1. Introduction
In today’s society, infectious diseases are constantly threatening human health. Therefore, understanding the outbreak mechanism and transmission mode of infectious diseases will help shed light on future prevention and treatment strategies. When the first case of AIDS was covered in America in June 1981, more and more people were infected and even dying of AIDS throughout the 1980s, and there is still a large population of AIDS patients today [
1]. In 2003, an outbreak of Severe Acute Respiratory Syndrome (SARS) occurred in Guangdong Province, China, characterized by its high infectivity and rapid disease progression, eventually leading to outbreaks in various regions of China and some cities worldwide [
2]. In 2009, a widespread outbreak of H1N1 influenza occurred in the United States and rapidly spread to other countries and regions [
3]. In March 2013, H7N9 was first discovered in eastern China [
4]. A flare-up of H7N9 avian influenza quickly gained wide attention as new cases and high infection-related death rates were not controlled. At the end of 2019, COVID-19 rapidly swept the world, becoming one of the most serious diseases in human history [
5]. An increasing number of sudden infectious diseases are emerging, some of which have become endemic, continuing to harm humans. In contrast, others have evolved into more insidious and harmful viruses through the continuous mutation of viruses.
Infectious disease modeling is a vital field that investigates the transmission routes and factors influencing the spread of emerging infectious diseases. With the rapid development of the global economy and the increasingly convenient means of transportation, population movements and people spreading the virus along the way are recognized as critical factors in disease transmission, which has attracted extensive attention from researchers. Perrin et al. [
6] emphasized that the global dissemination of the HIV-1 pandemic fundamentally revolves around travel, implying the significance of travel in disease transmission. Air travel is also considered pivotal in spreading infectious diseases, as discussed by Mangili and Gendreau [
7], who explored the potential for disease epidemics associated with air travel. In Beijing, China, Jia et al. [
8] conducted a study revealing the impact of population mobility on tuberculosis’s prevalence, further highlighting the role of population mobility in disease transmission. In addition, the study of Chan et al. [
9] revealed that person-to-person transmission of the novel coronavirus is possible within households, hospitals, and between cities. To better understand and predict transportation-related disease spread, Cui et al. [
10] proposed an epidemiological model that describes this transmission mode. Similarly, Liu et al. [
11] developed an SIQS infectious disease model incorporating transportation and entry screening, demonstrating the effectiveness of entry screening in mitigating transportation-related disease transmission. Many similar models of infectious diseases spread between two regions [
12,
13,
14,
15,
16]. In addition to considering patch models for two regions, many authors also consider patch models for multiple regions. In 2003, Arino et al. [
17] proposed a model for the spread of disease in a population of individuals traveling between
n cities. The results show that
is a threshold, when
, the disease disappears, and when
, the disease reaches epidemic levels in all connected cities. Gao and Ruan [
18] proposed a model with multiple patches to study how population movement affects the spread of malaria. Their results showed that travel between areas contributed to the spread of the disease in both regions. However, if travel rates continue to increase, the disease might disappear again in both areas. Sun et al. [
19] established a patch model reflecting the population flow between Hubei and other regions. They estimate the epidemic situation in Hubei based on the daily reported epidemic data from Hubei and other regions, as well as the data on the population flow between Hubei and other regions. Zhang et al. [
20] established a multi-patch model of HIV/AIDS with heterosexual transmission. The results indicate that if the disease vanishes in one region and spreads in another, it can spread or disappear in both regions depending on the individual mobility patterns.
Prosper et al. [
21] proposed an optimal control model for malaria and find that the effectiveness of vaccination programs at the population level can be improved by actively seeking out and treating asymptomatic infections. Kang et al. [
22] proposed a delayed avian influenza model incorporating slaughtering conditions. Their study indicates that optimal control, achieved by slaughtering susceptible (infected) avians and educating susceptible populations, can effectively minimize the number of infections in poultries and mankind. Moreover, the cost of implementing these control strategies can be minimized. Song et al. [
23] studied the impact of delayed vaccination and isolation on COVID-19 transmission and conclude that the best isolation rate minimizes the total number of infections and expenditure on disease control. Singh et al. [
24] have found that measures such as social distancing, lockdowns, and wearing masks can reduce the spread of the disease. These studies and models provide valuable insights and guidance for our in-depth understanding of the impact of population movements and traffic factors on disease transmission and how to control disease effectively.
For diseases such as tuberculosis, hepatitis A, hepatitis B, and influenza, the process of infection is unique because susceptible individuals do not immediately become ill after infection. Instead, they undergo an incubative period, where the pathogens replicate and spread within the host without showing noticeable symptoms. With this factor in mind, many researchers have proposed infectious disease models with delayed incubative periods in mathematical modeling and conducted extensive studies in this field [
25,
26,
27,
28,
29,
30,
31,
32]. Although many infectious disease models take into account the presence of incubative periods, we also need to be aware of the limitations of these models. The exact length of the incubative periods, variations in infectiousness, and interactions between individuals can all introduce uncertainty into the models. Therefore, further research is still needed to validate and improve the accuracy of these models by incorporating actual data and conducting field investigations. So it is also extremely interesting that we discuss the effect of time delay on measures of time-varying control.
