Submitted:
03 June 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
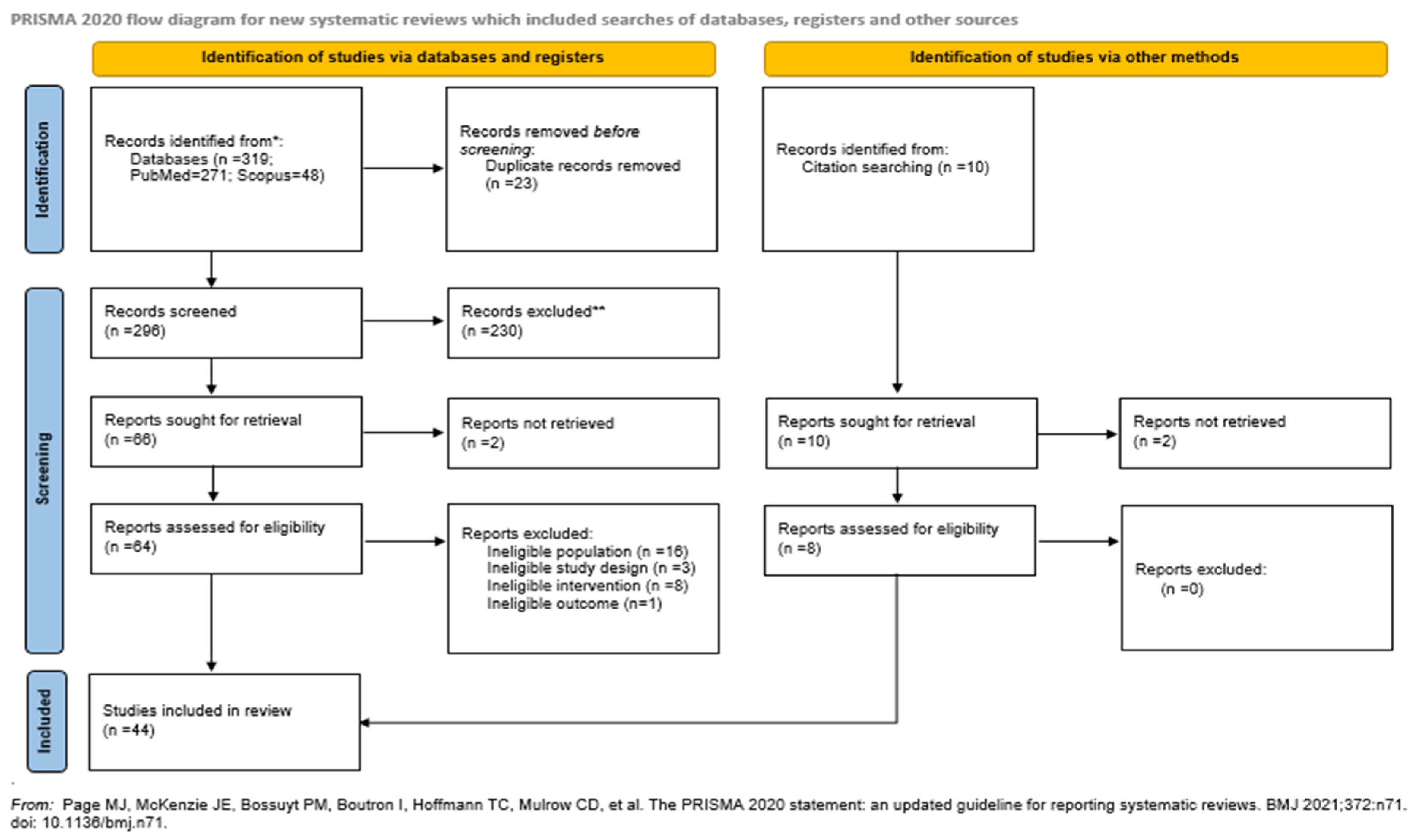
3.1. The selection Process of Included Studies
3.1.1. Preterm Birth
3.1.2. Miscarriage
3.1.3. Gestational Diabetes Mellitus (GDM)
3.1.4. Preeclampsia
3.1.5. Chorioamnionitis (CAT)
3.1.6. Ectopic Pregnancy
3.1.7. Preterm Premature Rapture of Membranes (PPROM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Law A, McCoy M, Lynen R, Curkendall SM, Gatwood J, Juneau PL, et al. The prevalence of complications and healthcare costs during pregnancy. J Med Econ [Internet]. 2015;18:533–41. [CrossRef]
- Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt) [Internet]. 2014;23:3–9. Available from: https://pubmed.ncbi.nlm.nih.gov/24383493. [CrossRef]
- Say L, Pattinson RC, Gülmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health [Internet]. 2004;1:3. Available from: https://pubmed.ncbi.nlm.nih.gov/15357863. [CrossRef]
- Paulo MS, Abdo NM, Bettencourt-Silva R, Al-Rifai RH. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front Endocrinol (Lausanne) [Internet]. 2021;12:691033. Available from: https://pubmed.ncbi.nlm.nih.gov/34956073. [CrossRef]
- Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertension Research [Internet]. 2016;40:213–20. [CrossRef]
- Otolorin E, Gomez P, Currie S, Thapa K, Dao B. Essential basic and emergency obstetric and newborn care: From education and training to service delivery and quality of care. International Journal of Gynecology & Obstetrics [Internet]. 2015;130. [CrossRef]
- Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int [Internet]. 2014/07/17. 2014;2014:297397. Available from: https://pubmed.ncbi.nlm.nih.gov/25136369. [CrossRef]
- Stupak A, Kwaśniewski W. Evaluating Current Molecular Techniques and Evidence in Assessing Microbiome in Placenta-Related Health and Disorders in Pregnancy. Biomolecules [Internet]. 2023;13:911. Available from: https://pubmed.ncbi.nlm.nih.gov/37371491. [CrossRef]
- Taddei CR, Cortez R V, Mattar R, Torloni MR, Daher S. Microbiome in normal and pathological pregnancies: A literature overview. American Journal of Reproductive Immunology [Internet]. 2018;80. [CrossRef]
- Lv L-J, Li S-H, Li S-C, Zhong Z-C, Duan H-L, Tian C, et al. Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Front Cell Infect Microbiol [Internet]. 2019;9:224. Available from: https://pubmed.ncbi.nlm.nih.gov/31297341. [CrossRef]
- Swati P, Thomas B, Vahab SA, Kapaettu S, Kushtagi P. Simultaneous detection of periodontal pathogens in subgingival plaque and placenta of women with hypertension in pregnancy. Arch Gynecol Obstet [Internet]. 2011;285:613–9. [CrossRef]
- Sobel JD. Is there a protective role for vaginal flora? Curr Infect Dis Rep [Internet]. 1999;1:379–83. [CrossRef]
- Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high d/l lactate ratio is consistent with bacteria being the primary source. Human Reproduction [Internet]. 2001;16:1809–13. [CrossRef]
- Kaewsrichan J, Peeyananjarassri K, Kongprasertkit J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunology & Medical Microbiology [Internet]. 2006;48:75–83. [CrossRef]
- Fortenberry JD. The uses of race and ethnicity in human microbiome research. Trends Microbiol [Internet]. 2013;21:165–6. [CrossRef]
