Introduction
Influenza A virus-related
Swine (H1N1) and
Avian (H5N1) flu infections are dangerous, widely prevalent infectious illnesses [
1]. An influenza outbreak in humans was brought on by a new strain of
Influenza A (H1N1) originating in pigs in April 2009 [
2]. It originated in Mexico, moved to the USA, and eventually to 208 other nations [
3].There were millions of instances worldwide as of December 2009 [
4]. There were 9596 fatalities worldwide, including 1445 of them in the United States [
5]. The World Health Organization (WHO) classified the
Swine flu as a category 6 pandemic in June 2009, making it abundantly evident that action and mitigation measures were required [
6]. Rarely do pandemics brought on by the H1N1 or H5N1 Influenza virus arise, although significant outbreaks in the winter months happen almost yearly [
7].
A pandemic happens when an
Influenza A virus variation carries a novel haemagglutinin to which no human-neutralizing antibodies have been found [
8]. There are only
Influenza viruses in the Orthomyxovirus family [
9]. The
Influenza virus has a segmented RNA genome, which makes mutations easier to develop [
10].
Influenza viruses are tiny in size [
11]. On several spikes, HA and NA are found [
12]. The annual number of deaths in the United States from influenza is estimated to be close to 36,000 [
13]. The medication included Zanamivir and Oseltamivir, which were successfully used to combat strains A, H5N1, and H1N1 during the worldwide pandemic flu outbreak [
14]. The
Influenza A vaccination (
H1N1 and
H5N1) was shown to be the primary means of prevention [
15]. Worldwide, two primary varieties of flu vaccinations are accessible: trivalent and quadrivalent vaccines [
16]. It was shown that humoral immunity, which produces neutralizing antibodies against HA, is the primary defense against many flu viruses [
17]. The current work set out to create a new bivalent LNP-mRNA flu vaccine that would target the HA and NA of Influenza A (H1N1 and H5N1), two strains of the virus that cause deadly flu illnesses every winter throughout the world.
Methodology
Ethical Statement
All pertinent national, institutional, and/or worldwide guidelines for the care and use of humans and animals were postdated in the current study. The ethical committee for human and animal handling at Cairo University (ECAHCU), the pharmacy faculty at the University of Cairo, Egypt, and the local authorities approved all procedures used in the study, including those involving humans and animals, following the Weatherall report's recommendations (approval number PXV359/2023). Every attempt was made to reduce the number of people and animals used in the study, as well as their suffering. Randomized human clinical trial registration number CTX628/2023 was obtained and authorized by EDA.
Source of Animal Models
A hundred male mice weighing between 40 and 50 grams were procured and approved for legalization by the Department of Pharmacology and Toxicology at Cairo University's College of Pharmacy in Egypt.
Collection of the Samples
A hundred blood samples were collected from Egyptian patients suffering from Influenza A (H1N1 and H5N1) viruses.
Inclusion Criteria for Animal Models
Adult male mice weighing 40-50 gm could be infected by Influenza A ( H1N1, H5N1) viruses after inoculation with an infectious dose of 0.6-3.0 TCID50 for each strain through an intranasal route of administration for exploding expression of viral proteins and exciting potent humoral immunity. Incubation time ranged from 3-5 days for the appearance of symptoms to fall out.
Exclusion Criteria
Young mice and Pregnant female mice.
Material
All chemical and biological components were acquired from Alnasr Pharmaceutical Company, Abo zabal Alkhanka, Qalyobia, Egypt, and Algomhoria Pharmaceutical Company, Cairo, Egypt.
Place and Date of the Study
Between January 2023 and May 2024, this study was conducted at Cairo University's pharmacy faculty in Egypt.
Type of Study
Screening experimental study.
Methods
The principle of manufacture of mRNA vaccine included polymerase chain reaction (PCR) used to clone genes of interest encoding Haemagglutinin (HA) and Neuraminidase (NA) of Influenza A virus strains (H5N1 and H1N1) known as Avian flu and Swine flu, respectively. The genes were then inserted using the EcoR1, BAM Hind II restriction endonuclease type II, and Ligase enzyme into the pET-21(+) transcription vector plasmid. On the other hand, the hybrid transcription vectors were mixed with T7 RNA polymerase and ribonucleotides within a test tube after being linearized using EcoR II. Open reading frames of genes encoding the spike proteins HA and NA of A (H1N1, H5N1) influenza were constructed and identified using nucleotide sequences deposited on the NCBI website and BLASTn software. TACATCCAGCCACAATTGGA was the forward primer utilized for the polymerase chain reaction (PCR) cloning of the cDNA of HA; while the reverse primer was TGTGTGCTCTTTTGGTCAGC. On the other hand, the forward primer for PCR cloning of NA was AGGGAGCCTTGCTGAATGAC; whereas the reverse primer used was ATCATTGGGGCGTGGATTGT. The polymerase chain (PCR) was exploited in the cloning process using a PCR SuperMix kit with Catalog number 10572014 (purchased from Invitrogen, USA). PCR steps included denaturation, annealing, and Extension. The four components (reagents and chemicals) were mixed in a centrifuge tube; and then inserted into the PCR apparatus. The procedure was run according to the PCR kit manufacturer's instructions. The reaction mixture comprised a segment of DNA sample, DNA primers, DNA polymerase, and nucleotide solution mixture containing adenine, thymine, cytosine, and guanine (A, T, C, G). During the denaturation cycle, the solution enclosing the reaction mixture was heated up to 94 ℃ using a thermal cycler; furthermore, the sample mixture was cooled to 50 ℃ allowing an annealing cycle to occur. Finally, the extension cycle comprising the extension of DNA occurred when the temperature cycled from 95 ℃ to 50 ℃ to 60 ℃. The 3 cycles were