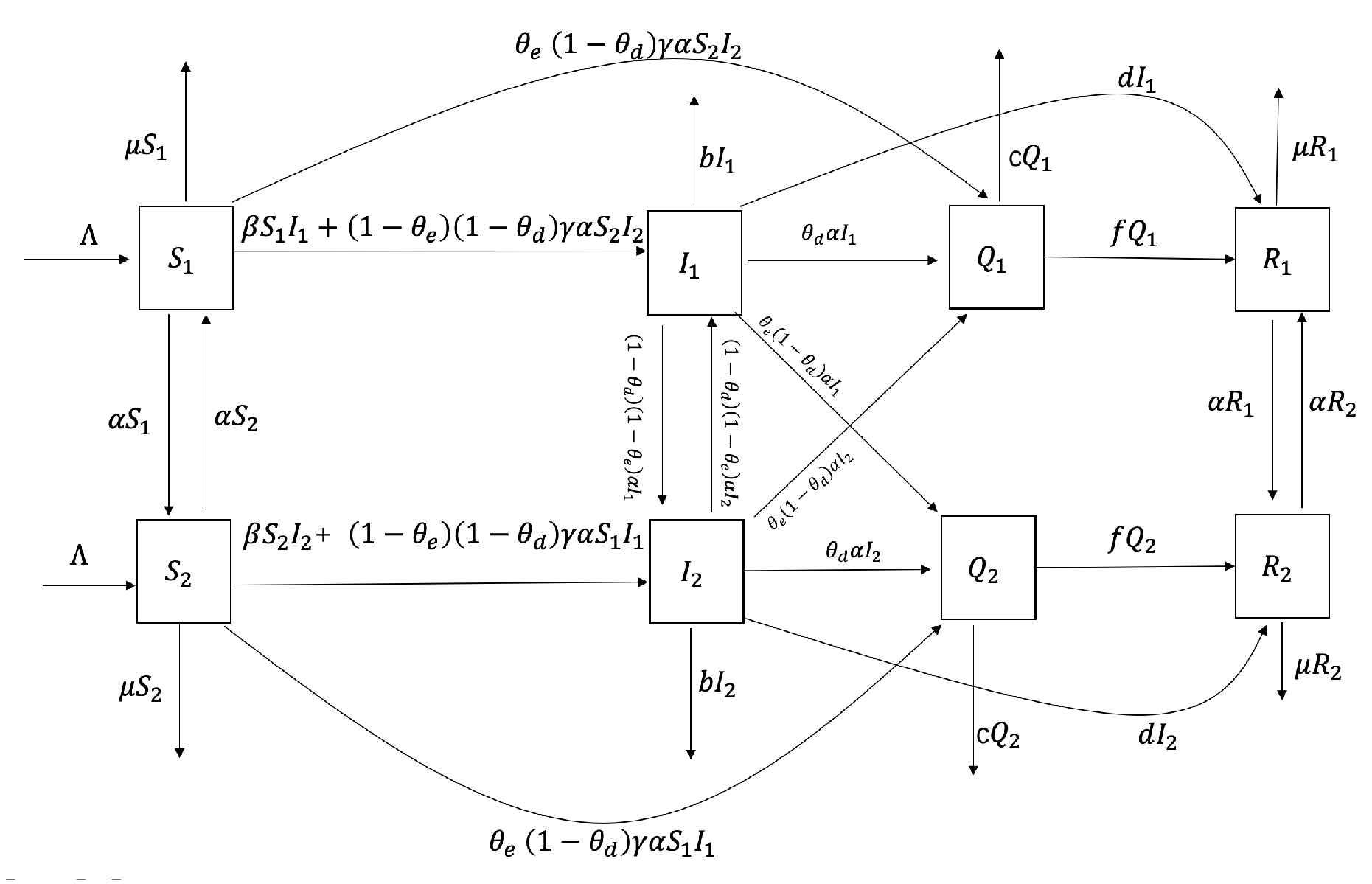
Major emerging infectious diseases differ from normalized infectious diseases, as they have uncertainty, stronger stealthiness, more destructive power, and more severe harm to society and the economy. Therefore, studying the characteristics of sudden outbreaks and epidemics of infectious diseases can help reduce the harm of infectious diseases. From the above revelation, relatively limited studies use actual data to explore transportation-related infections between two regions and how to control this spread effectively. The rest of this paper is organized as follows. In the next section, we establish an SIQR time-delayed infectious disease model for emerging infectious diseases that considers transportation-related infection and entry-exit screening. We count the basic reproduction number and demonstrate that the endemic equilibrium is sole, the global asymptotic stability of the disease-free equilibrium, and the persistence of the model (
1). In the section 3, we simulate the model using actual data from two regions in the USA and sensitivity analysis of
. We investigate optimal control strategies in the section 4, and the cost-effectiveness analysis results are presented in the section 5. Discussions and conclusions are provided in the last two sections.
4. Optimal Control Strategies
Owing to the previous
sensitivity analysis and combined with the reality of COVID-19 in America, we put our hands to seek an effective way to control COVID-19 infections.
aims to control the transmission rate of susceptible people, which is measured by preventive measures like wearing masks and reducing gatherings.
aims to control population migration, measured through policy interventions and city lockdowns.
aims to take medication and actively pursue treatment to enhance the natural recovery rate. With all the assumptions we mentioned above, the time-delayed model takes the control problem as follows:
Regarding variables and the above control system, considering that the spread of emerging infectious diseases is related to transportation-related infections between two regions, we consider reducing the number of patients who are not successfully detected in entry and exit screenings between the two regions.
Our objective function
is defined as
where the interval
signifies the time duration of the control program, the quantities
represents the weight constants of patients in region 1 and region 2 who were not successfully detected.
represents the transmission rate of the susceptible population. In every region
i, the susceptible and infected individuals move to region
j at a per capita rate
(
) and the per capita rate of natural recovery from disease. Constants
,
signify a measure of the relative cost of the interventions over the interval
. The expression
,
refers to the patients who are not successfully detected between two regions. Therefore, this term is included in the objective function. In addition, we should consider the costs of wearing masks, city lockdowns, and purchasing medications. Our goal is to find the optimal control
such as
the control set
U equals that
Through the Filippov–Cesari Existence Theorem and the Mangasarian Theorem [
49], a special method
which is the optimal control exists. After that, we can find this method in Pontryagin’s Minimum Principle [
50].
