due to a meningioma at thoracic level 4-5 and in a bad general condition with a KPS of 50 due to cardiovascular conditions. After emergency surgery she had to be admitted to an intensive care unit to further her condition due to further cardiac decompensation. Finally, she was dismissed to a nursery care facility after 18 days. Her neurological status indeed improved but remained very severe with a McCormick scale of 4. All these conditions are risk factors for nosocomial infection and prolonged LOS [
2,
22].
In comparison to other studies, we did not identify epidural hemorrhages as adverse events [
20], nor did we find any association between the localization of the tumor and complications [
8,
23]. Moreover, we noticed only one case of neurological deterioration after surgery, in a patient with a preoperative McCormick scale 3. Therefore, we could not verify any significant association for neurological deterioration [
20].
LOS was significantly lower in the cohort of patients with mild symptoms in comparison to those with more severe symptoms, and patients with favorable recovery stayed also for shorter period in hospital. Readmission within 90 days was reported one time due to a CFS leakage, which had to be treated operatively. This patient had a favorable outcome.
Intraoperative neurophysiological monitoring (IOM) was utilized in all included cases. Its role is not yet verified for intradural tumors [
2,
25], but may give the surgeon feedback on neurological conditions especially when spinal cord manipulation is required [
25,40]. We recommend using IOM for these procedures.
Minimal Invasive Surgery
Previous publication also showed very good QoL after resection of spinal meningiomas and other benign intra-dural tumors [
4,
8,
9,
10]. However, these cases series reported mostly on patients operated via a bilateral laminectomy as surgical approach. A unilateral surgical approach as less invasive was performed in most cases in the study. The main goal of minimal invasive spine surgery (MISS) is to minimize the collateral damage both locally and systematically, without reducing the effectiveness of the main goal of the surgical procedure, safe and complete resection in this case in this case [
35,
36]. The role and efficacy of minimal invasive spine surgery was shown in several studies in degenerative spinal conditions, showing similar effect on decompression of the spinal canal, with quicker recovery, less pain, and shorter LOS [
13,
37]. These results advocate that more extensive approaches are not required for the resection of spinal meningiomas [
38].
Previous publications showed also that these less invasive approaches were as effective as more invasive approaches to achieve GTR of spinal meningioma without additional side effects. GTR was achieved in 93.84% of all cases included in this study. Moreover, previous publications comparing both approaches show significantly less blood loss and lower LOS [
11]. More invasive surgical approaches do not seem to be required in most cases, even in ventral located and calcified meningiomas [
11,
27,
39,
40], and other intradural tumors [
41]. Furthermore, minimal invasive unilateral approaches seem to play a role in preventing CSF leakage, one of the more frequent possible complications of after surgery on intra-dural pathologies [
20,
22,
42,
43]. In this study we noticed one case of postoperative CSF leakage (1.54%). The enhanced recovery after minimal-invasive unilateral approaches may influence postoperative QoL and QI.
On the other hand, more extensive approaches including facetectomy are apparently required in order to achieve GTR on dumbbell tumors [
44,
45]. One patient in this study had to be operated on twice due to a dumbbell meningioma in the cervical spine. In the first surgery only, subtotal resection (STR) was achieved via a hemilaminectomy, and she had to undergo one more tumor resection due to progression after 5 years, again only achieving STR via hemilaminectomy.
Limitations
The study’s main limitation is the retrospective nature of the analysis. Hence, some of the analyzed scores were derived from medical reports with inherent limitations. Moreover, only a 60% of the patients were available to fill the questionnaire. The different time interval between surgery and recirculation of the questionnaires may also impact their validity. In addition, other medical conditions, such as cardio-vascular diseases, neurological and psychological disorders, may also affect the results of the questions. Further limitation is the lack of comparison between operative and non-operative cases.
5. Conclusions
Minimal-invasive resection of spinal meningiomas is safe and effective. Patients have very good outcome after surgery. Neurological, functional outcomes and quality of life highly depend on preoperative findings. The results of our study recommend resection of spinal meningiomas in an early stage of the disease when patients have mild symptoms, especially in case of cord compression. Larger registries and prospective studies should be performed to verify these results.
