Introduction
In the quest for generating environmentally benign and biocompatible materials for medical applications, carbon dots (CDs) are the greatest contender. The realization of CDs’ existence a few years ago has drawn a lot of interest to them because of their special, desirable qualities, which include low toxicity, photostability, water solubility, wavelength-tuneable emission, and functional ability [
1]. There are numerous types of CDs, and each has unique qualities appropriate for a range of uses. Graphitic carbon dots, graphitic carbon nitride dots, carbon black dots, amorphous carbon quantum dots (CQDs), polymeric dots, polymer/carbon hybrid dots, and co-doped (heteroatom) CDs are some examples [
2].
During the separation and purification of single-walled carbon nanotubes (SWCNTs) in 2004, Xu et al. unintentionally discovered CDs, which were initially known as “carbon nanoparticles. This discovery spurred further research in both the scientific and technological domains [
3]. The term “carbon quantum dots” was coined in 2006 by Sun et al., who successfully revealed a synthetic pathway to produce CDs with improved fluorescence emission [
4].
Renewable resources found in nature and it’s low-cost, has drawn the attention of researchers, which can be utilized to create green CDs due to their non-hazardous and environmentally favorable qualities. Carbon dots made from medicinal plants have therapeutic and environmental benefits. One feature that most other designed nanomaterials lack is the ability to be quickly eliminated through urine after treatment, which is something that carbon dots, one of the smallest biocompatible nanoparticles known to exist, can do. Because of their small size, they can be used independently or in combination with other nanotherapeutics to provide additional functional capacities that are advantageous for therapeutic applications [
5].
The functional capacities include;
Excellent heat-absorbing capabilities that are important for photothermal therapies.
They can transport drugs to different parts of the body that the drug alone could not reach.
It can be doped with juxtaposing agents for utilization in computerized tomography (CT) scanning or magnetic resonance imaging.
They can also act as a contrasting agent in photoacoustic imaging applications.
Utilized as a fluorescent probe that has low photobleaching tendencies to sense proteins, biomolecules, anions, and cations through particular interactions (electrostatic, π–π, electron transfer, covalent bonding, etc.). Their weak π–π conjugation interactions have a high binding affinity for hydrophobic molecules with ring structures, which may help to deliver medications to target tissues.
It is possible to synthesize hydrophilic or hydrophobic materials.
For increased functionality, they can be included in various nano/micro composites.
They can be tuned to act as an antioxidant or pro-oxidant depending on the final intended use. [
6,
7]
Green Precusors for Herbal Medicine Carbon Dots (HM-CDS)
The production of HM-CDs by the use of the green chemistry principle provides numerous benefits, including reduced chemical exposure, accessible cost, renewable source supply and minimized waste [
8]. When it comes to natural resources, plant components are more environmentally friendly than other materials because they are affordable, easily obtainable, safe, abundant, and regenerative, which promotes sustainability [
9]. It could be synthesized from roots, stems, leaves, fruits, flowers, and seeds. Furthermore, several low-value materials can be converted into useful materials with a high degree of biocompatibility through the synthesis of CDs from plant components.
Plant components rich in heteroatoms like nitrogen and sulfur are ideal starting materials for CDs, unlike other carbon sources that require additional heteroatom sources [
10]. In addition to addressing the pressing demand for large-scale CD manufacture, using plant parts as green resources encourages the creation of sustainable applications. Unlike chemical substances as carbon sources, plant parts contain a variety of carbohydrates, proteins, amino acids, and other biomolecules that supply enough elements for the surface functionality of CDs, negating the need for a separate reactant for doping, surface passivation, or post modification [
11].
In the meanwhile, green synthesis techniques are far more appropriate than physical and chemical procedures [
12]. Numerous synthesis techniques, including chemical oxidation, hydrothermal treatment, laser ablation, microwave treatment, and pyrolysis, have been used. A variety of plant materials have been used to synthesize CDs. As a result of employing such readily available natural precursors, scientists are still motivated to create new designs.
Fruits
Lemon peels, Prunus avium extract, cornstalk, corn bract, dried lemon peels, pulp-free lemon juice, citrus lemon peels, citrus sinensis peels, etc. are precursors that have been used in the development of CDs, as shown in (
Table 1). Among these precursors, acidic fruits, such as lemon peels, lemon juice, and citrus sinensis, were frequently chosen. This is because the juice extract is rich in sucrose, glucose, fructose, citric acid, and ascorbic acid, while the peels are mainly composed of proteins, fibers, and less of oils and antioxidants. Consequently, CDs from juice extract exhibit higher fluorescence properties than peels, due to the high acid and sugar contents that provide a considerable amount of carbon and hydrogen elements.
Table 1.
Synthesis of CDs from fruits.
Table 1.
Synthesis of CDs from fruits.