Theorem 4.
Suppose , and are the optimal control variables, and correspondingly , , , , , , , are the control system’s (8) optimal state variables, then there exists adjoint variable that satisfies the following adjoint equations:
Given the transversality conditions . Moreover, we provide the corresponding parameters below:
Proof. The control problem of the Hamilton function
H is defined as
where
is the adjoint variable satisfying
On
,
are indicator functions under the transversality conditions
, which fullfill
From the optimality equation,
Given that
is part of
U, we see
□
We present some numerical outcomes that belong to the optimality system. In order to determine the optimal control and the state system, we use the scheme proposed in [
47,
50], which is based on the forward-backward sweep scheme. Furthermore, many other algorithms describe approximation methods for obtaining optimal control [
51,
52]. We choose the figure from
Table 2 as the parameter values in the optimal control. The weights in the goal function
,
,
,
, we will explain these values in next section. The results in
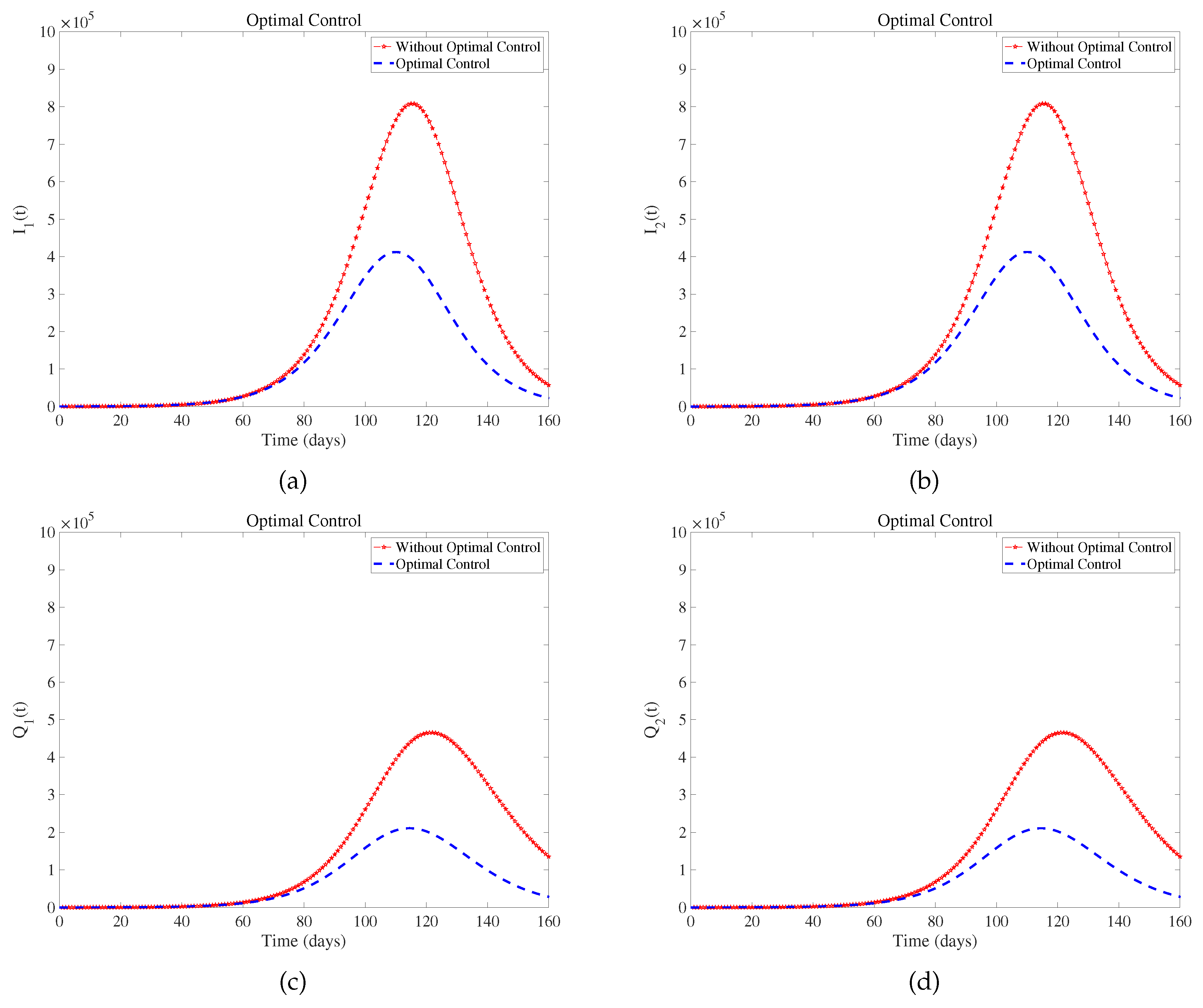
Figure 5 suggest that optimal control effectively controls the development and deterioration of the disease. In fact, the intensity of various measures implemented by the government is different, such as wearing masks. Combined with the prevention and control situation in the United States at that time[
53,
54,
55], we assume that the upper limit of these three measures is
.
