1. Introduction
Classical Swine Fever (CSF) is a highly contagious disease causing great losses in the porcine industry worldwide and, in particular, to developing countries [
1,
2]. The etiological agent is the classical swine fever virus, an RNA envelope virus from the Flaviviridae family. CSF is enzootic in areas of Central America and the Caribbean, South America, Southeast Asia and Eastern Europe. The control of the disease in endemic areas have been attempted through the application of modified live vaccines (MLV), however, the implementation of prolonged and imperfect vaccination programs has led to the reduction of the genetic diversity of the circulating strains of CSF and to a change in the pathogenicity of the novel, less virulent, persistent virus strains [
3,
4,
5,
6].
In Cuba, all pigs have been vaccinated with a lapinized MLV (Labiofam C strain) since 1965. For decades the country remained CSF free, but a viral outburst was detected in 1993 [
7]. Molecular epidemiology studies suggested that the reemergence of the disease was related to the escape of the highly virulent “Margarita” strain used in challenge experiments for vaccine lot release [
8].
Ten years after this reemergence the disease had become endemic in the country. The high rate of non-synonymous mutations found in the fragment of E2 sequence together with the tendency towards less virulent forms of the disease suggested that evolution of the virus has been driven by the selective pressure associated to a prolonged and inefficient vaccination program [
9]. Years later, it was demonstrated that the inability of the MLV in use to confer sterilizing immunity caused a bottleneck effect that facilitated the emergence of a viral population with new characteristics, lower virulence and pathogenicity as possible escape mutants to the NAb elicited by this vaccine [
5]. These authors identified six non-synonymous mutations located mainly in the B/C domain towards the N-terminus of the E2 protein. This domain has been pointed out by other researchers as the most variable within the protein and where mutations that allow the virus to escape from selective pressure are selected [
10,
11].
Subunit vaccines are now available which, in contrast to MLV, induce antibodies that can be theoretically distinguished from those generated by the natural virus (DIVA). The subunit vaccine Porvac
® was developed as an alternative to traditional MLV in endemic areas such as Cuba. Its active ingredient is the chimeric protein E2-CD154, formed by the external region of the E2 glycoprotein of the CSFV, coupled to the extracellular segment of the pig CD154 molecule. Porvac
® provides a very rapid onset of protection, similar to MLV [
12] and protects against the vertical transmission of the virus [
13].
However, the highly virulent “Margarita” strain of CSFV has been used in all challenge experiments conducted in Porvac
® vaccinated pigs. Since Porvac
® is being used as part of the control and elimination program of CSFV in Cuba, it is of great relevance to demonstrate its efficacy in a confrontation experiment against viral isolates circulating in our country. Many of these circulating isolates are of low or medium virulence [
5] and could have evolved to escape the selective pressure exerted by the MLV. The aim of this study was to evaluate whether the immunity conferred by Porvac
® is also able to protect pigs from the challenge with three autochthonous isolates of CSFV of low, medium or medium/high virulence.
2. Materials and Methods
2.1. Viruses and Cells
The three Cuban field CSFV strains used in the challenge experiments were isolated from pigs with atypical CSF symptomatology by the Virology Laboratory of Nacional Center for Animal Health (CENSA, Mayabeque, Cuba). Holguin_2009 (GenBank accession number HE584533) was isolated from Holguín province in 2009 [
5]. PR-11/10-3 (GenBank accession number KX576461) [
5,
14], also referred to as CSF1058 according to the nomenclature of the European Union Reference Laboratory for Classical Swine Fever (EURL-CSF), Hannover, Germany, and PR-2016 [
15] (GenBank accession number LT985811) were isolated from Pinar del Río province in 2010 and 2016, respectively. The three viruses were titrated by end point dilution in PK-15 cells using a Peroxidase Immunostaining Assay with the anti-E2 monoclonal antibody (Mab) CBSSE2.3 (Center for Genetic Engineering of Sancti Spíritus (CIGB-SS), Cuba) conjugated to horseradish peroxidase.
The pig kidney PK-15 cell line (ATCC CCL-33) was also used in the Neutralizing Peroxidase Linked Assay (NPLA) and Virus Isolation assays. It was grown in Dulbecco Modified Eagle Medium supplemented with 10% Fetal Calf Serum (Capricorn, Australia) and antibiotics 10,000 U/ mL of penicillin/10 mg/mL streptomycin (Sigma, USA).