| GREEN PRECURSOR |
METHOD OF PREPARATION |
REFERENCE |
| Orange juice |
Hydrothermal |
[13] |
| Watermelon peel |
Carbonization |
[14] |
| Sugarcane |
Hydrothermal, Carbonization |
[15] |
| Lemon juice |
Thermal decomposition |
[16] |
| Jackfruit juice |
Hydrothermal |
[17] |
| Grapefruit |
Hydrothermal |
[18] |
| Banana peel |
Hydrothermal |
[19] |
| Kiwi |
Hydrothermal, Carbonization |
|
Morus nigra (black mulberry) |
Hydrothermal |
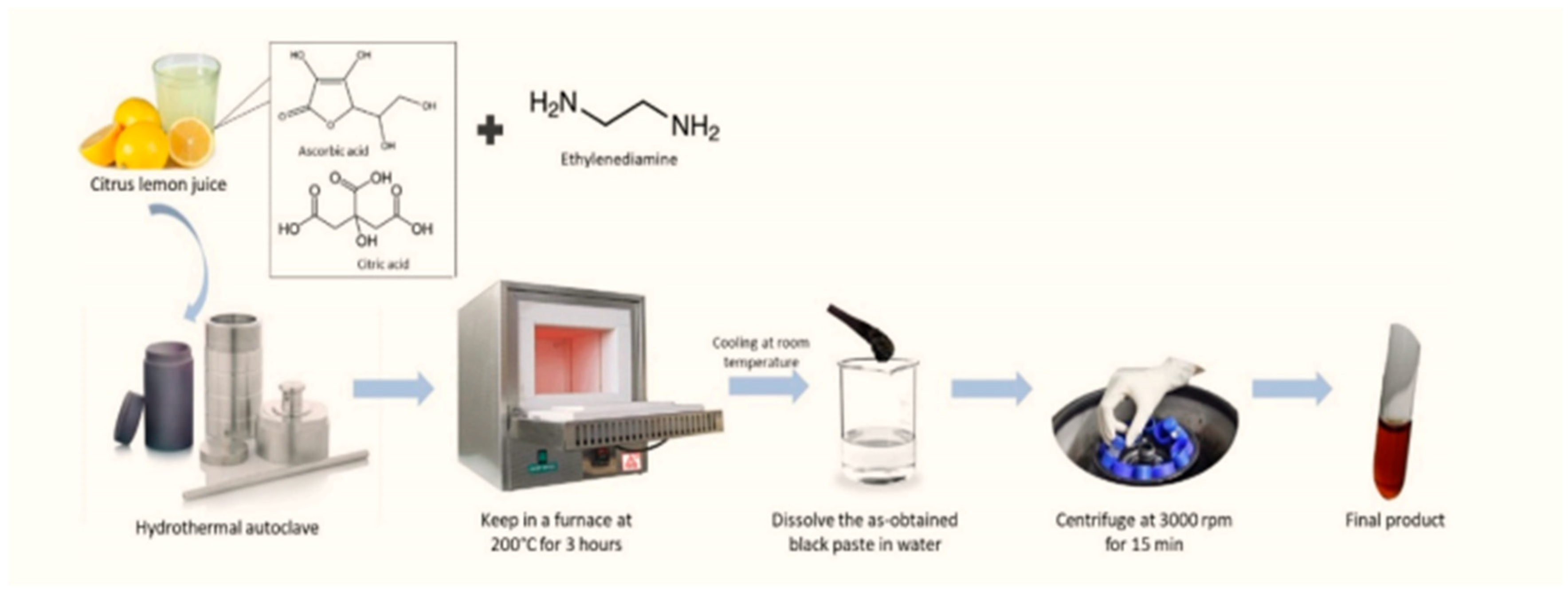
Figure 1.
Synthesis of CD from citrus lemon juice and ethylenediamine.
Figure 1.
Synthesis of CD from citrus lemon juice and ethylenediamine.
2. Vegetables
Vegetables can be potentially utilized to develop high-quality CDs, which has drawn tremendous attention. Various vegetables, such as tomato, carrot, radish, turmeric, cinnamon, red chili, black pepper, cauliflower and cabbage, have been reported, as shown in (
Table 2). Both green and non-green vegetables have many properties and their advantages. For instance, the family of green vegetables, such as celery leaves, lemon grass, cabbage, crown daisy leaf, and scallion, often contain many organic compounds, such as organic acids, amides, amino acids, proteins, saccharides, carbohydrates, chlorophyll, etc., which can bring good physical and chemical properties to CDs.
Non-green vegetables, such as tomato, red chili, turmeric, black pepper, cinnamon, and red beet, are plants rich in various bioactive compounds of lycopene, capsaicin, curcumin, piperine and cinnamaldehyde, respectively, which enable their application in the biomedical field. It was reported that bioactive compounds will partially remain inside or at the surface of the CDs after the hydrothermal process, leading to different photoluminescent and biomedical properties. Vasimalai et al. (2018) have demonstrated the uses of cinnamon, red chili, turmeric, and black pepper as CD precursors for biomedical applications [
20]. They found that black pepper CDs have the highest quantum yield of 43.6% due to the various functional groups present in the sample, namely O–H, C–H, C–O–N, C=O, C–O, and C–N vibrational stretching peaks.
Table 2.
Synthesis of CDs from vegetables.
Table 2.
Synthesis of CDs from vegetables.
| GREEN PRECURSOR |
METHOD OF PREPARATION |
SOLVENT |
FLUORESCENCE |
REFERENCE |
| Tomato juice |
Hydrothermal |
Water |
λem- 415 nm
λex- 340 nm |
[21] |
| Carrot |
Hydrothermal |
Water |
λem- 442–565 nm
λex- 360–520 nm |
[22] |
| Cauliflower |
Hydrothermal |
Ethylenediamine |
λem- 380 nm
λex- 325 nm |
[23] |
| Cabbage |
Solvothermal |
Anhydrous ethanol |
λem- 500 nm
and 678 nm
λex- 410 nm |
[24] |
| Scallion leaves |
Hydrothermal |
Water |
λem- 418 nm
λex- 320 nm |
[25] |
| Red beet |
Hydrothermal |
Water |
λem- 438 nm
λex- 350 nm |
[26] |
3. Flowers
Promising as carbon precursors, flowers including Tagetes erecta, water hyacinth, Osmanthus fragrans, rose blooms, and Selenicereus grandifloras have been utilized to bind metal ions and insecticides. The optical qualities of the synthesized CDs were assessed by Shekarbeygi et al. (2020) about the impact of rose pigments (blue, red, and yellow) and their extraction techniques (alcohol and aqueous) [29]. The results showed that rose colors and extraction procedures did not affect the quantum yields of CDs, since yields were nearly identical. Nevertheless, the thermal stability and emission wavelengths of the CDs were impacted by these rose pigments and extraction techniques.
Table 3.
Synthesis of CDs from flowers.