repeated nearly 40 times using a thermal cycler leading to the formation of millions of copies of a single short segment of DNA from one sample. These cDNA genes encoding HA and NA were selected for the utilization in the construction of lipid nanoparticles mRNA vaccine containing HA and NA mRNA encoding genes of the Swine and Avian flu using the invitro transcription technique. The procedure was performed using the Invitro MEGAscript T7 Transcription Kit Plus, purchased from Invitrogen, USA; as well as it was operated according to the manufacturer's instructions. Briefly, 10 µg of mRNA transcripts were generated per 1µg template DNA exploiting the MEGAscript T7 Transcription Kit. The frozen reagents were thawed, mixed, and centrifuged in a gyrator shaker (Corning gyrator shaker, Japan) for 3 minutes at 300 rpm. The enzymes and nucleotides were kept on ice. On the other side, the reaction buffer was maintained at room temperature. Furthermore, the reaction mixture was prepared at room temperature, and incubated at 37 ̊C (Sanyo aerobic incubator, Japan), pH 7.4 for 3 hours. Finally, the template DNA was separated using 2 U of DNAase; as well as protein impurities were removed utilizing 3 U proteinase K. The reaction was stopped by adding 2 U 0.5 EDTA at pH 8.5 then the incubation of the reaction mixture at 65 ̊C for 10 minutes. The purification of HA and NA mRNAs was made using reversed-phase high-performance liquid chromatography (RP-HPLC); as well as the purification was confirmed through the Northern blot technique. The mRNAs were then enclosed with solid lipid nanoparticles [LNPs] (20 mcg/ml of dimethyl dioctadecyl ammonium bromide lipid [DDAB]) with size 70 nm prepared using a hot microemulsion technique. The test LNP-mRNA vaccine formulation was equipped with a narrow size distribution as its polydispersity index (PDI) was 0.31. On the other hand, It possessed a high zeta potential of +28 mV. Lipid nanoparticles -mRNA A ( H1N1, H5N1) immunizing agent was formulated as a parenteral sterile suspension. Each 1 ml dose was formulated to contain 20 mcg mRNA of HA and NA, 20 mcg of DDAB, and 0.2 mg of aluminum hydroxide. Each dose; as well as besieged 5 mg of sodium chloride and 0.620 mg of sodium dihydrogen phosphate dihydrate. Immunogenicity was tested in animal models by injecting the purified mRNA in 100 mice by intraperitoneal route of administration. Later, Immunogenicity was well-tried in randomized human clinical trials phases 1/2.
Preclinical Trials (Animal Testing) Procedure
One hundred mice were challenged with the immunizing agent through an intraperitoneal route of administration; while the control group received the placebo through the same route of administration. After 15 days, both groups received graded infectious doses of Swine and Avian flu which ranged from 0.6 to 3 TCID50. The lethal dose was further detected during these protection tests to aid in the determination of the protection power of the test vaccine. The humoral immunity was determined using ELISA. On the other side, the flow cytometry technique using a flow cytometer was used to determine cell-mediated immunity by counting the different types of T lymphocytes specific to HA and NA antigens. The percentage of live and killed animal models was recorded during these protection tests to aid in the determination of immunogenicity and the efficacy of the test immunizing agent. As well as The outcomes were also compared with reference standards to assess the relative bioavailability and potency of the test vaccine. Finally, the acute toxicity of the immunizing agent was estimated using the up and down method for assay of acute toxicity.
Randomized Human Clinical Trials Phase 1/2 Procedure
One hundred human volunteers (test group) of different ages (5-60 years old) were challenged with the test vaccine containing 20 mcg mRNA of HA and NA through intramuscular injection; whereas the control group included 100 candidates who received the placebo intramuscularly. After 28 days, booster doses were given to all participants in this type of protection test.
Both groups were exposed to graded infectious doses (0.6-3 TCID50) of Swine and Avian flu through respiratory droplet transmission. Blood samples( 5-10 ml) were withdrawn using an injection needle (5 µm in diameter) from all participants in the randomized human clinical trials phases 1/2. These blood samples were further subjected to ELISA and flow cytometry techniques to determine the immunogenicity of the test vaccine. On the other hand, Graded quantities of serum (1/1280-1/10) from immunized individuals were transferred to normal volunteers who were then challenged with graded doses (0.6-3 TCID50) of the infectious agent of Swine and Avian flu. The highest dilution of serum efficacious at immunizing 50% of participants in this protection test (i.e. ED50%) was ascertained as a reference point of the efficaciousness of the vaccine. The LD50%, as well as the protection power of the immunizing agent, were determined through these protection tests.
Determination of Humoral Immunity of LNP-mRNA H1N1, H5N1 Flu Vaccine
ELISA was exploited for the detection of neutralizing antibodies. Procedures were applied using ELISA LumiKineTM Xpress 2.0 kits purchased from InvivoGen Europe, France. LumiKineTM Xpress 2.0 kits were sandwich ELISAs that required only a single 2-hour incubation at 37 ̊C (Sanyo aerobic incubator, Japan) and pH 7.3. The procedures of ELISA were followed by the ELISA kit manufacturer. Ten mcg/ml HA and NA Antigens were connected to the well bottom. Antibodies in the serum of the patient were attached to antigens. 5 µg/ml antibodies to human IgG were connected to the patient's IgG, and the antibodies to human IgG were enzyme-conjugated (5 mcg/ml Horseradish per-oxidase enzyme). 10 µl substrate for the enzyme was added, which changed color when acted upon by the enzyme. Enzyme activity was calculated by adding the substrate for the enzyme and estimating the color reaction in a UV spectrophotometer (UV1600PC, China) at 450nm wavelength.