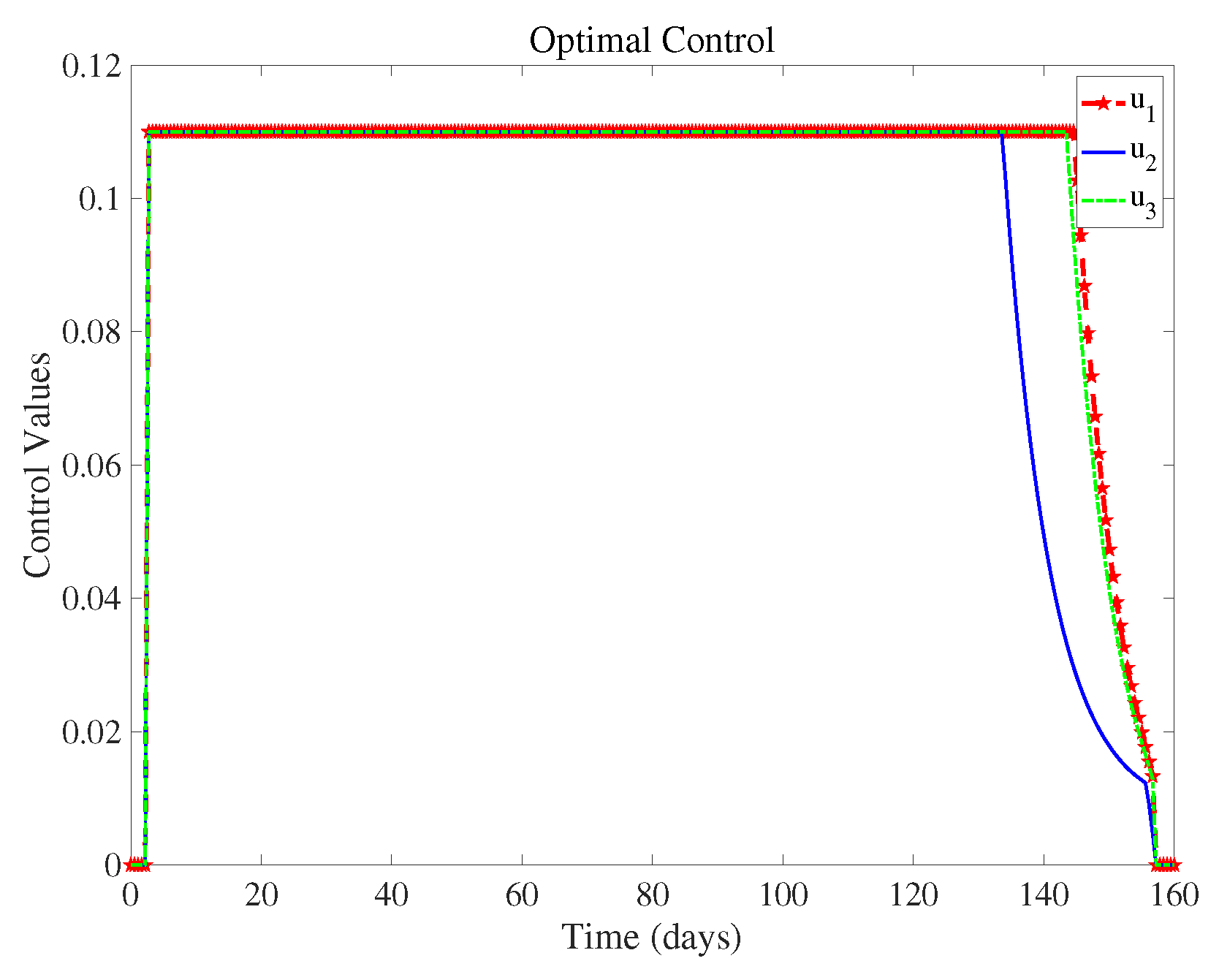
Figure 6 shows the control intensity of each measure in the control system over time, and we can see the magnitude of these three measures when they are taken at the same time.
(controlling population migration) can end about 20 days earlier than
(wearing masks) and
(take medication and actively pursue treatment). This means that we can reduce the control intensity of
(controlling population migration) sometime in advance when all three measures are implemented. Implementing various strategies has a certain cost, and we discuss the most cost-effective strategies in the next section.
5. Cost-Effectiveness Analysis
We use three ways, which are: infection averted ratio (IAR) [
56], average cost-effectiveness ratio (ACER) [
57], and incremental cost-effectiveness ratio (ICER) [
57] to complete the cost-effectiveness analysis. Below are the interpretations of the three ways:
(1) IAR can be denoted as
We regard the control way whose IAR is the highest as the economical choice [
57].
We assess the money which is used to perform a particular intervention strategy is
(3) ICER assess the variations in cost and health advantages of two distinct intervention strategies under the same limited resourses. Viewing strategies
m and
n as two viral control intervention strategies, then ICER is expressed as
The infection that is prevented with the controls during the time period
T is expressed as
Here,
is related to the per capita unit cost of the measures
. Let us take the example of Washington. Washington mandates wearing masks in public settings [
53]. However, due to cost and availability, individuals in low-income environments are likelier to use reusable cloth masks instead of disposable medical masks. There is still uncertainty in mask-wearing practices, such as the duration of wearing a mask [
58]. Therefore, we can reasonably assume that the daily cost of a mask for each person is approximately
dollars, which amounts to approximately
dollars per year.
Due to early stay-at-home orders in the United States, population mobility and migration decreased [
54], and local governments also provided subsidies to the public during the pandemic [
55]. We assume the average annual subsidy per person is approximately 80 dollars. As the prices of different drugs for treating and preventing COVID-19 vary [
59,
60], we assume that the annual cost for each person to purchase drugs for COVID-19 treatment is approximately 120 dollars.
Because of the specific numerical values given by the fourth column of
Table 3, it is clear that Strategy 1 (wearing a mask) is the most cost-effective measure.