Table 3.
Synthesis of CDs from flowers.
| GREEN PRECURSOR |
TECHNIQUE |
SOLVENT |
FLUORESCENCE |
REFERENCE |
| Water hyacinth |
Carbonization |
Phosphoric acid |
λem- 370 nm, λex- 300 nm |
[27] |
| Osmanthus fragrans |
Hydrothermal |
Water |
λem- 410 nm, λex- 340 nm |
[28] |
| Rose flowers: Blue |
Hydrothermal |
Water |
λex- 335 nm |
[29] |
| Tagetes erecta |
Solvothermal carbonization |
Water |
λem- 495 nm, λex- 420 nm |
[30] |
4. Leaves, Stem and Seeds
Leaves are considered superior carbon precursors due to their larger quantum yields compared to seeds and stems. Most notably, CDs made from Calotropis procera leaves had an outstanding quantum yield (71.95%) while containing no hazardous agents or surface passivation compounds [
30]. The functional groups (OH, N-H, C=O, and C=N bonds) generated from carbon precursors resulted in a high quantum yield. In the UV-Vis spectra of CDs (Figure 2), the synthesized CDs also exhibit a prominent peak around 320 nm, suggesting that this peak originated from the n → π* transition of C=O bonds over the CD surface.
Table 4.
Synthesis of CDs from leaves, stem and seeds.
Table 4.
Synthesis of CDs from leaves, stem and seeds.
| GREEN PRECURSOR |
TECHNIQUE |
SOLVENT |
FLUORESCENCE |
REFERENCE |
|
Ocimum sanctum leaves |
Hydrothermal |
Distilled water |
λem- 410 nm
λex- 340 nm |
[31] |
| Bamboo leaves |
Pyrolysis |
Sodium hydroxide and sodium hypochlorite |
λem- 425–475 nm |
[32] |
| Betel leaves |
Ammonia |
Hydrothermal |
λem- 402 nm
λex- 320 nm |
[33] |
5. Fungi/Bacteria Species
The conversion of fungi/bacteria species into a value-added product such as carbonaceous nanomaterials has contributed to green and sustainable improvement. Algal blooms, yogurt mushrooms, agarose waste have been previously chosen as a carbon resource (Table 5). The quantum efficiency can be attributed to the presence of different functional groups (–C=O, –OH, and N–H) on the surface of CDs.
| GREEN PRECURSOR |
TECHNIQUE |
SOLVENT |
FLUORESCENCE |
REFERENCE |
| Algal blooms |
Microwave |
Phosphoric acid |
λem- 438 nm
λex- 360 nm |
[34] |
| Mushroom |
Ultrapure water |
Hydrothermal |
λem- 440 nm
λex- 360 nm |
[35] |
| Yogurt |
Hydrochloric acid |
Pyrolysis |
λem- 420 nm
λex- 320 nm |
[36] |
Methods of Preparation
Over the past decade, CD development methodologies have been categorized into “top-down” and “bottom-up” approaches based on application. The “top-down” approach describes how to prepare CDs by breaking down large carbon precursor molecules into nanoscale particles, while the “bottom-up” approach describes how to create CDs from appropriate molecular precursors under specific circumstances [
37]. Modification functionalization, nanohybrids, and doping during the manufacture or post-treatment of carbon nanodots are a few of the chemical, electrochemical, and physical techniques that can be used to accomplish these goals [
38]. Research on the bioactivity of HM-CDs will require careful consideration of the hydrothermal and calcination methods, which are the most widely used for HM-CD preparation.
Hydrothermal Approach
Hydrothermal synthesis is environmentally friendly because it doesn’t require organic solvents [
38]. To maximize safety and decrease toxicity, CDs’ surface does not require extra passivation. To prepare, dried herbs are chopped into small pieces or ground into powder and dispersed in purified water. After ultrasonic treatment, the mixture is placed in a PTFE-lined stainless steel autoclave and heated to a predetermined temperature. The suspension had to be dialyzed using a dialysis bag for several days and then again through a 0.22 μm cellulose membrane to purify the CDs. The characteristics of HM-CDs are affected by the reaction temperature. Usually, the temperature of a hydrothermal reaction is between 100 and 200°C [
39].
Pyrolysis Method/Carbonization
Apart from hydrothermal synthesis, another common technique is high-temperature pyrolysis. Natural organic materials undergo high-temperature procedures such as heating, dehydration, degradation, and carbonization to progressively turn them into CDs while operating under vacuum or inert gas [
40]. Procedure is simple, free of solvents, cost-effective, and suitable for large production. Initially, the herbs are put in a crucible and burned at a certain temperature in a muffle furnace. Following a crushing and boiling process in ultrapure water, the burnt medication is removed from the top liquid layer.
To purify the CDs, the solution was filtered through a 0.22 μm microporous membrane and dialyzed using dialysis bags over many days. The success rate of the high-temperature calcination preparation procedure is mostly influenced by carbonization. Carbonization by stir-frying and carbonization by calcining (sometimes called wok-covering calcining) are the two primary traditional ways of carbonation. Stir-frying is a method of carbonizing medications that involves heating the substance in a preheated container at high or moderate heat until it burns black on the exterior and turns reddish-brown within. This method is primarily employed for root medications, such as cortical peony, ginger, and rhubarb. It works well with light or loose drugs (such as Nodus Nelumbinis Rhizomatis, Radix Rehmanniae, and Juncus efsus) that are easily carbonized [
41].
Microwave-Assisted Method
Using energy transmission to induce the breakage of chemical bonds is the concept behind microwave carbonization. Because of its easier operation, response time is significantly reduced and preparation efficacy is boosted [
42]. High processing precision, low contamination, and a variety of uses for light-textured charcoal herbs are the benefits of this approach. Furthermore, microwave-assisted hydrothermal synthesis has been reported as an alternative to conventional hydrothermal synthesis [
43]. Compared to the hydrothermal technique, M-CDs needed a much shorter time (5–15 min), and the particle size was comparatively lower.