Statistics
All cultures were conducted in triplets. Their presentation was by means and standard deviation. One-way analysis of variance (p value≤.05) was used as a means for performing statistical analysis and also, statistical analysis was based on excel-spreadsheet-software. The F test was used in the present study.
Results
Two groups( test, negative control) of mice animal models were included in the present study. Each group consisted of 100 mice. The test bivalent mRNA
Swine and
Avian flu vaccine were given to the test groups through an intraperitoneal route of administration; while the negative control groups were administered the placebo intraperitoneally. One month later, repeated booster doses of the test vaccine and placebo were received by the test and negative control groups respectively. Both groups were challenged with the infectious agent through an intranasal route of transmission after 45 days after the beginning of the first vaccine dose. The Immunogenicity testing revealed that about 63%± 1% of animal models were killed. On the other hand randomized human clinical trials phases 1/2 revealed that nearly 60%± 3% of volunteers were protected from obtaining the lethal
Swine or
Avian flu infectious diseases. The count of killed animal models during preclinical trial phases reached 79 after receiving the placebo and infectious dose which ranged from 0.6-3 TCID50; whereas the lethal dose was detected to exceed 5 TCID50. On the other side, during the randomized human clinical trials phases 1/2, only 69 candidates from the negative control group who received the placebo manifested lower respiratory infections when they were subjected to 0.6-3 TCID50 infectious doses of the infectious agents (