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta T-A, Coarfa C, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One [Internet]. 2012/06/13. 2012;7:e36466–e36466. Available from: https://pubmed.ncbi.nlm.nih.gov/22719832. [CrossRef]
- Serrano MG, Parikh HI, Brooks JP, Edwards DJ, Arodz TJ, Edupuganti L, et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat Med [Internet]. 2019/05/29. 2019;25:1001–11. Available from: https://pubmed.ncbi.nlm.nih.gov/31142850. [CrossRef]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A [Internet]. 2010/06/03. 2011;108 Suppl 1:4680–7. Available from: https://pubmed.ncbi.nlm.nih.gov/20534435. [CrossRef]
- Abdulla SR, Kareem SR, Hasan AH. Vaginal Microbiota Profile in first-trimester miscarriages cases. Cell Mol Biol [Internet]. 2023;69:9–17. [CrossRef]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev [Internet]. 2016;5:210. Available from: https://pubmed.ncbi.nlm.nih.gov/27919275. [CrossRef]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet [Internet]. 2012;379:2162–72. [CrossRef]
- Baldwin EA, Walther-Antonio M, MacLean AM, Gohl DM, Beckman KB, Chen J, et al. Persistent microbial dysbiosis in preterm premature rupture of membranes from onset until delivery. PeerJ [Internet]. 2015;3:e1398–e1398. Available from: https://pubmed.ncbi.nlm.nih.gov/26644969. [CrossRef]
- Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol [Internet]. 2015 [cited 2024 Mar 15];6:164. Available from: /pmc/articles/PMC4451362/. [CrossRef]
- Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C, et al. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case–control study. BJOG: An International Journal of Obstetrics & Gynaecology [Internet]. 2018;126:349–58. [CrossRef]
- Blostein F, Gelaye B, Sanchez SE, Williams MA, Foxman B. Vaginal microbiome diversity and preterm birth: results of a nested case-control study in Peru. Ann Epidemiol [Internet]. 2019/12/05. 2020;41:28–34. Available from: https://pubmed.ncbi.nlm.nih.gov/31883841. [CrossRef]
- Ng S, Chen M, Kundu S, Wang X, Zhou Z, Zheng Z, et al. Large-scale characterisation of the pregnancy vaginal microbiome and sialidase activity in a low-risk Chinese population. NPJ Biofilms Microbiomes [Internet]. 2021;7:89. Available from: https://pubmed.ncbi.nlm.nih.gov/34930922. [CrossRef]
- Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci [Internet]. 2013/05/28. 2014;21:32–40. Available from: https://pubmed.ncbi.nlm.nih.gov/23715799. [CrossRef]
- Gulavi E, Mwendwa F, Atandi DO, Okiro PO, Hall M, Beiko RG, et al. Vaginal microbiota in women with spontaneous preterm labor versus those with term labor in Kenya: a case control study. BMC Microbiol [Internet]. 2022;22:270. Available from: https://pubmed.ncbi.nlm.nih.gov/36357861. [CrossRef]
- Stout MJ, Zhou Y, Wylie KM, Tarr PI, Macones GA, Tuuli MG. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol [Internet]. 2017/05/23. 2017;217:356.e1-356.e18. Available from: https://pubmed.ncbi.nlm.nih.gov/28549981. [CrossRef]
- Freitas AC, Bocking A, Hill JE, Money DM, Group VR. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome [Internet]. 2018;6:117. Available from: https://pubmed.ncbi.nlm.nih.gov/29954448. [CrossRef]