Highly heated sand has great thermal conductivity, preventing inhomogeneous heating of drugs, achieving desired energy quickly, and being low-cost and simple to manufacture. This method can be used to make most charcoal-based medicines, such as Nodus Nelumbinis Rhizomatis, Sanguisorba officinalis, and Fructus Crataegi; however, it is not suitable for light, friable, or non-separable medicines [
44].
Solvothermal Method
The solvothermal method has advantages over hydrothermal fabrication in that it requires only less complex equipment and is less expensive for the creation of CDs [
45]. In contrast to the hydrothermal approach, one or more solvents sealed with Teflon and fitted with a steel autoclave were used in place of the water solution [
46]. High pressure and temperature were used in the reaction between the solvent and the raw carbon source mixes. The produced CDs were then used in bioimaging applications because of their excellent photostability and low toxicity.HM-CDs can be prepared using a variety of techniques, many of which are similar to standard carbon dot synthesis techniques. These techniques are similar in that they all focus on controlling the carbon source and reaction conditions to produce carbon dots. But what makes this unique is the use of natural materials as the carbon source used in the HM-CD manufacturing process. Herbal materials contain organic compounds such as polysaccharides, proteins, and polyphenols, which can break down into carbon dots at high temperatures. The properties and therapeutic benefits of herbal materials are taken into account during the HM-CD production process. Maintaining the active ingredients in herbal materials and turning them into carbon dots entails choosing the right extraction techniques, solvents, and reaction conditions.
Characterization of Carbon Dots
To improve knowledge of the mechanism associated with the unique physical features of CDs, characterization is required. Various spectroscopic techniques, such as ultraviolet-visible (UV-vis) spectroscopy and Fourier-transform infrared spectroscopy (FTIR), X-ray diffusion (XRD), zeta potential, quantum yield analysis, transmission electron microscopy (TEM), and high-resolution electron microscopy (HRTEM) are used.
UV-Vis Spectroscopy Technique
When assessing the optical characteristics of CDs, UV-vis spectroscopy is typically advised since different techniques of synthesizing CDs typically provide absorption peaks that differ in strength from one another [57]. The basis of UV-visible spectroscopy is the idea that different spectra are produced when chemical substances absorb ultraviolet or visible light. Spectroscopy relies on the way light and matter interact. A spectrum is created when a material absorbs light through excitation and de-excitation processes. To separate CDs with various sizes and shapes, C-dots were fractionated using high-performance liquid chromatography and gel electrophoresis. A characteristic π-π* transition peak, or absorption band peak, is often found in most CDs and is centered around the UV region of 250–300 nm.
FTIR Measurement
According to Singh et al. [57], CDs typically consist of carbon, hydrogen, nitrogen, and oxygen. Depending on the specific production method, the surface of CDs often consists of a variety of functional groups, such as hydroxyl, carboxyl, carbonyl, ether, or epoxy groups. As a result, FTIR may be used to identify the CDs’ surface functional groups [58]. The basic idea behind FTIR analysis is that light is absorbed at different frequencies by the bonds that unite various components. An infrared spectrometer is then used to measure the light using FTIR analysis, yielding an infrared spectrum as an output.
Electron Microscopy Approaches
Electron microscopy techniques are widely utilized to characterize nanoparticles in science, material science, medicines, and other areas of study and development. Several researchers have identified scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as primary approaches for CD visualization because they can provide significant information on the morphology, particle size, crystalline organization, and size distribution of C-dots. Standard SEM, images are created by scanning the surface of the CD sample with a focussed electron beam that can interact with the atoms of the CD sample and excite signals that include information about the surface topography and composition of CDs. However, TEM is more precise when CD measurements exceed SEM’s resolution, which is between 1 and 20 nm. This is because, in comparison to SEM, TEM has a higher resolving power (~0.2 nm), making it more suited to identify small-size particles. TEM creates images by transmitting high-energy electron beams through the CD sample [59].
XRD
Another essential structural tool for characterizing CDs is XRD, which can provide important information about particle size and purity while also investigating the crystalline structure of CDs. The crystalline carbon cores of CDs can also be evaluated by XRD, together with the unit cell dimensions that correspond to the crystal spacing [57]. Constructive interference is the result of the interaction between the monochromatic X-rays and the carbon dot sample. When X-rays are used to excite the electrons, a diffraction pattern is produced that preserves the regular spatial arrangement. The typical structure of nanomaterials is subsequently revealed by detecting, processing, and counting these diffracted X-rays.
Zeta Potential
The zeta potential is another important technique for determining the effective electric charge on the surface of nanoparticles. Analyzing the stability of the colloidal system and the surface impacts of nanoparticles is crucial because it influences both the initial absorption of nanoparticles onto the cell membrane and their toxicity [60]. Zeta potential, as depicted in Figure 6, is the electrical potential in the interfacial double layer at the sliding plane point. The potential difference between the dispersion medium and the fluid’s stationary layer that is affixed to the particle layer is known as the zeta potential (Figure 6). The potential stability of the colloidal system is indicated by the size of the zeta potential.
According to Sivasankaran et al. [60], a larger zeta potential value suggests system stability, whereas positive and negative zeta potential signs reflect nanoparticle surface charges, with nanoparticles with low zeta potential values aggregating. For instance, materials with zeta potentials between -10 and +10 mV are viewed as neutral, nanoparticles with zeta potentials greater than +30 mV are classified as strongly cationic, and materials with zeta potentials less than -30 mV are classified as strongly anionic [61].
Applications of CDs
CDs have several features, including controllable PL emission, superior biocompatibility, low toxicities, and excellent photoinduced electron transfer, making them ideal for various applications such as sensors, bioimaging, drug administration, photocatalysis, and so on.