According to the ACER values in column 5 of
Table 3, Strategy 1 is the lowest, so the most cost-effective Strategy is Strategy 1 (wearing masks).
Next, we calculate the ICER value, where we rank the number of people we want to avoid infection in increasing order.
The results are presented in
Table 3. Compared with the ICER value of Strategy 6, Strategy 5 is significantly superior. Therefore, Strategy 5 is removed from the control list. Similarly, in sequence, we can eliminate strategies 6, 7, 4, 3, and 2. Detailed calculation process can be found in .
Therefore, to sum up the analysis, we can know that when one considers the following control measures: (wearing masks ), (policy interventions and city lockdowns), (taking medication and actively seeking treatment). (wearing masks) is the least incremental cost-effective, providing the optimal cost over all other strategies on a large scale.
6. Discussion
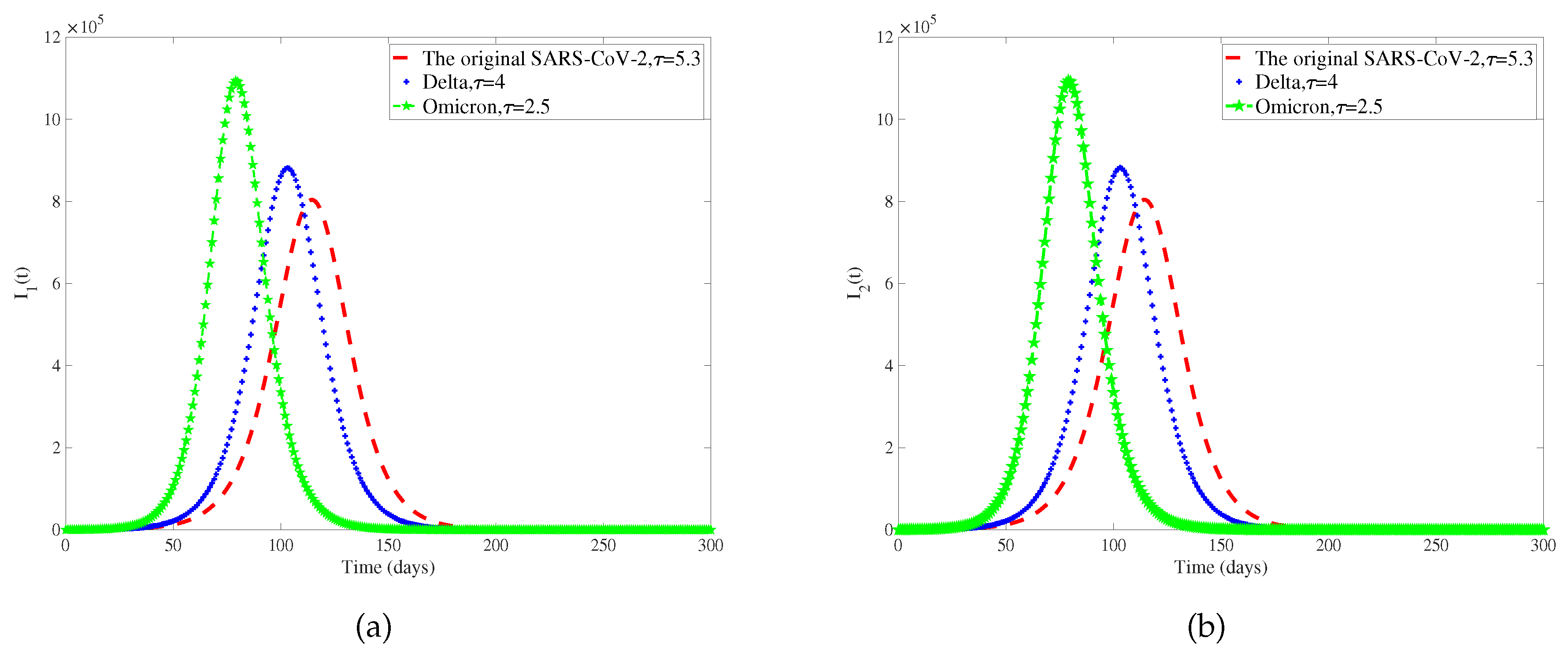
Considering that different genotypes of viruses have different incubative periods, we select different viruses circulating in different periods of COVID-19. The original SARS-CoV-2, Delta, and Omicron incubation periods are 5.3 days, 4 days, and 2.5 days [