2.2. Porvac® Vaccine
Porvac® subunit vaccine was kindly provided by the Center for Genetic Engineering and Biotechnology of Camagüey (Camagüey, Cuba). The active principle of this vaccine is the chimeric protein E2-CD154, formed by the fusion of the extracellular region of E2 glycoprotein of CSFV “Margarita” strain and the extracellular segment of swine CD154 molecule. A lentivirus-based gene delivery system was used to generate a stable recombinant HEK 293 cell line (ATCC CRL1573) for the expression E2-CD154, as previously described. E2-CD154 protein was formulated in MontanideTM ISA50 V2 (SEPPIC, La Garenne-Colombes, France) using a 60/40 proportion of aqueous/oil phase and a SD-41 homogenizer (IKA, Königswinter, Germany) under Good Manufacturing Practice (GMP) conditions. The concentration of E2-CD154 in the final emulsion was 25 μg/mL.
2.3. Experimental Animals
The trials were conducted under high containment conditions following the animal welfare regulations and standards from EU Directive 2010/63/EU (EU Directive 2010/63, Official J. of EU) and Good Clinical Practices [
16]. Twenty-four nine-week-old crossbred Duroc/Yorkshire swine (25–30 kg) were purchased from a CSF-free, non-vaccinated herd at National Center for Animal Production, (CENPALAB, Cuba). The animals were given 2 Kg/per animal/per day of food and water
ad libitum. The pigs in the paddocks were tagged with visible identification marks (notches).
2.4. Experimental Design, Immunization Schedule and Viral Challenge
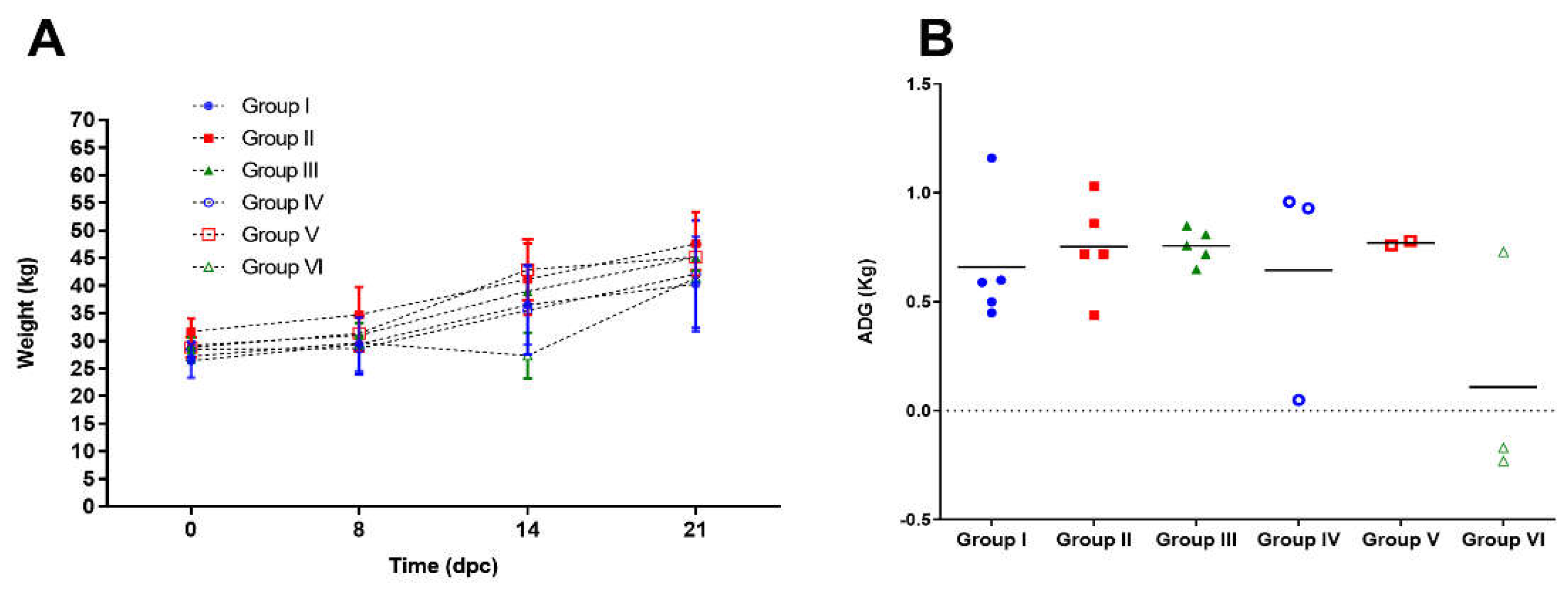
The animals were randomly allocated into six experimental groups:
Group 1: 5 animals vaccinated with Porvac® and challenged with isolate PR-11/10–3.
Group 2: 5 animals vaccinated with Porvac® and challenged with isolate Holguín_2009.
Group 3: 5 animals vaccinated with Porvac® and challenged with isolate PR-2016.
Group 4: 3 non-vaccinated control animals challenged with isolate PR-11/10–3.