CDS in Sensing
Detecting metal ions, anions, and molecules has been one of the key uses for CDs, with fluorescence sensors being one of these applications [
46].
CDS in Bioimaging
Because of their minimal toxicity, CDs have been deemed biocompatible fluorescent dyes for in vivo imaging rather than pharmacological compounds. CDs are a better option since they can be altered with different functional groups to provide the right PL emission. Numerous research have reported on the bioimaging potentials of CDs in recent years [
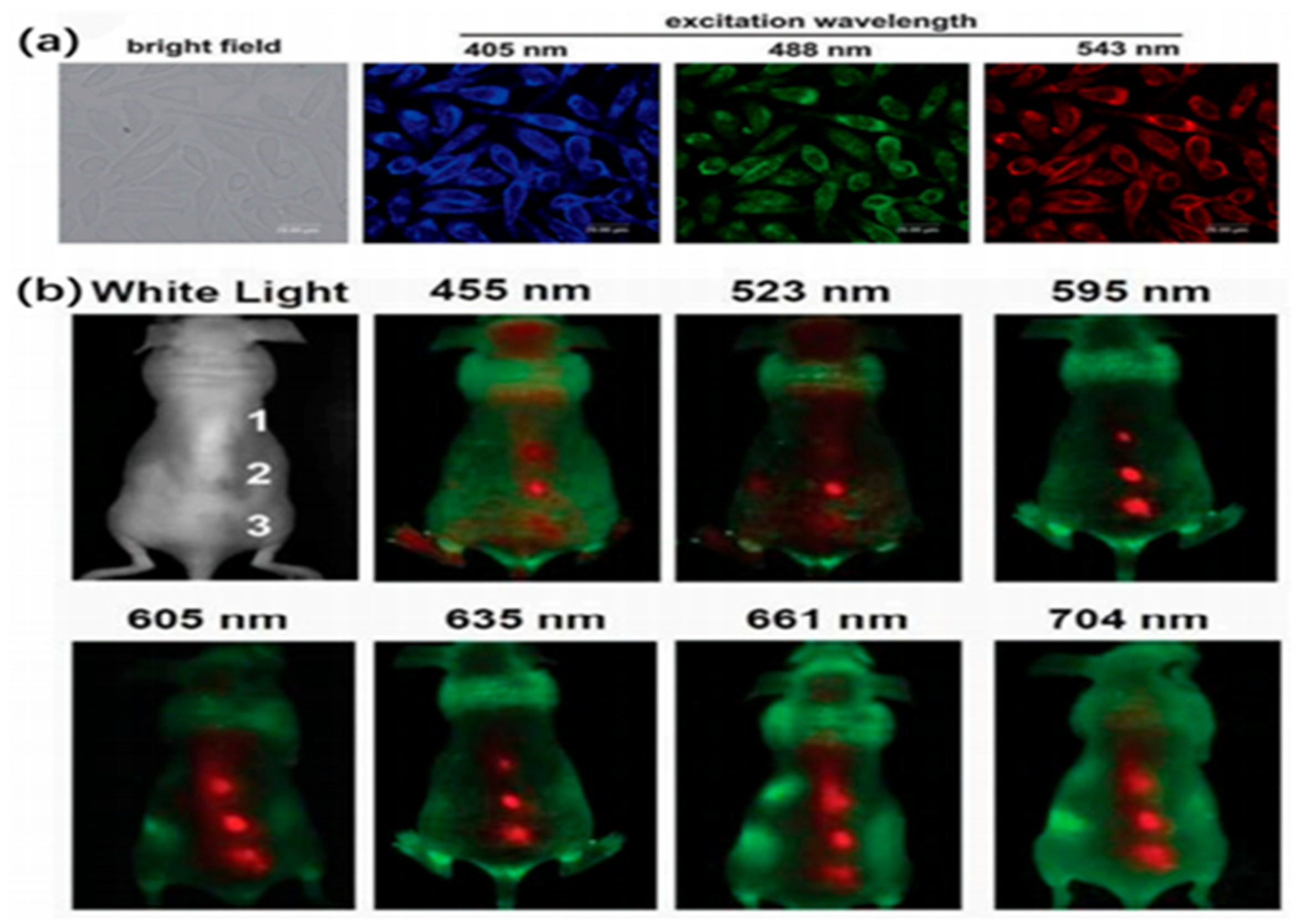
47]. Strongly absorptive CDs can make up for decreased fluorescence yield in bioimaging. Typically, CDs’ PL emission was adjusted to a wider wavelength range to increase signal-to-noise ratio (SNR). That is, because of the tissue backdrop in the NIR “water window,” PL emission of CDs in the NIR region was particularly important and crucial for in vivo optical imaging. Intracellular imaging has also been performed using Hella cells, HepG2 cells, MCF-7 cells, pancreatic progenitor cells, and human lung cancer cells. According to Zhai et al., CD-incubated L929 cells exhibit strong and consistent blue, green, and red PL emission at excitation wavelengths of 405 nm, 488 nm, and 543 nm (Figure 9) [
48].
Figure 2.
a. in vivo images of L929 cells which injected with CDs under different excitation wavelength, 62. Copyright 2012, Royal Society of Chemistry. b. in vivo fluorescent images of CDs that injected into mouse. 63. Copyright 2011, John Wiley and Sons.
Figure 2.
a. in vivo images of L929 cells which injected with CDs under different excitation wavelength, 62. Copyright 2012, Royal Society of Chemistry. b. in vivo fluorescent images of CDs that injected into mouse. 63. Copyright 2011, John Wiley and Sons.