Swine,
Avian flu). No symptoms of respiratory infections were detected at infectious doses which ranged from 0.1 to 0.5 TCID50 in randomized human clinical trials phases 1/2. The count of helper lymphocytes type 2 ( TH2) was predominant in participants who received the test mRNA vaccine against Avian and Swine flu as indicated in
Table 2. On the other hand,
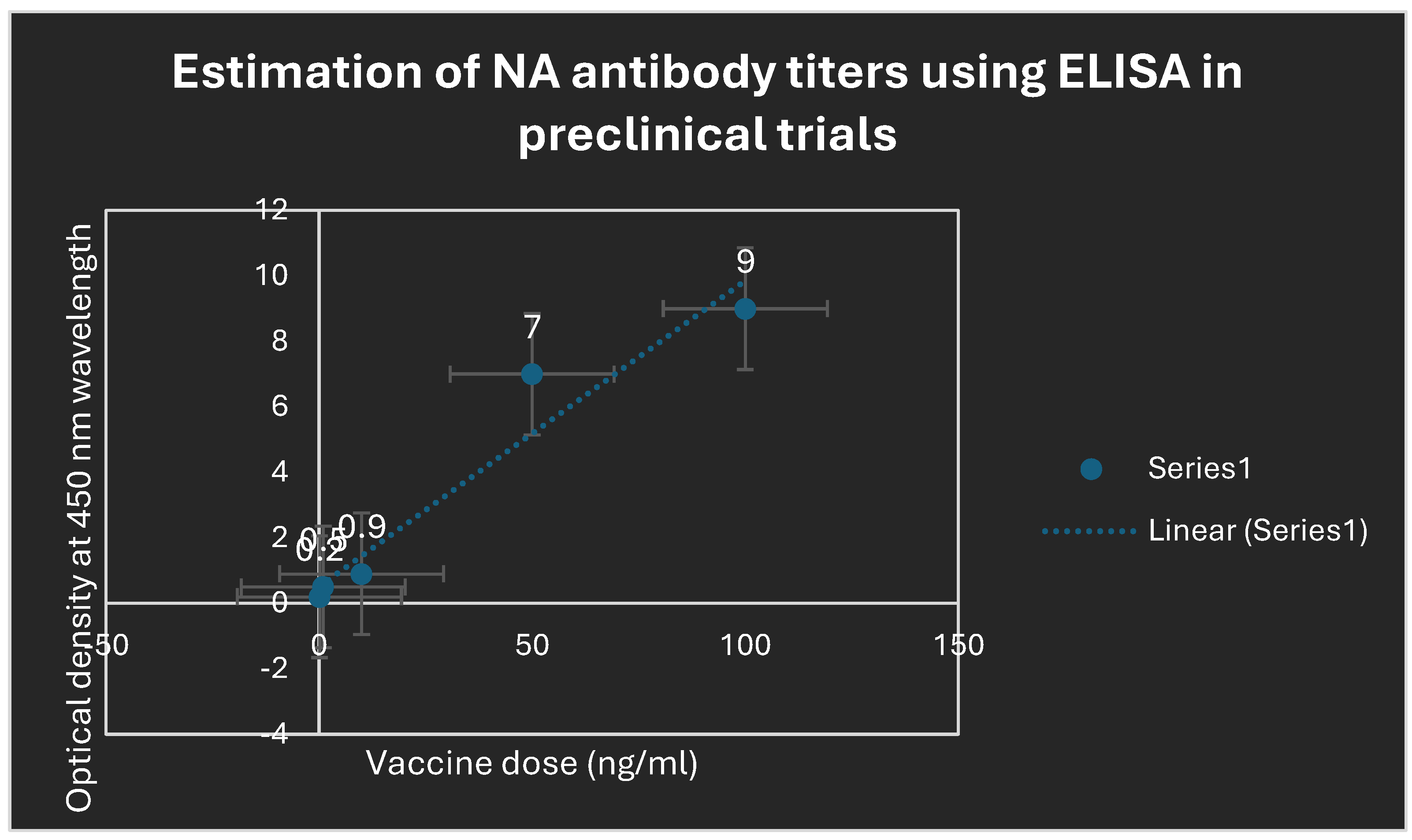
Table 1 revealed that CD+4 type 2 lymphocytes were major prophylactic defense during preclinical trial phases. Potent neutralizing antibodies to NA were detected to play an essential role in the prevention of infection during animal testing as indicated in
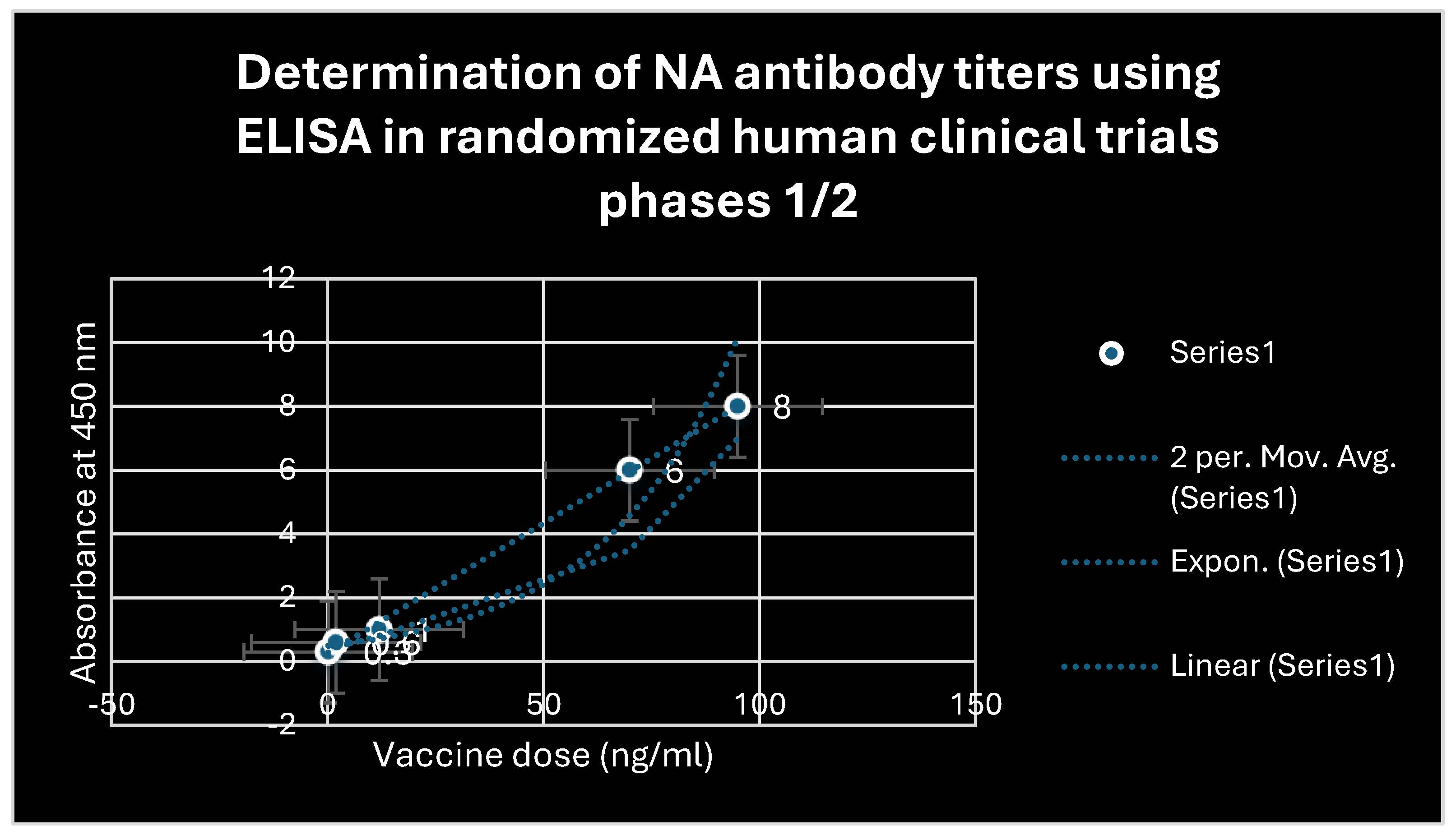
Figure 4. Also during the randomized human clinical trials phases 1/2, potent prophylactic antibodies to NA were detected as confirmed in
Figure 5. The counts of CD+8 lymphocytes were negligible in clinical trials phases 1/2 as well as during animal testing of immunogenicity of the test vaccine. The 3D structure of HA and NA antigens are shown in
Figure 2 and
Figure 3, respectively. Slight pain and redness at the site of vaccine injection as well as mild fever were detected as adverse effects during immunogenicity testing in all preclinical trials phases and randomized human clinical trials phases 1/2.