Author Contributions
Conceptualization, M.S. and D.S.; methodology, M.S. and D.S.; software, E.M. and W.Sa.; validation, M.S., W.Sa. and M.G.; formal analysis, M.S.; investigation, X.X.; resources, M.S., E.M. and W.Sa.; data curation, M.S., E.M., and W.Sa.; writing—original draft preparation, M.S.; writing—review and editing, M.S., W.Sa., E.M., S.S., D.S., M.G., W.St. and B.B.; visualization, M.S., D.S., S.S., and M.G.; supervision, M.S., W.St. and B.B.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Münster, Germany (reference number 2021-714-f-S, February 15th, 2022).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Study data can be obtained by contacting the corresponding author schwakem@uni-muenster.de
Conflicts of Interest
Michael Schwake reports financial support from Silony Medical (Leinfelden-Echterdingen, Germany) and Spineart (Frankfurt, Germany) and grants from Stryker (Duisburg, Germany) and Johnson and Johnson (Norderstedt, Germany). Stephanie Schipmann reports consultant activities for NxDevelopment (Kentucky, USA). Marco Gallus receives funding from the German Research Foundation (Bonn, Germany). The other authors have no personal, financial, or institu-308 tional interest in any of the drugs, materials, or devices described in this article.
References
- Capo, G.; Moiraghi, A.; Baro, V.; Tahhan, N.; Delaidelli, A.; Saladino, A.; Paun, L.; DiMeco, F.; Denaro, L.; Meling, T.R.; et al. Surgical Treatment of Spinal Meningiomas in the Elderly (≥75 Years): Which Factors Affect the Neurological Outcome? An International Multicentric Study of 72 Cases. Cancers 2022, 14, 4790. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Adeli, A.; Sporns, P.; Ewelt, C.; Schmitz, T.; Sicking, J.; Hess, K.; Cäcilia Spille, D.; Paulus, W.; Stummer, W.; et al. Spinal Meningiomas - Risks and Potential of an Increasing Age at the Time of Surgery. Journal of Clinical Neuroscience 2018, 57, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Setzer, M.; Vatter, H.; Marquardt, G.; Seifert, V.; Vrionis, F.D. Management of Spinal Meningiomas: Surgical Results and a Review of the Literature. Neurosurg Focus 2007, 23. [Google Scholar] [CrossRef]
- Pettersson-Segerlind, J.; Fletcher-Sandersjöö, A.; Tatter, C.; Burström, G.; Persson, O.; Förander, P.; Mathiesen, T.; Bartek, J.; Edström, E.; Elmi-Terander, A. Long-Term Follow-Up and Predictors of Functional Outcome after Surgery for Spinal Meningiomas: A Population-Based Cohort Study. Cancers 2021, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Schwake, M.; Suero Molina, E.; Stummer, W. Markers for Identifying and Targeting Glioblastoma Cells during Surgery. J Neurol Surg A Cent Eur Neurosurg 2019, 80. [Google Scholar] [CrossRef] [PubMed]
- Spille, D.C.; Lohmann, S.; Schwake, M.; Spille, J.H.; Alsofy, S.Z.; Stummer, W.; Brokinkel, B.; Schipmann, S. Can Currently Suggested Quality Indicators Be Transferred to Meningioma Surgery?-A Single-Centre Pilot Study. J Neurol Surg A Cent Eur Neurosurg 2022. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Sletvold, T.P.; Wollertsen, Y.; Schwake, M.; Raknes, I.C.; Miletić, H.; Mahesparan, R. Quality Indicators and Early Adverse in Surgery for Atypical Meningiomas: A 16-Year Single Centre Study and Systematic Review of the Literature. Brain & Spine 2023, 3, 101739. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, F.; Setzer, M.; Marquardt, G.; Keil, F.; Dubinski, D.; Bruder, M.; Seifert, V.; Behmanesh, B. Functional Outcome and Morbidity after Microsurgical Resection of Spinal Meningiomas. Neurosurg Focus 2021, 50, 1–7. [Google Scholar] [CrossRef]