3. CDS in Drug Delivery
Because of their strong fluorescence, low toxicity, chemical inertness, and exceptional biocompatibility, CDs were thought to be a multipurpose drug delivery system medium. For therapeutic purposes, the particular medication was transported and removed at a specified location. Karthik et al., for instance, placed medication onto nitrogen-doped CDs via a covalent bond. The drug-loaded CDs aggregated and traveled to the cancer cell’s nucleus and cytoplasm. Irradiation was used to release the medication at the designated locations. As seen in Figure 10 [
49]., Ding and colleagues produced BCDs by utilizing genomic DNA as a carbon source to evaluate medication delivery.
Application of Herbal Medicine-Derived Carbon Dots
1. Hemostasis
The carbonization of herbal remedies has a long history and a plethora of clinical evidence supporting its use in the treatment of hemorrhagic. The majority of herbal medications had CDs, which were shown to have low toxicity, good water solubility, and biocompatibility. This was discovered through research on CDs identified in herbal medicines such as Schizonepetae Spica Carbonisata 50 Cirsii Japonici Herba Carbonisata [51], and Pollen Typhae Carbonisata [52].
2. Bacterial Infection
Fungi, bacteria, parasites, and viruses all have the potential to cause catastrophic illnesses. Using CDs as a potential nanomaterial, the identification and inactivation of multiple bacterial species in photosensitizers (PS) has been accomplished [53]. One of the most prevalent dental lesions is persistent endodontic infections (PEIs), which are linked to Enterococcus faecalis (E. faecalis) biofilms. A study was done to develop CDs derived from fucoidan (FD) for the treatment of PEIs. In vitro experiments have demonstrated that FD-CDs have a beneficial inhibitory effect on Enterococcus faecalis and its biofilms by contributing to the production of both intracellular and extracellular reactive oxygen species and altering the permeability of the bacterium. The removal of E. faecalis biofilms from root canals and dentin tubules by FD-CDs is significant because it has considerable promise for the treatment of PEIs [55].
3. Anti Inflammatory
HM-CDs have also gained extensive research attention in the treatment of inflammatory diseases due to their distinctive advantages, such as great biocompatibility, photostability, and inherent targeting of functional groups. Wang et al. synthesized a novel Mulberry Silkworm Cocoon-CDs (MSC- CDs) based on MSC [55]. To assess the anti-inflammatory bioactivity of MSC-CDs, the authors of this work creatively applied three conventional experimental models of inflammation. The results showed that MSC-CDs possess significant anti-inflammatory activity, which may be related to the inhibition of inflammatory factors IL-6 and TNFα expression, providing a reference for further investigation of the potential pharmacodynamic basis of MSC-CDs.
4. Cancer
More powerful and unique anti-tumor effects are provided by herbal medications, some of which can be used in conjunction with radiation therapy to minimize side effects and increase effectiveness. Similarly, CDs made with herbal medicine have a lot of promise for use in cancer therapies. It is anticipated that the combination of CDs and herbal medication will improve therapeutic efficacy, accelerate tumor accumulation, and lessen adverse effects related to anti-cancer treatment. Li et al. created novel CDs (G-CDs) based on ginger, which they discovered could have a potent inhibitory effect on HepG2 cell development by upregulating the expression of the p53 gene in cancer cells and raising intracellular ROS levels. Li & al. were inspired by curcumin [56]. Significant anti-hepatocellular carcinoma activity was also demonstrated by GCDs in vivo. In solid tumors, this action was able to accumulate at the tumor site through the enhanced permeation retention (EPR) effect.
Conclusions and Future Perspectives
This study summarized current CD research, including green precursor sources, synthesis routes, characterization techniques, and biomedical applications. Fruits, vegetables, flowers, leaves, fungus, and bacteria can all be sources of HM-CD development. The “top-down” and “bottom-up” approaches are the two main categories into which CD preparation can be divided. Pyrolysis, hydrothermal, solvothermal, and microwave irradiation are various processes for the production of CDs.
In the meantime, a variety of technologies were reviewed that were used to characterize CDs, including the UV-vis spectroscopy method, FTIR, TEM, XRD, and zeta potential measurement. The purpose of characterizing CDs is to comprehend the mechanism associated with their properties, which were also covered in this review. There will inevitably be obstacles in the way of improving quantum yield, luminescence, and electrochemical performance, as well as a lack of information regarding the mechanism of CD synthesis, which researchers should take into consideration. To get around these limitations, more research in this area will need to be established.
Nonetheless, the majority of current research that examined the biological uses of CDs in the literature used in vitro cell lines and animal models as their primary research methods. To evaluate CD biological activity, toxicity, and blood circulation features and combine them into multifunctional platforms for biomedical application, more in vitro, in vivo, and pre-clinical research must be carried out. We therefore confidently think that more and more creative and promising uses for these increasingly important carbon nanoparticles, as well as more straightforward, affordable, and unique green production processes and promising features of CDs, will be continually discovered.
A great consideration on selectivity and sensitivity of green source-derived CDs has significant sensing on hazardous metals like Cr6+, Pb2+, Hg2+, and Fe3+ as well as on biomolecules, nitro compounds, pesticides, etc. The broad mechanism of the formation of CDs is still not fully understood. In the near future, green CDs must be prepared using underutilized, low-cost, and undervalued sources.
References
- Hasan, A.M.M.; Reza, A.; Islam, M.; Susan, A.B.H. Carbon dots as nano-modules for energy conversion and storage. Mater. Today Commun. 2021, 29, 102732. [Google Scholar] [CrossRef]
- Hai, K.; Feng, J.; Chen, X.W.; Wang, J.H. Tuning the optical properties of graphene quantum dots for biosensing and bioimaging. J. Mater. Chem. B 2018, 6, 3219–3234. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Xu D, Lin Q, Chang HT. Recent advances and sensing applications of carbon dots. Small Methods. 2020 Apr;4(4):1900387.
- Z. M. Markovic, M. Labudová, M. Danko, D. Matijašević, M. Mičušík, V. Nádaždy, M. Kováčová, A. Kleinová, Z. Špitalský, V. Pavlović, D. D. Milivojević, M. Medić, B. M. T. Marković, ACS Sustainable Chem. Eng. 2020, 8, 16327.