Group 5: 3 non-vaccinated control animals challenged with isolate Holguín_2009.
Group 6: 3 non-vaccinated control animals challenged with isolate PR-2016.
The pigs in groups 1, 2 and 3 were immunized twice by intramuscular injection with 2 ml of the vaccine (50 µg of E2-CD154 antigen). The first immunization was performed at day 0 on the right side of the neck, and the second at day 21 on the left side, using 18 G X 1-inch needles in compliance with good veterinary clinical practices. Control animals (Groups 4, 5 and 6) were immunized with mock formulation (Montanide ISA50V2, SEPPIC, France, emulsified with phosphate buffer saline).
Seven days after the second injection, all piglets were intranasally challenged with 2 x10
3 TCID
50 of each CSFV strain. Clinical signs were scored daily for 21 days after challenge according to Mittelholzer et al., [
17] with some modifications as described elsewhere [
12]. The samples of heparinized blood and serum were taken at challenge and at 7, 14, and 21 days post-challenge. Blood samples were taken by ophthalmic venous sinus punctures using sterile tubes, with and without anti-coagulant (VACUATTE
® Greiner bio-one, Frickenhausen, Germany). The blood samples were placed at room temperature for 2 h and then kept overnight at 2–8 °C to allow for serum extraction.
The protocol was approved and supervised by the Ethical Committee of CENPALAB.
2.5. Neutralizing Antibodies Detection
The serum samples were screened for the ability to neutralize the cell culture adapted “Margarita” CSFV strain in a Neutralizing Peroxidase-Linked Assay (NPLA), following the recommendations of WOAH [
18]. This assay has been approved for its routine application to Porvac
® batch release.
2.6. Detection of Antibodies Directed against the Erns Protein of CSFV
The commercial ELISA test PrioCHECK® CSFV Erns (Applied Biosystem, USA) was used to detect the presence of antibodies against Erns protein of CSFV in the sera of the piglets after the viral challenge. This is a competition ELISA, based on the blocking of the binding of Erns MAbs to the Erns protein. The assay was conducted according to the manufacturer’s instructions. A sample was regarded as positive for Erns antibodies if the percent of inhibition was greater than 40%.
2.7. Viral Isolation
Heparinized blood samples were taken at day 3, 7, 14, 21 and 28 days post-challenge (dpc) for viral isolation. The protocol described in the Manual of World Organization for Animal Health was followed [
18]. Briefly, tonsils and spleens were collected at the end of the experiment at 28 dpc. Tissues (approximately 1 cm
2) were macerated in 1 mL of DMEM (Sigma, St Louis, MO, USA) supplemented with 5% fetal calf serum, penicillin (100 IU), and streptomycin (100 μg). The homogenates were suspended in 4 mL of DMEM and allowed to settle for 1h at room temperature. Afterward, the samples were centrifuged at 1200 rpm for 15 min. and the supernatant transferred and preserved in cryovials (Sigma-Aldrich, USA) at -80ºC. Viral isolation was performed in PK-15 cells through two serial passages in 48 wells microplates and the third passage onto 96 wells microplates, with six replicates for each sample. Virus was detected with the anti-E2 Mab CBSSE2.3 (CIGB-SS, Cuba) conjugated to horseradish peroxidase, followed by incubation with 3-amino-9-ethyl carbazole (AEC) and hydrogen peroxide. A control curve of with the “Margarita” strain of CSFF with 1000, 100, 10 and 1 TCID
50 diluted in serum was run in every assay. For the assay to be valid, it should detect at last 10 TCID
50 of this virus.
2.8. Statistical Analysis
The normal distribution of the data was assessed by Kolmogorov–Smirnov and D’Agostino–Pearson tests. The Kruskal–Wallis test was used to compare antibody titers among groups of animals and Dunn’s multiple comparisons test to look for individual differences among groups. Mann-Whitney test was applied to compare antibody titers between two experimental groups. The statistical package GraphPad Prism 6 was used for all of the analysis (GraphPad Prism for Windows, Version 6.01, GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was considered when p < 0.05.
4. Discussion
Between 2001 and 2003, ten years after the beginning of the CSF epizooty in Cuba, several strains were isolated from animals with clinical signs corresponding to chronic forms of the disease. In these isolates, the presence of a rate of non-synonymous nucleotide mutations was observed in the same fragment of the partial E2 gene sequence with respect to the ancestral strain “Margarita”[
9]. The results of this and other studies suggested that the evolution of the virus in Cuba could have been driven by a positive selection pressure associated with inefficient vaccination programs with the lapinized MLV [
9].