42,
61]. We only discuss the influence of the length of the incubative period on the number of patients, so other parameters in the model are determined, and
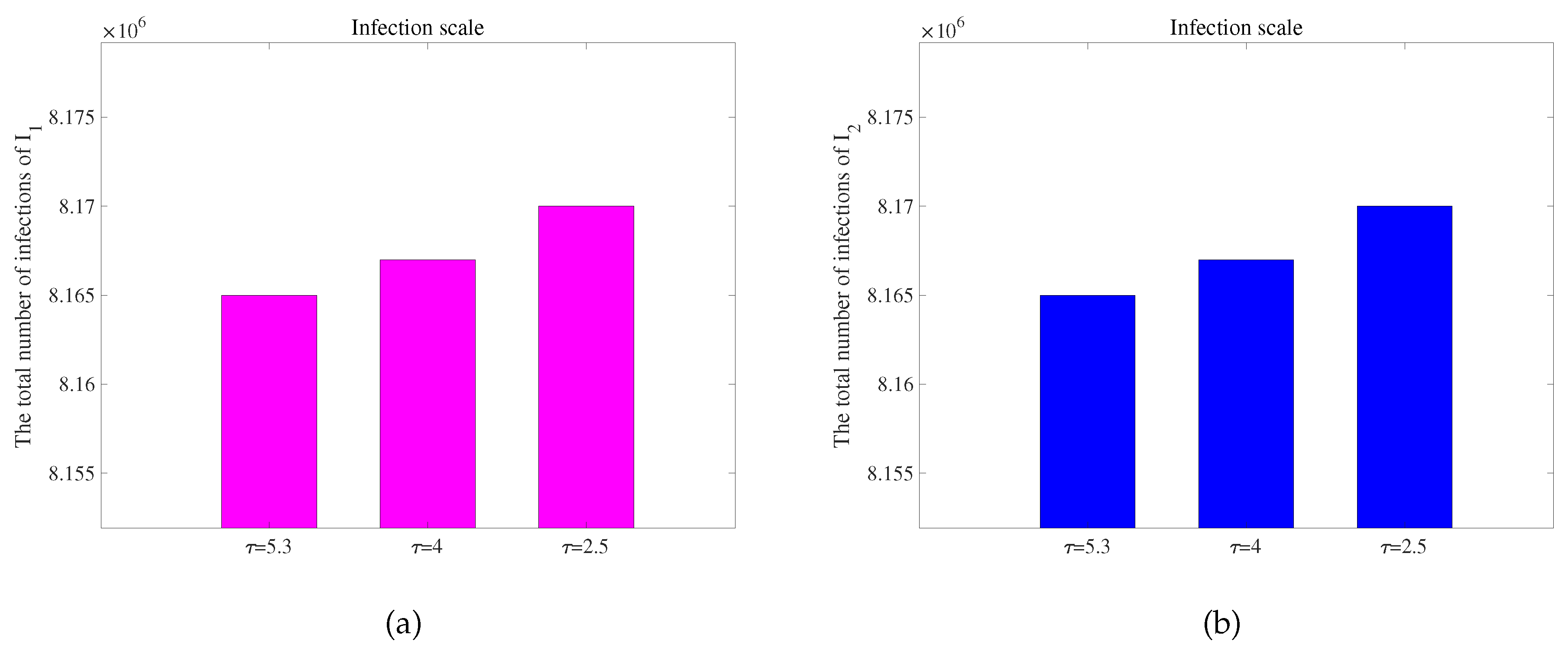
Figure 7 is obtained. To observe the extent of infection for different incubative periods, we have extracted data on the total quantity of infected individuals in 300 days with different incubative periods in two regions from
Figure 7, as shown in
Figure 8. We obtain conclusions similar to those of [
62]. As the disease incubative period becomes shorter, the disease tends to end earlier, leading to a larger scale of infection.
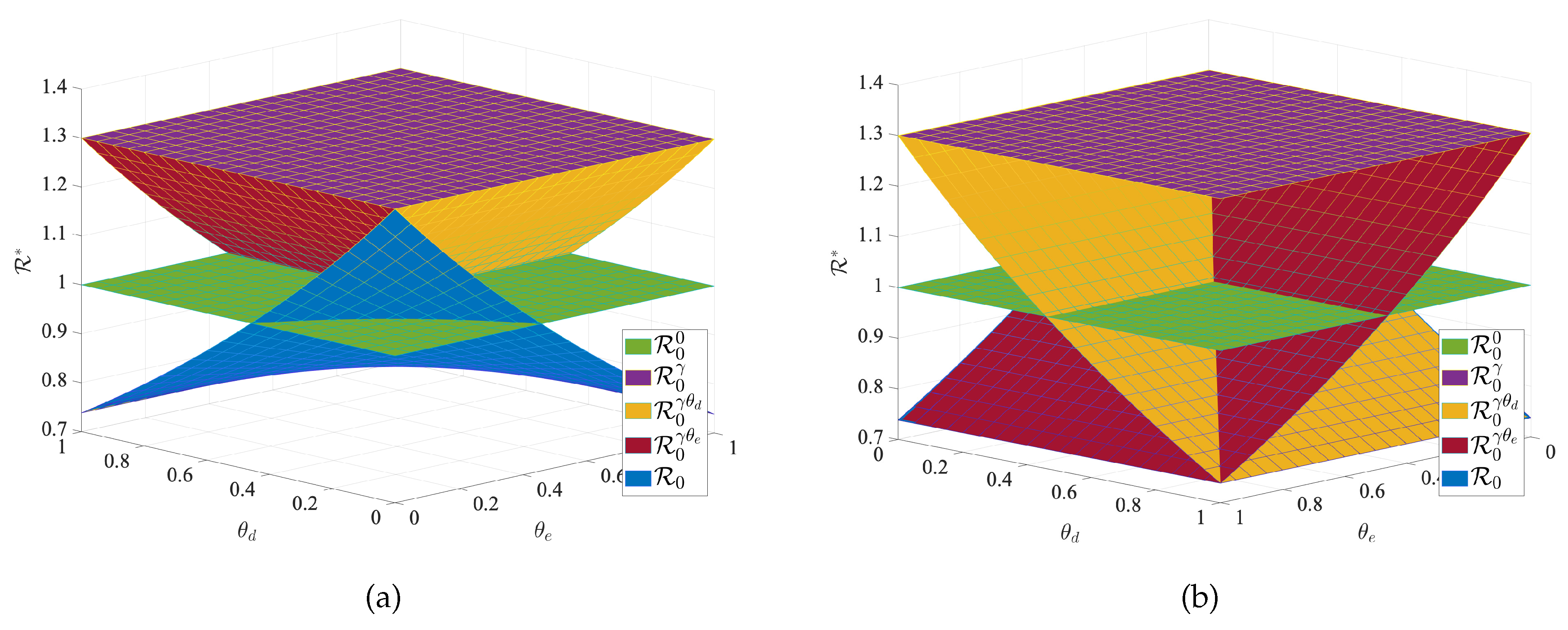
To verify the effectiveness of transportation-related infection and entry-exit screenings, we take the below four reproduction numbers into consideration,
The
is the basic reproduction number for each region when no individuals are moving between regions, and
is the basic reproduction number when there is transportation-related infection.