- Y. Chong, C. Ge, G. Fang, X. Tian, X. Ma, T. Wen, W. G. Wamer, C. Chen, Z. Chai, J.-J. Yin, ACS Nano 2016, 10, 8690.
- P. K. Pandey, Preeti, K. Rawat, T. Prasad, H. B. Bohidar, J. Mater. Chem. B 2020, 8, 1277. R. Prasad, N. K. Jain, A. S. Yadav, M. Jadhav, N. N. V. Radharani, M. Gorain, G. C. Kundu, J. Conde, R. Srivastava, ACS Appl. Bio Mater. 2021, 4, 1693.
- Kang C, Huang Y, Yang H, Yan XF, Chen ZP. A review of carbon dots produced from biomass wastes. Nanomaterials. 2020;10(11):2316. [CrossRef]
- Wang R, Lu K-Q, Tang Z-R, Xu Y-J. Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis. J Mater Chem A. 2017;5(8):3717–3734. [CrossRef]
- X. Jiang, D. Qin, G. Mo, J. Feng, C. Yu, W. Mo and B. Deng, J. Pharm. Biomed. Anal., 2019, 164, 514–519.
- S. Chahal, N. Youse and N. Tufenkji, ACS Sustainable Chem. Eng., 2020, 8, 5566–5575.
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple One-Step Synthesis of Highly Luminescent Carbon Dots from Orange Juice: Application as Excellent Bio-Imaging Agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile Synthesis of Fluorescent Carbon Dots Using Watermelon Peel as a Carbon Source. Mater. Lett. 2012, 66, 222–224.
- Du, F.; Zhang, M.; Li, X.; Li, J.; Jiang, X.; Li, Z.; Hua, Y.; Shao, G.; Jin, J.; Shao, Q.; et al. Economical and Green Synthesis of Bagasse-Derived Fluorescent Carbon Dots for Biomedical Applications. Nanotechnology 2014, 25, 315702.
- Gharat, P.M.; Pal, H.; Dutta Choudhury, S. Photophysics and Luminescence Quenching of Carbon Dots Derived from Lemon Juice and Glycerol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 14–21.
- Rajendran, K.; Rajendiran, N. Bluish Green Emitting Carbon Quantum Dots Synthesized from Jackfruit (Artocarpus Heterophyl-lus) and Its Sensing Applications of Hg (II) and Cr (VI) Ions. Mater. Res. Express 2018, 5, 24008.
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal Green Synthesis of Magnetic Fe3O4 -Carbon Dots by Lemon and Grape Fruit Extracts and as a Photoluminescence Sensor for Detecting of E. Coli Bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 481–493.
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable Synthesis of Carbon Quantum Dots from Banana Peel Waste Using Hydrothermal Process for in Vivo Bioimaging. Phys. E LowDimens. Syst. Nanostruct. 2021, 126, 114417. [Google Scholar] [CrossRef]
- Zhao Y, Jing S, Peng X, Chen Z, Hu Y, Zhuo H, Sun R, Zhong L. Synthesizing green carbon dots with exceptionally high yield from biomass hydrothermal carbon. Cellulose. 2020 Jan;27:415-28.
- Miao, H.; Wang, L.; Zhuo, Y.; Zhou, Z.; Yang, X. Label-Free Fluorimetric Detection of CEA Using Carbon Dots Derived from Tomato Juice. Biosens. Bioelectron. 2016, 86, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Park, M.; Park, S.J.; Zhang, Y.; Akanda, M.R.; Park, B.Y.; Kim, H.Y. Green Synthesis of Fluorescent Carbon Dots from Carrot Juice for in Vitro Cellular Imaging. Carbon Lett. 2017, 21, 61–67. [Google Scholar] [CrossRef]
- Tafreshi, F.A.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive Fluorescent Detection of Pesticides in Real Sample by Using Green Carbon Dots. PLoS ONE 2020, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Tang, C.; Li, T.; Tong, X.; Tong, C.; Guo, Y.; Gao, Q.; Wu, L.; Shi, S. Dual-Emissive Carbon Dots for Dual-Channel Ratiometric Fluorometric Determination of PH and Mercury Ion and Intracellular Imaging. Microchim. Acta 2020, 187, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, B.; Zhuang, Q.; Wang, Y.; Luo, X.; Xie, Y.; Zhou, D. Green Synthesis of Fluorescent Nitrogen–Sulfur Co-Doped Carbon Dots from Scallion Leaves for Hemin Sensing. Anal. Lett. 2020, 53, 1704–1718. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Bai, H.; Jia, P.; Zhao, Y.; Liu, Y.; Wang, L.; Zhuang, Y.; Yue, T. Fluorescent Detection of Tetracycline in Foods Based on Carbon Dots Derived from Natural Red Beet Pigment. LWT 2022, 157, 113100. [Google Scholar] [CrossRef]
- Deka, M.J.; Dutta, P.; Sarma, S.; Medhi, O.K.; Talukdar, N.C.; Chowdhury, D. Carbon Dots Derived from Water Hyacinth and their Application as a Sensor for Pretilachlor. Heliyon 2019, 5, e01985. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wan, Y.; Zhang, K.; Fu, Q.; Wang, L.; Zeng, J.; Xia, Z.; Gao, D. Green Synthesis of Carbon Dots Using the Flowers of Osmanthus fragrans (Thunb.) Lour. as Precursors: Application in Fe3+ and Ascorbic Acid Determination and Cell Imaging. Anal. Bioanal. Chem. 2019, 411, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Shekarbeygi, Z.; Farhadian, N.; Khani, S.; Moradi, S.; Shahlaei, M. The Effects of Rose Pigments Extracted by Different Methods on the Optical Properties of Carbon Quantum Dots and Its Efficacy in the Determination of Diazinon. Microchem. J. 2020, 158, 105232. [Google Scholar] [CrossRef]