The lower virulence of the circulating viruses makes the clinical picture mild, often undetectable, although they can cause deficiencies in the growth of the animals and predisposition to other diseases due to the immunosuppression associated to CSFV. In endemic areas, where infected animals do not show clinical signs indicative of CSFV infection, they can maintain low viral loads for long periods of time. This facilitates viral circulation in the field and increases the likelihood that new viral variants will be selected as a result of immune system pressure over a prolonged time [
19].
Porvac
® is a subunit vaccine whose active ingredient is the E2 protein fused to the pig CD154 antigen. The E2 gene of the “Margarita” strain was used for the construction of the HEK293 cell line expressing E2-CD154. For this reason the sequence of the vaccine E2 antigen is very close to that of the circulating viruses in the country, since the most accepted hypothesis about the origin of CSF zoonosis in Cuba, supported by the results of molecular epidemiology, is an escape of the “Margarita” strain used in the MLV challenge tests [
8].
The aim of this study was to test whether pigs immunized with Porvac® resisted challenge with three Cuban isolates, with low, or medium virulence, and which possibly have evolved to escape the immune response induced by the lapinized MLV.
Figure 6 shows the sequence alignment of the field isolates studied in this work and the “Margarita” strain. Only the E2 protein fragment included in the vaccine antigen E2-CD154, which does not include the transmembrane region, is shown.
The PR-11/10-3 isolate [
5,
14] has nine amino acid differences from the “Margarita” strain within this region. Three of these substitutions (G761R, L763S and I780V) are located within the B/C domain, the main target of neutralizing antibodies on the E2 protein [