is the value of the basic reproduction number for transportation-related infection and entry screening, while
is the one for transportation-related infection and exit screening, the
is the basic reproduction number for model (
1) with transportation-related infection and entry-exit screenings. In order to consider the effects of
and
on the disease, we take the basic reproduction number
with no population movement between the two cities as the base. We fix all the parameters except
and
and observe the change in the basic reproduction number. We obtain
Figure 9 of
with respect to
and
. The results in
Figure 9 indicate that transportation-related infections increase the basic reproduction number and enhance disease transmission. Entry or exit screening can inhibit disease transmission by reducing the basic reproduction number.
7. Conclusion
We have established a time-delayed SIQR epidemiological model for infectious diseases with transportation-related infections between two regions. Although many diseases, such as SARS and influenza, can be studied using this model, we focus on using COVID-19 as a specific example to consider our model. Despite the end of the COVID-19 pandemic, it is still essential to remain vigilant and understand how to prevent such emerging diseases in real-time.
We obtain some useful conclusions. Model (
1) has a unique global asymptotically stable disease-free equilibrium when
and a unique endemic equilibrium exists when
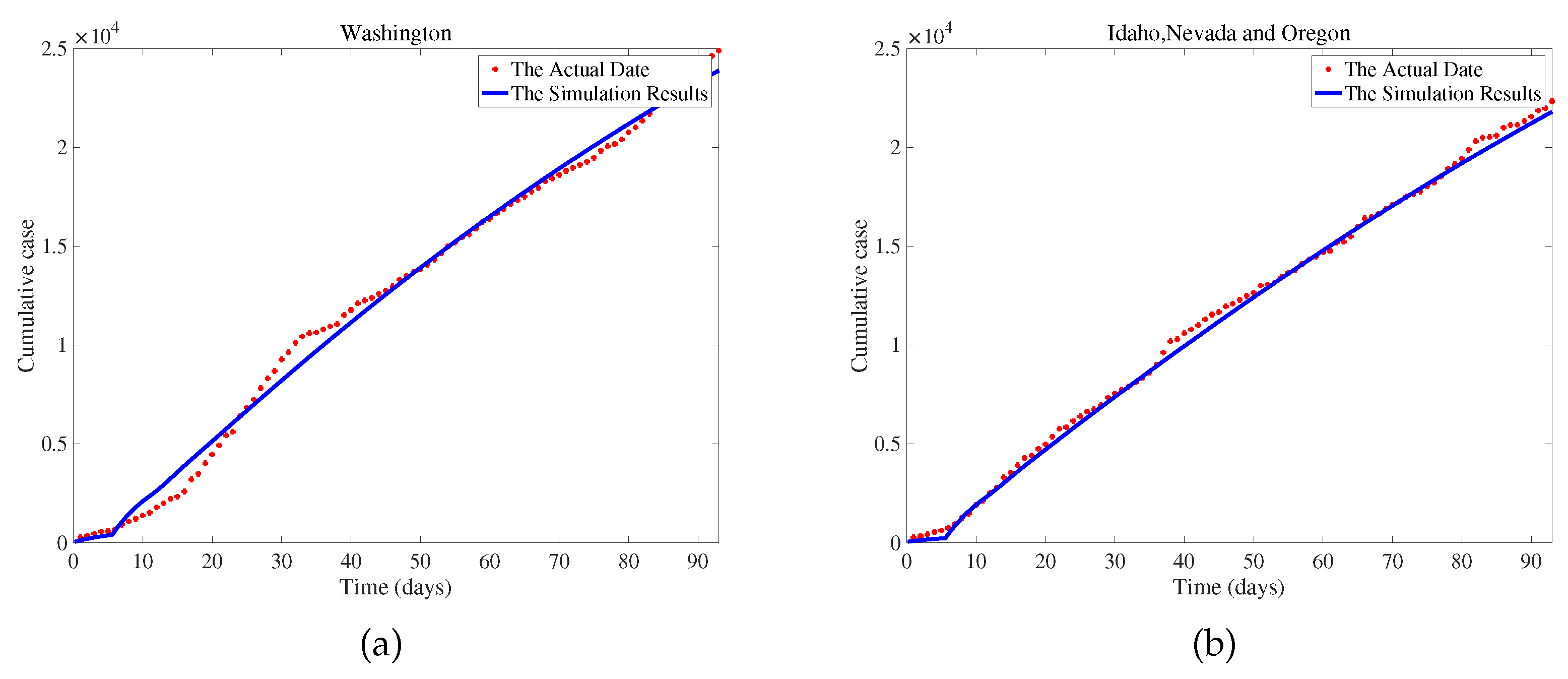
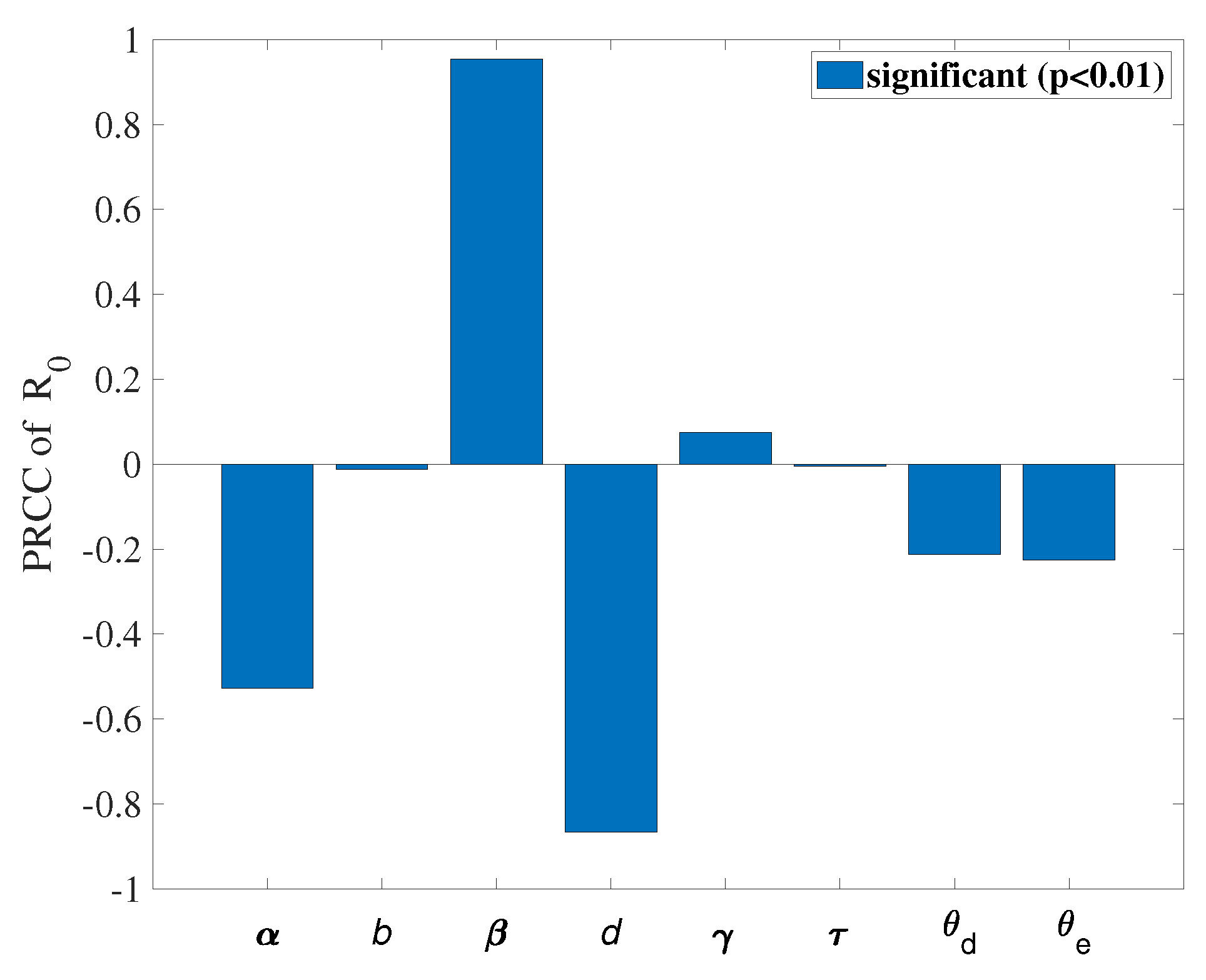
. We use COVID-19 data from Washington, USA, between June 10, 2020, and September 10, 2020, to fit the model and obtain a set of optimal parameters. We conduct a sensitivity analysis on the basic reproduction number (
) and obtain that
,
, and
d are more sensitive parameters compared to other parameters. Based on these sensitive parameters, we propose three control measures: (1) Wearing masks; (2) Policy interventions and city lockdowns; (3) Taking medication and actively pursuing treatment. Considering infections between regions through travel, we formulate an optimal control problem and find an optimal solution to reduce the number of undetected patients traveling between the two regions. We use Pontryagin’s minimum principle to obtain the optimal solution to achieve our goal. Subsequently, we obtain some numerical results using an optimal control approach based on the forward-backward sweep scheme. The results indicate that the control strategies help reduce infections. We can reduce the control intensity of