- Odogwu NM, Chen J, Onebunne CA, Jeraldo P, Yang L, Johnson S, et al. Predominance of Atopobium vaginae at Midtrimester: a Potential Indicator of Preterm Birth Risk in a Nigerian Cohort. mSphere [Internet]. 2021;6:e01261-20. Available from: https://pubmed.ncbi.nlm.nih.gov/33504666. [CrossRef]
- Brown RG, Marchesi JR, Lee YS, Smith A, Lehne B, Kindinger LM, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med [Internet]. 2018 [cited 2024 Mar 15];16. Available from: /pmc/articles/PMC5782380/. [CrossRef]
- Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci U S A [Internet]. 2017/08/28. 2017;114:9966–71. Available from: https://pubmed.ncbi.nlm.nih.gov/28847941. [CrossRef]
- Chang D-H, Shin J, Rhee M-S, Park K-R, Cho B-K, Lee S-K, et al. Vaginal Microbiota Profiles of Native Korean Women and Associations with High-Risk Pregnancy. J Microbiol Biotechnol [Internet]. 2020;30:248–58. Available from: https://pubmed.ncbi.nlm.nih.gov/31838792. [CrossRef]
- Shin H, Wu J, Nelson D, Dominguez-Bello M. The Gestational Vaginal Microbiome and Spontaneous Preterm Birth among Nulliparous African American Women. Am J Perinatol [Internet]. 2016;33:887–93. [CrossRef]
- Dunlop AL, Satten GA, Hu Y-J, Knight AK, Hill CC, Wright ML, et al. Vaginal Microbiome Composition in Early Pregnancy and Risk of Spontaneous Preterm and Early Term Birth Among African American Women. Front Cell Infect Microbiol [Internet]. 2021;11:641005. Available from: https://pubmed.ncbi.nlm.nih.gov/33996627. [CrossRef]
- Stafford GP, Parker JL, Amabebe E, Kistler J, Reynolds S, Stern V, et al. Spontaneous Preterm Birth Is Associated with Differential Expression of Vaginal Metabolites by Lactobacilli-Dominated Microflora. Front Physiol [Internet]. 2017;8:615. Available from: https://pubmed.ncbi.nlm.nih.gov/28878691. [CrossRef]
- de Freitas AS, Dobbler PCT, Mai V, Procianoy RS, Silveira RC, Corso AL, et al. Defining microbial biomarkers for risk of preterm labor. Braz J Microbiol [Internet]. 2019/07/22. 2020;51:151–9. Available from: https://pubmed.ncbi.nlm.nih.gov/31332740. [CrossRef]
- Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome [Internet]. 2017;5:6. Available from: https://pubmed.ncbi.nlm.nih.gov/28103952. [CrossRef]
- Petricevic L, Domig KJ, Nierscher FJ, Sandhofer MJ, Fidesser M, Krondorfer I, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep [Internet]. 2014;4:5136. Available from: https://pubmed.ncbi.nlm.nih.gov/24875844. [CrossRef]
- Payne MS, Newnham JP, Doherty DA, Furfaro LL, Pendal NL, Loh DE, et al. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am J Obstet Gynecol [Internet]. 2021;224:206.e1-206.e23. [CrossRef]
- Fettweis JM, Serrano MG, Sheth NU, Mayer CM, Glascock AL, Brooks JP, et al. Species-level classification of the vaginal microbiome. BMC Genomics [Internet]. 2012 [cited 2024 Mar 15];13:S17. Available from: /pmc/articles/PMC3535711/. [CrossRef]
- Kumar M, Murugesan S, Singh P, Saadaoui M, Elhag DA, Terranegra A, et al. Vaginal Microbiota and Cytokine Levels Predict Preterm Delivery in Asian Women. Front Cell Infect Microbiol [Internet]. 2021;11:639665. Available from: https://pubmed.ncbi.nlm.nih.gov/33747983. [CrossRef]
- Menard J, Fenollar F, Henry M, Bretelle F, Raoult D. Molecular Quantification ofGardnerella vaginalisandAtopobium vaginaeLoads to Predict Bacterial Vaginosis. Clinical Infectious Diseases [Internet]. 2008;47:33–43. [CrossRef]
- Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun [Internet]. 2019;10:1305. Available from: https://pubmed.ncbi.nlm.nih.gov/30899005. [CrossRef]
- Liao J, Shenhav L, Urban JA, Serrano M, Zhu B, Buck GA, et al. Microdiversity of the vaginal microbiome is associated with preterm birth. Nat Commun [Internet]. 2023;14:4997. Available from: https://pubmed.ncbi.nlm.nih.gov/37591872. [CrossRef]
- Wylie KM, Wylie TN, Cahill AG, Macones GA, Tuuli MG, Stout MJ. The vaginal eukaryotic DNA virome and preterm birth. Am J Obstet Gynecol [Internet]. 2018/05/05. 2018;219:189.e1-189.e12. Available from: https://pubmed.ncbi.nlm.nih.gov/29738749. [CrossRef]
- Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. The Lancet [Internet]. 2021;397:1658–67. [CrossRef]
- Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med [Internet]. 2013;11:154. Available from: https://pubmed.ncbi.nlm.nih.gov/23803387. [CrossRef]
- Devall AJ, Coomarasamy A. Sporadic pregnancy loss and recurrent miscarriage. Best Practice & Research Clinical Obstetrics & Gynaecology [Internet]. 2020;69:30–9. [CrossRef]
- Gryaznova M, Kozarenko O, Smirnova Y, Burakova I, Syromyatnikov M, Maslov A, et al. Cervical and Vaginal Microbiomes in Early Miscarriages and Ongoing Pregnancy with and without Dydrogesterone Usage. Int J Mol Sci [Internet]. 2023;24:13836. Available from: https://pubmed.ncbi.nlm.nih.gov/37762139. [CrossRef]
- Shahid M, Quinlivan JA, Peek M, Castaño-Rodríguez N, Mendz GL. Is there an association between the vaginal microbiome and first trimester miscarriage? A prospective observational study. Journal of Obstetrics and Gynaecology Research [Internet]. 2021;48:119–28. [CrossRef]
- Al-Memar M, Bobdiwala S, Fourie H, Mannino R, Lee YS, Smith A, et al. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG [Internet]. 2019/10/31. 2020;127:264–74. Available from: https://pubmed.ncbi.nlm.nih.gov/31573753. [CrossRef]
- Sun D, Zhao X, Pan Q, Li F, Gao B, Zhang A, et al. The association between vaginal microbiota disorders and early missed abortion: A prospective study. Acta Obstet Gynecol Scand [Internet]. 2022/07/24. 2022;101:960–71. Available from: https://pubmed.ncbi.nlm.nih.gov/35871770. [CrossRef]
- Nelson DB, Hanlon AL, Wu G, Liu C, Fredricks DN. First Trimester Levels of BV-Associated Bacteria and Risk of Miscarriage Among Women Early in Pregnancy. Matern Child Health J [Internet]. 2015;19:2682–7. [CrossRef]
- Nelson DB, Bellamy S, Nachamkin I, Ness RB, Macones GA, Allen-Taylor L. First trimester bacterial vaginosis, individual microorganism levels, and risk of second trimester pregnancy loss among urban women. Fertil Steril [Internet]. 2007/04/16. 2007;88:1396–403. Available from: https://pubmed.ncbi.nlm.nih.gov/17434499. [CrossRef]
- Zhang F, Zhang T, Ma Y, Huang Z, He Y, Pan H, et al. Alteration of vaginal microbiota in patients with unexplained recurrent miscarriage. Exp Ther Med [Internet]. 2019/03/04. 2019;17:3307–16. Available from: https://pubmed.ncbi.nlm.nih.gov/30988706. [CrossRef]
- Jiao X, Zhang L, Du D, Wang L, Song Q, Liu S. Alteration of vaginal microbiota in patients with recurrent miscarriage. J Obstet Gynaecol (Lahore) [Internet]. 2021;42:248–55. [CrossRef]