10,
11]. The first of these sites responds to a selective pressure according to previous analysis performed with several Cuban CSF isolates. Interestingly, this change has been fixed in the population from 2001 to 2011, therefore, it must represent an important adaptive advantage for the virus [
5]. This isolate is characterized by presenting the insertion of a polyuridine track in the 3′URL of the virus, which has been associated with its low virulence [
14].
The Holguín_2009 isolate has three amino acid changes with respect to “Margarita” in the 66 amino acid region of the E2 that has been sequenced [
5]. The first two changes are identical to those of isolate PR-11/10-3 (G761R, L763S) and the third is the conservative V767A substitution. All these changes are located in the B/C domain.
The complete sequence of the E2 protein from isolate PR-2016 is known. This strain presents 7 amino acid changes in relation to the ancestral strain “Margarita”, and two of these substitutions (G761R and D884N) have been identified as selective pressure points according to the analysis performed by Coronado et al., [
15]. The first of these changes, within the B/C domain, also appears in the two other isolates. Finally, the conservative I780V substitution within the B/C domain is common with isolate PR-11/10-3.
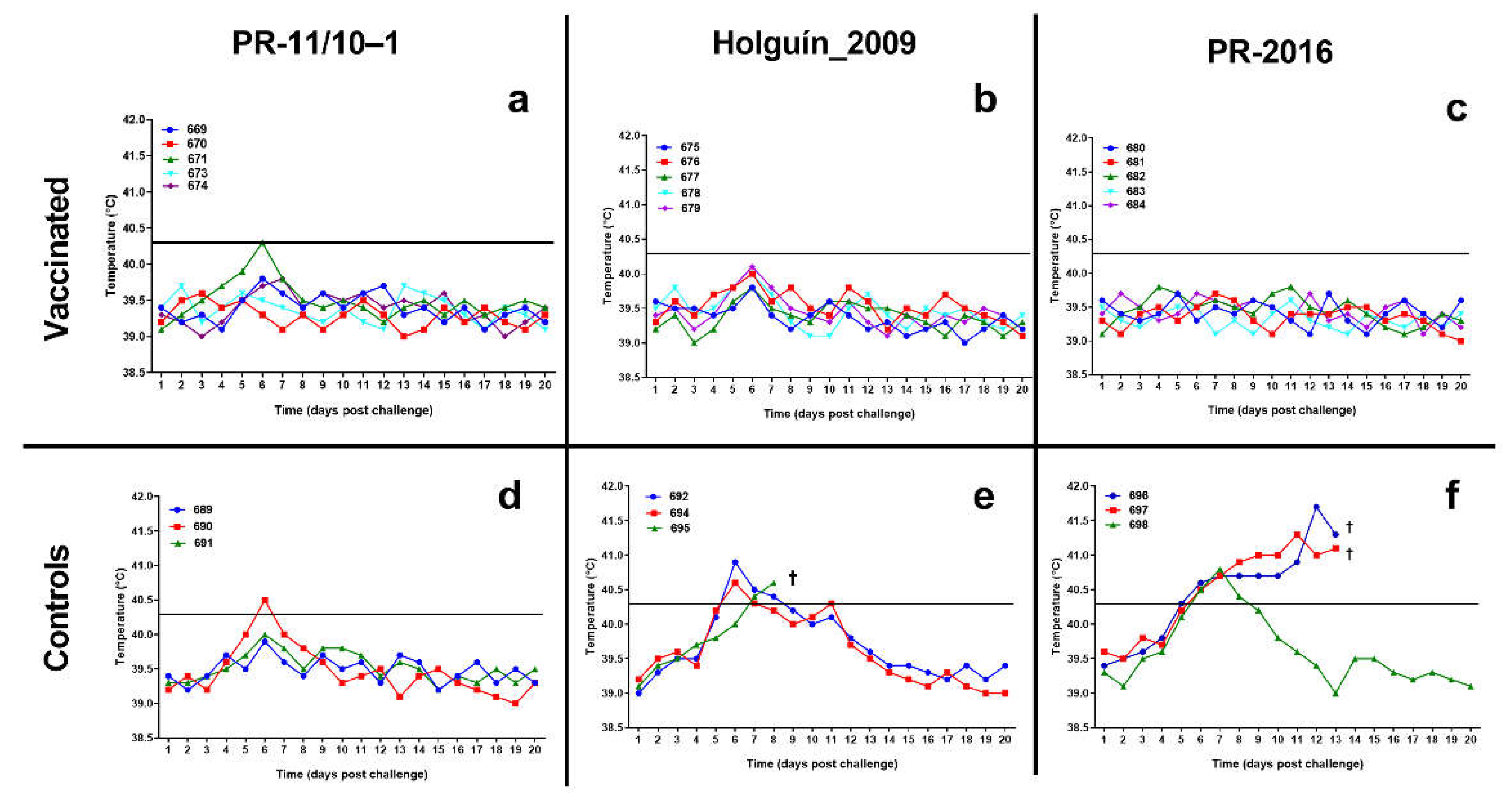
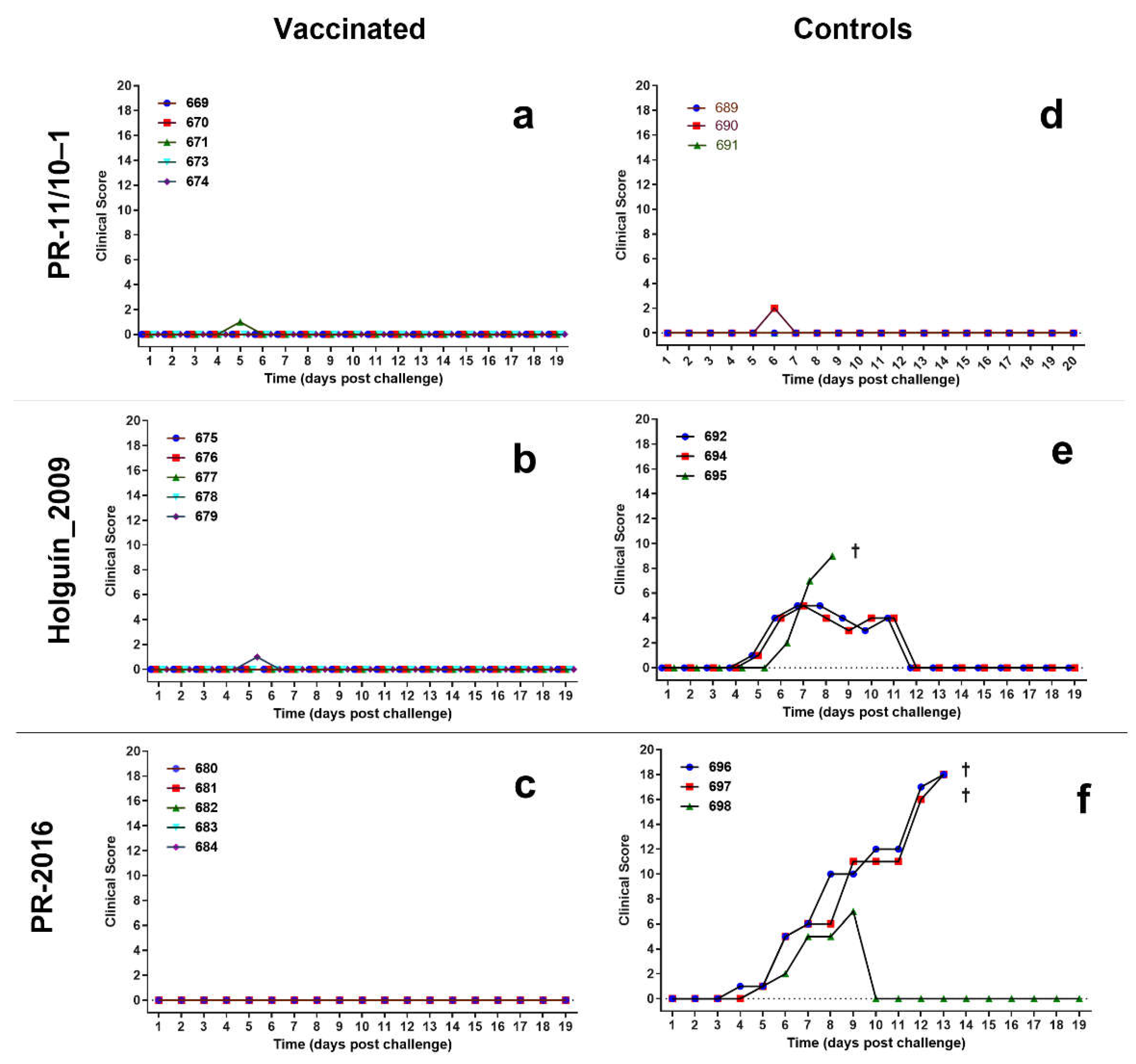
The clinical outcome after infection with these strains in unvaccinated control animals confirmed previous results by Coronado et al., [
15], who reported the low virulence of isolate PR-11/10-3, as well as the moderate virulence of isolate Holguin_2009.