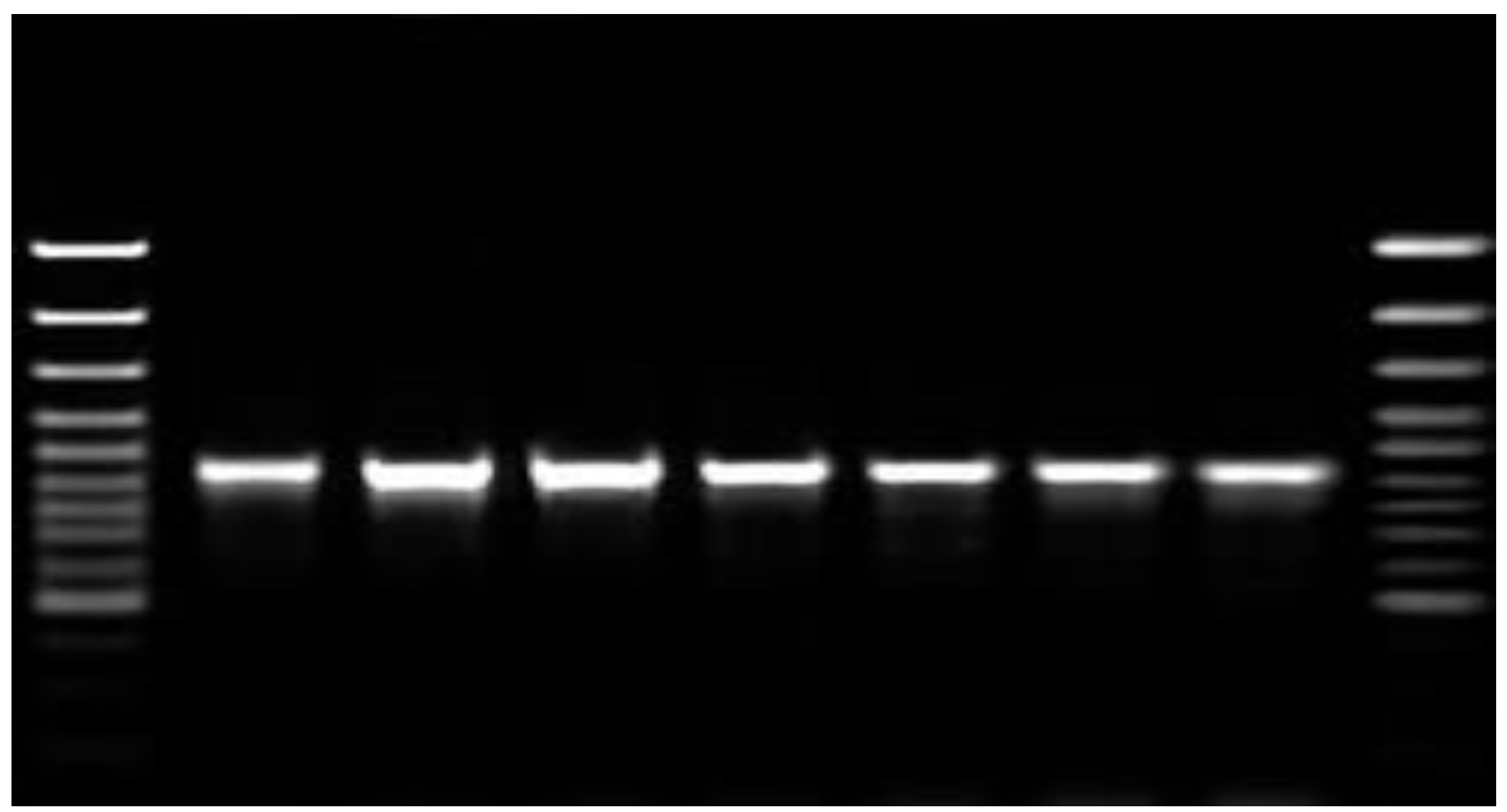
Figure 1 shows the purification of mRNA transcripts of HA and NA using the Northern blot technique. During the randomized human clinical trials phases 1/2, the determination of passive immunizes against
Avian and
Swine flu infectious diseases was achieved using preformed antibodies separated from previously infected patients. It was found that 1/40 HA antibody titers were detected to be the highest dilution which prevented the infections with
Swine flu; whereas
Avian flu infections were hindered using 1/160 HA antibody titers as indicated in
Table 5. Antibodies that were produced after the administration of the mRNA vaccine were of IgM2 and IgG1 types. No secretory IgA antibodies were detected in samples collected from nasal or oral swabs after the challenge with the booster test vaccine doses. As shown in
Table 3 and
Table 4, Potent neutralizing immunoglobulins to HA were detected using ELISA to be the major contributor in the prevention of infectious diseases of I
nfluenza A H1N1 and
H5N1 strains.
Figure 1.
It depicts the purification of mRNA transcripts of HA using the Northern blot technique. The degree of purity was approximately 63%.
Figure 1.
It depicts the purification of mRNA transcripts of HA using the Northern blot technique. The degree of purity was approximately 63%.