- Ghosh, S.; Gul, A.R.; Park, C.Y.; Kim, M.W.; Xu, P.; Baek, S.H.; Bhamore, J.R.; Kailasa, S.K.; Park, T.J. Facile Synthesis of Carbon Dots from Tagetes erecta as a Precursor for Determination of Chlorpyrifos via Fluorescence Turn-off and Quinalphos via Fluorescence Turn-on Mechanisms. Chemosphere 2021, 279, 130515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green Synthesis of Carbon Dots from Ocimum sanctum for Effective Fluorescent Sensing of Pb2+ Ions and Live Cell Imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z.; et al. Carbon-Quantum-Dots-Loaded Ruthenium Nanoparticles as an Efficient Electrocatalyst for Hydrogen Production in Alkaline Media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef] [PubMed]
- Kalanidhi, K.; Nagaraaj, P. Facile and Green Synthesis of Fluorescent N-Doped Carbon Dots from Betel Leaves for Sensitive detection of Picric Acid and Iron Ion. J. Photochem. Photobiol. A Chem. 2021, 418, 113369. [Google Scholar]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurthy, P. Outright Green Synthesis of Fluorescent Carbon Dots from Eutrophic Algal Blooms for in Vitro Imaging. ACS Sustain. Chem. Eng. 2016, 4, 4724–4731. [Google Scholar] [CrossRef]
- Zulfajri, M.; Liu, K.C.; Pu, Y.H.; Rasool, A.; Dayalan, S.; Huang, G.G. Utilization of Carbon Dots Derived from Volvariella Volvacea Mushroom for a Highly Sensitive Detection of Fe3+ and Pb2+ Ions in Aqueous Solutions. Chemosensors 2020, 8, 47. [Google Scholar] [CrossRef]
- Moonrinta, S.; Jamnongsong, S.; Sampattavanich, S.; Kladsomboon, S.; Sajomsang, W.; Paoprasert, P. Synthesis of Biocompatible Carbon Dots from Yogurt and Gas Vapor Sensing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 378, 012005. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Li W, Wang S, Li Y, et al.. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr Polym. 2017;175:7–17. [CrossRef]
- Tang S, Zhang H, Mei L, et al.. Fucoidan-derived carbon dots against Enterococcus faecalis biofilm and infected dentinal tubules for the treatment of persistent endodontic infections. J Nanobiotechnology. 2022;20(1):321. [CrossRef]
- Zheng X, Qin K, He L, et al.. Novel fluorescent nitrogen-doped carbon dots derived from Panax notoginseng for bioimaging and high selectivity detection of Cr6. Analyst. 2021;146(3):911–919. [CrossRef]
- In I, Park SY, Lim D, et al. Correction to simple microwave-assisted synthesis of amphiphilic carbon quantum dots from A3/B2 polyamidation monomer set. ACS Appl Mater Interfaces. 2018;10(3):3153. [CrossRef]
- Liu H, He Z, Jiang LP, Zhu JJ. Microwave-assisted synthesis of wavelength-tunable photoluminescent carbon nanodots and their potential applications. ACS Appl Mater Interfaces. 2015;7(8):4913–4920. [CrossRef]
- Shen Z, Zhang C, Yu X, et al.. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J Mater Chem C. 2018;6(36):9636–9641. [CrossRef]
- Deng, Y.F.; Qian, J.; Zhou, L.H. Solvothermal Synthesis and Inkjet Printing of Carbon Quantum Dots. Chem. Select 2020, 5, 14930–14934. [Google Scholar] [CrossRef]
- Chung S, Revia RA, Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2021;33(22):e1904362. [CrossRef]
- Dias, C.; Vasimalai, N.; PSárria, M.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and Bioimaging Potential of Fruit-Based Carbon Dots. Nanomaterials 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Du, F.; Liu, P.; Chen, Z.; Shen, J. DNA–Carbon Dots Function as Fluorescent Vehicles for Drug Delivery. ACS Appl.Mater. Interfaces 2015, 7, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Sun Z, Lu F, Cheng J, et al.. Haemostatic bioactivity of novel Schizonepetae Spica Carbonisata-derived carbon dots via platelet counts elevation. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S308–S317. [CrossRef]
- Wang Y, Kong H, Liu X, et al.. Novel carbon dots derived from cirsii japonici herba carbonisata and their haemostatic effect. J Biomed Nanotechnol. 2018;14(9):1635–1644. [CrossRef]
- Yan X, Zhao Y, Luo J, et al.. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J Nanobiotechnology. 2017;15(1):60. [CrossRef]
- Sun Y, Zhang M, Bhandari B, Yang C. Recent development of carbon quantum dots: biological toxicity, antibacterial properties and application in foods. Food Rev Int. 2022;38(7):1513. [CrossRef]
- Tang S, Zhang H, Mei L, et al.. Fucoidan-derived carbon dots against Enterococcus faecalis biofilm and infected dentinal tubules for the treatment of persistent endodontic infections. J Nanobiotechnology. 2022;20(1):321. [CrossRef]
- Wang X, Zhang Y, Kong H, et al.. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif Cells Nanomed Biotechnol. 2020;48(1):68–76. [CrossRef]
- Li CL, Ou CM, Huang CC, et al.. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J Mater Chem B. 2014;2(28):4564–4571. [CrossRef]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon quantum dots: Synthesis, characterization and biomedical applications. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Chen, P.-C.; Periasamy, A.P.; Chen, Y.-N.; Chang, H.-T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Hu, Q.; Gong, X.; Liu, L.; Choi, M.M.F. Characterization and analytical separation of fluorescent carbon nanodots. J. Nanomater. 2017, 2017, 1–23. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Human Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In Vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).