In the present study, control pigs infected with PR-11/10-3 did not manifest clinical signs of CSF at any time, other than transient fever in one of the animals. However, the positive virus isolation from the lungs of the three pigs at the time of sacrifice evidenced that the infection was effective.
The virulence of the Holguín_2019 strain in this study can be described as moderate, since the three animals showed clinical signs of CSF, although two of them reverted spontaneously to remain asymptomatic for the rest of the study, while the third died at 9 dpc. From the two pigs that survived the challenge it was possible to isolate the virus.
The virulence of the PR-2016 isolate was unknown. The results obtained in this trial suggest that this isolate is of moderate to high virulence as all three controls manifested clinical signs of CSF and two of them died at 13 dpc.
In this experiment, a dose of 2 x 10
3 TCID
50 was used by the intranasal route in the challenge. This dose is lower than that recommended by WOAH for challenge testing of CSF vaccines. Higher doses of virus in the challenge could lead to a more acute clinical picture than that observed here, however, the intranasal infection of 5 days old pigs with 2.5 x 10
4 TCID
50 of the PR-11/10-3 did not produce clinical signs of CSF in a previous study [
20]. Although tonsils are considered the main center for viral replication, our previous experience with low virulence isolates suggest they are more readily isolated from the lungs; this observation was confirmed here for the pigs challenged with isolates PR-11/10-3 and Holguín_2016 where it was possible to isolate the virus mostly from the lungs and not from other target organs.
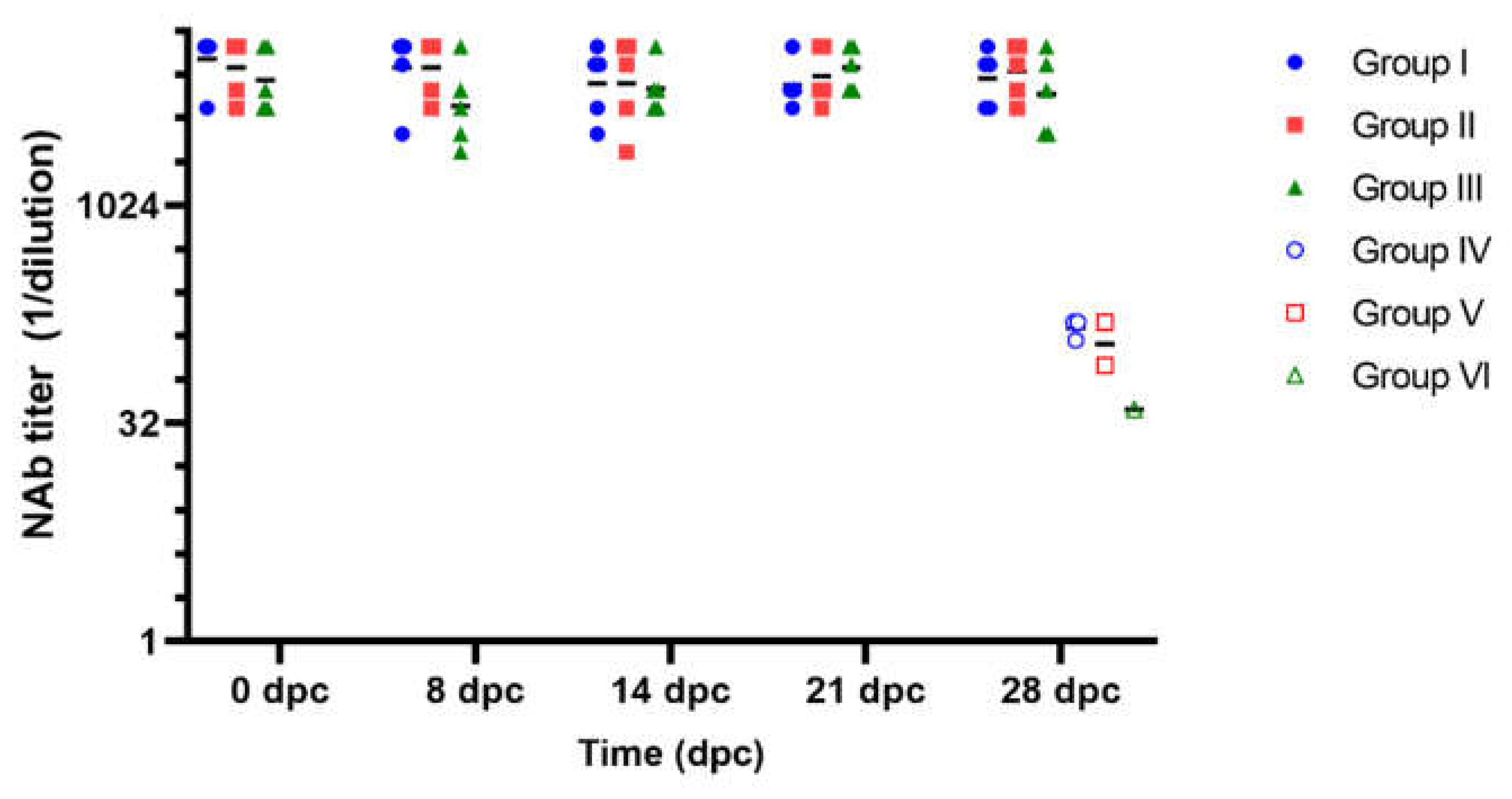