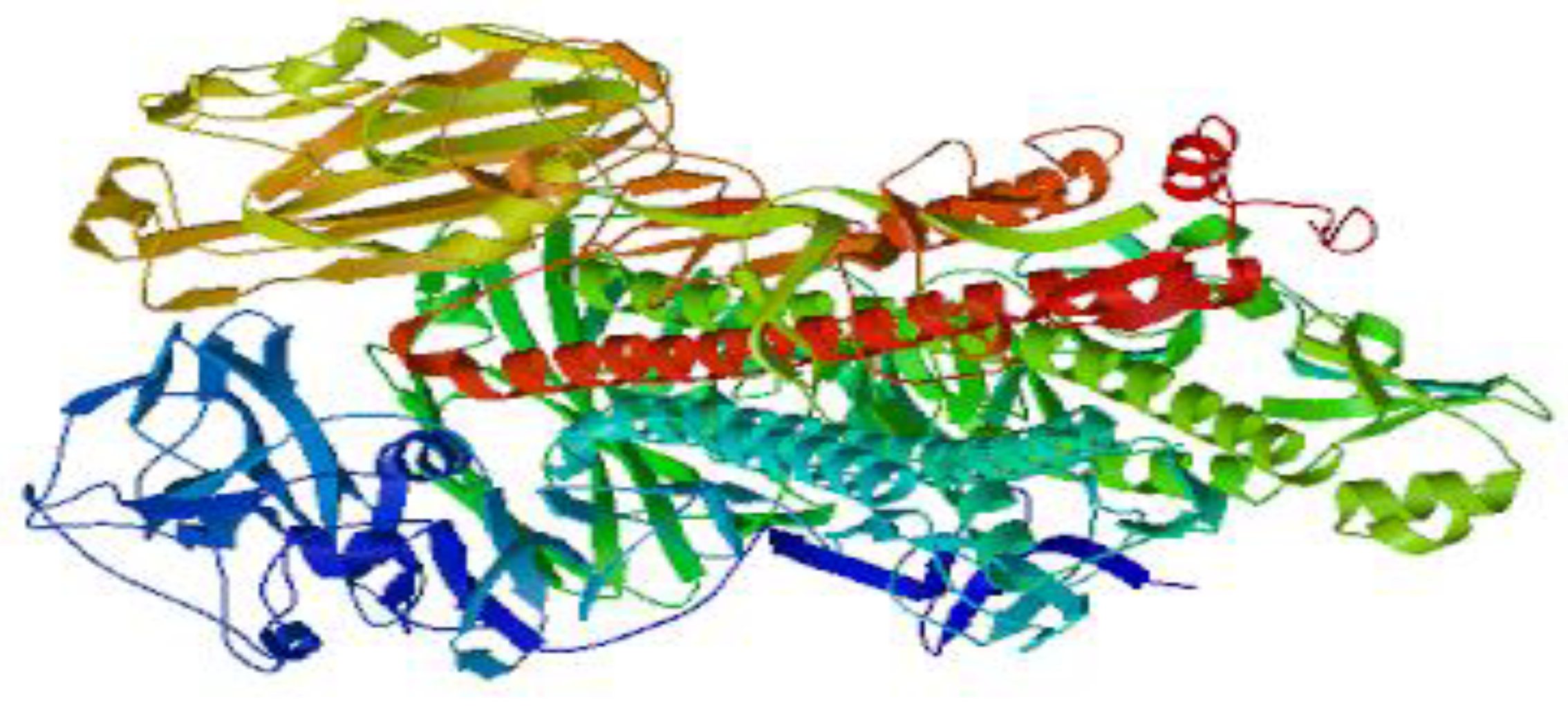
Figure 2.
It shows the 3D structure of NA of Avian flu which elicited the production of neutralizing antibodies in the present study. The 3D structure was designed using SWISS-MODEL software.
Figure 2.
It shows the 3D structure of NA of Avian flu which elicited the production of neutralizing antibodies in the present study. The 3D structure was designed using SWISS-MODEL software.
Figure 3.
It displays the 3D structure of HA of Swine flu which evoked the manufacture of neutralizing antibodies in the attendant work. The 3D structure was configured using SWISS-MODEL software.
Figure 3.
It displays the 3D structure of HA of Swine flu which evoked the manufacture of neutralizing antibodies in the attendant work. The 3D structure was configured using SWISS-MODEL software.
Figure 4.
Determination of antibody titers to NA antigen using ELISA. Potent protect-ant antibodies to NA were elicited due to the previous administration of the Swine and Avian bivalent LNP-mRNA flu vaccine.
Figure 4.
Determination of antibody titers to NA antigen using ELISA. Potent protect-ant antibodies to NA were elicited due to the previous administration of the Swine and Avian bivalent LNP-mRNA flu vaccine.
Figure 5.
Estimation of antibody titers to NA using ELISA after administration of the test mRNA vaccine during randomized human clinical trials phases 1/2. Novel potent neutralizing antibodies to NA were detected.
Figure 5.
Estimation of antibody titers to NA using ELISA after administration of the test mRNA vaccine during randomized human clinical trials phases 1/2. Novel potent neutralizing antibodies to NA were detected.
Table 1.
The count of T lymphocytes in animal testing ( preclinical trials) phases:.
Table 1.
The count of T lymphocytes in animal testing ( preclinical trials) phases:.
| Count of T lymphocytes (cell/mm3) |
CD+4( TH2) cells |
CD+8 (TC) cells |
P-value |
| Before immunization |
300±7 |
150±5 |
P≤0.05 |
| After immunization |
368±10 |
157±2 |
P≤0.01 |
Table 2.
The investigation of T lymphocyte count during randomized human clinical trials phases 1/2:.
Table 2.
The investigation of T lymphocyte count during randomized human clinical trials phases 1/2:.
| Count of T lymphocytes (cell/mm3) |
CD+4( TH2) cells |
CD+8 (TC) cells |
P-value |
| Before vaccination |
502±3 |
267±5 |
P≤0.01 |
| After vaccination |
617±5 |
270±8 |
P≤0.05 |
Table 3.
Assessment of antibody titers to HA antigen using ELISA in preclinical trials stages at P value ≤0.05:.
Table 3.