- Soyer Caliskan C, Yurtcu N, Celik S, Sezer O, Kilic SS, Cetin A. Derangements of vaginal and cervical canal microbiota determined with real-time PCR in women with recurrent miscarriages. J Obstet Gynaecol (Lahore) [Internet]. 2022;42:2105–14. [CrossRef]
- Peuranpää P, Holster T, Saqib S, Kalliala I, Tiitinen A, Salonen A, et al. Female reproductive tract microbiota and recurrent pregnancy loss: a nested case-control study. Reprod Biomed Online [Internet]. 2022;45:1021–31. [CrossRef]
- Fan T, Zhong X-M, Wei X-C, Miao Z-L, Luo S-Y, Cheng H, et al. The alteration and potential relationship of vaginal microbiota and chemokines for unexplained recurrent spontaneous abortion. Medicine [Internet]. 2020;99:e23558–e23558. Available from: https://pubmed.ncbi.nlm.nih.gov/33371084. [CrossRef]
- Liu F-T, Yang S, Yang Z, Zhou P, Peng T, Yin J, et al. An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women with Recurrent Spontaneous Abortion. Microbiol Spectr [Internet]. 2022/05/23. 2022;10:e0046222–e0046222. Available from: https://pubmed.ncbi.nlm.nih.gov/35604131. [CrossRef]
- Ponzo V, Fedele D, Goitre I, Leone F, Lezo A, Monzeglio C, et al. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients [Internet]. 2019;11:330. Available from: https://pubmed.ncbi.nlm.nih.gov/30717458. [CrossRef]
- Hasain Z, Mokhtar NM, Kamaruddin NA, Mohamed Ismail NA, Razalli NH, Gnanou JV, et al. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front Cell Infect Microbiol [Internet]. 2020;10:188. Available from: https://pubmed.ncbi.nlm.nih.gov/32500037. [CrossRef]
- Cortez R V, Taddei CR, Sparvoli LG, Ângelo AGS, Padilha M, Mattar R, et al. Microbiome and its relation to gestational diabetes. Endocrine [Internet]. 2018;64:254–64. [CrossRef]
- Zheng N-N, Guo X-C, Lv W, Chen X-X, Feng G-F. Characterization of the vaginal fungal flora in pregnant diabetic women by 18S rRNA sequencing. European Journal of Clinical Microbiology & Infectious Diseases [Internet]. 2013;32:1031–40. [CrossRef]
- Kuklina E V, Ayala C, Callaghan WM. Hypertensive Disorders and Severe Obstetric Morbidity in the United States. Obstetrics & Gynecology [Internet]. 2009;113:1299–306. [CrossRef]
- Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European Journal of Obstetrics & Gynecology and Reproductive Biology [Internet]. 2013;170:1–7. [CrossRef]
- Lin C-Y, Lin C-Y, Yeh Y-M, Yang L-Y, Lee Y-S, Chao A, et al. Severe preeclampsia is associated with a higher relative abundance of Prevotella bivia in the vaginal microbiota. Sci Rep [Internet]. 2020;10:18249. Available from: https://pubmed.ncbi.nlm.nih.gov/33106556. [CrossRef]
- Suzuki S. Association between clinical chorioamnionitis and histological funisitis at term. J Neonatal Perinatal Med [Internet]. 2019;12:37–40. [CrossRef]
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol [Internet]. 2015;213:S29–52. Available from: https://pubmed.ncbi.nlm.nih.gov/26428501. [CrossRef]
- Guo X, Hong X, Qian H, Qiao D, Wang B, Yu H. Relationship between vaginal microbiota and chorioamnionitis: A prospective cohort study. Microb Pathog [Internet]. 2024;186:106458. [CrossRef]
- Houser M, Kandalaft N, Khati NJ. Ectopic pregnancy: a resident’s guide to imaging findings and diagnostic pitfalls. Emerg Radiol [Internet]. 2021;29:161–72. [CrossRef]