- Viereck, M.J.; Ghobrial, G.M.; Beygi, S.; Harrop, J.S. Improved Patient Quality of Life Following Intradural Extramedullary Spinal Tumor Resection. J Neurosurg Spine 2016, 25, 640–645. [Google Scholar] [CrossRef]
- Newman, W.C.; Berry-Candelario, J.; Villavieja, J.; Reiner, A.S.; Bilsky, M.H.; Laufer, I.; Barzilai, O. Improvement in Quality of Life Following Surgical Resection of Benign Intradural Extramedullary Tumors: A Prospective Evaluation of Patient-Reported Outcomes. Neurosurgery 2021, 88, 989–995. [Google Scholar] [CrossRef]
- Said, W.; Maragno, E.; Leibrandt, L.; Spille, D.; Schipmann, S.; Stummer, W.; Gallus, M.; Schwake, M. A Retrospective Cohort Study Evaluating the Comparative Effectiveness of Unilateral Hemilaminectomy and Bilateral Laminectomy in the Resection of Spinal Meningiomas. Oper Neurosurg (Hagerstown) 2024. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.M.; Eggert, H.R.; Laborde, G.; Seeger, W. Microsurgical Unilateral Approaches for Spinal Tumour Surgery: Eight Years’ Experience in 256 Primary Operated Patients. Acta Neurochir (Wien) 1989, 100, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Schöller, K.; Alimi, M.; Cong, G.T.; Christos, P.; Härtl, R. Lumbar Spinal Stenosis Associated With Degenerative Lumbar Spondylolisthesis: A Systematic Review and Meta-Analysis of Secondary Fusion Rates Following Open vs Minimally Invasive Decompression. Neurosurgery 2017, 80, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.J.; Hadar, E.J. Long-Term Follow-up Review of Patients Who Underwent Laminectomy for Lumbar Stenosis: A Prospective Study. J Neurosurg 1998, 89, 1–7. [Google Scholar] [CrossRef]
- Simpson, D. The Recurrence of Intracranial Meningiomas after Surgical Treatment. J Neurol Neurosurg Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.T.; Pynsent, P.B. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000, 25, 2940–2953. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H. The Neck Disability Index: State-of-the-Art, 1991-2008. J Manipulative Physiol Ther 2008, 31, 491–502. [Google Scholar] [CrossRef] [PubMed]
- McCormick, P.C.; Torres, R.; Post, K.D.; Stein, B.M. Intramedullary Ependymoma of the Spinal Cord. J Neurosurg 1990, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Maurischat, C.; Ehlebracht-König, I.; Kühn, A.; Bullinger, M. [Structural Validity of the Short Form 36 (SF-36) in Patients with Rheumatic Diseases]. Z Rheumatol 2005, 64, 255–264. [Google Scholar] [CrossRef]
- Jankovic, D.; Kalasauskas, D.; Othman, A.; Brockmann, M.A.; Sommer, C.J.; Ringel, F.; Keric, N. Predictors of Neurological Worsening after Resection of Spinal Meningiomas. Cancers 2023, 15, 5408. [Google Scholar] [CrossRef]