In addition to being positive for virus isolation, five of the six control animals tested were also positive for anti-Erns antibodies, an antigen not included in the vaccine. Only one of the controls challenged with isolate PR-11/10-3 was negative for anti-Erns antibodies. In three other animals it was not possible to run this test because they died early with clinical signs of CSF. These results suggest that the anti-Erns antibody test can be helpful to discriminate vaccinated from infected animals (DIVA).
In a previous study, only 3 out of 10 5-days old piglets infected with this same isolate PR-11/10-3 seroconverted to E
rns [
20]. Our experiment was conducted in older, nine weeks old pigs, and this difference in age could account for the divergent results found in the frequency of E
rns seroconversion against this isolate. Previous results for other groups had suggested that the virulence of the infecting viral strain has a marked influence on the frequency and timing of E
rns seroconversion [
21].
The use of E
rns ELISAs to discriminate vaccinated from infected pigs has been controversial. Early evaluations concluded that those ELISAs are less sensitive than conventional CSF E2 antibody ELISAs [
22,
23]. However, although the sensitivity of these ELISA is not optimal, they can still be useful when applied in a herd basis to a given number of animals [
23]. An indirect ELISA, with more than 95% sensitivity, together with a second confirmatory ELISA to rule out false positive sera against other pestiviruses was reported as a viable tool for DIVA [
24]. The sensitivity of anti-E
rns tests to discriminate in the field between infected and vaccinated animals will largely depend on the frequency of circulation in the region of these very low virulence isolates capable of generating a silent infection, both in terms of clinical signs of the disease and in the absence of seroconversion to the E
rns antigen.