(controlling population migration) sometime in advance when all three measures (
,
,
) are implemented. Additionally, due to a decrease in the number of infected individuals, there is a relative reduction in isolating patients in hospitals, which aids in reducing the required medical resources for significant emergent diseases. We compare the three control strategies using three methods: Infection avoidance ratio (IAR), Average cost-effectiveness ratio (ACER), and Incremental cost-effectiveness Ratio (ICER). Strategy 1 (wearing masks) is the most cost-effective measure. Therefore, in the face of some major emerging infectious diseases, such as SARS, COVID-19, and other diseases. We can all encourage and require people to wear masks.
Finally, we discuss the effects of varying the disease’s incubative period and entry-exit screening on the disease. We observe that a shorter incubative period result in a shorter disease duration but leads to a larger scale of infection, and the peaks are reduced. This has a certain guiding role in the face of many major emerging infectious diseases between two regions. We can formulate some effective prevention and control measures according to the length of the disease’s incubative period, which can reduce the large-scale spread of the disease to a certain extent. Transportation-related infections increase the basic reproduction number () and enhance disease spread. Entry-exit screening have the ability to curb disease spread by lowering the basic reproduction number. Indeed, our model also has many factors that have not been considered. For instance, when the total populations of the two regions in the model are different, and the migration rates between these regions also vary, what is the impact on the spread of this disease? There are many uncertain factors in disease transmission, so what influence do they have on the disease? The risk of reinfection in recovered patients, differences in disease detection coverage in different regions, and transmission in n regions are all questions that deserve to be explored in the future.