Assessment of antibody titers to HA antigen using ELISA in preclinical trials stages at P value ≤0.05:.
| Vaccine concentration (ng/ml) |
Optical density at 450 nm |
| 0.2 |
0.2 |
| 10 |
0.73 |
| 20 |
0.8 |
| 40 |
1.2 |
| 80 |
4.00 |
| 100 |
6.57 |
Table 4.
Compartmentalization of antibody titers to HA antigen using ELISA in randomized human clinical trials stages 1/2 at P values ≤ 0.01:.
Table 4.
Compartmentalization of antibody titers to HA antigen using ELISA in randomized human clinical trials stages 1/2 at P values ≤ 0.01:.
| Vaccine concentration (ng/ml) |
Absorbance at 520 nm |
| 0.3 |
0.3 |
| 15 |
0.72 |
| 45 |
0.91 |
| 75 |
3.03 |
| 90 |
5.13 |
| 100 |
7.08 |
Table 5.
Detection of passive immunity in protection tests using ELISA technique after injection of different concentrations of preformed antibody titers to HA antigens during randomized human clinical trials phases 1/2:.
Table 5.
Detection of passive immunity in protection tests using ELISA technique after injection of different concentrations of preformed antibody titers to HA antigens during randomized human clinical trials phases 1/2:.
| HA antibody titer |
Percentage of immunization against Swine flu infection (%) |
Percentage of prophylaxis versus Avian flu infection (%) |
| 1/1280 |
0 |
0 |
| 1/640 |
7 |
9 |
| 1/320 |
20 |
43 |
| 1/160 |
35 |
100 |
| 1/80 |
40 |
100 |
| 1/40 |
100 |
100 |
| 1/10 |
100 |
100 |
Discussion
Swine (H1N1)
and Avian flu (H5N1) cause outbreaks globally every winter. The present study was concerned with the development of LNP-mRNA bivalent vaccines containing HA and NA antigens of
Influenza AH1N1 and
Influenza A H5N1. During the preclinical trials, the protection power of the test vaccine estimated through protection tests reached around 63%; while it was about 60% in randomized human clinical trials phases 1/2. The test vaccine evoked humoral immunity during the immunogenicity and protection tests performed through the induction of potent neutralizing antibodies to HA and NA surface protein antigens in Swine and Avian flu. The humoral immunity was detected using ELISA during the animal testing stage as well as the randomized human clinical trials phases 1/2. TH2 ( CD+4 Type II) lymphocytes were observed to be the prototype of the cell-mediated immunity evoked during protection tests. TH2 lymphocytes mediated the manufacture of neutralizing antibodies to NA and HA antigens. The major types of Immunoglobulins were found to be IgM2 after the first exposure to the infectious agents. On the other side, they were IgG1type following the repeated exposure to the same infectious agent. It was detected that no secretory IgA immunoglobulins were produced due to the absence of oral and/or nasal route of administration of the test vaccine. Only IgA antibodies are produced if the test vaccine was given orally or intranasally. On the other hand, the cell-mediated immunity was detected to be weakly stimulated via the test vaccine. A few counts of T lymphocytes (helper and cytotoxic T lymphocytes) were detected using a flow cytometer apparatus. The booster dose of the test vaccine in addition to AL(OH)
3 improved the immunogenicity and efficacy during randomized human clinical trials phase 1/2 by approximately 3%, due to enhancing the production of neutralizing antibodies to NA and HA surface protein antigens of Influenza A H1N1 and Influenza A H5N1. According to Jordan K et al., 2023 study, the current quadrivalent Flu vaccine led to an overall 70% immunogenicity; [
18] while in the present study, the test vaccine led to nearly 60% immunogenicity. No herd immunity was obtained from the test vaccine, owing to the deficiency of the secretion of secretory IgA antibodies in GIT and/or RT. The limits of the present study comprised a low sample size. In a comparison with the current flu vaccines in the market, the test vaccine was found to be recommended to be given to ages over 2 years; while the current flu vaccines could be utilized in ages above 6 months [
19].
Conclusion
The present study was a promising one since the evolution of novel LNP-mRNA bivalent Swine and Avian flu vaccines occurred. The outbreaks of these infectious agents were detected to be diminished during the current work.
Funding
This study was funded by the single author, Prof. Mohammed Kassab.
Data availability
Data used was provided within the manuscript.
Consent for publication
The author permits the publisher the right to publish the study.
Consent to participate
All informed consents were obtained from all the participants in the present study.
Ethical consideration
All pertinent national, institutional, and/or worldwide guidelines for the care and use of humans and animals were postdated in the current study. The ethical committee for human and animal handling at Cairo University (ECAHCU), the pharmacy faculty at the University of Cairo, Egypt, and the local authorities approved all procedures used in the study, including those involving humans and animals, per the Weatherall report's recommendations (approval number PXV359/2023). Every attempt was made to reduce the number of people and animals used in the study, as well as their suffering. Randomized human clinical trial registration number CTX628/2023 was obtained and authorized by EDA.
Conflict of interest and competing interests
There are no conflicts of interest and competing interests.