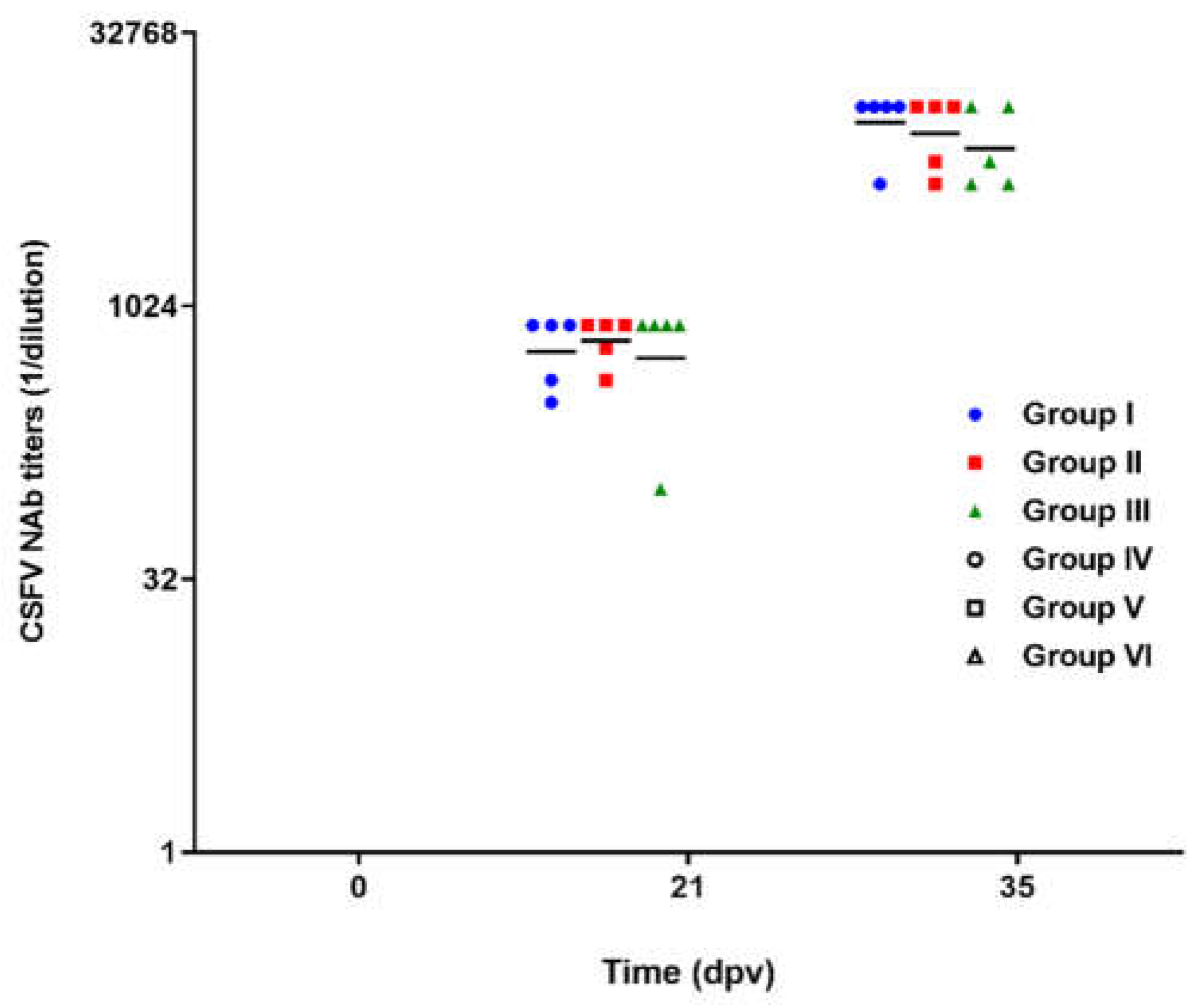
Animals vaccinated with Porvac® seroconverted from the first immunization and developed elevated NAb titers after the booster. None of these vaccinated animals showed clinical signs of CSF after challenge with the three Cuban isolates. Virus could not be isolated from blood or organ samples, nor did they develop anti-Erns antibodies.
These results strongly suggest that Porvac® is able to protect pigs from infection with these three field isolates, which have low, medium or medium-high virulence and some amino acid changes in the E2 protein relative to the vaccine strain. In this experiment, it was not possible to measure the viral load of pigs with the real-time RT-PCR assay, with greater sensitivity to detect virus remnants in tissues or blood. However, given its extreme sensitivity, RT-PCR can render positive results, with high CT values, in the absence of replicative virus; hence, virus isolation is the ultimate technique to demonstrate the existence of replicative CSFV in sera and organs. In the particular case of isolate PR-11/10-3, further studies with a larger number of animals and controls and longer follow-up time are required to have a more conclusive answer on the protection conferred by Porvac® against this very low virulence isolate. It would be also of interest to test if animals infected very early in life with this isolate could mount an immune response after Porvac® vaccination, after a chronic silent infection has been established.
The ability of Porvac® to protect against low, medium and high virulence autochthonous CFSV isolates supports its use in a vaccination campaign for the control and gradual elimination of CSF in Cuba.
Author Contributions
Conceptualization, YS-P, PR-M, CLP and MS-P; methodology, YS-P, DP-P, PN-V, MKM-O, TS-G, ES-R; formal analysis, YS-P, MP-R, CAD, CLP and MS-P; investigation, YS-P, DP-P, PN-V, TS-G, MK-M, ES-O, MV-H; resources, CLP; writing—original draft preparation YS-P, CAD; writing—review and editing, CAD, YS-P, DP-P, PR-M, TS-G, MKM-O, PN-V, ES-R, MV-H, CLP, MS-P; supervision, MS-P; project administration, MS-P.; funding acquisition, MS-P. All authors have read and agreed to the published version of the manuscript.