- Schwake, M.; Ricchizzi, S.; Krahwinkel, S.; Maragno, E.; Schipmann, S.; Stummer, W.; Gallus, M.; Holling, M. Resection of Intramedullary Hemangioblastoma: Timing of Surgery and Its Impact on Neurological Outcome and Quality of Life. Medicina 2023, 59, 1611. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj, V.G.; Pettersson-Segerlind, J.; Fletcher-Sandersjöö, A.; Edström, E.; Elmi-Terander, A. Current Knowledge on Spinal Meningiomas-Surgical Treatment, Complications, and Outcomes: A Systematic Review and Meta-Analysis (Part 2). Cancers 2022, 14, 6221. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Banat, M.; Schuss, P.; Güresir, E.; Vatter, H.; Scorzin, J. Age at Diagnosis and Baseline Myelomalacia Sign Predict Functional Outcome After Spinal Meningioma Surgery. Front Surg 2021, 8. [Google Scholar] [CrossRef]
- Raco, A.; Pesce, A.; Toccaceli, G.; Domenicucci, M.; Miscusi, M.; Delfini, R. Factors Leading to a Poor Functional Outcome in Spinal Meningioma Surgery: Remarks on 173 Cases. Neurosurgery 2017, 80, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Jesse, C.M.; Alvarez Abut, P.; Wermelinger, J.; Raabe, A.; Schär, R.T.; Seidel, K. Functional Outcome in Spinal Meningioma Surgery and Use of Intraoperative Neurophysiological Monitoring. Cancers 2022, 14, 3989. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Menezes, A.H.; Shimizu, K.; Woodroffe, R.W.; Helland, L.C.; Hitchon, P.W.; Howard, M.A. Differences and Characteristics of Symptoms by Tumor Location, Size, and Degree of Spinal Cord Compression: A Retrospective Study on 53 Surgically Treated, Symptomatic Spinal Meningiomas. J Neurosurg Spine 2020, 32, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Boström, A.; Bürgel, U.; Reinacher, P.; Krings, T.; Rohde, V.; Gilsbach, J.M.; Hans, F.J. A Less Invasive Surgical Concept for the Resection of Spinal Meningiomas. Acta Neurochir (Wien) 2008, 150, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Harati, A.; Satopää, J.; Mahler, L.; Billon-Grand, R.; Elsharkawy, A.; Niemelä, M.; Hernesniemi, J. Early Microsurgical Treatment for Spinal Hemangioblastomas Improves Outcome in Patients with von Hippel-Lindau Disease. Surg Neurol Int 2012, 3. [Google Scholar] [CrossRef]
- Butenschoen, V.M.; Gloßner, T.; Hostettler, I.C.; Meyer, B.; Wostrack, M. Quality of Life and Return to Work and Sports after Spinal Ependymoma Resection. Sci Rep 2022, 12. [Google Scholar] [CrossRef]
- Goodwin, C.R.; Price, M.; Goodwin, A.N.; Dalton, T.; Versteeg, A.L.; Sahgal, A.; Rhines, L.D.; Schuster, J.M.; Weber, M.H.; Lazary, A.; et al. Gender and Sex Differences in Health-Related Quality of Life, Clinical Outcomes and Survival after Treatment of Metastatic Spine Disease. Spine (Phila Pa 1976) 2023. [Google Scholar] [CrossRef]
- Bond, M.R.; Versteeg, A.L.; Sahgal, A.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Fehlings, M.G.; Lazary, A.; Clarke, M.J.; et al. Surgical or Radiation Therapy for the Treatment of Cervical Spine Metastases: Results From the Epidemiology, Process, and Outcomes of Spine Oncology (EPOSO) Cohort. Global Spine J 2020, 10, 21–29. [Google Scholar] [CrossRef]
- Guarino, A.; Polini, C.; Forte, G.; Favieri, F.; Boncompagni, I.; Casagrande, M. The Effectiveness of Psychological Treatments in Women with Breast Cancer: A Systematic Review and Meta-Analysis. J Clin Med 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Rick, O.; Dauelsberg, T.; Kalusche-Bontemps, E.M. Oncological Rehabilitation. Oncol Res Treat 2017, 40, 772–777. [Google Scholar] [CrossRef]
- Boersma, I.; Miyasaki, J.; Kutner, J.; Kluger, B. Palliative Care and Neurology: Time for a Paradigm Shift. Neurology 2014, 83, 561–567. [Google Scholar] [CrossRef]