References
- Minozzi, S.; Lytras, T.; Gianola, S.; Gonzalez-Lorenzo, M.; Castellini, G.; Galli, C.; Cereda, D.; Bonovas, S.; Pariani, E.; Moja, L. Comparative efficacy and safety of vaccines to prevent seasonal influenza: A systematic review and network meta-analysis. EClinicalMedicine. 2022, 46, 101331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veronika, A.A.; Thirugnanasampanthar, S.S.; Konstantinidis, M.; Dourka, J.; Ghassemi, M.; Neupane, D.; Khan, P.; Nincic, V.; Corry, M.; Robson, R.; Parker, A.; Soobiah, C.; Sinclair, A.; Doyon-Plourde, P.; Gil, A.; Siu, W.; Moqueet, N.; Stevens, A.; English, K.; Florez, I.D.; Yepes-Nuñez, J.J.; Hutton, B.; Muller, M.; Moja, L.; Straus, S.; Tricco, A.C. Trivalent and quadrivalent seasonal influenza vaccine in adults aged 60 and older: a systematic review and network meta-analysis. BMJ Evid Based Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2018, 2, CD004876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jefferson, T.; Rivetti, A.; Di Pietrantonj, C.; Demicheli, V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2018, 2, CD004879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Fougerolles, T.R.; Baïssas, T.; Perquier, G.; Vitoux, O.; Crépey, P.; Bartelt-Hofer, J.; Bricout, H.; Petitjean, A. Public health and economic benefits of seasonal influenza vaccination in risk groups in France, Italy, Spain, and the UK: state of play and perspectives. BMC Public Health. 2024, 24, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Zoellner, Y.; Gradl, B.; Palache, B.; Medema, J. Establishing the health and economic impact of influenza vaccination within the European Union 25 countries. Vaccine 2006, 24, 6812–6822. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Szucs, T.D. Influenza vaccination coverage rates in 5 European countries: a population-based cross-sectional analysis of the seasons 02/03, 03/04 and 04/05. Infection. 2007, 35, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Ruef, C. Influenza vaccination in Europe--still a long way to go. Infection. 2007, 35, 299. [Google Scholar] [CrossRef] [PubMed]
- Influenza vaccine 2011-2012. Med Lett Drugs Ther. 2011, 53, 81–83. [PubMed]
- Katayose, M.; Hosoya, M.; Haneda, T.; Yamaguchi, H.; Kawasaki, Y.; Sato, M.; Wright, P.F. The effectiveness of trivalent inactivated influenza vaccine in children over six consecutive influenza seasons. Vaccine. 2011, 29, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Heinonen, S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine. 2011, 29, 7529–7534. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Wu, Z.; Zhong, S.; Li, M.; Shu, Y. Factors influencing the immunogenicity of influenza vaccines. Hum Vaccin Immunother. 2021, 17, 2706–2718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldstein, L.R.; Matrajt, L.; Elizabeth Halloran, M.; Keitel, W.A.; Longini, I.M., Jr.; H5N1 Vaccine Working Group. Extrapolating theoretical efficacy of inactivated influenza A/H5N1 virus vaccine from human immunogenicity studies. Vaccine 2016, 34, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Campbell, J.D.; Frey, S.E.; Edwards, K.M.; Keitel, W.A.; Kotloff, K.L.; Berry, A.A.; Graham, I.; Atmar, R.L.; Creech, C.B.; Thomsen, I.P.; Patel, S.M.; Gutierrez, A.F.; Anderson, E.L.; El Sahly, H.M.; Hill, H.; Noah, D.L.; Bellamy, A.R. Effect of Varying Doses of a Monovalent H7N9 Influenza Vaccine With and Without AS03 and MF59 Adjuvants on Immune Response: A Randomized Clinical Trial. JAMA. 2015, 314, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, Dickey M, Stapleton JT, Edupuganti S, Spearman P, Ince D, Noah DL, Hill H, Bellamy AR; DMID 13-0032 H7N9 Vaccine Study Group. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 2014, 312, 1409–1419. [CrossRef] [PubMed]
- Belshe RB, Frey SE, Graham IL, Anderson EL, Jackson LA, Spearman P, Edupuganti S, Mulligan MJ, Rouphael N, Winokur P, Dolor RJ, Woods CW, Walter EB, Chen WH, Turley C, Edwards KM, Creech CB, Hill H, Bellamy AR; National Institute of Allergy and Infectious Diseases–Funded Vaccine and Treatment Evaluation Units. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA. 2014, 312, 1420–1428. [CrossRef] [PubMed]
- Chada, K.E.; Forshee, R.; Golding, H.; Anderson, S.; Yang, H. A systematic review and meta-analysis of cross-reactivity of antibodies induced by oil-in-water emulsion adjuvanted influenza H5N1 virus monovalent vaccines. Vaccine. 2017, 35, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Murchu, E.O.; Comber, L.; Hawkshaw, S.; Marshall, L.; O'Neill, M.; Teljeur, C.; Harrington, P.; Carnahan, A.; Pérez-Martín, J.J.; Robertson, A.H.; Johansen, K.; Jonge, J.; Krause, T.; Nicolay, N.; Nohynek, H.; Pavlopoulou, I.; Pebody, R.; Penttinen, P.; Soler-Soneira, M.; Wichmann, O.; Ryan, M. Systematic review of the efficacy, effectiveness and safety of cell-based seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev Med Virol. 2023, 33, e2332. [Google Scholar] [CrossRef] [PubMed]
- OMurchu, E.; Comber, L.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O'Neill, M.; Ryan, M.; Teljeur, C.; Carnahan, A.; Pérez, J.J.; Robertson, A.H.; Johansen, K.; Jonge, J.; Krause, T.; Nicolay, N.; Nohynek, H.; Pavlopoulou, I.; Pebody, R.; Penttinen, P.; Soler-Soneira, M.; Wichmann, O.; Harrington, P. Systematic review of the efficacy, effectiveness and safety of MF59® adjuvanted seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev Med Virol. 2023, 33, e2329. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).