- Ruan X-F, Zhang Y-X, Chen S, Liu X-R, Zhu F-F, Huang Y-X, et al. Non-Lactobacillus-Dominated Vaginal Microbiota Is Associated With a Tubal Pregnancy in Symptomatic Chinese Women in the Early Stage of Pregnancy: A Nested Case-Control Study. Front Cell Infect Microbiol [Internet]. 2021;11:659505. Available from: https://pubmed.ncbi.nlm.nih.gov/34307190. [CrossRef]
- Nunes V, Cross J, Speich JE, Morgan DR, Strauss 3rd JF, Ramus RM. Fetal membrane imaging and the prediction of preterm birth: a systematic review, current issues, and future directions. BMC Pregnancy Childbirth [Internet]. 2016;16:387. Available from: https://pubmed.ncbi.nlm.nih.gov/27938341. [CrossRef]
- Yan C, Hong F, Xin G, Duan S, Deng X, Xu Y. Alterations in the vaginal microbiota of patients with preterm premature rupture of membranes. Front Cell Infect Microbiol [Internet]. 2022;12. [CrossRef]
- Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci Transl Med [Internet]. 2012;4. [CrossRef]
- Amabebe E, Anumba DOC. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front Med (Lausanne) [Internet]. 2018;5:181. Available from: https://pubmed.ncbi.nlm.nih.gov/29951482. [CrossRef]
- Ma Y, Li Y, Liu Y, Cao L, Han X, Gao S, et al. Vaginal Microbiome Dysbiosis is Associated with the Different Cervical Disease Status. Journal of Microbiology [Internet]. 2023;61:423–32. [CrossRef]
- Lewis FMT, Bernstein KT, Aral SO. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstetrics & Gynecology [Internet]. 2017;129:643–54. [CrossRef]
- Tsonis O, Gkrozou F, Harrison E, Stefanidis K, Vrachnis N, Paschopoulos M. Female genital tract microbiota affecting the risk of preterm birth: What do we know so far? A review. European Journal of Obstetrics & Gynecology and Reproductive Biology [Internet]. 2020;245:168–73. [CrossRef]
- Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, et al. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. mBio [Internet]. 2016;7:e00781-16. Available from: https://pubmed.ncbi.nlm.nih.gov/27353757. [CrossRef]
- Cauci S, Hitti J, Noonan C, Agnew K, Quadrifoglio F, Hillier SL, et al. Vaginal hydrolytic enzymes, immunoglobulin A against Gardnerella vaginalis toxin, and risk of early preterm birth among women in preterm labor with bacterial vaginosis or intermediate flora. Am J Obstet Gynecol [Internet]. 2002;187:877–81. [CrossRef]
- Macklaim JM, Clemente JC, Knight R, Gloor GB, Reid G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb Ecol Health Dis [Internet]. 2015;26:27799. Available from: https://pubmed.ncbi.nlm.nih.gov/26282697. [CrossRef]
- Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, et al. Effects of Probiotics on the Recurrence of Bacterial Vaginosis. J Low Genit Tract Dis [Internet]. 2014;18:79–86. [CrossRef]
- Dugoua J-J, Machado M, Zhu X, Chen X, Koren G, Einarson TR. Probiotic Safety in Pregnancy: A Systematic Review and Meta-analysis of Randomized Controlled Trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. Journal of Obstetrics and Gynaecology Canada [Internet]. 2009;31:542–52. Http://dx.doi.org/. [CrossRef]
- Zhu B, Tao Z, Edupuganti L, Serrano MG, Buck GA. Roles of the Microbiota of the Female Reproductive Tract in Gynecological and Reproductive Health. Microbiol Mol Biol Rev [Internet]. 2022/10/12. 2022;86:e0018121–e0018121. Available from: https://pubmed.ncbi.nlm.nih.gov/36222685. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