- Mummaneni, P.V.; Park, P.; Shaffrey, C.I.; Wang, M.Y.; Uribe, J.S.; Fessler, R.G.; Chou, D.; Kanter, A.S.; Okonkwo, D.O.; Mundis, G.M.; et al. The MISDEF2 Algorithm: An Updated Algorithm for Patient Selection in Minimally Invasive Deformity Surgery. J Neurosurg Spine 2019, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Debono, B.; Wainwright, T.W.; Wang, M.Y.; Sigmundsson, F.G.; Yang, M.M.H.; Smid-Nanninga, H.; Bonnal, A.; Le Huec, J.C.; Fawcett, W.J.; Ljungqvist, O.; et al. Consensus Statement for Perioperative Care in Lumbar Spinal Fusion: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. Spine J 2021, 21, 729–752. [Google Scholar] [CrossRef] [PubMed]
- Dobran, M.; Paracino, R.; Nasi, D.; Aiudi, D.; Capece, M.; Carrassi, E.; Lattanzi, S.; Rienzo, A.D.I.; Iacoangeli, M. Laminectomy versus Unilateral Hemilaminectomy for the Removal of Intraspinal Schwannoma: Experience of a Single Institution and Review of Literature. J Neurol Surg A Cent Eur Neurosurg 2021, 82, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.L.; Aryan, H.E.; Chi, J.; Parsa, A.T.; Ames, C.P. Modified Paramedian Transpedicular Approach and Spinal Reconstruction for Intradural Tumors of the Cervical and Cervicothoracic Spine: Clinical Experience. Spine (Phila Pa 1976) 2007, 32. [Google Scholar] [CrossRef]
- Onken, J.; Obermüller, K.; Staub-Bartelt, F.; Meyer, B.; Vajkoczy, P.; Wostrack, M. Surgical Management of Spinal Meningiomas: Focus on Unilateral Posterior Approach and Anterior Localization. J Neurosurg Spine 2018, 30, 308–313. [Google Scholar] [CrossRef]
- Thakur, J.; Ulrich, C.T.; Schär, R.T.; Seidel, K.; Raabe, A.; Jesse, C.M. The Surgical Challenge of Ossified Ventrolateral Spinal Meningiomas: Tricks and Pearls for Managing Large Ossified Meningiomas of the Thoracic Spine. J Neurosurg Spine 2021, 35, 516–526. [Google Scholar] [CrossRef]
- Liao, D.; Li, D.; Wang, R.; Xu, J.; Chen, H. Hemilaminectomy for the Removal of the Spinal Tumors: An Analysis of 901 Patients. Front Neurol 2022, 13, 1094073. [Google Scholar] [CrossRef] [PubMed]
- Krahwinkel, S.; Schipmann, S.; Spille, D.; Maragno, E.; Al Barim, B.; Warneke, N.; Stummer, W.; Gallus, M.; Schwake, M. The Role of Prolonged Bed Rest in Postoperative Cerebrospinal Fluid Leakage After Surgery of Intradural Pathology—A Retrospective Cohort Study. 2023. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Krahwinkel, S.; Gallus, M.; Schipmann, S.; Maragno, E.; Neuschmelting, V.; Perrech, M.; Müther, M.; Lenschow, M. Does Early Mobilization Following Resection of Spinal Intra-Dural Pathology Increase the Risk of Cerebrospinal Fluid Leaks?-A Dual-Center Comparative Effectiveness Research. Medicina (Kaunas) 2024, 60, 171. [Google Scholar] [CrossRef] [PubMed]
- Müther, M.; Lüthge, S.; Gerwing, M.; Stummer, W.; Schwake, M. Management of Spinal Dumbbell Tumors via a Minimally Invasive Posterolateral Approach and Carbon Fiber-Reinforced Polyether Ether Ketone Instrumentation: Technical Note and Surgical Case Series. World Neurosurg 2021, 151, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Maragno, E.; Gallus, M.; Schipmann, S.; Spille, D.; Al Barim, B.; Stummer, W.; Müther, M. Minimally Invasive Facetectomy and Fusion for Resection of Extensive Dumbbell Tumors in the Lumbar Spine. Medicina (Kaunas) 2022, 58, 1613. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).